Abstract
Background: Acorus calamus (Sweet flag) is a known herbal drug commonly used in traditional medicine. Our aim was to perform seasonal and altitudinal phytochemical screening to assess the antioxidant activity of the essential oils in the rhizome and leaves of A. calamus from three different altitudes. Methods: Phytochemical screening was performed using GC/MS analysis and in vitro antioxidant assay was done by different methods. Results: The essential oils mainly contained α-asarone, β-asarone (35.3–90.6%), and Z-isoelemicin (1.7–7.3%) as the major constituents, besides linalool, Z-methyl isoeugenol, shyobunone, kessane, etc. All the oils exhibited vast molecular diversity in terms of quantitative ingredients. All essential oils were studied for their antioxidant activity by different methods, including their effect on the DPPH radical-scavenging activity, reducing power, and chelating properties of Fe2+. The oils isolated in all the different seasons exhibited antioxidant activity as a function of concentration, with IC50 values ranging from 475.48 ± 0.08 to 11.72 ± 0.03 compared to standards. Conclusion: From the results obtained it can be inferred that the herb may be a good source of bioactive compounds and can work as an antioxidant to prevent oxidative deterioration in food. The data provide a basis for its in-situ investigation for judicious exploitation.
Keywords: Acorus calamus, antioxidant activity, phenylpropanoids, asarone, essential oil
1. Introduction
Acorus is a genus of wetland monocot flowering plants distributed in North America and northern and eastern Asia, and naturalised in southern Asia and Europe from ancient cultivation [1,2,3,4,5,6]; they grow as herbs with perennial tuberous thick rhizomes in wetlands, particularly marshes [7]. The species are used in traditional medicine for the treatment of epilepsy, mental ailments, chronic diarrhoea, dysentery, bronchial catarrh, intermittent fevers, and glandular and abdominal tumours [8]. Plant diversity has considerable importance as a source of pharmaceutically active substances [9]. The natural antioxidants from plants can protect the human body from the attack of free radicals and retard the progress of many chronic diseases [10,11]. Natural antioxidants are generally classified as phenols, including flavonoids, phenolic acids and volatile compounds [7]. Acorus calamus is a traditional indigenous herb generally used in the treatment of cough, bronchitis, gout, tumours, haemorrhoids, skin diseases, numbness, and general debility [12,13]. It possesses a wide range of pharmacological activities, such as anti-diabetic [14], central nervous system depressant [15], anti-inflammatory [16], antioxidant [17], antispasmodic [18], antibacterial [19], antifungal [20], and cardiovascular [21] and insecticidal agent [22]. It has been reported by different workers that medicinal plants show a remarkable variation of active ingredients during different seasons; this is widely attributed to variations in environmental variables such as temperature and rainfall [23,24]. Besides its uses in traditional medicine, A. calamus has been used for digestive problems such as gas, bloating, colic, and poor digestive function; because of its rich ethnobotanical history, the herb is also used in the Ayurveda and Uniani systems of medicine. A number of bioactive constituents, viz., 2-allyl-5-ethoxy-4-methoxyphenol, 4-terpineol, lysidine, epieudesmin, spathulenol, furylethyl ketone, borneol, nonanoic acid, 2,2,5,5-tetramethyl-3-hexanol, galgravin, bornyl acetate, retusin, (9E,12E,15E)-9,12,15-octadecatrien-1-ol, geranylacetate, butyl butanoate, sakuranin, camphor, acetic acid, isoelemicin, acetaphenone, α-ursolic acid, dehydroabietic acid, methyl ether, isoeugenol, apigenin 4,7-dimethylether, linalool, dehydrodiisoeugenol, elemicin and linolenic acid, 1 beta,7 alpha(H)-cadinane-4 alpha,6 alpha,10 alpha-triol (1), 1 alpha,5 beta-guaiane-10 alpha-O-ethyl-4 beta,6 beta-diol (2), and 6 beta,7 beta(H)-cadinane-1 alpha,4 alpha,10 alpha-triol (3) have been reported in A. calamus [25,26,27]. The phenyl propanoids, sesquiterpenes, monoterpenes, xanthone glycosides, flavones, lignans, and steroids from Acorus calamus have been reported to possess various pharmacological activities such as insecticidal, larvicidal, antibacterial, mutagenic, cytotoxic, hepatoprotective, anticonvulsant, neuroleptic, smooth muscle relaxant, and smooth muscle stimulant activity [28]. The plant A. calamus has also been reported to be used in treating central nervous system abnormalities and normalizing the appetite, and various pharmacological activities like hepatoprotective, antidiabetic, antiproliferative, immunosuppressive, antidiarrhoeal, hypolipidemic, anti-spasmodic, and anti-proliferative have been reportedin extracts [29,30].
The essential oil composition of A. calamus rhizomes and concomitant antibacterial and antihelmintic activity has already been reported by our group [31,32]. However keeping in mind the above statements, the present study reports the results obtained on the seasonal and altitudinal diversity of essential oil components among three accessions of A. calamus from the Himalayan region of Uttarakhand in India. Publishing these data will help to generate a database of this plant and facilitate its more judicious and scientific exploitation in the future.
2. Material and Methods
2.1. Plant Material
Fresh samples of leaves and rhizomes of A. calamus were collected in different seasons (during 2014/2015) from three different altitudes of the Uttarakhand Himalayas in India. The plant was identified by Dr. D.S Rawat, a plant taxonomist at the Department of Biological Sciences, G.B., Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India. The GPS coordinates of the three locations were: INDIA, Uttarakhand, Pithoragarh district, Pithoragarh near Govt PG College, along stream, 29°34′59.17″ N, 80°11′49.46″ E, 1570 m a.s.l., July 2014, Archna Parki s.n., GBPUH specimen Acc. No. 910. Naini Tal district, Bhimtal, along stream, 29°20′58.61″ N, 79°33′14.53″ E, 1340 m a.s.l., July 2014, Archna Parki s.n., GBPUH Acc. No. 911 and Udham Singh Nagar district, near CBSH Pantnagar, in drainage channel, 29°01′27.2″ N, 79°29′27.2″ E, 236 m a.s.l., July 2014, Archana Parki s.n., GBPUH Acc. No. 912.
2.2. Extraction of Essential Oils
Fresh leaves/rhizomes of A. calamus were collected from their natural habitat and tested in the phytochemistry research lab. To extract essential oil, the plant material (500 g) was crushed and separately subjected to hydro-distillation in Clevenger apparatus for 3 h using the apparatus described in the European Pharmacopoeia [33].
2.3. Gas Chromatography and Gas Chromatography/Mass Spectrometry
GC and GC/MS analyses of all the oils were performed on a Thermo Fischer GC apparatus using an DB5-5MS fused-silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) equipped with a flame ionization (FID) detector. GC/MS analysis of the different essential oil samples were performed using a GC MS-QP 2010, in the following conditions. Column DB-5 (30 m × 0.25 mm i.d.; 0.25 μm film thickness; J&W Scientific, Agilent, Santa Clara, CA, USA); carrier gas: helium, with a flow rate of 1 mL/min; injection temperature: 250 °C; oven temperature programme: initial temperature 80 °C, isothermal for 2 min, RAMP 7 °C/min, final temperature 280 °C, and isothermal for 10 °C/min. Ionization mode: EI (70 eV), mass range: 40–6500 amu. The compounds were identified with the help of NIST-MS, FFNSC Wiley Library, and comparing the data with literature reports and GC retention indices [34].
2.4. Antioxidant Activity
To analyse the in vitro antioxidant property, the essential oils of A. calamus were subjected to the following methods.
2.4.1. DPPH Radical Scavenging Activity
This is a quick method to study the scavenging ability of the antioxidants [35]. Briefly, the tested samples (5–25 μL/mL) were added to 5 mL of a 0.004% methanol solution of DPPH. Finally, the absorbance was read against a blank at 517 nm after 30 min of incubation at room temperature. Ascorbic acid was used as the standard antioxidant. Inhibition of free radical by DPPH in percent (IC %) was calculated using the equation. IC% = (A0 − At)/A0 × 100, where A0 = the absorbance value of the control sample, At = the absorbance value of the test sample, and IC = inhibitory concentration. The radical scavenging activities of essential oils were discussed in terms of their IC50 values.
2.4.2. Reducing Power
The reducing power of essential oils was determined by the method developed earlier [36]. In brief, varying concentrations of tested samples (5–25 μL/mL) were mixed with 2.5 mL of phosphate buffer (200 mM, pH = 6.6) and 2.5 mL of 1% potassium ferricyanide, K3 [FeCN6]. The mixtures were incubated for 20 min at 50 °C. After incubation, 2.5 mL of trichloroacetic acid was added to the mixtures, followed by centrifugation at 650 rpm for 10 min. The upper layer (1 mL) was mixed with 5 mL distilled water and 1 mL of 0.1% ferric chloride and the absorbance of the resultant solution was measured at 700 nm. Reducing power % = (A0 − At)/A0 × 100, where A0 = the absorbance value of the control sample and At = the absorbance value of the test sample. The percent of chelating ability was plotted against concentrations and using a standard (gallic acid). The reducing potential of essential oils was discussed in terms of their RP50 values.
2.4.3. Metal Chelating Activity
The chelation of Fe2+ by essential oils was evaluated using the method developed earlier [37]. In brief, 0.1 mL of 2 mM FeCl2·4H2O, 0.2 mL of 5 mM ferrozine, and 4.7 mL of methanol were added to different concentrations of a test sample (5–25 μL/mL). The solutions were mixed and allowed to react for 10 min. The absorbance was determined at 562 nm; IC% = (A0 − At)/A0 × 100, where A0 = the absorbance value of the control sample and At = the absorbance value of the test sample. The percent of chelating ability was plotted against the concentration, and the standard curve was drawn using a standard antioxidant (EDTA). The metal chelating ability of essential oils was discussed in terms of their IC50 values.
3. Results and Discussion
A noticeable variation was observed in the percentage yield of the hydro distilled essential oils (EOs) samples, taken at four seasons (winter, spring, summer, and autumn), The essential oil was pale yellow with a characteristic odour, and produced an irritating sensation in the eyes. The yields of essential oils in different seasons were 0.02–1.3% for leaves and 1.2–4.8% w/v for rhizomes. However, in previous reports the yields of essential oils in A. gramineus and A. calamus from different regions have been reported to range from 1.0 to 3.5% [31,38,39]. In the present study, the yield showed the highest percentage (4.8%) during the summer in all accessions, whereas during autumn and winter the yields obtained were only up to 0.02%.
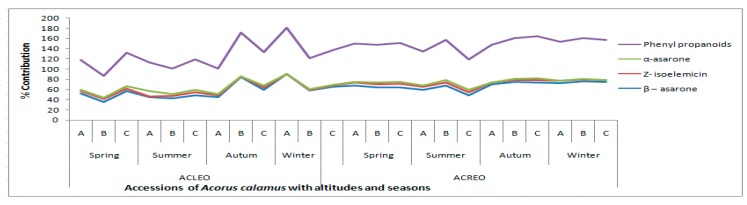
GC/MS showed marked variation in the major ingredients of oils prepared in spring, summer, autumn, and winter, respectively. The composition of the essential oils differed quantitatively and qualitatively according to the time of collection. A detailed comparative analysis of all the oils with different seasons has been recorded in Table 1, with phenyl propanoids in Table 2 and Figure 1, while the class composition is in Table 3 and Table 4.
Table 1.
Comparative chart on seasonally GC/MS analysis of Acorus calamus leaves and rhizome essential oils.
| S. N. | Compounds | RI | ACLEO | ACREO | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEASONS | ||||||||||||||||||||||||||
| SPRING | SUMMER | AUTUMN | WINTER | SPRING | SUMMER | AUTUMN | WINTER | |||||||||||||||||||
| Sites of Collection | Sites of Collection | |||||||||||||||||||||||||
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | |||
| 1 | bornyl acetate | 948 | - | 0.1 | - | - | 1.0 | - | - | - | - | - | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - | - |
| 2 | limonene | 1031 | 0.8 | - | - | - | 0.2 | 0.2 | 0.1 | t | 0.5 | 1.9 | 1.3 | 0.8 | - | - | - | - | - | - | - | - | - | - | ||
| 3 | trans-β ocimene | 1050 | - | t | - | - | 0.2 | 0.1 | t | - | 1.3 | 3.0 | 0.8 | 0.5 | 0.9 | - | 1.9 | 2.0 | 0.1 | 0.5 | - | 1.2 | 1.9 | 0.3 | ||
| 4 | linalool | 1096 | 9.2 | 5.8 | 0.9 | 0.5 | 7.2 | 6.4 | 3.2 | 0.7 | 7.3 | 1.9 | 5.6 | 2.6 | 0.6 | 0.8 | - | 1.8 | 1.5 | 6.4 | 0.3 | 0.5 | 0.2 | 0.8 | 0.4 | - |
| 5 | shyobunone | 1324 | 9.3 | 7.6 | 5.0 | 0.4 | 7.0 | 6.6 | 7.1 | 2.5 | 6.0 | - | 6.9 | 8.8 | 4.4 | 4.2 | 4.7 | 5.1 | 3.6 | 6.6 | 3.3 | 2.7 | 2.8 | 5.5 | 4.5 | 8.0 |
| 6 | α-copaene | 1376 | - | t | - | - | 0.2 | - | - | - | - | - | - | - | - | t | - | - | 0.1 | - | - | t | - | - | - | - |
| 7 | β elemene | 1390 | - | - | - | 0.2 | 0.1 | - | - | 0.2 | - | - | - | - | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.6 | - | - | - | - | - | |
| 8 | aristolene | 1416 | - | 2.0 | - | - | - | - | - | - | - | - | 1.1 | 0.4 | 1.0 | 1.6 | - | - | 2.0 | - | 0.2 | 1.0 | 0.6 | 0.7 | ||
| 9 | E caryophyllene | 1418 | 2.0 | 1.8 | 1.9 | 0.4 | 1.7 | - | 3.1 | 0.5 | 2.0 | - | 2.0 | 1.8 | - | - | - | - | - | 1.6 | - | - | - | - | - | - |
| 10 | calarene | 1432 | - | - | - | - | - | - | 8.2 | 2.3 | 0.7 | 1.9 | 2.9 | 0.5 | - | 4.2 | 0.6 | 0.4 | 2.2 | 0.9 | 1.9 | |||||
| 11 | α-humulene | 1440 | 0.7 | 1.1 | 0.5 | 0.2 | 0.8 | 0.5 | 1.4 | 0.2 | 0.3 | - | 0.9 | 0.6 | - | - | - | - | - | - | - | - | - | - | - | - |
| 12 | α-muurolene | 1478 | - | - | 0.2 | - | 0.1 | 0.1 | 0.3 | - | - | - | - | - | - | - | 0.1 | - | - | 0.1 | - | - | - | - | 1.0 | - |
| 13 | germacrene D | 1480 | 0.4 | 0.1 | 0.4 | - | 0.6 | 0.5 | 0.3 | - | 0.3 | - | 0.3 | 0.2 | - | 0.1 | - | - | - | 0.5 | - | - | - | - | - | 0.1 |
| 14 | Z-methyl isoeugenol | 1492 | 2.8 | 2.8 | 2.2 | 0.4 | 2.3 | 1.6 | 3.3 | 0.5 | 2.1 | - | 2.2 | 4.7 | 5.1 | 3.4 | 3.8 | 6.3 | 4.6 | 1.6 | 5.0 | 9.0 | 4.1 | 7.4 | 4.5 | 4.9 |
| 15 | viridiflorene | 1496 | - | 0.4 | 1.2 | 0.1 | 1.4 | 1.5 | 0.9 | - | - | - | - | - | - | - | - | - | - | 1.5 | - | - | - | - | - | - |
| 16 | dehydroxy-isocalamendiol | 1497 | 6.1 | - | - | - | - | - | - | - | - | - | - | 4.8 | - | - | - | - | - | - | - | - | 2.3 | - | - | 1.4 |
| 17 | δ-cadinene | 1524 | 0.3 | 1.1 | 1.1 | 0.3 | 1.3 | 0.3 | 0.8 | 0.3 | - | - | - | 0.4 | 0.4 | 0.3 | 0.6 | 0.3 | - | - | 0.3 | - | - | 0.4 | 0.2 | |
| 18 | kessane | 1528 | 1.0 | 3.2 | 0.1 | - | 2.1 | 0.1 | 2.0 | 0.3 | 0.3 | - | 1.9 | 0.5 | 0.7 | 0.5 | 0.3 | 1.5 | 0.4 | 0.1 | 0.2 | - | - | 0.5 | 0.5 | |
| 19 | α-cadinene | 1538 | - | 1.0 | - | - | - | 0.2 | - | - | - | - | - | - | - | - | - | - | 0.3 | - | - | - | - | - | - | |
| 20 | α-calacorene | 1545 | - | 0.2 | 0.2 | - | 0.2 | - | 0.2 | - | - | - | - | - | 0.6 | 0.2 | 0.4 | 0.3 | 0.1 | - | - | 0.5 | - | - | - | - |
| 21 | α-elemol | 1547 | - | - | - | - | 0.7 | - | 0.7 | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 22 | β-calacorene | 1548 | - | - | - | - | - | - | - | - | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 | 0. | - | - | - | - | - | - | - |
| 23 | elemicin | 1554 | - | - | - | 0.2 | - | - | - | 2.1 | - | - | - | - | 0.9 | 1.2 | - | 0.9 | 1.6 | 1.0 | - | 0.7 | - | - | 0.6 | - |
| 24 | spathulenol | 1575 | - | 0.4 | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||||||
| 25 | caryophyllene oxide | 1581 | - | - | 1.3 | 1.0 | 0.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| 26 | β-asarone | 1617 | 52.2 | 35.3 | 56.5 | 44.4 | 42.4 | 48.6 | 44.6 | 84.3 | 59.4 | 90.6 | 57.6 | 65.2 | 68.0 | 64.4 | 64.3 | 59.5 | 67.7 | 48.6 | 70.0 | 74.5 | 73.7 | 73.1 | 76.6 | 74.9 |
| 27 | asaronaldehyde | 1620 | - | 4.3 | 0.6 | 0.9 | 2.9 | 0.1 | 2.1 | - | - | - | - | - | 0.2 | 1.0 | 0.6 | - | - | 0.1 | - | - | - | - | - | |
| 28 | Z-isoelemicin | 1644 | 4.5 | 6.3 | 5.5 | 1.8 | 4.8 | 6.0 | 4.1 | 1.7 | 4.6 | - | 2.5 | 3.0 | 5.7 | 6.0 | 7.3 | 5.5 | 6.7 | 6.0 | 3.8 | 4.6 | 5.0 | 4.0 | 3.3 | 3.4 |
| 29 | α-asarone | 1676 | 2.0 | 1.8 | 4.3 | 10.5 | 3.7 | 5.2 | 2.2 | - | 3.1 | 0.2 | 0.7 | 0.2 | 1.3 | 3.4 | 3.9 | 2.7 | 4.4 | 5.2 | 0.3 | 1.5 | 3.7 | 0.1 | 0.7 | 0.8 |
| 30 | 2,4,6-trimethoxyacetophenone 3 methyl |
1701 | - | 0.4 | 0.6 | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 31 | aspidinol | 1833 | - | - | - | 0.4 | - | - | 1.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 32 | 2,4,5-trimethoxybenzoic acid | 1927 | - | 2.3 | 0.6 | - | - | - | 1.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 33 | phytol | 1941 | 2.3 | 2.8 | 1.1 | 0.6 | 0.8 | 0.4 | - | - | - | - | - | 1.2 | - | - | - | - | - | |||||||
| 34 | palmitic acid | 1984 | - | - | - | 13.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| total | 91.3 | 78 | 85.4 | 76.9 | 81.9 | 78.8 | 80.4 | 93.4 | 86.7 | 95.9 | 93.1 | 94.4 | 93.4 | 88.8 | 88.7 | 89.4 | 92.7 | 81.5 | 92.7 | 94.9 | 92.4 | 92.7 | 95.9 | 96.6 | ||
| SD (±) | 9.2 | 6.3 | 10.0 | 8.0 | 7.5 | 8.7 | 7.7 | 14.9 | 10.6 | 15.7 | 10.3 | 11.7 | 11.6 | 11.1 | 11.0 | 10.2 | 11.6 | 8.4 | 12.2 | 13.2 | 13.2 | 12.5 | 13.1 | 13.1 | ||
T = trace (0.05%), A = Pithoragarh location (hilly region), B = Bhimtal location (sub hilly region), C = Pantnagar location (tarai region), ACLEO = A. calamus leaves essential oil, ACREO = A. calamus rhizome essential oil ACREO.
Table 2.
Phenylpropanoids in ACLEO and ACREO in different seasons from different altitudes.
| SN | Phenyl Propanoids | RI | ACLEO | ACREO | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEASONS | ||||||||||||||||||||||||||
| SPRING | Summer | AUTUMN | WINTER | SPRING | SUMMER | AUTUMN | WINTER | |||||||||||||||||||
| Sites of Collection | Sites of Collection | |||||||||||||||||||||||||
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | |||
| 1 | β-asarone | 1617 | 52.2 | 35.3 | 56.5 | 44.4 | 42.4 | 48.6 | 44.6 | 84.3 | 59.4 | 90.6 | 57.6 | 65.2 | 68.0 | 64.4 | 64.3 | 59.5 | 67.7 | 48.6 | 70.0 | 74.5 | 73.7 | 73.1 | 76.6 | 74.9 |
| 2 | Z-isoelemicin | 1644 | 4.5 | 6.3 | 5.5 | 1.8 | 4.8 | 6.0 | 4.1 | 1.7 | 4.6 | - | 2.5 | 3.0 | 5.7 | 6.0 | 7.3 | 5.5 | 6.7 | 6.0 | 3.8 | 4.6 | 5.0 | 4.0 | 3.3 | 3.4 |
| 3 | α-asarone | 1676 | 2.0 | 1.8 | 4.3 | 10.5 | 3.7 | 5.2 | 2.2 | - | 3.1 | 0.2 | 0.7 | 0.2 | 1.3 | 3.4 | 3.9 | 2.7 | 4.4 | 5.2 | 0.3 | 1.5 | 3.7 | 0.1 | 0.7 | 0.8 |
| 4 | Phenyl propanoids | 58.7 | 43.4 | 66.3 | 56.7 | 50.9 | 59.8 | 50.9 | 86 | 67.1 | 90.8 | 60.8 | 68.4 | 75 | 73.8 | 75.5 | 67.7 | 78.8 | 59.8 | 74.1 | 80.6 | 82.4 | 77.2 | 80.6 | 79.1 | |
A = Pithoragarh location (hilly region), B = Bhimtal location (sub hilly region), C = Pantnagar location (tarai region), ACLEO = A. calamus leaves essential oil, ACREO = A. calamus rhizome essential oil ACREO.
Figure 1.
Phenylpropanoids in ACLEO and ACREO in different seasons from different altitudes.
Table 3.
Classes of compounds identified in ACLEO from four seasons.
| Class | SPRING | SUMMER | AUTUMN | WINTER | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | A | B | C | |
| Hydrocarbons | [4.2] | [7.7] | [6.8] | [1.2] | [7.8] | [3.1] | [7.9] | [4.2] | [3.1] | [3.2] | [15.7] | [4.2] |
| Monoterpenoids | 0.8 | - | - | - | 0.4 | 0.2 | 0.2 | - | 0.5 | 3.2 | 4.3 | 1.6 |
| Sesquiterpenoids | 3.4 | 7.7 | 6.8 | 1.2 | 7.4 | 2.9 | 7.7 | 4.2 | 2.6 | - | 11.4 | 2.6 |
| Oxygenated compounds | [28.4] | [26.9] | [12.3] | [18.8] | [23.2] | [15.9] | [21.6] | [8.1] | [16.5] | [1.9] | [16.6] | [21.8] |
| Monoterpenoids | 12 | 15.7 | 4.9 | 2.2 | 13.4 | 8.1 | 11.2 | 5.2 | 9.4 | 1.9 | 7.8 | 7.3 |
| Sesquiterpenoids | 16.4 | 11.2 | 7.4 | 16.6 | 9.8 | 7.8 | 10.4 | 2.9 | 7.1 | 8.8 | 14.5 | |
| Phenyl propanoids | 58.7 | 43.4 | 66.3 | 36.9 | 50.9 | 59.8 | 50.9 | 58.6 | 67.1 | 90.8 | 60.2 | 68.4 |
A = Pithoragarhlocation (hilly region), B = Bhimtal location (sub hilly region), C = Pantnagar location (tarai region).
Table 4.
Classes of compounds identified in ACREO from four seasons.
| Class | SPRING | SUMMER | AUTUMN | WINTER | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | A | B | C | |
| Hydrocarbons | [5.1] | [2.9] | [3.8] | [7.5] | [3.1] | [4.7] | [6.7] | [1.4] | [0.6] | [4.4] | [4.8] | [3.2] |
| Monoterpenoids | 0.5 | 0.9 | 1.9 | 2.0 | 0.1 | 0.5 | 1.2 | 1.9 | 0.3 | |||
| Sesquiterpenoids | 4.6 | 2.0 | 3.8 | 5.6 | 1.1 | 4.6 | 6.2 | 1.4 | 0.6 | 3.2 | 2.9 | 2.9 |
| Oxygenated compounds | [11.0] | [9.9] | [9.4] | [14.7] | [10.2] | [16.0] | [8.8] | [12.2] | [9.4] | [14.2] | [9.9] | [14.3] |
| Monoterpenoids | 5.9 | 5.2 | 4.4 | 8.1 | 6.2 | 8.1 | 5.3 | 9.5 | 4.3 | 8.2 | 4.9 | 4.9 |
| Sesquiterpenoids | 5.1 | 4.7 | 5.0 | 6.6 | 4.0 | 7.9 | 3.5 | 2.7 | 5.1 | 6.0 | 5.0 | 9.4 |
| Phenyl propanoids | 75.9 | 75 | 75.5 | 68.6 | 80.4 | 60.8 | 74.1 | 81.3 | 82.4 | 77.2 | 81.2 | 79.1 |
A = Pithoragarhlocation (hilly region), B = Bhimtal location (sub hilly region), C = Pantnagar location (tarai region).
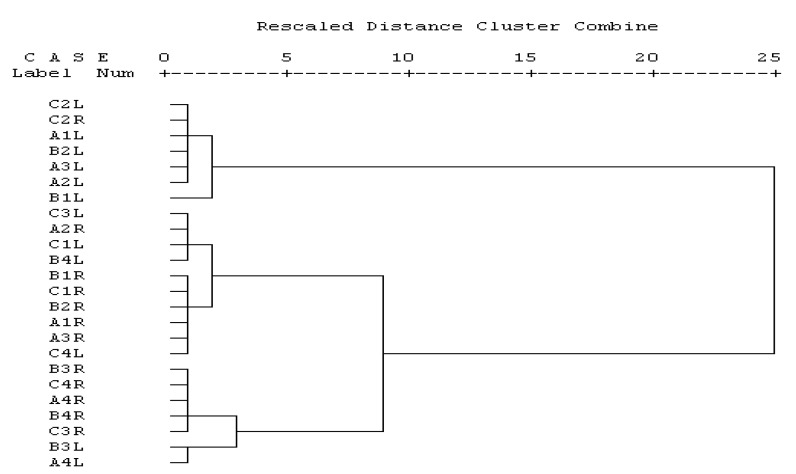
The oils were dominated by phenylpropanoids, and were detected in all the accessions in all seasons but in different quantities. Interestingly, the β-asarone content was highest in winter (57.0–90.6%) and lowest in summer, except Bhimtal, in which spring has the lowest content; whereas the α-asarone content was highest in summer and lowest in winter in all the accessions. On the basis of major components (phenyl propanoids) analysis by cluster analysis, as in Figure 2, it was observed that there were little difference in principal components like α-asarone, Z-isoelemicin, β-asarone, etc., both altitude-wise and season-wise.
Figure 2.
Dandrogram of major compounds (phenyl propanoids) in ACLEO and ACREO prepared Software SPSS, ward method with square Euclidean distance measure. Where 1 = spring, 2 = summer, 3 = autumn, 4 = winter seasons, L = A. calamus leaves essential oils (ALEO), R = A. calamus rhizomes essential oils (RLEO).
Over 78–96% of constituents were identified in both ACLEO and ACREO. The major constituents identified in both the oils in all four seasons were trans-methyl isoeugenol, Z-isoelimicin, α-asarone, β-asarone, etc., but with different respective yield. Both ACLEO and ACREO exhibited similar qualitative diversity in terms of terpenoid composition, but their quantity varied in different seasons. However, the constituents, viz., limonene, α-humulene, 2,4,5-trimethoxy benzoic acid, and heptadecanol, could be detected only in ACLEO, whereas elimicin and β-calacorene were detected only in ACREO.
The seasonal variation in the chemical composition of essential oils might be due to different altitudes, environmental conditions, and the developmental stage/season of the plant materials, which is in agreement with results reported earlier [40,41,42]. In terms of class composition, the monoterpenoids in ACLEO from all three accessions in four different seasons ranged from 0.2 to 15.7%, mainly represented by limonene, β oscimene, linalool, bornyl acetate, etc. The sesquiterpenoids ranged from 2.6 to 16.6% with E caryophyllene, β elemene, α copaene, calarene, Z methyl isoeugenol, α humulene, spathulenol, germacrene D, α cadenene, α calacorene, etc. The marker class of, phenyl propanoids, ranged from 36.9 to 90.8%, mainly represented by α asarone, β asarone, Z isoelimicin, elimicin, etc. (Table 2 and Table 3). Similarly, in ACREO the monoterpenoids ranged from 0.1 to 9.5%, and sesquiterpenoids from 0.6 to 9.4%, while phenyl propanoids ranged from 60.8 to 82.4% in three accessions for the four seasons. The essential oil composition of rhizomes from our laboratory has already been reported. The essential oil was found to possess significant antibacterial and antihelmintic activity [31,32]. It has also been reported that asarone and sesquiterpenoids were the major constituents in two phylogenetically different accessions [38]. Patra and Mitra [43] and Tamas et al. [44] have also reported acoramone and phenylpropane derivatives like α-asarone, β-asarone, γ-asarone, isoeugenol, and methyl ether in essential oils. The components reported earlier were also identified, besides some other constituents, but in different quantities. Seasonal along with altitudinal variation in both rhizomes (ACREO) and the aerial part (ACLEO) are reported here for the first time, demonstrating the qualitative and quantitative diversity of constituents in the essential oils of A. calamus.
All the essential oils isolated in different seasons exhibited DPPH radical scavenging activity in a dose-dependent manner (5 µL/mL–25 µL/mL) (Table 5). The radical scavenging potential of ACREO and ACLEO from three altitudes in the form of their IC50 values in four different seasons was observed as Spring: ACBTREO > ACPGLEO > ACBTLEO > ACPNLEO > ACPNREO > ACPGREO; Summer: ACPNREO > ACPGREO > ACBTREO > ACPGLEO > ACPNLEO > ACBTLEO; Autumn: ACPNREO > ACPNLEO > ACBTREO > ACPGLEO > ACBTLEO > ACPGREO; Winter: ACPNREO > ACBTREO > ACPNLEO > ACPGLEO > ACPGREO > ACBTLEO, respectively, compared to the standards, BHT (IC50 = 28.37 µg/mL) > catechin (IC50 = 28.48 µg/mL). The antioxidant power of EOs might be attributed to their hydrogen donating ability to DPPH free radicals.
Table 5.
Antioxidant activity in terms of IC50 values for leaves and rhizome essential oils.
| S.N. | Sample Name | DPPH IC50 Value (µg/mL) ± SD | Reducing RP50 Value (µg/mL) ± SD | Chelating IC50 Value (µg/mL) ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seasons | |||||||||||||
| SPRING | SUMMER | AUTUMN | WINTER | SPRING | SUMMER | AUTUMN | WINTER | SPRING | SUMMER | AUTUMN | WINTER | ||
| 1 | ACPGREO | 198.06 h ±0.07 |
41.46 c ±0.66 |
107.52 g ±3.96 |
78.03 f ±3.30 |
137.14 b ±1.39 |
72.89 b ±0.01 |
52.30 a ±3.99 |
65.32 a,b ±0.15 |
34.82 c ±0.27 |
68.53 a ±0.99 |
206.75 e ±4.30 |
24.00 c ±0.12 |
| 2 | ACPGLEO | 59.20 d ±0.36 |
59.36 f ±0.81 |
83.07 e ±2.73 |
70.36 e ±2.80 |
168.97 b ±0.89 |
320.11 e ±2.47 |
227.63 d ±4.36 |
350.98 f ±3.88 |
15.24 a ±0.06 |
13.08 a ±0.13 |
158.10 b ±11.56 |
25.78 d ±0.12 |
| 3 | ACPNREO | 188.36 g ±3.59 |
41.35 c ±0.10 |
59.02 c ±1.26 |
37.31 c ±0.19 |
475.48 e ±28.19 |
77.72 b ±0.19 |
71.54 b ±0.12 |
325.83 e ±0.04 |
47.25 f ±0.32 |
41.29 b ±0.37 |
187.41 d,e ±10.59 |
30.04 f ±0.25 |
| 4 | ACPNLEO | 116.22 f ±3.14 |
78.71 g ±2.83 |
75.53 d ±2.11 |
58.71 d ±0.71 |
414.19 d ±17.8 |
245.77 d ±7.68 |
131.70 c ±1.25 |
201.25 d ±4.74 |
29.78 b ±0.22 |
11.72 c ±0.03 |
153.13 b ±13.55 |
26.94 e ±0.20 |
| 5 | ACBTREO | 49.67 c ±1.53 |
51.42 e ±0.65 |
82.52 e ±1.88 |
38.52 c ±0.62 |
326.87 c ±11.83 |
72.24 b ±0.18 |
71.08 b ±0.10 |
84.97 b ±0.22 |
44.98 e ±1.34 |
84.64 c ±2.25 |
193.54 e ±9.28 |
18.21 a ±0.03 |
| 6 | ACBTLEO | 83.94 e ±1.97 |
46.36 d ±0.94 |
96.17 f ±2.85 |
98.27 g ±4.44 |
382.27 d ±2.92 |
161.65 c ±0.82 |
73.98 b ±0.19 |
133.34 c ±18.88 |
30.88 b ±0.14 |
16.84 d ±0.59 |
164.46 b,c ±9.19 |
19.82 b ±0.07 |
| 7 | BHT * | 27.74 b ±0.05 |
27.74 b ±0.04 |
27.74 b ±0.04 |
28.37 a ±0.20 |
- | - | -- | - | - | - | - | - |
| 8 | Catechin * | 18.53 a ±0.15 |
18.53 a ±0.15 |
18.53 a ±0.15 |
28.48 b ±0.17 |
71.74 b ±1.39 |
71.74 b ±0.49 |
71.74 b ±0.49 |
71.74 a,b ±0.49 |
- | - | - | - |
| 9 | Gallic acid | - | - | - | - | 56.14 b ±1.39 |
56.07 a ±0.91 |
56.07 a ±0.91 |
56.07 a ±0.91 |
- | - | - | - |
| 10 | EDTA | - | - | - | - | - | - | - | - | 38.74 d ±0.33 |
38.74 e ±0.33 |
38.74 a ±0.33 |
38.74 g ±0.33 |
| 11 | Citric acid | - | - | - | - | - | - | - | - | 45.57 e ±0.33 |
45.57 f ±0.33 |
45.57 a ±0.33 |
45.57 h ±0.33 |
ACPGREO = Acorus calamus Pithoragarh rhizome essential oil, ACPGLEO = Acorus calamus Pithoragarh leaves essential oil, ACPNREO = Acorus calamus Pantnagar rhizome essential oil, ACPNLEO = Acorus calamus Pantnagar leaves essential oil, ACBTREO = Acorus calamus Bhimtal rhizome essential oil, ACBTLEO = Acorus calamus Bhimtal leaves essential oil. - = Not applicable, Values are means of three replicates ± SD, Within a column, mean values followed by the same letter are not significantly different according to Tukey’s test (p < 0.05).
In terms of Fe3+ reducing activity at selected dose levels of 5–25 µg/mL, the EOs exhibited dose dependent reducing power activity. The RP50 values in different seasons for all the EOs were observed in the order of Spring: ACPGREO > ACPGLEO > ACBTREO > ACBTLEO > ACPNLEO > ACPNREO; Summer ACBTREO > ACPGREO > ACPNREO > ACBTLEO > ACPNLEO > ACPGLEO; Autumn ACPGREO > ACBTREO > ACPNREO > ACBTLEO > ACPNLEO > ACPGLEO; Winter ACPGREO > ACBTREO > ACBTLEO > ACPNLEO > ACPNREO > ACPGLEO. However, the RP50 of standards were observed in the order gallic acid (RP50 = 56.07 µg/mL) > catechin (RP50 = 71.74 µg/mL) (Table 5).
Similarly, the dose-dependent response for chelating activity for all the Eos (Table 5) exhibited the following order of IC50 values in different seasons: Spring ACPGLEO > ACPNLEO > ACBTLEO > ACPGREO > ACBTREO > ACPNREO; Summer ACPNLEO > ACPGLEO > ACBTLEO > ACPNREO > ACPGREO > ACBTREO; Autumn ACPNLEO > ACPGLEO > ACBTLEO > ACPNREO > ACBTREO > ACPGREO; and Winter ACBTREO > ACBTLEO > ACPGREO > ACPGLEO > ACPNREO. The IC50 for standard EDTA and citric acid were IC50 = 38.74 µg/mL and 45.57 µg/mL, respectively, under the same experimental conditions (Table 5).
The EOs from A. calamus exhibited good in vitro antioxidant activity, which might be because of the mixture of essential oils containing mono and sesquiterpenoids and the synergetic effects of the constituents. This is proven by a report that says that antioxidant capacity is affected by other bioactive compounds and could involve synergistic effects [45]. Several reports have shown in vitro antioxidant properties of many natural products, including essential oils. Antioxidants are believed to be directly anti mutagenic. In vitro physicochemical assays characterize most of them as antioxidants. However, the published report shows that in eukaryotic cells, essential oils can act as pro-oxidants, affecting inner cell membranes and organelles like mitochondria, and are usually non-genotoxic, and hence the beneficial effects of essential oils are due to pro-oxidants’ effects on the cellular level [46]. The anticancer and antioxidant properties of certain medicinal herbs are used to treat trauma over a longer period of time, which is promising. Acorus calamus extracts and essential oils have been reported to possess anticancer and anti-angiogenic effects on cancer cells [47,48,49], which might be due to its antioxidant activity. In folk medicine the herb A. calamus has been used as a wound healing agent for many years, which has been proven scientifically by reporting the significant wound-healing activity of aqueous extracts in the animal model of excise wound healing, and anti-inflammatory activity in vitro [50].
It has been reported that essential oils containing linalool and the corresponding acetate play a major role in terms of anti-inflammatory activity [51]. The compounds shyobunone and isoshyobunone, isolated from essential oils, have been reported to possess insecticidal and repellant activity against Lasioderma serricorne (LS) and Tribolium castaneum (TC) [52]. Z methyl isoeugenol is used in perfumes as a flavouring agent [53]. Elimicin and caryophyllene have been reported to possess anti-inflammatory, antimicrobial, and analgesic activity [54,55,56]. Palmitic acid, regardless of obesity, impairs leptin and insulin’s ability to regulate food intake and body weight. In addition, it has been reported that fatty acids containing palmitic acid and its ester possess significant antifungal and antibacterial activity [57,58]. Various essential oil components present in essential oils like linalool, 1,8-cineol, caryophyllene, α humulene, and asarone have been reported to possess antioxidant activity [59,60,61]. Components like linalool, asarone, α humulene, and caryophyllene oxide are also present in essential oils. Based on the reported data, it can be inferred that the antioxidant activity of A. calamus essential oil is because of these compounds, besides the synergetic effects of other constituents in the oil.
4. Conclusions
On the basis of our results, we can conclude that seasonal fluctuations periodically impact on the production of the constituents in medicinal plants, and also likely influence their therapeutic efficiency. Our study reveals that A. calamus plants show a rhythmic increase in oil production throughout the growing season and decline towards the winter. Hence, late summer can be the best time for collecting A. calamus plants. The present study reveals the presence of bioactive compounds, the antioxidant activity, and the free radical scavenging activity of A. calamus. Thus, this study supports the use of A. calamus against various ailments. More bioactive compounds present in EOs of A. calamus may warrant further characterisation.
Acknowledgments
The authors are thankful to the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), New Delhi, Govt. of India for financial assistance. Thanks are due to D. S. Rawat, plant taxonomist, Department of Biological Science, GBPUAT Pantnagar for identifying the plant, while the Advanced Instrumentation Research Facility, Jawaharlal Nehru University, New Delhi is thankfully acknowledged for the gas chromatography/mass spectrometry analysis.
Author Contributions
Archana Parki and Pinky Chaubey are pursuing their PhD degrees under the supervision of Om Prakash. Anil K. Pant and Ravendra Kumar helped in studying the antioxidant activity.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Govaerts R. World Checklist of Selected Plant Families Database in Access: 1-216203. The Board of Trustees of the Royal Botanic Gardens; Kew, UK: 2003. [Google Scholar]
- 2.Govaerts R., Frodin D.G. World Checklist and Bibliography of Araceae (and Acoraceae) Royal Botanic Gardens; Kew, UK: 2002. 560p. [Google Scholar]
- 3.Acuna U.M., Atha D.E., Ma J., Nee M.H., Kennelly E.J. Antioxidant capacities of ten edible North American plants. Phytother. Res. 2002;16:63–65. doi: 10.1002/ptr.1031. [DOI] [PubMed] [Google Scholar]
- 4.Pushu C. Acorus Linnaeus, Sp. Pl. 1: 324. 1753. Volume 23. Fl. China; Beijing, China: 2010. pp. 1–2. [Google Scholar]
- 5.Boyce P.C., Sookchaloem D., Hetterscheid W.L.A., Gusman G., Jacobsen N., Idei T., Nguyen V.D. Flora of Thailand. Volume 11. The Forest Herbarium, National Park, Wildlife and Plant Conservation Department; Bangkok, Thailand: 2012. pp. 101–325. [Google Scholar]
- 6.Nooteboom H.P., editor. Flora Malesiana. Volume 20. Noordhoff-Kolff N.V.; Djakarta, Indonesia: 2011. pp. 1–61. [Google Scholar]
- 7.Keddy P.A. Wetland Ecology: Principles and Conservation. 2nd ed. Cambridge University Press; Cambridge, UK: 2010. Chapter 1. [Google Scholar]
- 8.Paithankar V.V., Belsare S.L., Charde R.M., Vyas J.V. Acorus calamus: An overview. Int. J. Biomed. Sci. 2011;2:518–529. doi: 10.7439/ijbr.v2i10.174. [DOI] [Google Scholar]
- 9.Thomas E., Vandebroek I., Goetghebeur P., Sanca S., Arrázola S., Van Damme P. The relationship between plant use and plant diversity in the Bolivian Andes, with special reference to medicinal plant use. Hum. Ecol. 2008;36:861–879. doi: 10.1007/s10745-008-9208-z. [DOI] [Google Scholar]
- 10.Katalinic V., Mozina S.S., Generalic I., Skroza D., Ljubenkov I., Klancnik A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitisvinifera L. Varieties. Int. J. Food Prop. 2013;16:45–60. doi: 10.1080/10942912.2010.526274. [DOI] [Google Scholar]
- 11.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 12.Parab R.S., Mengi S.A. Hypolipidemic activity of Acorus calamus L. in rats. Fitoterapia. 2002;73:451–455. doi: 10.1016/S0367-326X(02)00174-0. [DOI] [PubMed] [Google Scholar]
- 13.Shukla R., Kumar A., Prasad C.S., Srivastava B., Dubey N.K. Efficacy of Acorus calamus L. leaves and rhizome on mortality and reproduction of Callosobruchu schinensis L. (Coleoptera: Bruchidae) Appl. Entomol. Zool. 2009;44:241–247. doi: 10.1303/aez.2009.241. [DOI] [Google Scholar]
- 14.Prisilla D.H., Balamurugan R., Shah H.R. Antidiabetic activity of methanol extract of Acoruscalamus in STZ induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012;2:S941–S946. doi: 10.1016/S2221-1691(12)60341-4. [DOI] [Google Scholar]
- 15.VengadeshPrabu K., George T., VinothKumar R., Nancy J., Kalaivani M., Vijayapandi P. Neuromodulatory effect of Acrous calamus leaves extract on dopaminergic system in mice. Int. J. Pharm. Res. 2009;1:1255–1259. [Google Scholar]
- 16.Kim H., Han T.H., Lee S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 2009;122:149–156. doi: 10.1016/j.jep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Subathraa K., Poonguzhali T.V. In vitro studies on antioxidant and free radical scavenging activities of aqueous extract of Acorus calamus L. Int. J. Curr. Sci. 2012;1:69–73. [Google Scholar]
- 18.Gilani A.U., Shah A.J., Ahmad M., Shaheen F. Antispasmodic effect of Acorus calamus L. is mediated through calcium channel blockade. Phytother. Res. 2006;20:1080–1084. doi: 10.1002/ptr.2000. [DOI] [PubMed] [Google Scholar]
- 19.Manikandan S., Devi R.S., Srikumar R., Ayyappan R., Thangaraj R., Jegadeesh R., Hariprasath L. In-vitro antifungal activity of aqueous and ethanolic extracts of Acorus calamus L. Int. J. PharmTech Res. 2010;2:57–59. [Google Scholar]
- 20.Begum J., Yusuf M., Chowdhury J.U., Khan S., Anwar M.N. Antifungal activity of forty higher plants against phytopathogenic fungi. Bang. J. Microbol. 2007;24:76–78. doi: 10.3329/bjm.v24i1.1245. [DOI] [Google Scholar]
- 21.Shah A.J., Gilani A.H. Aqueous-methanolic extract of sweet flag (Acorus calamus) possesses cardiac depressant and endothelial-derived hyperpolarizing factor-mediated coronary vasodilator effects. J. Nat. Med. 2012;66:119–126. doi: 10.1007/s11418-011-0561-7. [DOI] [PubMed] [Google Scholar]
- 22.Nalamwar V.P., Khadabadi S.S., Aswar P.B., Kosalge S.B., Rajurkar R.M. In Vitro licicidal activity of different extracts of Acorus calamus Linn. (Araceae) rhizome. Int. J. Pharm. Res. 2009;1:96–100. [Google Scholar]
- 23.Ahmad I., Ahmad M.S.A., Ashraf M., Hussain M., Ashraf M.Y. Seasonal variation in some medicinal and biochemical ingredients in Mentha longifolia (L.) Huds. Pak. J. Bot. 2011;43:69–77. [Google Scholar]
- 24.Szakiel A., Pączkowski C., Henry M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011;10:471–491. doi: 10.1007/s11101-010-9177-x. [DOI] [Google Scholar]
- 25.Balakumbahan R., Rajamani K., Kumanan K. Acorus calamus: An overview. J. Med. Plant Res. 2008;4:2740–2745. [Google Scholar]
- 26.Dong W., Yang D., Lu R. Chemical Constituents from the Rhizome of Acorus calamus L. Planta Med. 2010;76:454–457. doi: 10.1055/s-0029-1186217. [DOI] [PubMed] [Google Scholar]
- 27.Imam H., Riaz Z., Azhar M., Sofi G., Hussain A. Sweet flag (Acorus calamus Linn.): An incredible medicinal herb. Int. J. Green Pharm. 2013;7:288–296. doi: 10.4103/0973-8258.122053. [DOI] [Google Scholar]
- 28.Divya G., Gajalakshmi S., Mythili S., Sathiavelu A. Pharmacological activities of A. calamus: A Review. Asian J. Bichem. Pharm. Res. 2011;1:57–64. [Google Scholar]
- 29.Palani S., Raja S., Kumar R.P., Venkadesan D., Devi D., Sivaraj A., Kumar B.S. Therapeutic efficacy of antihepatotoxic and antioxidant activities of Acorus calamus on acetaminophen-induced toxicity in rat. Int. J. Integr. Biol. 2009;7:39–44. [Google Scholar]
- 30.Singh R., Sharma P.K., Malviya R. Pharmacological Properties and Ayurvedic value of Indian Buch Plant (Acorus calamus): A Short Review. Adv. Biol. Res. 2011;5:145–154. [Google Scholar]
- 31.Joshi N., Prakash O., Pant A.K. Essential oil composition and in vitro antibacterial activity of rhizome essential oil and beta-asarone from A. calamus L. collected from lower Himalayan region of Uttarakhand. J. Essnt. Oil Bear. 2012;15:32–37. doi: 10.1080/0972060X.2012.10644016. [DOI] [Google Scholar]
- 32.Kumar R., Prakash O., Pant A.K., Hore S.K., Chanotiya C.S., Mathela C.S. Compositional variations and anthelmintic activity of essential oils from rhizomes of different wild populations of A. calamus L. and its major component, beta-asarone. Nat. Prod. Commun. 2009;4:275–278. [PubMed] [Google Scholar]
- 33.Maisonneuve S.A., Ruffine S. European directorate for the quality of medicines. Eur. Pharm. 1975;3:68–71. [Google Scholar]
- 34.Adams R.P. Identification of Essential Oils by Gas Chromatography Quadrupole Mass Spectroscopy. Allured; Carol Stream, IL, USA: 2001. [Google Scholar]
- 35.Sethi S., Prakash O., Pant A.K. Essential oil composition, antioxidant assay and antifungal activity of essential oil and various extracts of Alpinia allughas (Retz.) Roscoe leaves. Cogent Chem. 2015;1:1079349. doi: 10.1080/23312009.2015.1079349. [DOI] [Google Scholar]
- 36.Jeena G.S., Punatha H., Prakash O., Chandra M., Kushwaha K.P.S. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom) Ind. J. Nat. Prod. Res. 2016;5:56–61. [Google Scholar]
- 37.Kunwar G., Prakash O., Chandra M., Pant A.K. Chemical composition, antifungal and antioxidant activities of Perilla frutescens (L.) syn. P. ocimoides L. collected from different regions of Indian Himalaya. Asian J. Tradit. Med. 2013;8:88–98. [Google Scholar]
- 38.Sugimoto N., Kiuchi F., Mikage M., Mori M., Mizukami H., Tsuda Y. DNA profiling of Acorus calamus chemotypes differing in essential oil composition. Biol. Pharm. Bull. 1999;22:481–485. doi: 10.1248/bpb.22.481. [DOI] [PubMed] [Google Scholar]
- 39.Gretsusnikova T., Koel M., Orav A. Comparison of the Essential Oil Composition of Acorus Calamus Obtained by Supercritical Carbon Dioxide Extraction and Hydrodistillation Methods. International Symposium on Supercritical Fluids; Arcachon, France: 2009. p. 301. [Google Scholar]
- 40.Lohani H., Andola H.C., Chauhan N., Bhandari U. Variations of Essential oil composition of Acorus calamus: From Uttarakhand Himalaya. J. Pharm. Res. 2012;5:1246–1247. [Google Scholar]
- 41.Kulevanova S., Ristić M., Stafilov T., Dorevski K., Ristov T. Essential oil analysis of some taxa of genera Thymus L.-environment influences. Bull. Chem. Technol. Maced. 1996;15:33–38. [Google Scholar]
- 42.Ebrahimi S.N., Hadian J., Mirjalili M.H., Sonboli A., Yousefzadi M. Essential oil composition and antibacterial activity of Thymus caramanicus at different phenological stages. Food Chem. 2008;110:927–931. doi: 10.1016/j.foodchem.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 43.Patra A., Mitra A.K. Constituents of Acoruscalamus: Structure of acoramone. Carbon-13 NMR spectra of cis- and trans-asarone. J. Nat. Prod. 1981;44:668–669. doi: 10.1021/np50018a007. [DOI] [Google Scholar]
- 44.Tamas M., Oprean R., Roman L. Identification and quantitative determination of beta-asarone in essential oil and extracts of Acorus calamus L. Farm (Bucharest) 1996;44:13–21. [Google Scholar]
- 45.Sanchez M.C., Larrauri J.A., Saura C.F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999;32:407–412. doi: 10.1016/S0963-9969(99)00097-6. [DOI] [Google Scholar]
- 46.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 47.Antony M., Gayathri R., Vishnu P.V. Apoptotic Activity of Acorus calamus on oral cancer cell Lines. Int. J. Pharm. Sci. Rev. Res. 2017;44:30–32. [Google Scholar]
- 48.Rajkumar V., Guha G., Kumar A.R., Mathew L. Evaluation of cytotoxic Potential of Acorus calamus Rhizome. Ethnobot. Leafl. 2009;13:832–839. [Google Scholar]
- 49.Haghighi S.R., Asadi M.H., Akrami H., Baghizadeh A. Anti-carcinogenic and anti-angiogenic properties of the extracts of Acorus calamus on gastric cancer cells. Avicenna J. Phytomed. 2017;7:145–156. [PMC free article] [PubMed] [Google Scholar]
- 50.Shi G.B., Wang B., Wu Q., Wang T.C., Wang C.L., Sun X.H., Zong W.T., Zong W.T., Yan M., Zhao Q.C., et al. Evaluation of the wound-healing activity and anti-inflammatory activity of aqueous extracts from Acorus calamus L. Pak. J. Pharm. Sci. 2014;27:91–95. [PubMed] [Google Scholar]
- 51.Peana A.T., D’Aquila P.S., Panin F., Serra G., Pippia P., Moretti M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721–726. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 52.Chen H.P., Yang K., Zheng L.S., You C.X., Cai Q., Wang C.F. Repellant andinsecticidal activities of shyobunone and isoshyobunone derived from the essential oil of Acorus calamus rhizomes. Pharmacogn. Mag. 2015;11:675–681. doi: 10.4103/0973-1296.165543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Surburg H., Panten J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses. John Wiley and Sons; Hoboken, NJ, USA: 2006. [Google Scholar]
- 54.Legault J., Pichette A. Potentiating effect of beta-caryophyllene on anticanceractivity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007;59:1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 55.Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: A shortreview. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guarda A., Rubilar J.F., Miltz J., Galotto M.J. The antimicrobial activity ofmicroencapsulated thymol and carvacrol. Int. J. Food Microbol. 2011;146:144–150. doi: 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Benoit S.C., Kemp C.J., Elias C.F., Abplanalp W., Herman J.P., Migrenne S., Lefevre A., Cruciani-Guglielmacci C., Magnan C., Yu F., et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J. Clin. Investig. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agroramoorthy G., Chandrasekaran M., Venkatesalu V., Hsu M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbol. 2007;38:739–742. doi: 10.1590/S1517-83822007000400028. [DOI] [Google Scholar]
- 59.Papachristos D.P., Karamanoli K.I., Stamopoulos D.C., Spiroudi M.U. The relationship between the chemical composition of three essential oils and their insecticidal activity against Acanthoscelides obtectus (Say) Pest Manag. Sci. 2004;60:514–520. doi: 10.1002/ps.798. [DOI] [PubMed] [Google Scholar]
- 60.Leela N.K., Sapna V.P. Clove. Chem. SPI. 2008;8:146–165. [Google Scholar]
- 61.Gonzalez-Rivera J., Duce C., Falconieri D., Ferrari C., Ghezzi L., Piras A., Tine M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. Technol. 2016;33:308–318. doi: 10.1016/j.ifset.2015.12.011. [DOI] [Google Scholar]