Abstract
Mesenchymal stromal cells are a population of undifferentiated multipotent adult cells possessing extensive self-renewal properties and the potential to differentiate into a variety of mesenchymal lineage cells. They express broad anti-inflammatory and immunomodulatory activity on the immune system and after transplantation can interact with the surrounding microenvironment, promoting tissue healing and regeneration. For this reason, mesenchymal stromal cells have been widely used in regenerative medicine, both in preclinical and clinical settings. Another clinical application of mesenchymal stromal cells is the targeted delivery of chemotherapeutic agents to neoplastic cells, maximizing the cytotoxic activity against cancer cells and minimizing collateral damage to non-neoplastic tissues. Mesenchymal stem cells are home to the stroma of several primary and metastatic neoplasms and hence can be used as vectors for targeted delivery of antineoplastic drugs to the tumour microenvironment, thereby reducing systemic toxicity and maximizing antitumour effects. Paclitaxel and gemcitabine are the chemotherapeutic drugs best loaded by mesenchymal stromal cells and delivered to neoplastic cells, whereas other agents, like pemetrexed, are not internalized by mesenchymal stromal cells and therefore are not suitable for advanced antineoplastic therapy. This review focuses on the state of the art of advanced antineoplastic cell therapy and its future perspectives, emphasizing in vitro and in vivo preclinical results and future clinical applications.
Keywords: drug loading, drug delivery, mesenchymal stromal cell, paclitaxel
1. Introduction
The ultimate goal of cancer chemotherapy is to optimize patient outcomes by increasing the drug concentration in the target tissues, thereby enhancing therapeutic efficacy, while simultaneously decreasing the exposure of healthy cells and tissues to reduce toxicity. Nanomedicines shift the tissue distribution of chemotherapeutic drugs, thereby significantly reducing the dose-limiting adverse effects while maintaining or even improving their efficacy [1].
Synthetic lipid- or polymer-based carrier systems or natural carriers like extracellular vesicles, viruses, bacteria and cells have been employed as drug carriers [2]. Mesenchymal stromal cells (MSC) are a population of undifferentiated multipotent adult cells possessing extensive self-renewal properties and the potential to differentiate into a variety of mesenchymal lineage cells [3,4]. They express broad anti-inflammatory and immunomodulatory activity on the immune system and after transplantation can interact with the surrounding microenvironment, promoting tissue healing and regeneration. For this reason, MSC have been widely used in the field of regenerative medicine, both in preclinical and clinical settings [5,6]. More recently, they have been advocated as natural carriers for targeted delivery of chemotherapeutic agents to neoplastic cells, maximizing the cytotoxic activity against cancer cells, and minimizing collateral damage to normal tissues [7]. In the present review, we describe MSC drug loading and delivery as potential new tool in clinical practice for oncologists, with specific regard to the most used chemotherapeutic agents like paclitaxel, gemcitabine, and pemetrexed.
2. Mesenchymal Stromal Cells
MSC are undifferentiated multipotent adult cells defined as plastic-adherent, fibroblast-like cells possessing extensive self-renewal properties and the in vivo and in vitro potential to differentiate into osteogenic, chondrogenic, and adipogenic lineages when cultured in specific inducing media [3]. MSC have an immune phenotype evading the host immune system, thus allowing allogenic transplantation without immunosuppression [8]. After transplantation into host tissues, MSC can interact with the surrounding microenvironment, stimulating tissue healing and regeneration by “cross talking” with other cells within the damaged tissue [9].
Initially discovered in bone marrow, MSC can be isolated from a wide spectrum of adult and foetal tissues like umbilical cord, adipose tissue, periosteum, tendon, dental pulp, cornea, thymus, spleen, brain, liver, placenta, and synovial and amniotic fluids [3]. Besides their potential to differentiate into adipocytes, osteoblasts, and chondroplasts, MSC can also differentiate into other mesodermal, endodermal, and ectodermal lineages, such as cardiomyocytes, skeletal myocytes, endothelial cells, tenocytes and hepatocytes, neuronal cells, photoreceptor cells, insulin-producing cells, epidermal and sebaceous duct cells, and renal tubular epithelial cells [10]. MSC are also able to migrate to sites of injury and engraftment, responding to chemokines, cytokines, and growth factors [11] and exerting local reparative effects mainly via the paracrine secretion of anti-inflammatory and wound-healing soluble factors [12].
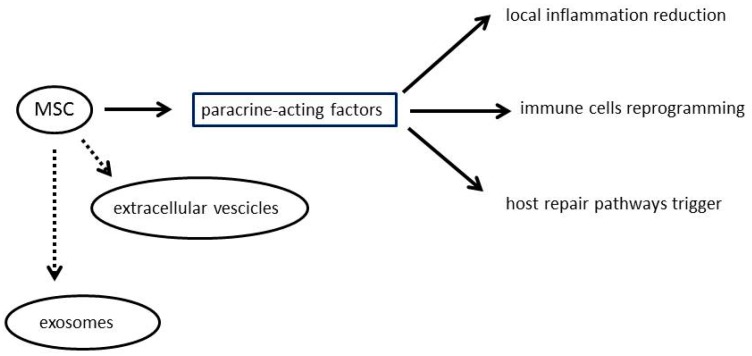
Thanks to these characteristics, MSC are being exploited as an experimental therapy for a wide spectrum of human diseases. Current knowledge indicates that MSC effectively impact on disease via the secretion of paracrine-acting factors to reduce local inflammation, reprogramme immune cells, and trigger host repair pathways. It was recently discovered that MSC also produce extracellular vesicles—including exosomes—carrying as cargo mRNAs, microRNAs, and proteins inducing non-autonomous therapeutic changes within the damaged host tissue [13] (Figure 1).
Figure 1.
Potential mechanisms of mesenchymal stromal cells (MSC) actions.
3. Drug Loading and Drug Delivery by Mesenchymal Stem Cells
New therapeutic approaches to the cell-based delivery of chemotherapeutic drugs have been widely explored thanks to the capacity of MSC to migrate and engraft into tumours after intravenous administration [14]. After exposure to high doses of chemotherapeutic drugs like paclitaxel, MSC have been shown to accumulate intracellularly and deliver the antineoplastic agents without any genetic modifications, thereby decreasing tumour proliferation [15].
Many different methods of drug delivery have been described in the last decade, including immunoconjugates for targeting tumour-specific antigens, nanoparticles, and genetically modified stem cells; glycoengineering protocols to induce expression of non-natural azide groups on the surface of MSCs without altering their viability or tumour homing capacities have been reported, as well as nano-engineered MSCs were prepared by treating human MSCs with drug-loaded polymeric nanoparticles [16,17,18,19].
However, non-modified MSC are probably the best choice for anticancer drug delivery as they readily adapt to culture conditions and home to pathological tissues when injected in vivo and possess intrinsic antineoplastic activity [15]. This technique, however, is still experimental; only experiments on cellular lines or small animals have been performed until now, although with very promising results. For an experienced biologist, the procedure is quite easy, and it is not time consuming or expensive.
On the one hand, MSC hold great promise for oncology because they release active soluble factors and play an effective immunomodulatory role. They can also cross the blood brain barrier, thus representing a potential therapeutic tool for adult and paediatric brain tumours [20,21]. On the other, the issue of whether MSC cross-talk with the tumour microenvironment boosts tumour suppression or instead favours tumour growth remains unsettled [22]. For this reason, further experimental and preclinical studies are needed before switching MSC application as drug carriers to clinical practice. In fact, due to international regulatory dispositions and mainly to the lack of sufficient preclinical data, no clinical application has been performed until now.
Several hypotheses have been put forward to explain MSC antineoplastic activity, including inhibition of proliferation-related signalling pathways, angiogenesis suppression, and cell cycle inhibition [23,24,25].
Paclitaxel and gemcitabine are the chemotherapeutic drugs best loaded by mesenchymal stromal cells and delivered to neoplastic cells. Although pemetrexed has shown promising results in vivo for malignant mesothelioma therapy, the drug is not internalized by MSC and hence cannot benefit from this method for the time being.
The three lead compounds currently used in anticancer therapy, paclitaxel, gemcitabine and pemetrexed, have shown a wide range of activity focusing on different targets and still represent the first-line chemotherapy, especially against solid tumours.
4. Paclitaxel
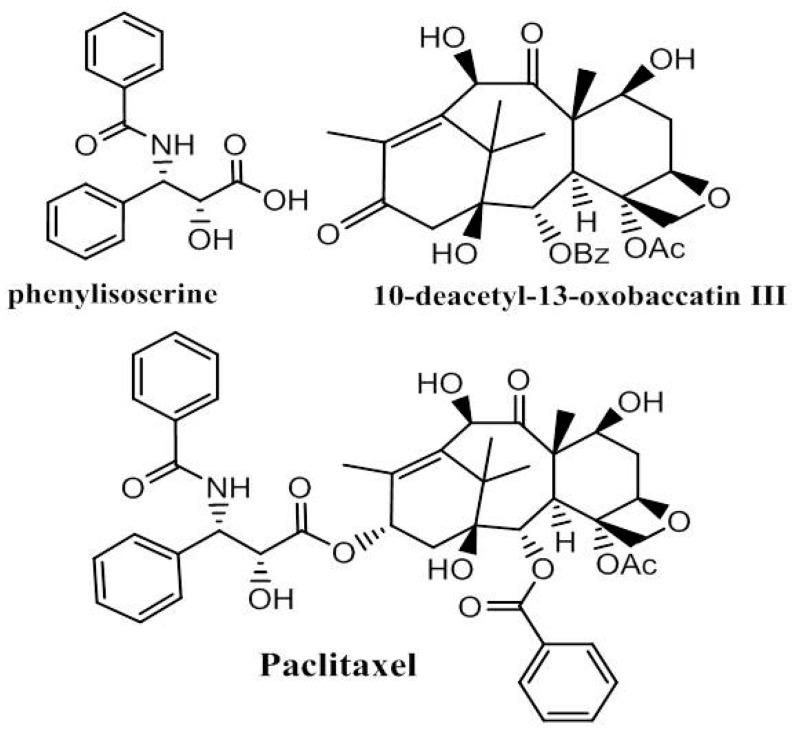
Among the plant-derived drugs used in the treatment of an enormous array of pathologies, paclitaxel (Figure 2) plays a significant role in cancer therapy, eliciting its activity by binding to the β-tubulin subunits in microtubules and influencing the depolarized/polarized equilibrium. The discovery of this antineoplastic agent from Taxus brevifolia [26] inspired the search for new taxoids chemically extracted from plant components. Paclitaxel is characterized by a chiral lateral chain (N-benzoyl-phenyl-isoserine group) and a taxoid ring. Both groups are necessary for the drug’s biological activity. The different synthetic approaches studied involve semi-synthesis in which the chiral lateral chain, obtained through bio- or organometallic catalysis [27,28,29,30], reacts with the baccatin III core structure isolated by Taxus species [31,32,33,34] (Figure 2).
Figure 2.
Paclitaxel obtained by a coupling reaction between (2R,3S)-N-benzoyl-3-phenylisoserine and the baccatin III core structure.
5. Gemcitabine
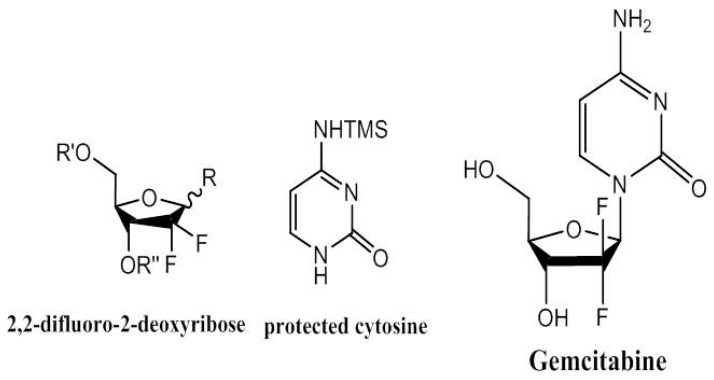
Gemcitabine [35] (Figure 3) is a nucleoside analogue formed by a deoxy-difluorinated d-ribose in combination with a pyrimidine base (cytosine). The drug’s activity relies on the inhibition of ribonucleotide reductase and DNA synthesis against different types of solid tumours. Many gemcitabine synthesis strategies have been employed, especially those building enantioenriched nucleosides starting from natural sugars. This strategy bypasses the need for anomeric activation at the oxygen atom by synthesis of enantioselective prefabricated building blocks in which an appropriate leaving group, necessary during the coupling reaction with the nucleobase, is selectively introduced in the de novo synthetic sequence [36,37,38,39]. Other approaches also focused on modifying either the substituted nucleoside or the nucleobase [40,41,42].
Figure 3.
Gemcitabine obtained by a combination of protected and activated pentose and a substituted cytosine base.
6. Pemetrexed
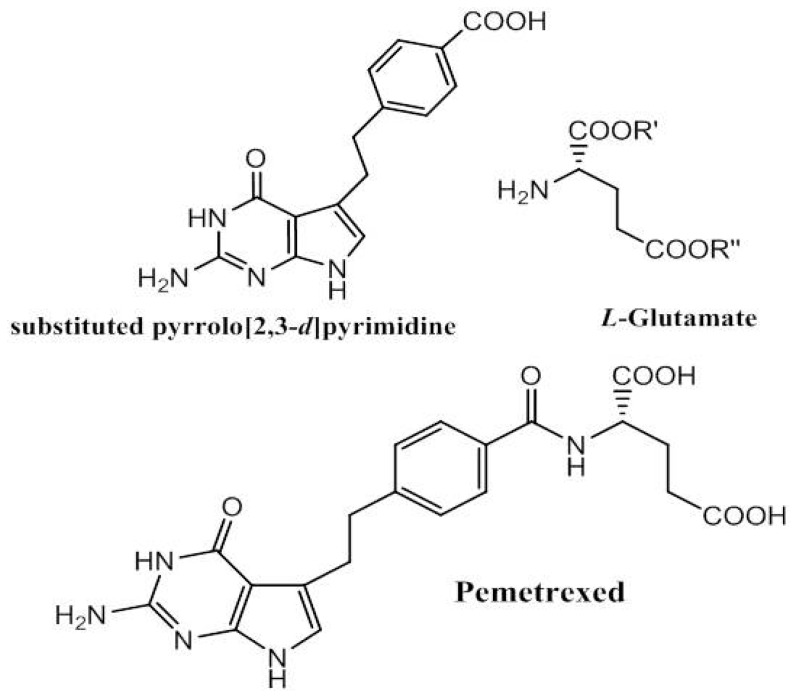
Pemetrexed (Figure 4) is a multi-target inhibitor of folate-dependent enzymes and plays a crucial role in blocking DNA and RNA replication by nucleobase biosynthesis. The folate metabolism inhibitors have antineoplastic activity especially in treating haematologic and solid tumours [43,44]. The synthetic pathway was mainly developed to enhance the efficiency and yield of the total synthesis starting from 2,6-diamino-4(3H)-pyrimidinone through different condensation steps to the last peptide coupling with chiral glutamate [45,46,47,48]. Different structure-activity relationship modifications have been implemented either on substituted pyrrolo [2,3-d] pyrimidine [49,50,51] or in the bridge between this substance and the benzoyl ring in the side chain [52].
Figure 4.
Pemetrexed synthetic pathway: 2,6-diamino-4(3H)-pyrimidinone as starting material to obtain substituted pyrrolo [2,3-d] pyrimidine, which reacts in a conventional peptide coupling with glutamate.
7. Other Drugs Potentially Deliverable by MSC
Although paclitaxel and gemcitabine are the most common antineoplastic drugs potentially deliverable by MSC, in the last decade, several other toxic compounds have been experimentally tested.
Kosaka et al. reported that MSCs expressing cytosine deaminase and concurrent 5-fluorocytosine administration could improve the survival of rats bearing 9 L gliomas [53]. Ryu et al. demonstrated that MSCs loaded with herpes simplex virus type I thymidine kinase may increase the survival of glioma-bearing mice [54]. Li et al. showed that silica nanorattle-doxorubicin particles could be anchored to MSCs in a system called “nanoparticulate patches” [55]. Loaded cells could migrate towards U251 cancer cells both in vitro and in vivo. Roger et al. demonstrated that marrow-isolated adult multilineage inducible cells (MIAMI cells) containing ferrociphenol could induce cytotoxicity in U87MG glioma cells in vitro via a transwell system assay [56]. In a report from Rachakatla et al., neural progenitor cells were loaded with magnetic nanoparticles and delivered to mice suffering from malignant melanoma. Using an alternating magnetic field, hyperthermia was induced, and significant tumor decrease was observed [57].
Present results combining stem cells and nanoparticles for the induction of toxicity toward neoplastic cells demonstrate a promising proof-of-concept, but more work needs to be developed to confirm that stem cells effectively improve the efficacy of free-standing nanoparticle systems.
8. Clinical Perspectives
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer, being the fourth leading cause of cancer death in the United States [58]. PDAC resistance to radiotherapy and chemotherapy represents a major limit to the efficacy of these treatments, resulting in an extremely disappointing overall five-year survival rate (about 5%) [59]. The current standard first-line treatment for locally advanced and metastatic PDAC is gemcitabine (GCB) and 5-fluorouracil, although a clinical response is obtained in only 10% of cases, so that novel therapies are urgently needed [60]. It has been experimentally demonstrated that GCB-loaded MSC can integrate into the tumour mass and deliver much higher concentrations of the chemotherapeutic drug than intravenous injection, thus acting as a “Trojan horse” for drug delivery [14].
Glioblastoma—also known as glioblastoma multiforme (GBM)—is the most aggressive brain cancer, representing 15% of brain tumours [60]. Treatment typically involves surgery followed by chemotherapy and radiation, but despite maximum treatment GBM usually recurs, the most common length of survival following diagnosis being 12 to 15 months with less than 3% to 5% of people surviving longer than five years [61]. MSC can migrate to and colonize GBM tumour xenografts when administered systemically or injected directly into the brain [22]. The MSC-tumour cell interaction resulted in significantly longer animal survival, reduced tumour volume, and impaired cell proliferation and vascularization. These preclinical results support the possible clinical use of MSC to treat GBM [22].
Melanoma—also known as malignant melanoma (MM)—is a malignant tumour developing from melanocytes of the skin or other tissues. Surgery plays a curative role for local disease, whereas immunotherapy, biologic therapy, radiation, or chemotherapy may improve survival for metastatic patients, although five-year survival rates drop from 98% among patients localized disease to 17% among those in whom spread has occurred. Paclitaxel-loaded MSC (PTX-MSC) have effectively inhibited lung metastasis formation in a murine B16 melanoma model, thus representing a potential therapeutic model for MM lung metastases [62].
Multiple myeloma is a neoplasm suppressing osteoblastogenesis by bone marrow mesenchymal stromal cells (BM-MSC). The role of MSC for multiple myeloma treatment is widely debated because of the contradictory results on MSC’s ability to inhibit or stimulate cancer growth. MSC could serve as vehicles for targeted delivery of anti-tumour agents into bone marrow. It has been experimentally demonstrated that PTX-loaded BM-MSC are active on the proliferation of a human myeloma cell line, expressing an intense suppression of myeloma cell growth. This suggests that drug-loaded MSC could represent an effective method to deliver the chemotherapeutic agent into the bone marrow [63].
Malignant pleural mesothelioma (MPM) is a cancer related to asbestos exposure whose incidence is expected to peak in 2020–2025 in Europe and Japan. The current standard first-line treatment is a platinum-based doublet containing a third-generation antifolate like pemetrexed (PMX) or ralitrexed. There are no approved second-line treatments for MPM as its prognosis is extremely poor, making it a disease setting to test new drugs. Initial experimental observations showed that PMX is not internalized and released by MSC. However, based on previous results with PTX-primed MSC, further experimental studies demonstrated that PTX-loaded MSC showed an excellent capacity to inhibit MPM cell proliferation, hitherto representing the best in vitro combination (drug + cells) for MPM therapy [7,64] (Table 1).
Table 1.
Possible oncologic indications for clinical use of MSC.
| Tumor | Standard Care | MSC Action |
|---|---|---|
| Pancreatic ductal adenocarcinoma | Gemcitabine and 5-fluorouracil | “Trojan horse” |
| Glioblastoma multiforme | Surgery followed by chemotherapy and radiation | Reduction of tumour volume, impairment of cell proliferation and vascularization. |
| Malignant melanoma | Surgery and immunotherapy or biologic therapy and radiation or chemotherapy | Inhibition of lung metastasis in a murine melanoma model |
| Multiple myeloma | Chemotherapy | Intense suppression of myeloma cell growth |
| Malignant mesothelioma | Platinum-based doublet containing a third-generation antifolate + surgery + radiotherapy | PTX-loaded MSC strongly inhibit MPM cell proliferation |
9. Conclusions
MSC antineoplastic drug loading and drug delivery represents a promising new field of chemotherapy and advanced cell therapy for oncological diseases, in particular those with a poor prognosis. Successful experimental tests on models of pancreatic adenocarcinoma, glioblastoma, melanoma, multiple myeloma and malignant pleural mesothelioma have yielded very encouraging in vitro results. Phase I clinical studies are now required for further confirmation of the feasibility and efficacy of advanced cell therapy. Adipose-derived MSC as well as nanoparticles may represent—in the future—the new frontiers of drug loading and delivery.
Acknowledgments
Anne Prudence Collins edited the English text.
Abbreviations
| MSC | mesenchymal stromal cells |
| PDAC | pancreatic ductal adenocarcinoma |
| GCB | gemcitabine |
| GBM | glioblastoma multiforme |
| PTX | paclitaxel |
| BM | bone marrow |
| MPM | malignant pleural mesothelioma |
| PMX | pemetrexed |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chauhan V.P., Jain R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Meel R., Fens M.H.A.M., Vader P., van Solinge W.W., Eniola-Adefeso O., Schiffelers R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control. Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 3.Petrella F., Rizzo S., Borri A., Casiraghi M., Spaggiari L. Current Perspectives in Mesenchymal Stromal Cell Therapies for Airway Tissue Defects. Stem Cells Int. 2015;2015:7. doi: 10.1155/2015/746392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P., Petrella F., Nicosia L., Bellomi M., Rizzo S. Molecular Imaging of Stem Cell Transplantation for Liver Diseases: Monitoring, Clinical Translation, and Theranostics. Stem Cells Int. 2016;2016:8. doi: 10.1155/2016/4058656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrella F., Toffalorio F., Brizzola S., De Pas T.M., Rizzo S., Barberis M., Pelicci P., Spaggiari L., Acocella F. Stem Cell Transplantation Effectively Occludes Bronchopleural Fistula in an Animal Model. Ann. Thorac. Surg. 2014;97:480–483. doi: 10.1016/j.athoracsur.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Petrella F., Acocella F., Barberis M., Bellomi M., Brizzola S., Donghi S., Giardina G., Giordano R., Guarize J., Lazzari L., et al. Airway Fistula Closure after Stem-Cell Infusion. N. Engl. J. Med. 2015;372:96–97. doi: 10.1056/NEJMc1411374. [DOI] [PubMed] [Google Scholar]
- 7.Petrella F., Coccè V., Masia C., Milani M., Salè E.O., Alessandri G., Parati E., Sisto F., Pentimalli F., Brini A.T., et al. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed. Pharmacother. 2017;87:755–758. doi: 10.1016/j.biopha.2017.01.118. [DOI] [PubMed] [Google Scholar]
- 8.Igura K., Zhang X., Takahashi K., Mitsuru A., Yamaguchi S., Takahashi T.A. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–553. doi: 10.1080/14653240410005366-1. [DOI] [PubMed] [Google Scholar]
- 9.Baiguera S., Jungebluth P., Mazzanti B., Macchiarini P. Mesenchymal stromal cells for tissue-engineered tissue and organ replacements. Transpl. Int. 2012;25:369–382. doi: 10.1111/j.1432-2277.2011.01426.x. [DOI] [PubMed] [Google Scholar]
- 10.Kyurkchiev D., Bochev I., Ivanova-Todorova E., Mourdjeva M., Oreshkova T., Belemezova K., Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding D.-C., Shyu W.-C., Lin S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y., Chen L., Scott P.G., Tredget E.E. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 13.Phinney D.G., Pittenger M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 14.Bonomi A., Sordi V., Dugnani E., Ceserani V., Dossena M., Coccè V., Cavicchini L., Ciusani E., Bondiolotti G., Piovani G., et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy. 2015;17:1687–1695. doi: 10.1016/j.jcyt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Pessina A., Bonomi A., Coccè V., Invernici G., Navone S., Cavicchini L., Sisto F., Ferrari M., Viganò L., Locatelli A., et al. Mesenchymal Stromal Cells Primed with Paclitaxel Provide a New Approach for Cancer Therapy. PLoS ONE. 2011;6:e28321. doi: 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mooney R., Weng Y., Garcia E., Bhojane S., Smith-Powell L., Kim S.U., Annala A.J., Aboody K.S., Berlin J.M. Conjugation of pH-responsive nanoparticles to neural stem cells improves intratumoral therapy. J. Control. Release. 2014;191:82–89. doi: 10.1016/j.jconrel.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y., Morshed R., Cheng S.-H., Tobias A., Auffinger B., Wainwright D.A., Zhang L., Yunis C., Han Y., Chen C.-T., et al. Nanoparticle-Programmed Self-Destructive Neural Stem Cells for Glioblastoma Targeting and Therapy. Small. 2013;9:4123–4129. doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layek B., Sadhukha T., Prabha S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomaterials. 2016;88:97–109. doi: 10.1016/j.biomaterials.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Sadhukha T., O’Brien T.D., Prabha S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release. 2014;196:243–251. doi: 10.1016/j.jconrel.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Park H.J., Shin J.Y., Kim H.N., Oh S.H., Song S.K., Lee P.H. Mesenchymal stem cells stabilize the blood–brain barrier through regulation of astrocytes. Stem Cell Res. Ther. 2015;6:187. doi: 10.1186/s13287-015-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Fei Y., Xu C., Zhao Y., Pan Y. Bone marrow mesenchymal stem cells ameliorate neurological deficits and blood-brain barrier dysfunction after intracerebral hemorrhage in spontaneously hypertensive rats. Int. J. Clin. Exp. Pathol. 2015;8:4715–4724. [PMC free article] [PubMed] [Google Scholar]
- 22.Pacioni S., D’Alessandris Q.G., Giannetti S., Morgante L., Coccè V., Bonomi A., Buccarelli M., Pascucci L., Alessandri G., Pessina A., et al. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res. Ther. 2017;8:53. doi: 10.1186/s13287-017-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khakoo A.Y., Pati S., Anderson S.A., Reid W., Elshal M.F., Rovira I.I., Nguyen A.T., Malide D., Combs C.A., Hall G., et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao L., Xu Z.-L., Zhao T.-J., Ye L.-H., Zhang X.-D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Qiao L., Xu Z., Zhao T., Zhao Z., Shi M., Zhao R.C., Ye L., Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 26.Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 27.Wilding B., Vesela A.B., Perry J.J.B., Black G.W., Zhang M., Martinkova L., Klempier N. An investigation of nitrile transforming enzymes in the chemo-enzymatic synthesis of the taxol sidechain. Org. Biomol. Chem. 2015;13:7803–7812. doi: 10.1039/C5OB01191D. [DOI] [PubMed] [Google Scholar]
- 28.Qian Y., Xu X., Jiang L., Prajapati D., Hu W. A Strategy to Synthesize Taxol Side Chain and (−)-epi Cytoxazone via Chiral Bronsted Acid-Rh2(OAc)4 Co-catalyzed Enantioselective Three-Component Reactions. J. Org. Chem. 2010;75:7483–7486. doi: 10.1021/jo101559p. [DOI] [PubMed] [Google Scholar]
- 29.Rimoldi I., Pellizzoni M., Facchetti G., Molinari F., Zerla D., Gandolfi R. Chemo- and biocatalytic strategies to obtain phenylisoserine, a lateral chain of Taxol by asymmetric reduction. Tetrahedron Asymmetry. 2011;22:2110–2116. doi: 10.1016/j.tetasy.2011.11.017. [DOI] [Google Scholar]
- 30.Torssell S., Somfai P. 1,3-Dipolar Cycloadditions of Carbonyl Ylides to Aldimines: Scope, Limitations and Asymmetric Cycloadditions. Adv. Synth. Catal. 2006;348:2421–2430. doi: 10.1002/adsc.200600324. [DOI] [Google Scholar]
- 31.Kasaei A., Mobini-Dehkordi M., Mahjoubi F., Saffar B. Isolation of Taxol-Producing Endophytic Fungi from Iranian Yew Through Novel Molecular Approach and Their Effects on Human Breast Cancer Cell Line. Curr. Microbiol. 2017;74:702–709. doi: 10.1007/s00284-017-1231-0. [DOI] [PubMed] [Google Scholar]
- 32.Nasiri J., Naghavi M.R., Alizadeh H., Moghadam M.R.F. Seasonal-based temporal changes fluctuate expression patterns of TXS, DBAT, BAPT and DBTNBT genes alongside production of associated taxanes in Taxus baccata. Plant Cell Rep. 2016;35:1103–1119. doi: 10.1007/s00299-016-1941-y. [DOI] [PubMed] [Google Scholar]
- 33.Dang P.H., Nguyen H.X., Duong T.T.T., Tran T.K.T., Nguyen P.T., Vu T.K.T., Vuong H.C., Phan N.H.T., Nguyen M.T.T., Nguyen N.T., et al. α-Glucosidase Inhibitory and Cytotoxic Taxane Diterpenoids from the Stem Bark of Taxus wallichiana. J. Nat. Prod. 2017;80:1087–1095. doi: 10.1021/acs.jnatprod.7b00006. [DOI] [PubMed] [Google Scholar]
- 34.Li C., Qiu Y., Li X., Liu N., Yao Z. Biological evaluation of new antitumor taxoids: Alteration of substitution at the C-7 and C-10 of docetaxel. Bioorg. Med. Chem. Lett. 2014;24:855–859. doi: 10.1016/j.bmcl.2013.12.083. [DOI] [PubMed] [Google Scholar]
- 35.Hertel L.W., Kroin J.S., Misner J.W., Tustin J.M. Synthesis of 2-deoxy-2,2-difluoro-d-ribose and 2-deoxy-2,2′-difluoro-d-ribofuranosyl nucleosides. J. Org. Chem. 1988;53:2406–2409. doi: 10.1021/jo00246a002. [DOI] [Google Scholar]
- 36.Yang B., Jinnouchi A., Suemune H., Aso M. Difluoro-C4′-oxidized Abasic Site for Efficient Amine Modification in Biological Systems. Org. Lett. 2012;14:5852–5855. doi: 10.1021/ol302703m. [DOI] [PubMed] [Google Scholar]
- 37.Colombel S., Van Hijfte N., Poisson T., Leclerc E., Pannecoucke X. Addition of Electrophilic Radicals to 2-Benzyloxyglycals: Synthesis and Functionalization of Fluorinated α-C-Glycosides and Derivatives. Chem. Eur. J. 2013;19:12778–12787. doi: 10.1002/chem.201302070. [DOI] [PubMed] [Google Scholar]
- 38.Hamon N., Quintiliani M., Balzarini J., McGuigan C. Synthesis and biological evaluation of prodrugs of 2-fluoro-2-deoxyribose-1-phosphate and 2,2-difluoro-2-deoxyribose-1-phosphate. Bioorg. Med. Chem. Lett. 2013;23:2555–2559. doi: 10.1016/j.bmcl.2013.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang G., Chen H., Zhao D., Ding D., Sun M., Kou L., Luo C., Zhang D., Yi X., Dong J., et al. Combination of l-Carnitine with Lipophilic Linkage-Donating Gemcitabine Derivatives as Intestinal Novel Organic Cation Transporter 2-Targeting Oral Prodrugs. J. Med. Chem. 2017;60:2552–2561. doi: 10.1021/acs.jmedchem.7b00049. [DOI] [PubMed] [Google Scholar]
- 40.Peifer M., Berger R., Shurtleff V.W., Conrad J.C., MacMillan D.W.C. A General and Enantioselective Approach to Pentoses: A Rapid Synthesis of PSI-6130, the Nucleoside Core of Sofosbuvir. J. Am. Chem. Soc. 2014;136:5900–5903. doi: 10.1021/ja502205q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown K., Weymouth-Wilson A., Linclau B. A linear synthesis of gemcitabine. Carbohydr. Res. 2015;406:71–75. doi: 10.1016/j.carres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z., Ku T.C., Seley-Radtke K.L. Thiophene-expanded guanosine analogues of Gemcitabine. Bioorg. Med. Chem. Lett. 2015;25:4274–4276. doi: 10.1016/j.bmcl.2015.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor E.C., Kuhnt D., Shih C., Rinzel S.M., Grindey G.B., Barredo J., Jannatipour M., Moran R.G. A dideazatetrahydrofolate analog lacking a chiral center at C-6: N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5yl)ethyl[benzoyl]-l-glutamic acid is an inhibitor of thymidylate synthase. J. Med. Chem. 1992;35:4450–4454. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 44.Taylor E.C., Liu B. A simple and concise synthesis of LY231514(MTA) Tetrahedron Lett. 1999;40:4023–4026. doi: 10.1016/S0040-4039(99)00676-0. [DOI] [Google Scholar]
- 45.Taylor E.C., Liu B. A New and Efficient Synthesis of Pyrrolo[2,3-d]pyrimidine Anticancer Agents: Alimta (LY231514, MTA), Homo-Alimta, TNP-351, and Some Aryl 5-Substituted Pyrrolo[2,3-d]pyrimidines. J. Org. Chem. 2003;68:9938–9947. doi: 10.1021/jo030248h. [DOI] [PubMed] [Google Scholar]
- 46.Qi H., Wen J., Li L., Bai R., Chen L., Wang D. An Efficient Synthesis of Pemetrexed Disodium. J. Heterocycl. Chem. 2015;52:1565–1569. doi: 10.1002/jhet.2164. [DOI] [Google Scholar]
- 47.Palacios F., Vicario J., Aparicio D. Efficient Synthesis of 1-Azadienes Derived from α-Aminoesters. Regioselective Preparation of α-Dehydroamino Acids, Vinylglycines, and α-Amino Acids. J. Org. Chem. 2006;71:7690–7696. doi: 10.1021/jo061140f. [DOI] [PubMed] [Google Scholar]
- 48.McKerrow J.D., Al-Rawi J.M.A., Brooks P. Use of Diphenyliodonium Bromide in the Synthesis of Some N-Phenyl α-Amino Acids. Synth. Commun. 2010;40:1161–1179. doi: 10.1080/00397910903051259. [DOI] [Google Scholar]
- 49.Zaware N., Kisliuk R., Bastian A., Ihnat M.A., Gangjee A. Synthesis and evaluation of 5-(arylthio)-9H-pyrimido[4,5-b]indole-2,4-diamines as receptor tyrosine kinase and thymidylate synthase inhibitors and as antitumor agents. Bioorg. Med. Chem. Lett. 2017;27:1602–1607. doi: 10.1016/j.bmcl.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian C., Zhang Z., Zhou S., Yuan M., Wang X., Liu J. Synthesis, Antifolate and Anticancer Activities of N5-Substituted 8,10-Dideazatetrahydrofolate Analogues. Chem. Biol. Drug Des. 2016;87:444–454. doi: 10.1111/cbdd.12681. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y., Li M., Zhang H., Yuan J., Zhang C., Zhang K., Guo H., Zhao L., Du Y., Wang L., et al. Design, synthesis and biological evaluation of 6-substituted pyrrolo[2,3-d]pyrimidines as dual inhibitors of TS and AICARFTase and as potential antitumor agents. Eur. J. Med. Chem. 2016;115:245–256. doi: 10.1016/j.ejmech.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell-Ryan S., Wang Y., Raghavan S., Ravindra M.P., Hales E., Orr S., Cherian C., Hou Z., Matherly L.H., Gangjee A. Discovery of 5-Substituted Pyrrolo[2,3-d]pyrimidine Antifolates as Dual-Acting Inhibitors of Glycinamide Ribonucleotide Formyltransferase and 5-Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase in De Novo Purine Nucleotide Biosynthesis: Implications of Inhibiting 5-Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase to AMPK Activation and Antitumor Activity. J. Med. Chem. 2013;56:10016–10032. doi: 10.1021/jm401328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosaka H., Ichikawa T., Kurozumi K., Kambara H., Inoue S., Maruo T., Nakamura K., Hamada H., Date I. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012;19:572–578. doi: 10.1038/cgt.2012.35. [DOI] [PubMed] [Google Scholar]
- 54.Ryu C.H., Park K.Y., Kim S.M., Jeong C.H., Woo J.S., Hou Y., Jeun S.-S. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem. Biophys. Res. Commun. 2012;421:585–590. doi: 10.1016/j.bbrc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 55.Li L., Guan Y., Liu H., Hao N., Liu T., Meng X., Fu C., Li Y., Qu Q., Zhang Y., et al. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS Nano. 2011;5:7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 56.Roger M., Clavreul A., Huynh N.T., Passirani C., Schiller P., Vessières A., Montero-Menei C., Menei P. Ferrociphenol lipid nanocapsule delivery by mesenchymal stromal cells in brain tumor therapy. Int. J. Pharm. 2012;423:63–68. doi: 10.1016/j.ijpharm.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 57.Rachakatla R.S., Balivada S., Seo G.-M., Myers C.B., Wang H., Samarakoon T.N., Dani R., Pyle M., Kroh F.O., Walker B., et al. Attenuation of Mouse Melanoma by A/C Magnetic Field after Delivery of Bi-Magnetic Nanoparticles by Neural Progenitor Cells. ACS Nano. 2010;4:7093–7104. doi: 10.1021/nn100870z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raimondi S., Maisonneuve P., Lowenfels A.B. Epidemiology of pancreatic cancer: An overview. Nat. Rev. Gastroenterol. Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 59.Li D., Xie K., Wolff R., Abbruzzese J.L. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 60.Hines O.J.R. Howard A, Pancreatic surgery. Curr. Opin. Gastroenterol. 2008;24:603–611. doi: 10.1097/MOG.0b013e32830b112e. [DOI] [PubMed] [Google Scholar]
- 61.Wee C.W., Kim E., Kim T.M., Park C.K., Kim J.W., Choi S.H., Yoo R.E., Lee S.T., Kim I.H. Impact of interim progression during the surgery-to-radiotherapy interval and its predictors in glioblastoma treated with temozolomide-based radiochemotherapy. J. Neurooncol. 2017;134:169–175. doi: 10.1007/s11060-017-2505-x. [DOI] [PubMed] [Google Scholar]
- 62.Pessina A., Leonetti C., Artuso S., Benetti A., Dessy E., Pascucci L., Passeri D., Orlandi A., Berenzi A., Bonomi A., et al. Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J. Exp. Clin. Cancer Res. 2015;34:82. doi: 10.1186/s13046-015-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonomi A., Steimberg N., Benetti A., Berenzi A., Alessandri G., Pascucci L., Boniotti J., Coccè V., Sordi V., Pessina A., et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol. Oncol. 2016 doi: 10.1002/hon.2306. [DOI] [PubMed] [Google Scholar]
- 64.Facchetti G., Petrella F., Spaggiari L., Rimoldi I. Malignant Pleural Mesothelioma: State of the art and advanced cell therapy. Eur. J. Med. Chem. 2017;142:266–270. doi: 10.1016/j.ejmech.2017.07.063. [DOI] [PubMed] [Google Scholar]