Abstract
The phenolic composition of leaves from Phyllanthus acuminatus L., a plant commonly used in Costa Rica as traditional medicine, was studied using UPLC-ESI-MS on an enriched phenolic extract. A total of 20 phenolic compounds were identified, comprising eight flavonoids (two flavanones—pinocembrin isomers and six derivatives from apigenin, chrysin, quercetin, and kaempferol); seven ellagitannins, two flavan-3-ols (prodelphinidin B dimer and (epi)gallocatechin); and three phenolic acids (ellagic acid, trimethylellagic acid, and ferulic acid). All of these compounds are reported for the first time in P. acuminatus, while previously reported in the genus Phyllanthus. Antioxidant evaluation was performed for P. acuminatus phenolic extract obtaining DPPH results with a remarkably low IC50 value of 0.15 μg/mL. Also, cytotoxicity on gastric AGS and colon SW20 adenocarcinoma cell lines was evaluated, and highly promising results were obtained, with IC50 values of 11.3 μg/mL and 10.5 μg/mL, respectively. Furthermore, selectivity index values obtained when comparing cytotoxicity on normal Vero cells was SI > 20 for both cancer cell lines, indicating a particularly high selectivity. Additionally, Justicidin B, a metabolite extensively studied for its antitumoral activity, was isolated from a non-polar extract of P. acuminatus, and comparatively evaluated for both bioactivities. The DPPH value obtained for Justicidin B was moderate (IC50 = 14.28 μg/mL), while cytotoxicity values for both AGS (IC50 = 19.5 μg/mL) and SW620 (IC50 = 24.8 μg/mL) cell lines, as well as selectivity when compared with normal Vero cells (SI = 5.4 and 4.2 respectively), was good, but lower than P. acuminatus extract. These preliminary results suggest that P. acuminatus enriched phenolic extract containing flavonoids, ellagitannins, flavan-3-ols, and phenolic acids, reported for the first time in this plant, could be of interest for further cancer cytotoxicity studies to elucidate structure–bioactivity relationships, and the molecular mechanisms and pathways.
Keywords: P. acuminatus, UPLC, ESI-MS, flavonoids, ellagitannins, mass spectrometry, antioxidant, cytotoxicity
1. Introduction
The search for new drugs, or the combinatory effects of these, looking for a synergistic effect that can target several human health problems, is urged and mandatory. Efforts on synthetic medication to treat cancer and tumorigenesis, in recent years, have involved mainly the approach of changing functional groups on natural precursors in order to augment the potency of their effects. However, despite relative efficiency in treating targeted diseases, their use remains associated with important collateral effects.
Extracts of several botanical origins have been shown as promising resources for obtaining new isolated metabolites, as well as sources of mixtures of compounds with differential and synergistic effects at biochemical, cellular, and physiological levels. Synergic assessment of plant polyphenols, particularly flavonoids, has taught us that often centuries-old multi-drug combinations of traditional medicine are superior to the single modified constituent trends observed in recent literature and medical practice [1].
Phyllanthus acuminatus belongs to the most diversified genus of Phyllantaceae family sensu lato, which is widespread globally, and comprises circa 14 different species in Costa Rica [2], however, this plant has been particularly used in traditional medicine at local level [3]. P. acuminatus is used as icthioside piscicide, in order to hunt fishes [4], suggesting an important insight on its biological activity. In fact, both, antitumor and antimalarial activities have been reported [5]. Regarding metabolites, a majority of lignans has been reported in this plant, such as phyllanthocindiols, deacetylphyllanthostatins, and deacetylphyllanthosides [6], as well as phyllanthostatins and phyllantosides, including Justicidin B [7,8,9], with attributed potent antitumoral activity.
In recent literature, sufficient evidence on polyphenols has been reported to be considered a serious option for the management of non-communicable diseases, such as cancer and infections, supporting the potential use of complex multi-target approach to treat diseases with polyphenols. In fact, there is increasing evidence that these compounds have multiple molecular targets, modulate pro-inflammatory gene expression, also interacting with phospholipid membranes [10], and modulating pathways related to chronic inflammation and energy metabolism [11], among their studied anticancer activity [12].
The literature reported for polyphenols in P. acuminatus is scarce, as well as their antioxidant and cytotoxic effects. Most studies regarding antineoplastic activity in vivo and in vitro have been performed with the above-mentioned lignan metabolites. For instance, since the first study in murine lymphocyte leukemia P388 [7], among aryl naphthalene lignans, Justicidin B has been of special interest, because of its promising effects in cancer cells. For instance, this lignan showed strong cytotoxic effects on chronic myeloid leukemia (LAMA-8 and K-562) and chronic lymphoid leukemia (SKW-3) [13], and furthermore, it induced programmed cell death on breast cancer cell lines MDA-MB-231 and MCF-7 [14], and exhibited antiplatelet potency [15].
There are no specific reports on the ecological interaction of flavonoids from P. acuminatus and from Phyllanthus genus. However, flavonoids have been reported to have a role protecting plants against insect pests, influencing their behavior, growth, and development [16], and against UV damage, acting as screens that absorb UV radiation and transfer light energy to or from other molecules, via sensitization [17]; and to act as antipathogenic compounds that can be non-specific and result from their antioxidant properties [18].
This evidence and the scarcity of phenolic data lead us to perform a comparative study, aiming to obtain an enriched phenolic extract of P. acuminatus, in order to characterize its phenolic contents through UPLC-ESI-MS, and to evaluate its antioxidant properties and cytotoxic activity on gastric AGS and colon SW620 cancer cell lines, specially targeted by polyphenols [19]; as well as to isolate Justicidin B, reported as a highly potent antitumoral metabolite, in order to assess also its bioactivity effects, and to perform comparative data analysis.
2. Results and Discussion
2.1. Phenolic Profile by UPLC-ESI-MS Analysis
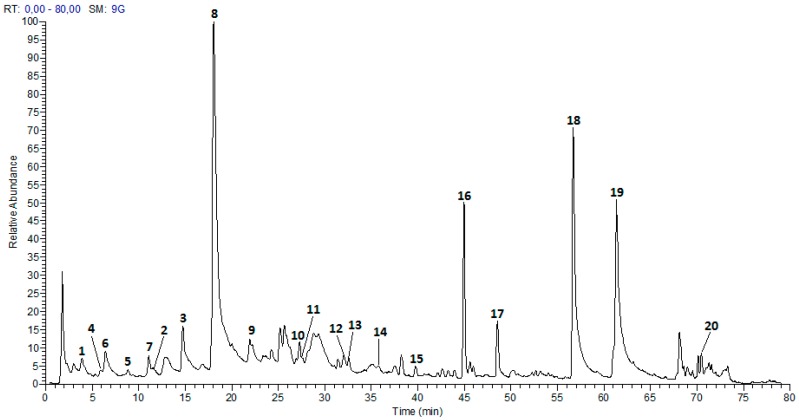
The extraction process, described in the Materials and Methods section, allowed us to obtain a phenolic enriched extract from P. acuminatus leaves. Table 1 summarizes the results of UPLC-ESI-MS analysis. Twenty compounds (Figure 1) were tentatively identified by comparing the fragmentation peaks with those reported in the literature, all of these compounds are reported for the first time in P. acuminatus, while previously reported in the genus Phyllanthus. As shown in Table 1, these compounds comprise eight flavonoids, including two flavanones—pinocembrin and six derivatives from apigenin, quercetin, and kaempferol; seven ellagitannins, two flavan-3-ols (prodelphinidin B dimer and (epi)gallocatechin); and three phenolic acids (ellagic acid, trimethylellagic acid, and ferulic acid).
Table 1.
Phenolic characterization of P. acuminatus extract.
| No. | Tenative Identification | tR (min) | λmax (nm) | [M − H]+ | Formula | Error (ppm) | MS2 |
|---|---|---|---|---|---|---|---|
| 1 | Gemin D | 3.89 | 216, 265 | 633.0717 | C27H21O18 | 1.738 | [633]: 275(18), 301(100), 249(15) |
| 2 | Phyllanemblinin B | 11.50 | 216, 278 | 633.0705 | C27H21O18 | 3.633 | [633]:463(26), 301(100), 275(15), 614(62), 615(24) |
| 3 | Corilagin | 14.72 | 221, 269 | 633.0701 | C27H21O18 | 4.265 | [633]: 463(27), 301(100), 275(15) |
| 4 | Prodelphinidin B dimer | 5.89 | 205, 270 | 609.1242 | C30H25O14 | −0.328 | [609]:305(50), 423(85), 441(100), 483(28), 591(18) |
| 5 | (epi)galocatequina | 8.84 | 205, 270 | 305.0657 | C15H13O7 | 1.311 | [305]: 125(24), 165(30), 219(77), 179(100), 261(41), 287(12), 247(13), 221(84), 167(10) |
| 6 | 1′,3′,5′-Trihydroxybenzene 1′-O-[4,6-(S)-HHDP]-β-Glucoside | 6.42 | 199, 271 | 589.0815 | C26H21O16 | −1.478 | [589]: 301(100) |
| 7 | 1′,3′,5′-Trihydroxybenzene 1′-O-[4,6-(S)-HHDP-β-Glucosyl-β-Glucosyl]-β-Glucoside | 11.09 | 204, 264 | 913.1857 | C45H37O21 | −3.285 | [913]: 625(100), 463(13) |
| 8 | Geraniin | 18.03 | 230, 276 | 951.0667 | C41H27O27 | −4.676 | [951]: 933(100) |
| 9 | Phyllanthusiin C | 21.92 | 222, 278 | 925.0905 | C40H29O26 | −4.540 | [925]: 301(100), 435(15)605(10)907(13) |
| 10 | quercertin-3-O-rutinósido | 27.28 | 219, 255, 349 | 609.1430 | C27H29O16 | 4.268 | [609]:343(8), 301(100), 300(39) |
| 11 | quercetin-3-O-hexoside | 27.58 | 221, 254, 347 | 463.0857 | C21H19O12 | 4.319 | [463]: 301(100), 300(35) |
| 12 | kaempferol-3-O-rutinoside | 31.95 | 221, 271 | 593.1487 | C27H29O15 | −2.866 | [593]: 285(100) |
| 13 | kaempferol-3-O-hexoside | 32.22 | 221, 265 | 447.0917 | C21H19O11 | 2.237 | [247]: 285(69), 284(100), 255(17), 327(18) |
| 14 | Ellagic acid | 35.75 | 221, 265 | 300.9979 | C14H5O8 | 1.661 | [301]: 257(100), 229(60), 301(28), 284(23), 185(28), 255(12), 201(11) |
| 15 | O-trimethyl ellagic acid | 39.74 | 222, 243, 353, 366 | 343.0443 | C17H11O8 | −3.207 | [343]: 328(100) |
| 16 | Apigenin derivative | 44.98 | 199, 227, 287 | 575.1381 | C27H27O14 | 3.477 | [575]: 515(80), 455(16), 371(11), 343(10), 311(100) |
| 17 | Chrysin derivative | 48.55 | 223, 289 | 559.1428 | C27H27O13 | 4.292 | [559]:499(100), 295(57) |
| 18 | Pinocembrin 7-O-[4″,6″-(S)-hexahydroxydibenzoyl]-b-d-glucopiranoside | 56.67 | 226, 282 | 719.1230 | C35H27O17 | −2.503 | [719]: 301(100) |
| 19 | Pinocembrin 7-O-[3″-O-galloyl-4″,6″-(S)-hexahydroxydibenzoyl]-β-d-glucopiranoside | 61.31 | 223, 282 | 871.1323 | C42H31O21 | −4.018 | [871]: 301(100), 569(13), 827(13) |
| 20 | Ferulic acid | 70.53 | 224 | 193.0490 | C10H9O4 | −3.698 | [193]: 178(70), 149(100), 134(62) |
Figure 1.
Chromatogram of Phyllanthus acuminatus enriched phenolic extract.
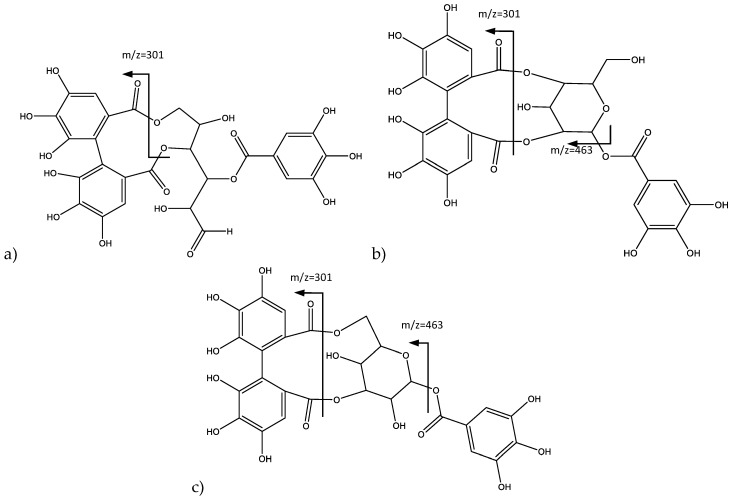
Peaks 1 (Rt = 3.89 min), 2 (Rt = 11.50 min), and 3 (Rt = 14.72 min) show negative molecular ion peaks [M − H]− at m/z 633.0717, 633.0705, and 633.0701 (C27H21O18), respectively. The identified isomers are all part of the galloyl-HDDP-glucose type [20], corresponding tentatively to three isomeric ellagitannins, gemin D, phyllanemblinin B, and corilagin, respectively, all of them previously reported in the genus Phyllanthus [6]. In fact, for peak 3, as shown in Figure 2a, the 463 amu (atomic mass units) fragment was consistent with the loss of a galloyl acid, and the 301 amu fragment was consistent with the loss of the galloyl-glucose, which is coincident with the fragmentation previously reported for corilagin (3) [21,22].
Figure 2.
Structure and fragments of: (a) Gemin D (1); (b) Pinocembrin (2); and (c) Coraligin (3).
On the other hand, Peak 2 also shows fragments at 463 amu and 301 amu, but additionally, an important peak is observed at 615 amu [M − H-H2O]. Thus, the loss of galloyl acid and galloyl-glucose, similar to corilagin, would correspond in turn to phyllanemblinin B (2) (Figure 2b). Peak 1 does not show the fragment at 463 amu attributed to the loss of galloyl bonded to a glucose, indicating the structure of gemin D (1) (Figure 2c), since this isomer does not have a glucose moiety and generates the loss of a fragment at 301 amu due to the structure bonded to the HHDP unit.
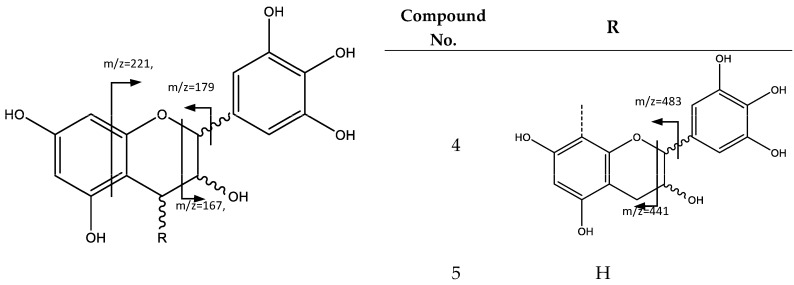
Peak 4 (Rt = 5.89 min) and peak 5 (Rt = 8.84 min) show negative molecular ion peaks [M − H]− at m/z 609.1242 (C30H25O14) and m/z 305.0657 (C15H13O7), respectively, corresponding to flavan-3-ol structures. In fact, peak 5 presents a fragmentation pattern common for an (epi)gallocatechin monomeric unit (5), because of characteristic fragments at m/z 261, 221, 219, 179, 167, 165, consistent with the loss of CO2, C4H4O2, C4H6O2, C6H6O3, C7H6O3, and C7H8O3, respectively. The loss of C4H4O2 and C4H6O2 occurs because of the fragmentation of the flavan-3-ol A ring. C6H6O3 loss is due to the fission of the heterocyclic ring, while neutral fragments C7H6O3 and C7H8O3 are released through a retro-Diels–Alder fission [23]. In turn, peak 4 corresponds to prodelphinidin B dimer (4), since the MS2 spectrum shows an ion at m/z 483, which is the product of the heterocyclic ring fission, while m/z 441 belongs to the retro-Diels–Alder fission, and further loss of water originates the fragment observed at m/z 423 [23], as shown in Figure 3.
Figure 3.
General structure and fragments of flavan-3-ols (epi)gallochatechin (5) and Prodelphinidin B dimer (4).
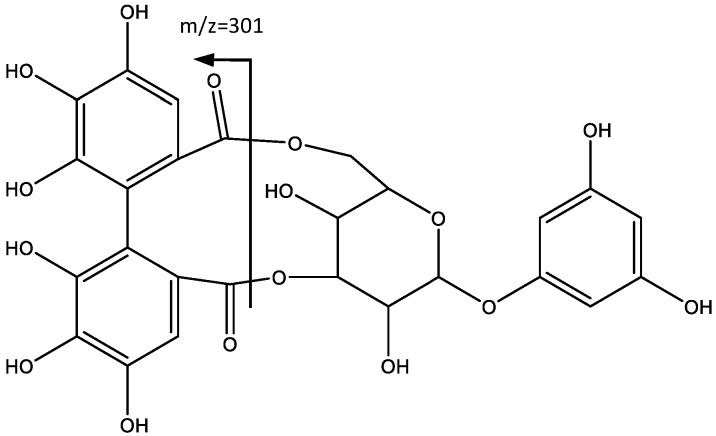
Peak 6 (Rt = 6.42 min) and peak 7 (Rt = 11.09 min) show negative molecular ion peaks [M − H]− at m/z 589.0815 (C26H21O16) and m/z 913.1857 (C45H37O21) respectively, with a similar fragmentation pattern in agreement with an ellagitannin structure, including 1,3,5-trihydroxybenzene and HHDP moieties. For instance, peak 6 shows (MS2 spectrum) a fragment at m/z = 301 [M − H-288], which undergoes further fragmentation in a pattern that matches that of the HHDP group [24,25]. The loss of 288 amu is consistent with a hexose bonded to an 1,3,5-trihydroxybenzene, which leads to the identification of the compound as 1′,3′,5′-trihydroxybenzene 1′-O-[4,6-(S)-HHDP]-β-glucoside (6), as shown in Figure 4 [26].
Figure 4.
Structure and fragmentation of 1′,3′,5′-trihydroxybenzene 1′-O-[4,6-(S)-HHDP]-β-glucoside (6).
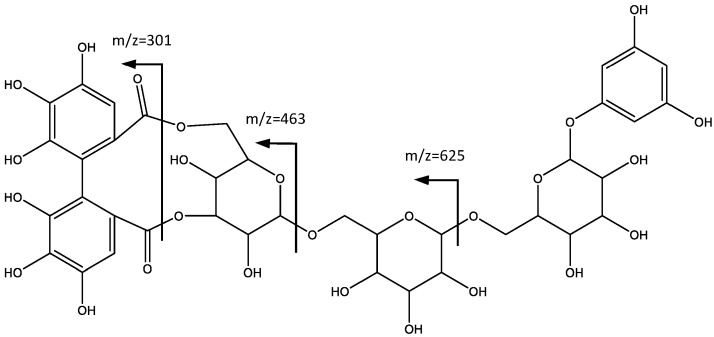
In a similar way, peak 7 shows fragments at m/z 301 that indicate the presence of the HHDP unit. As shown in Figure 5, the MS2 fragment at m/z 625 ([M − H]-288) is consistent with the loss of a glucose molecule bonded to a 1,3,5-trihydroxybenzene. Further losses of 162 amu at m/z 463 [M − H-288-162] and m/z 301 [M − H-288-162-162] are due to the loss of additional hexoses. Therefore, the compound is tentatively identified as 1′,3′,5′-trihydroxybenzene 1′-O-[4,6-(S)-HHDP-β-glucosyl-β-glucosyl]-β-glucoside (7).
Figure 5.
Structure and fragmentation of 11′,3′,5′-trihydroxybenzene 1′-O-[4,6-(S)-HHDP-β-glucosyl-β-glucosyl]-β-glucoside (7).
Peak 8 (Rt = 18.03 min) shows a negative molecular ion peak [M − H]− at m/z 951.0667, and it is tentatively assigned to Geraniin (8), ellagitannin with formula C21H28O27. In fact, as shown in Figure 6, the peak at m/z 933 corresponded to loss of H2O, and the fragments at m/z 915 and 897 match the successive loss of H2O, while the fragments at m/z 443 and 445 derive from the loss of galloyl and HDDP groups [27].
Figure 6.
Structure and fragments of Geraniin (8).
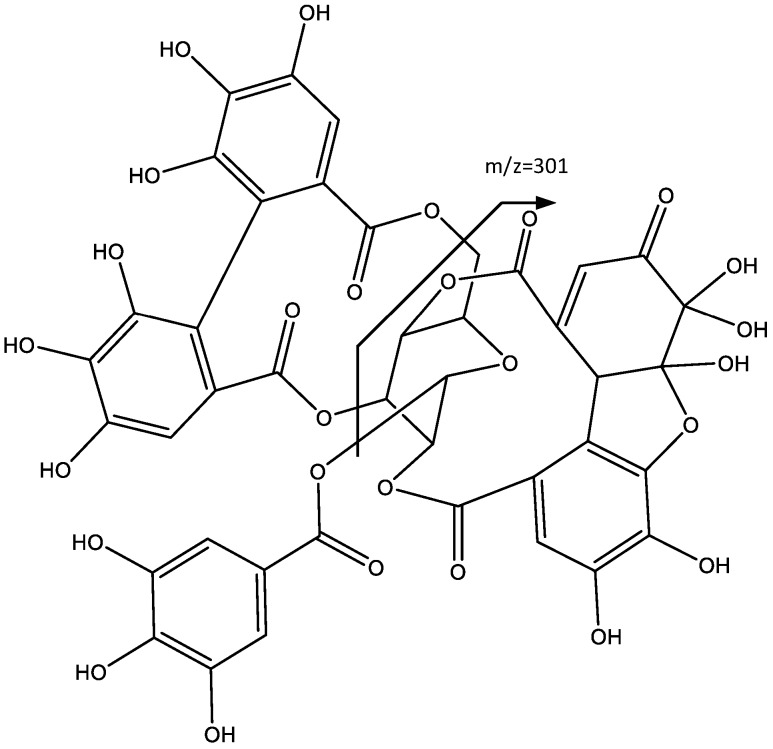
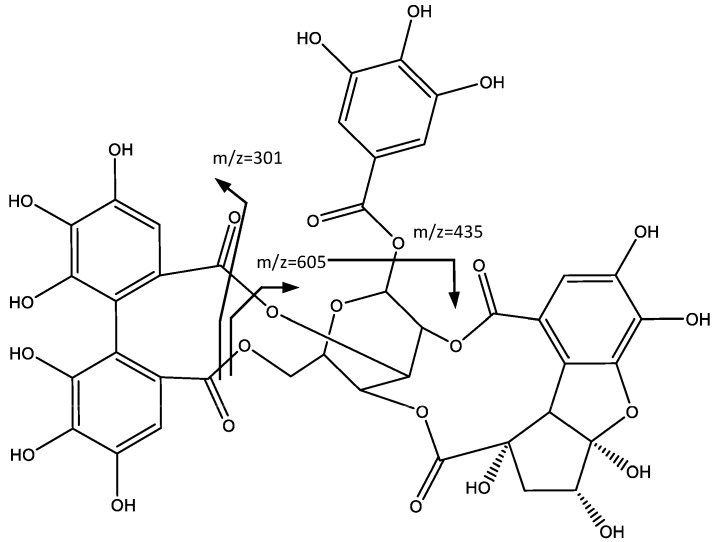
Peak 9 (Rt = 21.92 min) shows a negative molecular ion peak [M − H]− at m/z 925.0905, and it is tentatively assigned to ellagitannin Phyllanthusiin C (9), whereas the peak at m/z 907 is due to the water loss in the geminal alcohol, which after further loss of HDDP, forms the fragment at m/z 605, as shown in Figure 7. The additional loss of a galloyl group originates the fragment at m/z 435. The peak at m/z 301 corresponds to the ionized HDDP unit [25].
Figure 7.
Structure and fragments of Phyllantusin C (9).
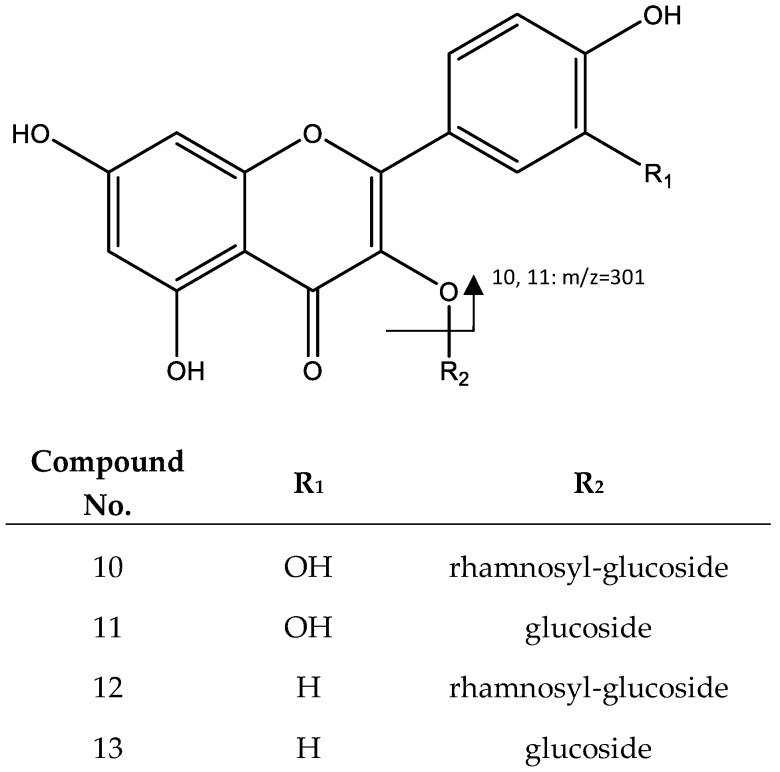
Two flavonoids correspond to peak 10 (Rt = 27.28) and peak 11 (RT = 27.58), which show a negative molecular ion peak [M − H]− at m/z 609.1430 and m/z 463.0857, respectively. Peak 10 was identified as quercetin-3-O-rutinoside (10), whose loss of the glycosylated unit yields the peak at m/z 301, followed by a fragmentation (Figure 8) with a pattern previously reported for the same compound [28], while peak 11 was assigned to quercetin-3-O-glucoside (11), because the main fragment corresponds to the loss of a hexose (m/z = 301, [M − H-162]) [25].
Figure 8.
Structure and fragments of quercetin derivatives (10), (11), and kaempferol derivatives (12), (13).
Peaks 12 and 13 were identified as kaempferol derivatives. For instance, Peak 12 (Rt = 31.95 min) shows a negative molecular ion peak [M − H]− at m/z 593.1487, and it was identified as kaempferol-3-O-rutinoside (12) [29]; and peak 13 (Rt = 32.22 min), which shows a negative molecular ion peak [M − H]− at m/z 447.0917, was identified as kaempferol-3-O-hexoside (13) [30]. Fragmentation for both molecules show the loss of glycosides generating signals that correspond to kaempferol (m/z 284 and 285), as shown in Figure 8.
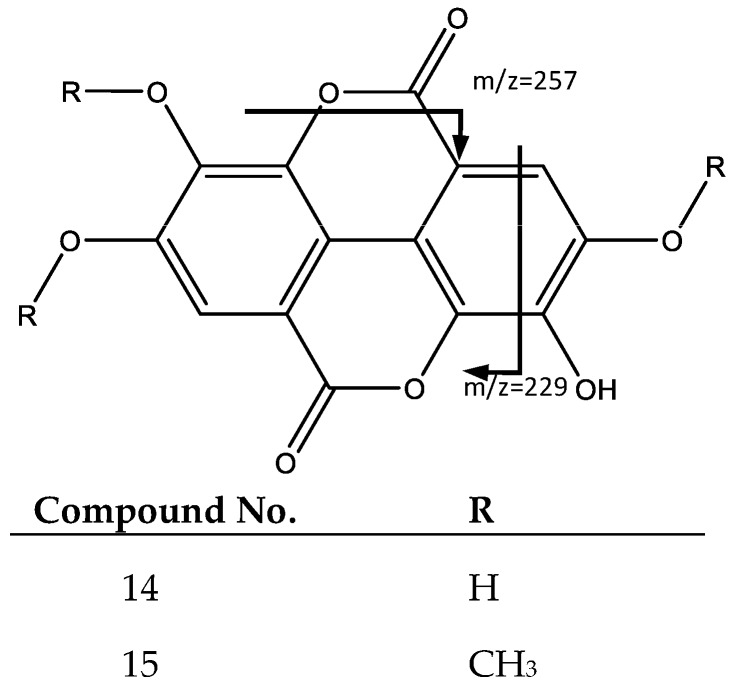
Peaks 14 and 15 have similar fragmentation patterns in agreement with an ellagic acid moiety, as shown in Figure 9. For instance, peak 14 (Rt = 35.75 min) corresponds to ellagic acid (14), which shows a negative molecular ion peak [M − H]− at m/z 300.9979, characterized by fragments at m/z 257 [M − H-CO2]− and m/z 229 [31]. In turn, Peak 15 (Rt = 39.74 min) shows a negative molecular ion peak [M − H]− at m/z 343.0443, and corresponds to O-trimethyl ellagic acid (15), whose fragments at m/z 328, 313, and 298 match the successive loss of methyl groups (–CH3) [32].
Figure 9.
Structure and fragments of ellagic acid (14) and O-trimethyl ellagic acid (15).
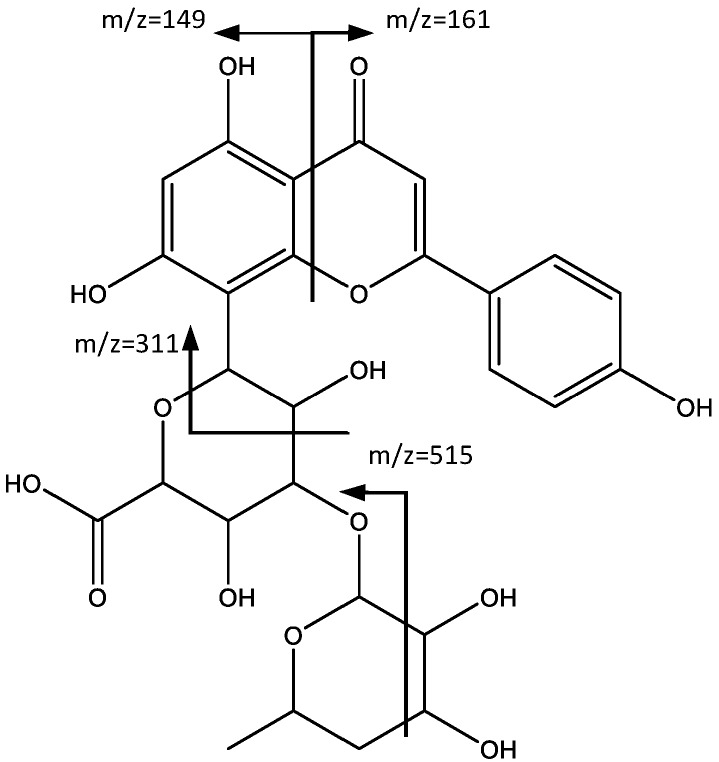
Peaks 16 (Rt = 44.98 min) and 17 (Rt = 48.55 min) correspond also to flavonoids, specifically to apigenin and chrysin derivatives. In fact, peak 16 showed a negative molecular ion [M − H]− at m/z 575.1381 (C27H27O14), and a fragment at m/z 515 corresponding to [M − H-60] which was previously reported [33] for this type of structure (Figure 10). According to Wu et al. [34], the fragment observed at m/z 311 corresponds to apigenin derivative (16). The fragment at m/z 293 arose from the successive loss of water. Two more peaks, observed at m/z 161 and m/z 149, originated from the fragment identified at m/z 311, due to the breakdown of the C ring, with each peak corresponding to one of the fragments generated.
Figure 10.
Structure and fragments of apigenin derivative (16).
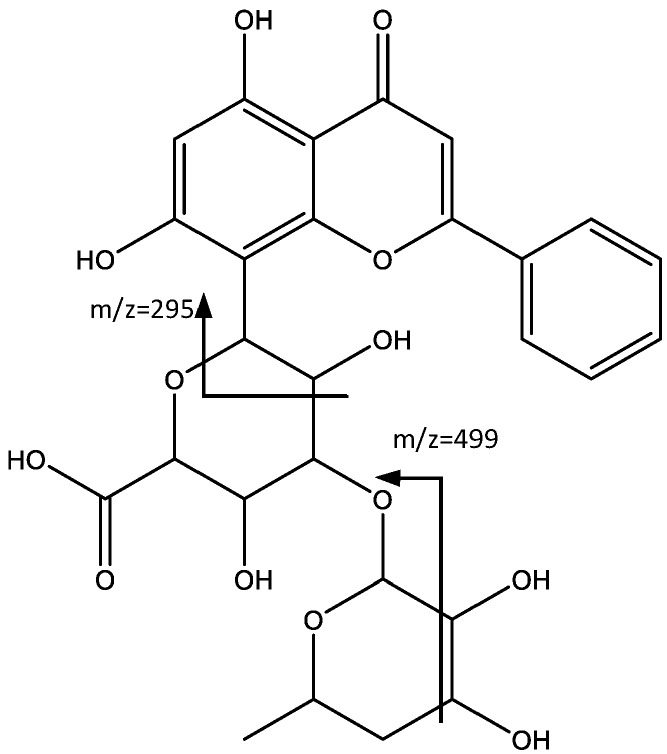
In a similar way, peak 17 shows a negative molecular ion peak [M − H]− at m/z 559.1428 (C27H27O13), which in turn shows a fragmentation pattern similar to the previous peak. For instance, the peak at m/z 499 originating from the loss of 60 amu [M − H-60] [33], and the peak at m/z 295, arises from a fragmentation similar to the one described by Wu et al. [34], with an oxygen in the aglycone instead of the glycoside, which leads to the tentative identification of chrysin (17) as aglycone (Figure 11).
Figure 11.
Structure and fragments of chrysin derivative (17).
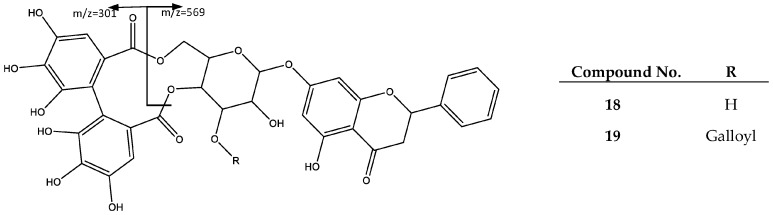
Peaks 18 and 19 were assigned to ellagitannin derivatives of flavanone pinocembrin, previously reported in the genus Phyllanthus [6]. In fact, peak 18 (Rt = 56.67 min) shows a negative molecular ion peak [M − H]− at m/z 719.1230 (C35H27O17), and was assigned to Pinocembrin 7-O-[4″,6″-(S)-hexahydroxydibenzoyl]-b-d-glucopiranoside (18), for which the main fragment at m/z = 301 corresponds to the HDDP group [35]. Peak 19 (Rt = 61.31 min) shows a negative molecular ion peak [M − H]− = 719.1230 (C42H31O21) consistent with Pinocembrin 7-O-[3″-O-galloyl-4″,6″-(S)-hexahydroxydibenzoyl]-β-d-glucopiranoside (19), which besides the fragment at m/z 301 previously explained, exhibits fragments at m/z 569 due to HDDP loss, and at m/z 827 after the loss of a CO2 molecule, as shown in Figure 12 [25].
Figure 12.
Structure and fragments of flavanone pinocembrin derivatives (18), (19).
Finally, Peak 20 (Rt = 70.53 min) shows a negative molecular ion peak [M − H]− at m/z 193.0490, and MS2 fragments at m/z 178 [M − H-H2O], 149 [M − H-CO2], and 134 [M − H-H2O-CO2], corresponding to ferulic acid [36].
2.2. Isolation and Characterization of Justicidin B
As described in the Methods and Materials section, fractioning with Sephadex LH-20 of the non-polar extract of P. acuminatus, allowed the isolation of a compound characterized by 1H-NMR, 13C-NMR and 2D-(HMBC, HSQC, COSY)-NMR data coincident with reports from the literature [37,38,39] on aryl naphthalene lignan Justicidin B (20) C21H16O6 (Figure 13), a metabolite previously reported in P. acuminatus [7] and extensively studied because of its high antitumoral potential against different cancer cell lines [40], thus enabling a comparison of bioactivities among this metabolite and the phenolic extract, as described in the following sections.
Figure 13.
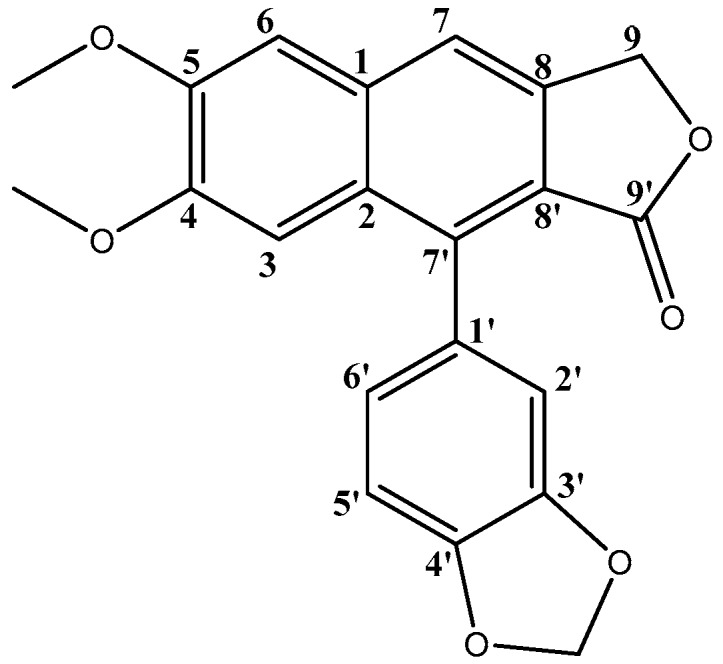
Justicidin B (20) structure.
2.3. Antioxidant Activity
DPPH is widely used as an indicator of antioxidant activity, although is not present naturally in the body, because it is a reliable assay that can give a preliminary appraisal of the antioxidant capacity of the agent under test [41]. As a second assay with better correlation to physiological radicals, our study used the ORAC method, which measures antioxidant scavenging activity against a peroxyl radical derived from 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH), a hydrophilic alkyl peroxyl radical similar to the ones formed in biological systems, particularly in the process of lipid peroxidation [42].
Results obtained for antioxidant activity evaluation of both P. acuminatus extract and isolated Justicidin B metabolite, as well as ascorbic acid used as positive control, through DPPH and ORAC methodologies, are summarized in Table 2.
Table 2.
Antioxidant activity of P. acuminatus phenolic extract and Justicidin B metabolite.
| Sample | DPPH 1,2 IC50 (μg/mL) ± SD | ORAC 1,2 (mmol TE/mg Extract) ± SD |
|---|---|---|
| P. acuminatus extract | 0.15 a ± 0.01 | 2.76 a ± 0.05 |
| Justicidin B | 14.28 b ± 0.30 | 0.95 b ± 0.02 |
| Ascorbic Acid | 3.74 c ± 0.05 | 1.62 c ± 0.07 |
1 Different superscript letters in the same column indicate differences are significant at p < 0.05 using ANOVA with a Tukey post hoc test; 2 Results represent average ± standard deviation from three independent runs for each sample (n = 3).
In both methodologies, P. acuminatus extract showed better results than Justicidin B and ascorbic acid, used as positive control. While no previous data has been reported for ORAC, the DPPH IC50 value of 14.28 µg/mL obtained for Justicidin B is in agreement with the moderate scavenging result previously reported in the literature in similar assay conditions [43]. Of special interest is the DPPH value for P. acuminatus extract (IC50 = 0.15 µg/mL), which is superior to DPPH values reported for Phyllanthus species in similar assay conditions, such as those reported for a phenolic-enriched extract of P. niruri (IC50 = 6.40 µg/mL) [44], and aqueous extracts of P. emblica (IC50 = 6.99–7.72 µg/mL) [45].
As described, the phenolic characterization of P. acuminatus extract indicated flavonoids and ellagitannins as main components, which have been previously reported as metabolites of interest for their antioxidant activity. For instance, flavones and ellagitannins have shown better antioxidant properties than other important phenolics, such as anthocyanins [46]; and, on the other hand, ellagitannins-enriched extracts exhibited greater inhibition than crude extracts from R. idaeus and R. chamaemorus [47].
2.4. Cytotoxicity Evaluation
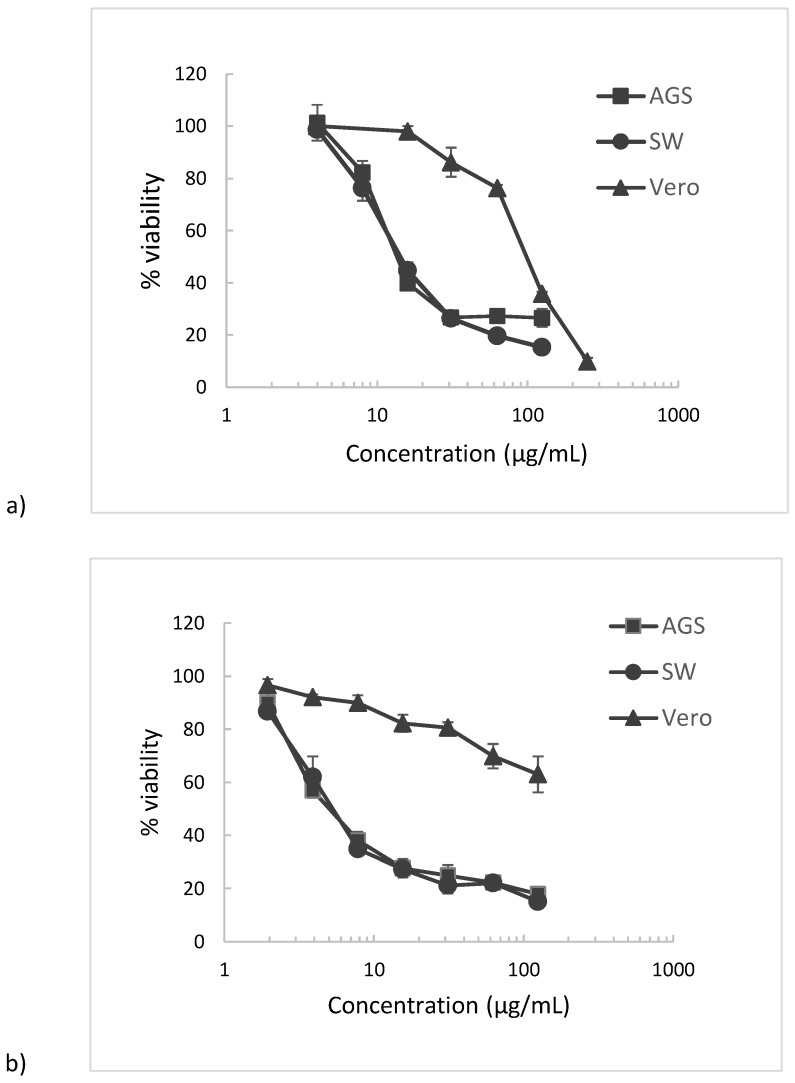
Table 3 summarizes the IC50 values for the cytotoxicity of P. acuminatus phenolic extract and Justicidin B on AGS human gastric adenocarcinoma, SW620 human colon adenocarcinoma, and Vero monkey normal epithelial kidney cell lines. IC50 values indicate that there is significant difference (one-way analysis of variance, ANOVA) between cytotoxicity values (p < 0.05) against gastric AGS adenocarcinoma cells and SW620 adenocarcinoma cells between P. acuminatus extract and Justicidin B metabolite, showing superior values for the plant extract. Also, Figure 14 shows dose-response curves.
Table 3.
Cytotoxicity of P. acuminatus extract and Justicidin B to gastric (AGS) and colon (SW620) adenocarcinoma cells as well as to control Vero cells.
| Sample | IC50 (µg/mL) ± S.D. 1 | ||
|---|---|---|---|
| AGS 2 | SW620 2 | Vero 2 | |
| P. acuminatus extract 3 | 11.3 a,* ± 0.7 (SI = 5.4) | 10.5 a,* ± 0.5 (SI = 20.1) | 226.6 a,◊ ± 4.2 |
| Justicidin B 3 | 19.5 b,* ± 2.2 (SI = 4.2) | 24.8 b,* ± 2.1 (SI = 21.5) | 104 b,◊ ± 6 |
1 Results are presented as mean ± SD of three independent experiments. 2 Different superscript letters in the same column indicate differences are significant at p < 0.05 using ANOVA with a Tukey post hoc test. 3 Different superscript signs in the same row indicate differences are significant at p < 0.05 using ANOVA with a Tukey post hoc test.
Figure 14.
Cytotoxicity dose–response curves 1 of (a) Justicidin B and (b) P. acuminatus extract treatments on AGS and SW620 tumor cell lines and Vero normal cell lines. 1 Results are presented as mean ± SD of three independent experiments.
In fact, the results obtained in the cytotoxic assay show that P. acuminatus extract exhibits a strong cytotoxic response in both AGS and SW620 cell lines (IC50 = 11.3 µg/mL and 10.5 µg/mL respectively). The isolated Justicidin B IC50 values indicate also good cytotoxicity (AGS: 19.5 µg/mL, SW: 24.8 µg/mL), however, the extract values are higher than those of Justicidin B for both in vitro cell phenotypes. Regarding normal Vero cells, ANOVA shows significant difference for both samples when comparing to cancer cells with lower cytotoxicity results, with the extract results indicating better values (IC50 = 226.6 µg/mL) compared to Justicidin B (IC50 = 104 µg/mL).
Regarding aryl naphthalene lignans, a similar study on Justicidin B using MTT assay with 72 h of incubation, showed moderate cytotoxicity on MDA-MB-231 and MCF-7 breast cancer cell lines (converted values of 38.91 and 14.09 μg/mL, respectively) [14] While no previous data has been reported for P. acuminatus extracts, the assessment of cytotoxicity has been performed for other species in the genus Phyllanthus. For instance, a study in similar MTT assay conditions performed on P. niruri phenolic extract indicated IC50 values of 113.2 µg/mL on the same cell line, AGS gastric, and 145.2 µg/mL on SW620 colon tumor cells [44], while on other adherent cell lines, MTT assay evaluation of hydro-methanolic extracts of P. amarus and P. virgatus on Hep G2 hepatic carcinoma, with measurements performed after 24 h of incubation, reported lack of cytotoxicity (IC50 > 250 µg/mL) [48].
Concerning the selectivity of the cytotoxic activity of samples between cancerous and normal cells, our results indicate significant differences (ANOVA, p < 0.05) between IC50 values for both samples in both AGS and SW620 adenocarcinoma cell lines, compared to IC50 values for normal Vero cells. When evaluating the selectivity index (SI), defined as the ratio of IC50 values of normal cells to cancer cells (AGS or SW620), P. acuminatus extract showed the highest selectivity result for SW620 colon cancer cells (SI = 21.5), followed closely for AGS gastric cancer cells (SI = 20.1), while Justicidin B results showed SI of 5.4 and 4.2 respectively, thus, lower selectivity values than P. acuminatus extract for both AGS and SW620 cancer cell lines. When comparing these selectivity results with reports using similar MTT assay conditions, Justicidin B displayed non-specific cytotoxicity in normal peripheral blood mononuclear cells (PMBC), HepG2 hepatoma, and PC3 prostrate tumor cell lines [40], while P. niruri phenolic extract showed selectivity values of 2.2 and 2.8, respectively, for AGS gastric and SW colon cancer cells [44]. Although selectivity (SI ≥ 3) and cytotoxicity (IC50 ≤ 20) results for P. acuminatus extract on AGS gastric and SW620 colon cancer cells fall within the parameters of the National Cancer Institute (NCI) [49,50] for plant extracts to be considered promising in the preliminary assay, further studies on mechanisms of action are needed.
The fact that polyphenols have control over several signaling pathways that affect different processes at cellular and tissue level, makes a synergic approach a conducive way to interpret the events mediated by polyphenolic profile. For instance, referring to the type of phenolic compounds present in the P. acuminatus extract, studies on quercetin and kaempferol flavonoid derivatives indicated that these compounds bioactivities—such as anti-oxidant, anti-inflammatory, and anti-proliferative—could act in a synergistic manner, and may repress carcinogenesis and cancer progression [51,52,53]. On the other hand, the flavone chrysin has been reported to induce apoptosis in several cancer cells [54], such as U87-MG malignant glioma [55], and U937 leukemia cells [56,57]. Also, flavone apigenin derivatives, such as vitexin, have been reported to induce apoptosis and inhibit autophagy on hepato-carcinoma cells (HCC) [58]. With respect to the other main group of polyphenolic compounds present in P. acuminatus extract, ellagitannins, such as pinocembrin derivatives, have shown to act on multiple molecular targets that are related to the inflammatory pathway in cancer cells [59]. In clinical studies, pomegranate juice rich in ellagitannins administered to patients with prostate cancer led to a decrease in the rate of rise of prostate specific antigen after primary treatment [60].
The diverse structure contents of P. acuminatus enriched phenolic extract could suggest the preliminary results on cytotoxicity and selectivity towards AGS gastric and SW620 colon cancer cells, are due to a multi-targeted, synergistic effect, however, further studies are needed to elucidate mechanisms of action.
3. Materials and Methods
3.1. Materials, Reagents, and Solvents
Phyllanthus acuminatus leaf plant material was acquired from a local Agricultural Producers Association in the Caribbean region of Costa Rica. The plant was identified with the support of the Costa Rican National Herbarium, and a voucher is deposited there. P. acuminatus material was rinsed in water and cut into small pieces. Subsequently, it was dried in a stove at 40 °C until completely dry, and after being ground, it was preserved at −5 °C. Among reagents, 2,2′-azobis(2-methyl-propionamidine)-dihydrochloride (AAPH), fluorescein, ascorbic acid, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin-EDTA solution and Sephadex LH-20 gel were provided by Sigma-Aldrich (St. Louis, MO, USA), while amphotericin B, penicillin–streptomycin, and Eagle’s minimum essential medium (MEM, 10% fetal bovine serum), were purchased from Life Technologies (Carlsbad, CA, USA). AGS human gastric adenocarcinoma, SW 620 human colorectal adenocarcinoma and Vero monkey normal epithelial kidney cell lines were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). DMSO was provided by Sigma-Aldrich (St. Louis, MO, USA), while MTBE, dichloromethane, chloroform, and methanol were purchased from Baker (Center Valley, PA, USA).
3.2. Extraction of P. acuminatus Secondary Metabolites
The process followed for obtaining a phenolic-enriched extract from P. acuminatus was formerly described on other plants by our group [61], involving different organic solvents to optimize separation of compounds in a preliminary effort for characterization. Briefly, plant dried material was first extracted in a mixture of methyl tert-butyl ether (MTBE) and methanol (MeOH) 90:10 (v/v) at 25 °C during 30 min in ultrasound. Afterwards, it was extracted for 24 h in order to obtain a non-polar extract of the samples. After filtration, the extraction was repeated once. The extracts were combined, and the solvents evaporated in a rotavapor to dryness, and subsequently washed with MeOH in order to extract any polyphenols. The residual plant material was extracted with MeOH at 25 °C during 30 min in ultrasound, and then extracted for 24 h. After filtration, the extraction was repeated twice. The three methanol extracts were combined with the previous MeOH washings, and were evaporated in a rotavapor to dryness. Finally, the dried extract was washed with hexane, MTBE and chloroform consecutively in order to obtain a phenolic rich extract.
On the other hand, the non-polar extract after the MeOH washings, was concentrated and dissolved in CH2Cl2/MeOH 70:30 (v/v) and extracted twice with water 50:50 (v/v). The aqueous phase was extracted twice with CH2Cl2 50:50 (v/v), and the organic phase was evaporated in a rotavapor to dryness. The extract was fractionated using Sephadex LH-20, allowing isolation of 24 mg (0.01%) of lignan 20, with the following NMR data. 1H-NMR (CDCl3) δ (ppm) 3.82 (s, 3H), 4.05 (s, 3H), 5.38 (d, J = 1.0 Hz, 2H), 6.06 (d, J = 1.5 Hz, 1H), 6.10 (d, J = 1.5 Hz, 1H), 6.83 (dd, J = 7.9, 1.7 Hz, 1H), 6.86 (d, J = 1.7 Hz, 1H), 6.97 (d, J = 7.9 Hz, 1H), 7.11 (s, 1H), 7.19 (s, 1H), 7.70 (s, 1H); and 13C-NMR (CDCl3) δ (ppm) 55.99 (MeO-C5), 56.22 (MeO-C4), 68.19 (C9), 101.40 (O–CH2–O), 106.01 (C6), 106.16 (C3), 108.38 (C5′), 110.73 (C6′), 118.42 (C7), 123.63 (C2′), 128.56 (C1′), 129.02 (C1), 133.33 (C2), 139.68 (C8′), 139.82 (C8), 147.70 (C4′), 147.74 (C3′), 150.23 (C5), 151.98 (C4), 170.12 C9′).
3.3. UPLC-ESI—MS Analysis
The UPLC-MS system used to analyze the phenolic composition of the P. acuminatus extract consisted of an LTQ Orbitrap XL mass spectrometer with an Accela 1250 binary Pump, a PAL HTC Accela TMO autosampler, a PDA detector (ThermoFisher Scientific, San Jose, CA, USA), and a G1316A column compartment (Agilent, Palo Alto, CA, USA). Separation was carried out on a Hypersil Gold AQ RP—C18 UHPLC column (200 mm × 2.1 mm i.d., 1.9 µm, Thermo Fisher Scientific, Waltham, Massachusetts) with an UltraShield pre-column filter (Analytical Scientific Instruments, Richmond, CA, USA) at a flow rate of 0.3 mL/min. Mobile phases A and B consist of a combination of 0.1% formic acid in water (v/v), and 0.1% formic acid in acetonitrile (v/v), respectively. The linear gradient is from 4% to 20% B (v/v) at 40 min, to 35% B at 70 min and to 100% B at 71 min, and held at 100% B to 75 min. The UV–vis spectra were acquired from 200–700 nm.
Negative electrospray ionization mode was used, and the conditions were set as follows: sheath gas, 70 (arbitrary units); aux and sweep gas, 15 (arbitrary units); spray voltage, 4.8 kV; capillary temperature, 300 °C; capillary voltage, 15 V; tube lens, 70 V. The mass range was from 100 to 2000 amu with a resolution of 30,000, FTMS AGC target at 2 × 105, FT-MS/MS AGC target at 1 × 105, isolation width of 1.5 amu, and max ion injection time of 500 ms. The most intense ion was selected for the data-dependent scan to offer their MS2 to MS5 product ions, respectively, with a normalization collision energy at 35%.
3.4. DPPH Radical-Scavenging Activity
A solution of 2,2-diphenyl-1-picrylhidrazyl (DPPH) (0.25 mM) was prepared using methanol as solvent. Next, 0.5 mL of this solution were mixed with 1 mL of sample or ascorbic acid used as positive control, at different concentrations, and incubated at 25 °C in the dark for 30 min. DPPH absorbance was measured at 517 nm. Blanks were prepared with 1 mL of each sample concentration and 0.5 mL of methanol instead of DPPH. The percentage of the radical-scavenging activity of the sample was plotted against its concentration to calculate IC50, which is the amount of sample required to reach the 50% radical-scavenging activity. The samples were analyzed in three independent assays each one in triplicate.
3.5. ORAC Antioxidant Activity
The ORAC (Oxygen Radical Absorbance Capacity) antioxidant activity was determined following a method previously described [62] using fluorescein as a fluorescence probe. Briefly, 0.05 g of each sample to be measured is mixed with 10 mL of methanol/HCl (1000:1, v/v) and, if needed, sonicated until complete dissolution, for 5 min. Afterwards, the mixture was centrifuged and filtered. The reaction was carried out in 75 mM phosphate buffer (pH 7.4) at 37 °C. The final assay mixture contained AAPH (12 mM), fluorescein (70 nM), and 20 µL of either Trolox (1–8 µM) or sample (extract or Justicidin B) at different concentrations. Blanks were prepared adding 20 µL of phosphate buffer instead of sample, and positive controls were prepared adding 20 µL of ascorbic acid at different concentrations, instead of sample. Fluorescence was recorded every minute for 98 min in black 96-well untreated microplates (Nunc, Denmark), using a Polarstar Galaxy plate reader (BMG Labtechnologies GmbH, Offenburg, Germany) with 485-P excitation and 520-P emission filters. Fluostar Galaxy software version 4.11-0 (BMG Labtechnologies GmbH, Offenburg, Germany) was used to measure fluorescence. Fluorescein was diluted from a stock solution (1.17 mM) in 75 mM phosphate buffer (pH 7.4), while AAPH and Trolox solutions were freshly prepared. Samples were evaluated in three independent experiments with different concentrations of each sample (or positive control) analyzed in triplicate.
Fluorescence values obtained were normalized to the curve of the blank (no antioxidant). The area under the fluorescence decay curve (AUC) was calculated from the normalized curves, and the net AUC was then established. Subsequently, regression equation between antioxidant concentration and net AUC was obtained. Finally, ORAC value was estimated by dividing the slope of the latter equation by the slope of the Trolox line. ORAC values were expressed as mmol of Trolox equivalents (TE)/g of extract.
3.6. Evaluation of Cytotoxicity
The AGS, SW620, and Vero cells were grown in MEM (10% FBS) in the presence of glutamine (2 mmol/L), penicillin (100 IU/mL), streptomycin (100 µg/L), and amphotericin B (0.25 µg/m), at 37 °C, in a humidified atmosphere (5% CO2). Briefly, 100 µL of 1.5 × 105 cells/mL (suspension) were seeded overnight into 96-well plates to reach 100% confluency. Subsequently, the cells were exposed for 48 h to 50 µL of samples in concentrations varying 1.5–500 µg/mL in MEM (DMSO 0.1% v/v). Controls to establish 100% of viability were prepared, adding 50 µL of MEM (DMSO 0.1% v/v) instead of samples. Afterwards, the medium was eliminated, cells were washed with PBS (100 µL) and incubated with 100 µL of a MTT solution (0.5 mg/mL, final concentration) in PBS, for 2 h at 37 °C. Then, MTT was removed, and the formazan crystals were dissolved in 100 µL of ethanol 95%. Absorbance was read at 570 nm in a microplate reader. DMSO was diluted in media in the same way as the extracts and incubated with the cells for 48 h to be used as control. Dose–response curves were established, and IC50 was calculated. Samples were tested in three independent experiments with different doses of each sample analyzed in triplicate.
4. Conclusions
This study represents the first detailed MS analysis of phenolic-enriched extract from P. acuminatus. Using UPLC-ESI-MS techniques, 20 phenolic compounds were identified, comprising eight flavonoids, (two flavanones—pinocembrin isomers and six derivatives from apigenin, chrysin, quercetin and kaempferol); seven ellagitannins, two flavan-3-ols (prodelphinidin B dimer and (epi)gallocatechin); and three phenolic acids (ellagic acid, trimethylellagic acid and ferulic acid). These findings constitute the first report on the diversity of phenolics in P. acuminatus. DPPH and ORAC antioxidant methods were evaluated both in the extract and the isolated aryl naphthalene lignan Justicidin B, with P. acuminatus extract showing a particularly high value (IC50 = 0.15 µg/mL). Based on these results, due to antioxidant properties of flavonoids resulting in antipathogenic effects that can be non-specific [18], further studies on these properties could be promising. P. acuminatus phenolic-enriched extract also showed cytotoxicity and selectivity on AGS gastric and SW620 colon adenocarcinoma cell lines with SI > 20 for both cell lines when compared to normal cells, with lower values (SI > 4) for Justicidin B. Since polyphenols could work in a synergistic manner, purification or fractioning of P. acuminatus phenolic extract could be of interest to further evaluate the structure–bioactivity relationship. Also, studies using solvents adequate for human health consumption, such as ethanolic or aqueous phenolic-enriched extracts, are important to assess the potential anticancer bioactivity of P. acuminatus phenolic extracts and components. The results on the cell lines studied could suggest potential health effects of P. acuminatus extract on gut-related diseases, considering that polyphenols are metabolized by the gut [19,63]. However, further research is required to understand the mechanisms of action and pathways.
Acknowledgments
This work was partially funded by a grant from FEES-CONARE (Ref 115B0653). Authors also thank financial support from the University of Costa Rica and the Technological Institute of Costa Rica. The authors thank Alonso Quesada from the Costa Rican National Herbarium for his support with the voucher.
Author Contributions
Mirtha Navarro, Elizabeth Arnaez and Ileana Moreira participated in the conception and design of the study. Mirtha Navarro, Silvia Quesada, Gabriela Azofeifa, Pei Chen, Felipe Vargas and Diego Alvarado were involved in technical work and interpretation of data. Elizabeth Arnaez and Ileana Moreira participated in plant collection, identification and initial plant treatment. Mirtha Navarro drafted the manuscript that was revised and approved by all the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Wagner H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia. 2011;82:34–37. doi: 10.1016/j.fitote.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Hammel B.E., Grayum M.H., Herrera C., Zamora N. Manual de Plantas de Costa Rica. Volume 5 Missouri Botanical Garden Press; St. Louis, MO, USA: 2010. [Google Scholar]
- 3.Arnaez E., Moreira I., Navarro M. Manejo Agroecológico de Nueve Especies de Plantas de Uso Tradicional Cultivadas en Costa Rica. FLACSO Latin American Institute; San Jose, Costa Rica: 2016. pp. 1–85. [Google Scholar]
- 4.Muñoz V., Sauvain M., Bourdy G., Callapa J., Rojas I., Vargas L., Tae A., Deharo E. The search for natural bioactive compounds through a multidisciplinary approach in Bolivia. Part II. Antimalarial activity of some plants used by Mosetene Indians. J. Ethnopharmacol. 2000;69:139–155. doi: 10.1016/S0378-8741(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 5.Pouvelle B., Farley P.J., Long C.A., Taraschi T.F. Taxol arrest the development of blood-stage Plasmodium falciparum in vitro and Plasmodium chabaudi adami in malaria-infected mice. J. Clin. Investig. 1994;94:413–417. doi: 10.1172/JCI117338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi X., Hua L., Gao K. Chemical Constituents of the Plants from the Genus Phyllanthus. Chem. Biodivers. 2014;11:364–395. doi: 10.1002/cbdv.201200244. [DOI] [PubMed] [Google Scholar]
- 7.Pettit G.R., Cragg G.M., Gust D., Peter Brown P. The isolation and structure of phyllanthostatins 2 and 3. Can. J. Chem. 1982;60:544–546. doi: 10.1139/v82-080. [DOI] [Google Scholar]
- 8.Pettit G.R., Cragg G.M., Suffness M.I. Antineoplastic agents. 104. Isolation and structure of the Phyllanthus acuminatus vahl (Euphorbiaceae) glycosides. J. Org. Chem. 1984;49:4258–4266. doi: 10.1021/jo00196a029. [DOI] [Google Scholar]
- 9.Pettit G.R. Evolutionary Biosynthesis of Anticancer Drugs. In: Ojima I., Vite G.D., Heinz K., editors. Anticancer Agents: Frontiers in Cancer Chemotherapy. American Chemical Society; Washington, DC, USA: 2001. pp. 16–42. [Google Scholar]
- 10.Funes L., Laporta O., Cerdán-Calero M., Micol V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids. 2010;163:190–199. doi: 10.1016/j.chemphyslip.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez-Aguilera A., Rull A., Rodriguez-Gallego E., Riera-Borrull M., Luciano-Mateo F., Camps J., Menéndez J., Joven J. Mitochondrial dysfunction: A basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediat. Inflamm. 2013:135698. doi: 10.1155/2013/135698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo W., Kong E., Meydani M. Dietary polyphenols, inflammation and cancer. Nutr. Cancer. 2009;61:807–810. doi: 10.1080/01635580903285098. [DOI] [PubMed] [Google Scholar]
- 13.Vasilev N., Elfahmi B.R., Kayser O., Momekov G., Konstantinov S., Ionkova I. Production of justicidin B, a cytotoxic arylnaphthalene lignan from genetically transformed root cultures of linum leonii. J. Nat. Prod. 2006;69:1014–1017. doi: 10.1021/np060022k. [DOI] [PubMed] [Google Scholar]
- 14.Momekov G., Konstantinov S., Dineva I., Ionkova I. Effect of justicidin B—A potent cytotoxic and pro-apoptotic arylnaphtalene lignan on human breast cancer-derived cell lines. Neoplasma. 2011;58:320–325. doi: 10.4149/neo_2011_04_320. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.C., Hsin W.C., Ko F.N., Huang Y.L., Ou J.C., Teng C.M. Antiplatelet arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod. 1996;59:1149–1150. doi: 10.1021/np960443+. [DOI] [PubMed] [Google Scholar]
- 16.Simmonds M.S. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/S0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 17.Dai G.H., Nicole M., Andary C., Martinez C., Bresson E., Boher B., Daniel J.F., Geiger J.P. Flavonoids accumulate in cell walls, middle lamellae and callose-rich papillae during an incompatible interaction between Xanthomonas campestris pv. malvacearum and cotton. Physiol. Mol. Plant Pathol. 1996;49:285–306. doi: 10.1006/pmpp.1996.0055. [DOI] [Google Scholar]
- 18.Sisa M., Bonnet S.L., Ferreira D., van der Westhuizen J.H. Photochemistry of flavonoids. Molecules. 2010;15:5196–5245. doi: 10.3390/molecules15085196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monagas M., Urpi-Sarda M., Sanchez-Patán F., Llorach R., Garrido I., Gómez-Cordoves C., Andres-Lacueva C., Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 20.Dincheva I., Badjakov I., Kondakova V., Dobson P., Mcdougall G., Stewart D. Identification of the phenolic components of bulgarian raspberry cultivars by LC-ESI-MS. Int. J. Agric. Sci. Res. 2013;3:127–138. [Google Scholar]
- 21.Yang B., Kortesniemi M., Liu P., Karonen M., Salminen J.P. Analysis of Hydrolyzable Tannins and Other Phenolic Compounds in Emblic Leafflower (Phyllanthus emblica L.) Fruits by High Performance Liquid Chromatography−Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2012;60:8672–8683. doi: 10.1021/jf302925v. [DOI] [PubMed] [Google Scholar]
- 22.Zhu M., Dong X., Guo M. Phenolic Profiling of Duchesnea indica Combining Macroporous Resin Chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules. 2015;20:22463–22475. doi: 10.3390/molecules201219859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dou J., Lee V.S., Tzen J.T., Lee M.R. Identification and Comparison of Phenolic Compounds in the Preparation of Oolong Tea Manufactured by Semifermentation and Drying Processes. J. Agric. Food Chem. 2007;55:7462–7468. doi: 10.1021/jf0718603. [DOI] [PubMed] [Google Scholar]
- 24.He W., Xia W., Chen J. Isolation and structure elucidation of phenolic compounds in Chinese olive (Canarium album L.) fruit. Eur. Food Res. Technol. 2008;226:1191–1196. doi: 10.1007/s00217-007-0653-5. [DOI] [Google Scholar]
- 25.Lee J.H., Johnson J.V., Talcott S.T. Identification of Ellagic Acid Conjugates and Other Polyphenolics in Muscadine Grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005;53:6003–6010. doi: 10.1021/jf050468r. [DOI] [PubMed] [Google Scholar]
- 26.Era M., Matsuo Y., Shii T., Saito Y., Tanaka T., Jiang Z.H. Diastereomeric Ellagitannin Isomers from Penthorum chinense. J. Nat. Prod. 2015;78:2104–2109. doi: 10.1021/acs.jnatprod.5b00439. [DOI] [PubMed] [Google Scholar]
- 27.Tuominen A., Sundman T. Stability and Oxidation Products of Hydrolysable Tannins in Basic Conditions Detected by HPLC/DAD–ESI/QTOF/MS. Phytochem. Anal. 2013;24:424–435. doi: 10.1002/pca.2456. [DOI] [PubMed] [Google Scholar]
- 28.Bresciani L., Calani L., Cossu M., Mena P., Sayegh M., Ray S., Del Rio D. (Poly)phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition. 2015;3:11–19. doi: 10.1016/j.phanu.2015.01.001. [DOI] [Google Scholar]
- 29.Savić I., Nikolić V.D., Savić I.M., Nikolić L.B., Jović M.D., Jović M.D. Quantitative analysis of the green tea extract using ESI-MS method. Adv. Technol. 2014;3:30–37. [Google Scholar]
- 30.Fabre N., Rustan I. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001;12:707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Cha S., Yeung E.S. Colloidal graphite-Assisted laser desorption/ionization MS and MS of small molecules. 2. Direct Profiling and MS imaging of small metabolites from fruits. Anal. Chem. 2007;79:6575–6584. doi: 10.1021/ac0706170. [DOI] [PubMed] [Google Scholar]
- 32.Sun J., Chen P. UHPLC/HRMS Analysis of African Mango (Irvingia gabonensis) Seeds, Extract and Related Dietary Supplements. J. Agric. Food Chem. 2012;60:8703–8709. doi: 10.1021/jf302703u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao B., Caulfield J.C., Hamilton M.L., Pickett J.A., Midega C.A.O., Khan Z.R., Wang J., Hooper A.M. Biosynthesis of natural and novel C-glycosylflavones utilizing recombinant Oryza sativa C-glycosyltransferase (OsCGT) and Desmodium incanum root proteins. Phytochemistry. 2016;125:73–87. doi: 10.1016/j.phytochem.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Wu L.Z., Zhang X.P., Xu X.D., Ding W.L. Characterization of aromatic glycosides in the extracts of Trollius species by ultra high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013;75:55–63. doi: 10.1016/j.jpba.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y.L., Chen C.C., Hsu F.L., Chen C.F. Two Tannins from Phyllanthus tenellus. J. Nat. Prod. 1998;61:523–524. doi: 10.1021/np970428k. [DOI] [PubMed] [Google Scholar]
- 36.Callipo L., Cavaliere C., Fuscoletti V., Gubbiotti R., Samperi R., Laganà A. Phenilpropanoate identification in young wheat plants by liquid chromatography/tandem mass spectrometry: Monomeric and dimeric compounds. J. Mass Spectrom. 2010;45:1026–1040. doi: 10.1002/jms.1800. [DOI] [PubMed] [Google Scholar]
- 37.Charlton J.L., Oleschuk C.J., Chee G.L. Hindered rotation in arylnaphthalene lignans. J. Org. Chem. 1996;61:3452–3457. doi: 10.1021/jo952048e. [DOI] [Google Scholar]
- 38.Okigawa M., Maeda T., Kawano N. The isolation and structure of three new lignans from Justicia procumbens linn. var. Leucantha honda. Tetrahedron. 1970;26:4301–4305. doi: 10.1016/S0040-4020(01)93074-1. [DOI] [Google Scholar]
- 39.Mohagheghzadeh A., Schmidt T.J., Alfermann A.W. Arylnaphthalene lignans from in vitro cultures of Linum austriacum. J. Nat. Prod. 2002;65:69–71. doi: 10.1021/np0102814. [DOI] [PubMed] [Google Scholar]
- 40.Hemmati S., Seradj H. Justicidin B: A Promising Bioactive Lignan. Molecules. 2016;21:820. doi: 10.3390/molecules21070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur I., Geetha T. Screening Method for antioxidants—A review. Mini Rev. Med. Chem. 2006;6:305–312. doi: 10.2174/138955706776073448. [DOI] [PubMed] [Google Scholar]
- 42.Ou B., Hampsch M., Flanagan J., Deemer E., Prior R., Huang D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002;50:2772–2777. doi: 10.1021/jf011480w. [DOI] [PubMed] [Google Scholar]
- 43.Rao Y.K., Geethangili M., Fang S.H., Tzeng Y.M. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: A comparative study. Food Chem. Toxicol. 2007;45:1770–1776. doi: 10.1016/j.fct.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Navarro M., Moreira I., Arnaez E., Quesada S., Azofeifa G., Alvarado D., Monagas M.J. Proanthocyanidin Characterization, Antioxidant and Cytotoxic Activities of Three Plants Commonly Used in Traditional Medicine in Costa Rica: Petiveria alliaceae L., Phyllanthus niruri L. and Senna reticulata Willd. Plants. 2017;6:50. doi: 10.3390/plants6040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iamsaard S., Arun S., Burawat J., Sukhorum W., Wattanathorn J., Nualkaew S., Sripanidkulchai B. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllantus emblica L.) aqueous extracts. J. Zheijiang Univ. Sci. B. 2014;15:405–408. doi: 10.1631/jzus.B1300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullen W., McGinn J., Lean M.E., MacLean M.R., Gardner P., Duthie G.G., Yokota T., Crozier A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002;50:5191–5196. doi: 10.1021/jf020140n. [DOI] [PubMed] [Google Scholar]
- 47.Kähkönen M., Kylli P., Ollilainen V., Salminen J.P., Heinonen M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012;60:1167–1174. doi: 10.1021/jf203431g. [DOI] [PubMed] [Google Scholar]
- 48.Poompachee K., Chudapongse N. Comparison of the antioxidant and cytotoxic activities of Phyllanthus virgatus and Phyllanthus amarus extracts. Med. Princ. Pract. 2012;21:24–29. doi: 10.1159/000331596. [DOI] [PubMed] [Google Scholar]
- 49.Ramasamy S., Wahab N.A., Abidin N.Z., Manickam S., Zakaria Z. Growth Inhibition of Human Gynecologic and Colon Cancer Cells by Phyllanthus watsonii through Apoptosis Induction. PLoS ONE. 2012;7:e34793. doi: 10.1371/journal.pone.0034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahavorasirikul W., Wiratchanee M., Vithoon V., Wanna C., Arunporn I., Kesara N. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement. Altern. Med. 2010;10:1–8. doi: 10.1186/1472-6882-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajesh E., Sankari L.S., Malathi L., Krupaa J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015;7:181–183. doi: 10.4103/0975-7406.155895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo G.L., Russo M., Spagnuolo C. The pleiotropic flavonoid quercetin: From its metabolism to the inhibition of protein kinases in chronic lymphocytic leukemia. Food Funct. 2014;5:2393–2401. doi: 10.1039/C4FO00413B. [DOI] [PubMed] [Google Scholar]
- 53.Men K., Duan X., Wei X.W., Gou M.L., Huang M.J., Chen L.J., Qian Z.Y., Wei Y.Q. Nanoparticle-delivered quercetin for cancer therapy. Anticancer Agents Med. Chem. 2014;14:826–832. doi: 10.2174/1871520614666140521122932. [DOI] [PubMed] [Google Scholar]
- 54.Khoo B., Chua S., Balaram P. Apoptotic Effects of Chrysin in Human Cancer Cell Lines. Int. J. Mol. Sci. 2010;11:2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parajuli P., Joshee N., Rimando A.M., Mittal S., Yadav A.K. In vitro anti-tumor mechanisms of various Scutellaria extracts and constituent flavonoids. Planta Med. 2009;75:41–48. doi: 10.1055/s-0028-1088364. [DOI] [PubMed] [Google Scholar]
- 56.Monasterio A., Urdaci M.C., Pinchuk I.V., Lopez-Moratalla N., Martinez-Irujo J.J. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr. Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 57.Woo K.J., Jeong Y.J., Park J.W., Kwon T.K. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem. Biophys. Res. Commun. 2004;325:1215–1222. doi: 10.1016/j.bbrc.2004.09.225. [DOI] [PubMed] [Google Scholar]
- 58.He J.D., Wang Z., Li S.P., Xu Y.J., Yu Y., Ding Y.J., Yu W.L., Zhang R.X., Zhang H.M., Du H.Y. Vitexin suppresses autophagy to induce apoptosis in hepatocellular carcinoma via activation of the JNK signaling pathway. Oncotarget. 2016;7:84520–84532. doi: 10.18632/oncotarget.11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heber D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008;269:262–268. doi: 10.1016/j.canlet.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 60.Pantuck A.J., Leppert J.T., Zomorodian N., Aronson W., Hong J., Barnard R.J., Seeram N., Liker H., Wang H., Elashoff R., et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 61.Navarro Hoyos M., Sánchez-Patán F., Murillo Masis R., Martín-Álvarez P.J., Zamora Ramirez W., Monagas M.J., Bartolomé B. Phenolic Assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, Stem, Bark and Wood Extracts. Molecules. 2015;20:22703–22717. doi: 10.3390/molecules201219875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davalos A., Gomez-Cordoves C., Bartolome B. Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) assay. J. Agric. Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Patan F., Monagas M., Moreno-Arribas M.V., Bartolome B. Determination of microbial phenolic acids in human faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011;59:2241–2247. doi: 10.1021/jf104574z. [DOI] [PubMed] [Google Scholar]