Abstract
MicroRNAs are widely involved in the pathogenesis of cardiovascular diseases through regulating gene expression via translational inhibition or degradation of their target mRNAs. Recent studies have indicated a critical role of microRNA-206 in myocardial ischaemia-reperfusion (I/R) injury. However, the function of miR-206 in myocardial I/R injury is currently unclear. The present study was aimed to identify the specific role of miR-206 in myocardial I/R injury and explore the underlying molecular mechanism. Our results revealed that the expression level of miR-206 was significantly decreased both in rat I/R group and H9c2 cells subjected to hypoxia/reoxygenation (H/R) compared with the corresponding control. Overexpression of miR-206 observably decreased infarct size and inhibited the cardiomyocyte apoptosis induced by I/R injury. Furthermore, bioinformatics analysis, luciferase activity and western blot assay proved that Gadd45β (growth arrest DNA damage-inducible gene 45β) was a direct target gene of miR-206. In addition, the expression of pro-apoptotic-related genes, such as p53, Bax and cleaved caspase3, was decreased in association with the down-regulation of Gadd45β. In summary, this study demonstrates that miR-206 could protect against myocardial I/R injury by targeting Gadd45β.
Keywords: apoptosis, cardiomyocyte, Gadd45β, ischaemia–reperfusion, miR-206
INTRODUCTION
Myocardial ischaemia-reperfusion (I/R) injury represents a cardiovascular damage or dysfunction after myocardial ischaemia or cardiac surgery in patients with coronary heart disease, which is a common cardiovascular problem and a major cause of death (Ke et al., 2016; Moens et al., 2005; Thind et al., 2015). The molecular mechanisms responsible for myocardial I/R injury have been studied for decades. Myocardial I/R injury is mediated by oxidative stress, intracellular Ca2+ overload, rapid restoration of physiological pH upon reperfusion, the mitochondrial permeability transition pore, and exaggerated inflammation (Hausenloy and Yellon, 2013; Wei et al., 2013; Yu et al., 2012; 2015). In addition, apoptosis plays a crucial role in the initiation and development of myocardial I/R injury (Baines, 2011; Eefting et al., 2004). Apoptosis causes loss of contractile cells, compensatory hypertrophy of myocardial cells, and reparative fibrosis (Swynghedauw, 1999).
MicroRNAs (miRNAs) are a large family of small noncoding RNAs that regulate gene expression through binding to their target mRNAs and subsequently lead to translational repression or degradation (Lee et al., 2003; Lin and Gregory, 2015). Numerous studies revealed that miRNAs are involved in various heart diseases, such as hypertrophy, remodelling, heart failure, and arrhythmia, suggesting the therapeutic potential for miRNA treatment of cardiovascular disease. Ectopic expression of miRNAs was also reported in I/R injury. As one of the most studied and best characterized miRNAs to date, miR-206 has been identified as a tumour suppressor in different types of cancers (Goren et al., 2014; Qin et al., 2013). Recently, miR-206 attracted considerable attention in the field of ischaemic heart disease (IHD). It was demonstrated that miR-206 is involved in high glucose-induced cardiomyocyte apoptosis through targeting Hsp60 (heat shock protein 60) (Shan et al., 2010). MiR-206 is involved in apoptotic cell death in myocardial infarction by posttranscriptional repression of IGF-1 (insulin-like growth factor-1) in a rat model (Shan et al., 2009). MiR-206 had a role in endothelial progenitor cells (EPCs) function and potential pathogenesis of coronary artery disease by targeting PIK3C2α (phosphoinositide-3-kinase class II alpha) (Tang et al., 2015). In addition, miR-206 significantly suppressed the viability and invasion of EPCs and promoted the apoptosis of EPCs in coronary artery disease patients (Wang et al., 2016). However, the role of miR-206 in myocardial I/R injury has never been illustrated.
In the present study, we established a rat model of myocardial I/R injury and cardiomyocyte hypoxia/reoxygenation (H/R) model to investigate the effect and underlying mechanism of miR-206 in myocardial I/R injury. Here, we found that miR-206 was significantly downregulated in rats’ myocardial tissues after I/R injury and H9c2 cells subjected to H/R. Ectopic expression of miR-206 decreased infarct size, creatine kinase (CK) and lactic acid dehydrogenase (LDH) activity and meanwhile inhibited the cardiomyocyte apoptosis induced by I/R injury. There had a same phenomenon in H9c2 cells subjected to H/R. Furthermore, we found that Gadd45β is one of the direct target genes of miR-206, and confirmed that miR-206 might exert its effects on the inhibition of cardiomyocyte apoptosis, CK and LDH activity by downregulating Gadd45β in H9c2 cells subjected to H/R. Thus, these results demonstrate that the miR-206 could protect against myocardial I/R injury by targeting Gadd45β.
MATERIAL AND METHODS
Cell culture and hypoxia/reoxygenation (H/R) model
The H9c2 cells were obtained from the Bank of the Chinese Academy of Sciences (China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, USA) containing 10% (v/v) foetal bovine serum (FBS, HyClone, USA) in a humidified atmosphere of 5% CO2 at 37°C. A modular incubator (Model 3131, Forma Scientific, USA) was used to induce hypoxia by exposing the H9c2 cells to 1% O2, 94% N2, and 5% CO2 for 24 h. After hypoxia, the cells were exposed to 95% air, 5% CO2, and 37°C for 12 h. Cells in the control group were cultured under normoxia.
Rat I/R model
All protocols were approved by the Ethics Committee on Animal Research at Shanghai Jiao Tong University School of Medicine in accordance with the Guide for Care and Use of Laboratory Animals published by the US NIH. Male Sprague-Dawley rats (280–300 g) were anesthetized with sodium pentobarbital (40 mg/kg) by intraperitoneal injection and fixed for endotracheal intubation by the use of a small animal respirator. The skin was disinfected with iodine after removing hair from praecordium. A longitudinal incision was made from the third to fourth ribs, separating the pectoralis major muscle, serratus anterior muscle and pectoralis minor muscle, and exposing the heart. Then, a 6-0 silk suture was placed around the l-2 cm of the root of the left anterior descending coronary artery (LAD). The suture was loosened after occlusion for 30 min, which was followed by 2 h reperfusion of LAD. Sham controls underwent the same procedures except that the LAD was not ligated. After reperfusion, the blood and heart samples were collected for further research (Yamamoto et al., 2005). All surgical procedures were carried out under aseptic conditions.
Quantitative real-time PCR
Total RNA from the myocardial and cell samples was extracted using Trizol Reagent (Invitrogen, USA) according to the manufacturer’s protocol. The cDNA was synthesized using the PrimeScript RT reagent (Takara, China). Briefly, 1 μg cDNA product was used for the amplification template in a 20 μl reaction mixture using SYBR Green PCR Master mix kit (Takara, China). PCR was performed on a 7500 fast real-time PCR System (Applied Biosystems, USA). GAPDH and U6 were used as internal controls for mRNAs and miRs, respectively. The primers sequences of miR-206, Gadd45β mRNA and corresponding controls are listed in Table 1. Relative expression levels of miR-206 and Gadd45β mRNA were calculated using the 2−ΔΔCt method. Each experiment was performed at least thrice.
Table 1.
Primers sequences of miR-206, U6, Gadd45β mRNA and GAPDH
| Gene | Primers sequences |
|---|---|
| miR-206 | F: 5′-GGGTGGAATGTAAGGAAGT-3′ |
| R: 5′-TGCGTGTCGTGGAGTC-3′ | |
| U6 | F: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| R: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ | |
| Gadd45β | F: 5′-GAGGCGGCCAAACTGATGAAT-3′ |
| R: 5′-CGCAGCAGAACGACTGGAT-3′ | |
| GAPDH | F: 5′-TATCGGACGCCTGGTTAC-3′ |
| R: 5′-CGTTCAAGTTGCCGTGTC-3′ |
Note: miR-206, microRNA-206; Gadd45β, growth arrest DNA damage-inducible gene 45β; GAPDH, glyceraldehyde phosphate dehydrogenase; F, forward; R, reverse.
Tansfection of Ad-miR-206 into the heart of rat in vivo
A left parasternal incision was made through the fourth and fifth ribs, and the pericardium was opened to expose the heart. A 26-gauge needle was used to inject 100 μl of Ad-miR-206 (1 × 109 PFU, Riobio, China) or Ad-Scramble (1 × 109 PFU, Riobio, China) into eight different sites of the left ventricular wall. The chest was closed after injection, and the rat was allowed to recover. A tracheotomy was performed and cannulated with a PE-90 catheter. Artificial respiration was provided by a respirator (Taimeng, China) with a tidal volume of 1 ml/100 g and a frequency of 60 strokes/min.
Infarct size determination
Briefly, 2% Evans blue dye (wt/vol, Sigma-Aldrich, USA) was injected into the vena cava to identify the area of myocardial perfusion at the end of the myocardial reperfusion. The left ventricle was isolated by removing the right ventricle and washing out the remaining blood. The left ventricle was then cut into 2-mm thick slices and stained with 1% tri-phenyltetrazolium chloride (TTC) for 10 min at 37°C. The red region was identified as the live area, whereas the infarct area was identified as the non-stained region. Finally, the AAR and infarct size were calculated, and the infarct size was expressed as a percentage of the AAR.
CK (creatine kinase) and LDH (lactic acid dehydrogenase) activity analysis
CK and LDH activities of serum and cell culture medium were detected with commercial kits (Jiancheng, China) according to the manufacturer’s instructions.
Oligonucleotides transfection
MiR-206 mimic (miR-206 sequence: 5′-ACAUGCUUCUUUA UAUCCUCAU-3′), mimic control (miR-con sequence: 5′-AAGAGGUAGGUAGAGAGCGCGU-3′), miR-206 inhibitor (anti-miR-206 sequence: 5′-AUGAGGAUAUAAAGAAGCAU GU-3′) and inhibitor control (anti-miR-con sequence: 5′-ACGCGCUCUCUACCUACCUCUU-3′) as well as small interfering RNAs (siRNA) of Gadd45β and scrambled siRNA were obtained from the Genepharma (Shanghai, China). H9c2 cells were seeded in six-well plates until 50% confluence and transiently transfected with these oligonucleotides using Lipo2000 (Invitrogen, USA) according to the manufacturer’s instructions. After 6 h, the supernatant was removed, and fresh medium was added. Cells were collected after culture for an additional 48 h for further studies.
Flow cytometry analysis
To detect the apoptosis of the cells, the cultured cardiomyocytes were digested with trypsin, washed, and dual-stained with AV and PI according to the manufacturer’s instructions. Then, the cells were analysed by flow cytometry on a BD FACSCalibur (Becton Dickinson Co., Becton Dickinson Co., USA).
Terminal dUTP nick end labelling (TUNEL) assay
A TUNEL kit (Roche, Germany) was used to assess the myocardial apoptosis following the manufacturer’s instructions. Briefly, tissue sections were deparaffinized and rehydrated then preprocessed with permeabilization solution freshly prepared with 0.1% Triton X–100 and 0.1% sodium citrate for 15 minutes. The TdT reaction was performed for 1 h at 37°C in a humidified chamber. Finally, sections were stained with DAPI for 5 min. Three slides from each block were evaluated for the percentage of apoptotic cells and four fields on each slide were examined at the border areas using a defined rectangular field area with 20× magnification. The apoptosis rate refers to the number of apoptotic cells divided by the total number of cardiomyocytes ×100%.
Luciferase reporter gene assay
To construct reporter vectors bearing miRNA-target sites, the wild-type or mutant Gadd45β mRNA 3′UTR sequence was amplified by polymerase chain reaction (PCR) and cloned into pGL3-promoterconstruct (Promega), thus obtaining the wild-type or mutant Gadd45β 3′UTR firefly luciferase reporter gene. The cardiomyocytes were co-transfected with 80 ng wild-type or mutant Gadd45β 3′UTR firefly luciferase reporter gene, 40 ng Renilla luciferase reference plasmid pRL-TK and miR-206 per controls (final concentration, 20 nM). Forty-eight hours after transfection, the luciferase activity was measured by a dual-luciferase reporter gene assay system (Promega, USA). The final data were the ratio of firefly fluorescent value to Renilla fluorescence value.
Western blot
Total protein was extracted from the myocardial tissues and cells. The proteins were separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to the nitrocellulose membranes (Bio-Rad, USA). The blots were then incubated with 5% milk to block the non-specific binding sites for 2 h. The membranes were incubated with the primary antibodies overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody (Abcam, USA). Finally, the ECL System (Bio-Rad, USA) was used to visualize the protein bands with ChemiDoc™ XRS Plus luminescent image analyser (Bio-Rad, USA).
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde and cut into 4-μm sections. After dewaxing and hydration, sections were incubated in 0.1% Triton for permeabilization. Sections were blocked with 3% BSA for 1 h at room temperature. Subsequently, sections were incubated with an antibody against Gadd45β (Abcam, USA) at 4°C overnight and then stained with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. Counterstaining was performed with haematoxylin.
Statistical analysis
Data are presented as the median ± SD. Student t-test was performed to compare differences in two groups. Comparisons among multiple samples were made by the Statistical Analysis System software (v.9.1.3; SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
MiR-206 was significantly down-regulated in rat myocardial tissues under I/R treatment and H9c2 cells subjected to H/R
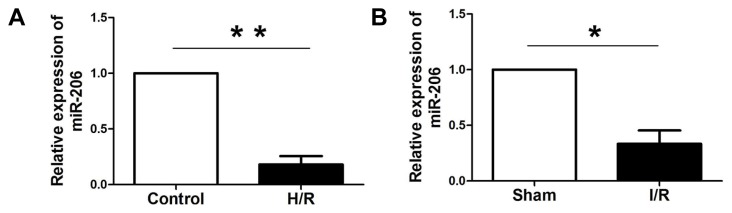
Quantitative real-time PCR was performed to detect the expression level of miR-206 in rat myocardial tissues under I/R treatment and H9c2 cells subjected to H/R. As noted in Figs. 1A and 1B, the level of miR-206 was notably decreased in rat myocardial tissues after I/R injury and H9c2 cells subjected to H/R compared with that in the sham and control groups, respectively.
Fig. 1. miR-206 was significantly down-regulated in rat myocardial tissue under I/R treatment and H9c2 cells subjected to H/R.
(A) Quantitative real-time PCR was performed to detect the expression of miR-206 in cardiomyocytes under normal and H/R condition. (B) The expression of miR-206 in myocardial tissue under I/R treatment and normal condition. The results were presented as the mean ± SD, n = 6, *P < 0.05, **P < 0.001.
MiR-206 decreased the infarct size and inhibited myocardial apoptosis induced by I/R
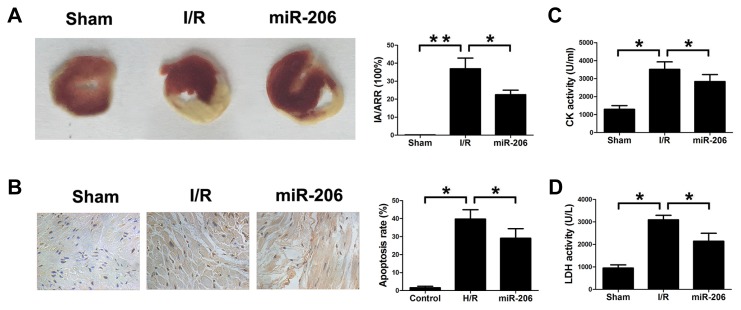
To investigate whether miR-206 exerts a protective role against myocardial I/R injury, we increased the expression of miR-206 by transfecting the heart of rat with Ad-miR-206 in vivo. Infarct size was indicated by the area at risk (IA/AAR). As shown in Fig. 2A, infarct size was significantly increased in I/R group compared with the sham group. In contrast, overexpression of miR-206 decreased the infarct size remarkably compared to the I/R group. Figure 2B shows that I/R markedly induced myocardial apoptosis compared with the sham group and overexpression of miR-206 inhibited the myocardial apoptosis induced by I/R injury. Furthermore, we evaluated the release of serum CK and LDH. As shown in Figs. 2C and 2D, the CK and LDH activity was observably increased in I/R group compared with the sham group. However, overexpression of miR-206 significantly attenuated the CK and LDH activity.
Fig. 2. miR-206 decreased infarct size and inhibited myocardial apoptosis in rat I/R model.
(A) Infarct myocardial tissue was stained by Evans Blue and TTC. Dark blue area indicates non-ischaemic tissue. Red-stained area indicates area at risk. White area indicates infracted tissue. The ratio of IA (infarct area) and AAR (area at risk) was calculated. (B) TUNEL assay was performed to evaluate myocardial apoptosis. (C) CK activity was detected with ELISA in different groups. (D) LDH activity was detected with ELISA in different groups. The results were presented as the mean ± SD, n = 6, *P < 0.05, **P < 0.001.
MiR-206 inhibited H9c2 cell apoptosis subjected to H/R
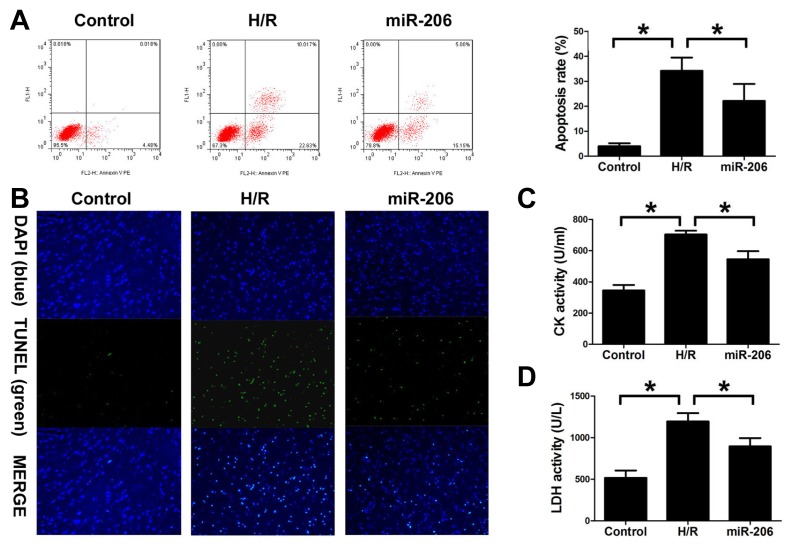
To determine whether miR-206 plays a role in the protection against H/R-induced cell injury, we established the H9c2 cells subjected to H/R model. Flow cytometry and TUNEL analysis both demonstrated that miR-206 mimic transfection significantly suppressed cell apoptosis of H9c2 induced by H/R injury (Figs. 3A and 3B). In addition, the CK and LDH activity of H9c2 cells culture medium was obviously increased in H/R group compared with control group. We also examined the effect of increased expression of miR-206 on the CK and LDH activity. In contrast, H/R-induced CK and LDH activity in H9C2 cells was significantly attenuated by miR-206 mimic transfection. (Figs. 3C and 3D). Our results suggest that overexpression of miR-206 may play a critical protective role in H/R-induced cellular injury.
Fig. 3. miR-206 inhibited apoptosis of H9c2 cells subjected to H/R.
(A) Flow cytometry was used to investigate apoptosis in H9c2 cells under normal and H/R conditions. (B) TUNEL was used to investigate H9c2 cell apoptosis under normal and H/R conditions. (C) CK activity of the cell supernatant was detected with ELISA in different groups. (D) LDH activity of cell supernatant was detected with ELISA in different groups. The results were presented as the mean ± SD, n = 6, *P<0.05.
MiR-206 directly targeted Gadd45β
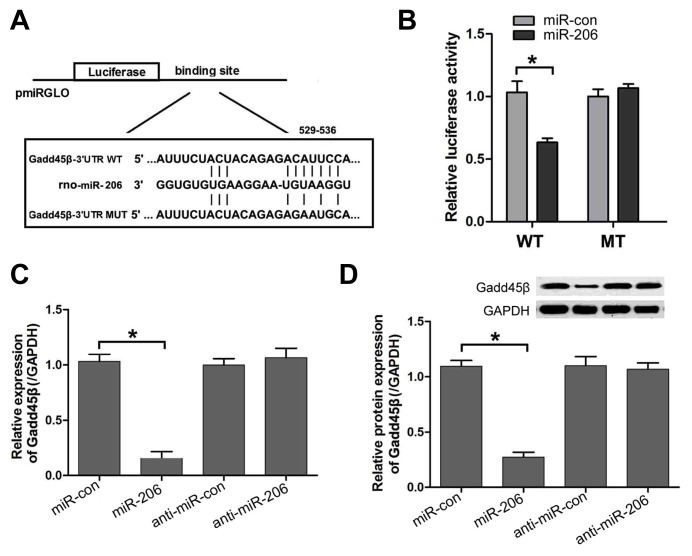
In order to clarify the molecular mechanism underlying miR-206 exerting its inhibitory effects on the cell apoptosis of cardiomyocytes after H/R injury, we predicted potential targets of miR-206 using the TargetScan online tool, and identified a conserved binding site for miR-206 in the 3′ UTR region of the Gadd45β gene. To determine whether miR-206 targeted Gadd45β through its 3′ untranslated region (3′UTR), a fragment of wild-type or mutant 3′UTR of Gadd45β was cloned into a firefly luciferase reporter plasmid (Fig. 4A). Luciferase activity assay results indicated that miR-206 attenuated luciferase activity of the reporter containing wild-type Gadd45β 3′UTR but did not change that of the reporter with mutations in the binding sites of Gadd45β 3′UTR (Fig. 4B). Furthermore, to verify miR-206 targeted Gadd45β under physiological conditions, we evaluated the mRNA and protein expression of Gadd45β in cells transfected with miR-206 mimics, mimics control, miR-206 inhibitor or inhibitor control. Our results revealed that overexpression of miR-206 significantly decreased the mRNA and protein expression of Gadd45β, whereas the miR-206 inhibitor had little effect on the mRNA and protein expression of Gadd45β (Figs. 4C and 4D). These findings suggested that miR-206 can directly target Gadd45β in H9c2 cells.
Fig. 4. miR-206 directly targets Gadd45β.
(A) The putative miR-206 binding sequence and the mutant sequence in the 3′UTR of Gadd45. (B) Luciferase reporter activities of vectors carrying luciferase gene and a fragment of Gadd45β 3′UTR containing the wild-type and mutant binding sites of miR-206. (C) Quantitative real-time PCR was performed to detect the expression of Gadd45β mRNA. (D) Western blot was used to detect the level of Gadd45β protein. The results were presented as the mean ± SD, n = 6, *P < 0.05.
Restoration of Gadd45β reversed the anti-apoptosis effect of miR-206 in H9c2 cells subjected to H/R
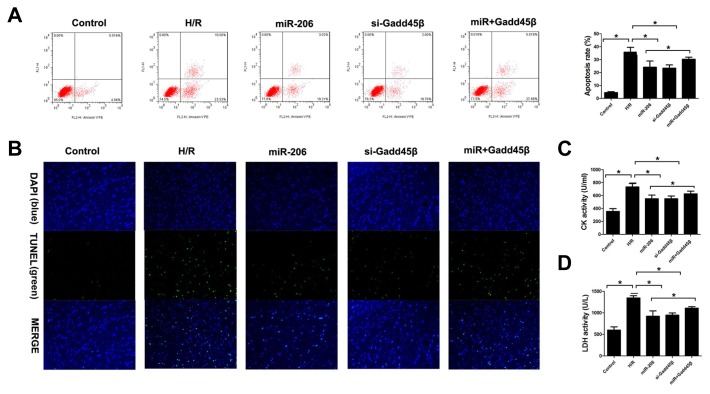
To further explore the role of Gadd45β in cardiomyocytes subjected to H/R injury, short hairpin RNA targeting Gadd45β mRNA (si-Gadd45β) was exploited to specifically inhibit the expression of Gadd45β. Interestingly, depletion of Gadd45β showed the same effect of overexpression of miR-206. Knock-down of Gadd45β expression attenuated cardiomyocyte apoptosis under H/R treatment. Moreover, co-transfection of miR-206 mimics and pcDNA3.1/Gadd45β in H9c2 cells increased the apoptosis rate of cardiomyocytes compared with the co-transfection of miR-206 mimics and pcDNA3.1 vector group (Figs. 5A and 5B). In addition, overexpression of miR-206 and knock-down of Gadd45β expression both inhibited the CK and LDH activity of cardiomyocytes subjected to H/R injury. In contrast, upregulated of Gadd45β expression significantly reversed the effect of overexpression of miR-206 (Figs. 5C and 5D). These results determined that miR-206 exerts its anti-apoptotic role through targeting Gadd45β.
Fig. 5. Restoration of Gadd45β reversed the anti-apoptosis effect of miR-206 in H9c2 cells subjected to H/R.
(A) Flow cytometry was used to investigate H9c2 cell apoptosis under normal and H/R conditions. (B) TUNEL was used to investigate H9c2 cell apoptosis under normal and H/R condition. (C) CK activity of the cell supernatant was detected with ELISA in different groups. (D) LDH activity of cell supernatant was detected with ELISA in different groups. The results were presented as the mean ± SD, n = 6, *P < 0.05.
The expression of Gadd45β was negatively correlated with expression of miR-206 and influenced the expressions of apoptosis-related genes
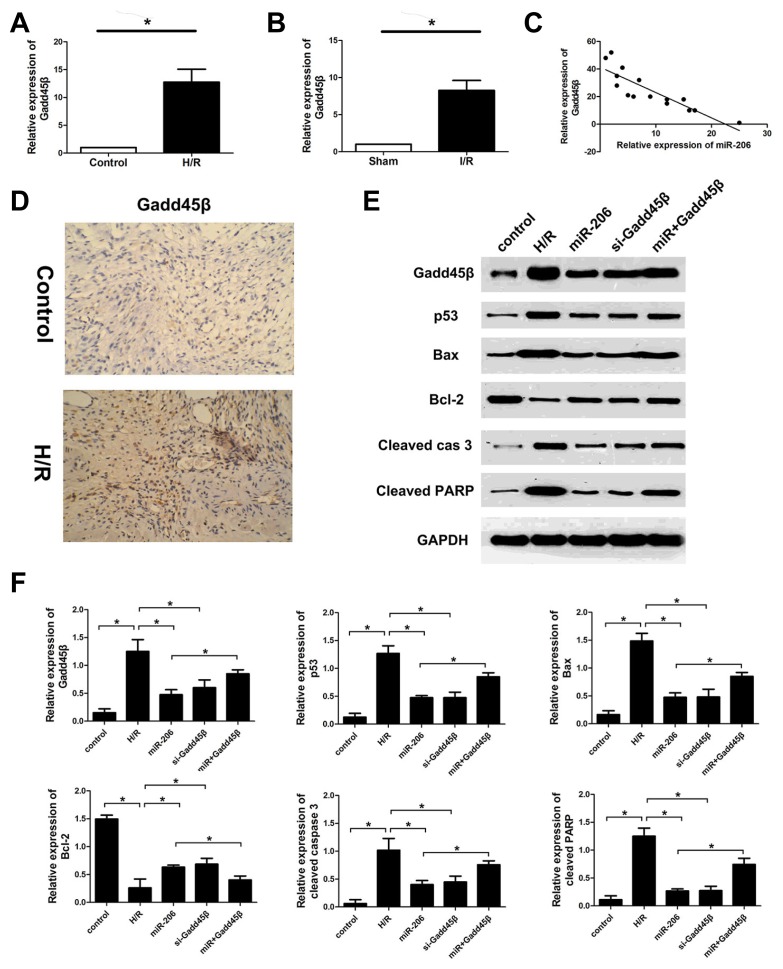
Given that Gadd45β exerts an essential role in cardiomyocyte apoptosis, we investigated the expression levels of Gadd45β in different myocardial tissues and H9c2 cells. The results revealed that the expression of Gadd45β was upregulated in myocardial tissues after I/R injury and H9c2 cells subjected to H/R, and meanwhile was negatively correlated with the level of miR-206 (Figs. 6A and 6C). Representative immunohistochemistry stain was shown in Fig. 6D. Our present results suggest that miR-206 inhibited the cardiomyocyte apoptosis induced by H/R injury through targeting Gadd45β. Furthermore, to investigate the mechanisms of miR-206 inhibition on the apoptosis of cardiomyocytes, we analyzed the effects of miR-206 on the expressions of apoptosis-related genes: p53, Bax, Bcl-2, cleaved caspase-3 and cleaved PARP (Poly (adenosine diphosphate-ribose) polymerase). The results indicated that increased expression of miR-206 by transfecting miR-206 mimics and downregulated expression of Gadd45β by transfecting si-Gadd45β in the H9c2 cells after H/R injury observably decreased the expression of pro-apoptotic proteins (p53, Bax, cleaved caspase-3 and cleaved PARP), however increased the expression of anti-apoptotic protein (Bcl-2) compared to the controls. Meanwhile, upregulated expression of Gadd45β by co-transfecting miR-206 mimics and pcDNA3.1/Gadd45β had an opposite phenomenon in the H9c2 cells after H/R injury (Figs. 6E and 6F).
Fig. 6. Gadd45β exhibits a negative correlation with miR-206 in H9c2 cells and influences the expression of apoptosis-related protein.
(A, B) Quantitative real-time PCR was performed to detect the expression of miR-206 in cardiomyocytes under normal and H/R condition. (C) The level of miR-206 is negatively correlated with the expression of Gadd45β. (D) Immunocytochemistry stain for Gadd45β in rat myocardial tissues subjected to normal and I/R treatment. (E, F) Western blot was used to evaluate the protein expression level. The results were presented as the mean ± SD, n = 6, *P < 0.05.
DISCUSSION
MiR-206 is located on chromosome 6 in a bicistronic cluster, which is specifically expressed in muscles and closely related to myogenic differentiation (Dai et al., 2016). An increasing number of studies have focused on the function of miR-206 in heart disease. MiR-206 is up-regulated by YAP (Yes-associated protein) and mediates YAP-induced hypertrophy and cardiomyocyte survival (Yang et al., 2015). MiR-206 overexpression and miR-206-mediated inhibition of TIMP-3 (tissue inhibitor of metalloproteinase-3) involve in enhancing left ventricular (LV) function and attenuating LV remodelling of chronically failing hearts induced by HMGB1 (High Mobility Group Box-1) injection (Limana et al., 2011). In addition, miR-206 accelerates cardiomyocyte apoptosis through regulating Hsp60 expression post-transcriptionally (Shan et al., 2010). In consideration of the potential effects of miR-206 in the diagnosis and treatment of heart disease, we investigated whether miR-206 exerts a role in myocardial ischaemia-reperfusion (I/R) injury in vivo and in vitro.
In the present study, the results indicated that the expression level of miR-206 is significantly decreased, however the rate of cardiomyocyte apoptosis is increased in myocardial tissues after I/R injury and H9c2 cells subjected to H/R compared to per controls, suggesting that miR-206 may play an essential role during myocardial I/R injury. Futhermore, overexpression of miR-206 obviously decreased infarct size of the heart in rat I/R model, and meanwhile attenuated cardiomyocyte apoptosis in H9c2 cells after H/R injury. Cell injury was also determined by the release of CK and LDH, and overexpression of miR-206 notably inhibited the CK and LDH activity. Therefore, our discovery demonstrated that increased expression of miR-206 significantly decreased myocardial infarct size and prevented cardiomyocyte apoptosis induced by myocardial I/R injury. To the best of our knowledge, this is the first report that miR-206 exerts a protective role in myocardial I/R injury.
MicroRNAs (miRNAs) are a class of small noncoding regulatory RNAs, which can regulate the target genes by promoting mRNA degradation or translation repression (Engels and Hutvagner, 2006). Thus, establishing the interrelationship of miRNA and its target genes may help us to better understand the molecular mechanism underlying myocardial I/R injury progression and provide potential therapeutic targets for the clinical treatment of myocardial I/R injury. The results revealed that Gadd45β is a direct target gene of miR-206 through luciferase reporter assay, and the levels of Gadd45β mRNA and protein are both negatively regulated by miR-206 in H9c2 cells. We also analysed the correlation between the expression of miR-206 and Gadd45β in myocardial tissues after I/R injury. It showed that miR-206 has a negative correlation with the Gadd45β level.
The Gadd45 (growth arrest and DNA damage-inducible 45) family includes Gadd45α, Gadd45β, and Gadd45γ, which are commonly implicated in stress signalling in response to physiological or environmental stressors, resulting in cell-cycle arrest, DNA repair, cell survival, senescence, and apoptosis (Hollander and Fornace, 2002; Salvador et al., 2013; Vairapandi et al., 2002). Gadd45β mediates apoptotic cardiomyocyte death during hypoxia, and knockdown of Gadd45β protects rats against ischaemic heart injury. These results suggest that Gadd45β is a critical mediator of ischaemia/hypoxia-induced apoptotic death in cardiomyocytes (Edwards et al., 2004). In this research, our results showed that knockdown of Gadd45β by transfecting si-Gadd45β can observably weaken the apoptosis in cardiomyocytes treated with H/R, and meanwhile overexpression of Gadd45β has a reverse phenomenon. Furthermore, the CK and LDH activities were decreased in si-Gadd45β group however were increased in overexpression Gadd45β group compared to per controls. Collectively, the data suggest that miR-206 inhibits the apoptosis of cardiomyocytes by negatively regulating Gadd45β.
Myocardial apoptosis contributes to myocardial I/R injury (Narula et al., 1998). p53 is a critical pro-apoptotic factor for myocardial apoptosis during myocardial I/R injury. And, inhibition of p53 expression is an important approach for attenuation of myocardial I/R injury (Matsusaka et al., 2006). p53 regulates and interacts with the apoptotic protein Bax. Bax acts as an antagonist against anti-apoptotic Bcl-2 (Vaseva and Moll, 2009; Zhou et al., 2010). In addition, endomorphin-1 postconditioning can reduce I/R injury and inhibit myocardial cell apoptosis by increasing the Bcl-2/Bax ratio and decreasing the expression of cleaved caspase-3 protein, LDH, IL-6 and CK-MB activities in a rat model (Zhang et al., 2016). The present study demonstrated that miR-206 regulates the expression of apoptosis-related protein such as p53, Bax, Bcl-2, cleaved Caspase-3, and cleaved PARP in cardiomyocytes after H/R injury by targeting Gadd45β. The data suggest that the mechanism by which miR-206 protects against myocardial I/R injury involve the prevention of p53-mediated apoptotic signalling.
In summary, our results reveal that miR-206 is significantly down-regulated in myocardial tissues after I/R injury, and demonstrate the effects of miR-206 on inhibiting the apoptosis of H9c2 cells subjected to H/R, at least partially by negatively regulating Gadd45β and p53-mediated apoptotic signal pathway, which suggests that overexpression of miR-206 protects against myocardial I/R injury, and then provides a possibility to treat I/R injury using a new strategy in the future.
ACKNOWLEDGEMENTS
The study was supported by The 2015 Zhejiang province medical and health science and technology Research fund (2015KYB387), The 2015 Zhejiang Province Traditional Chinese Medicine Scientific Research Fund (2015ZA203), The 2015 Zhejiang Province Traditional Chinese Medicine Scientific Research Fund (2016ZA191), Jiaxing cardiovascular key discipline fund (2014-F07), Zhejiang Province chronic diseases Base fund (2013-JX) and Jiaxing key innovation team fund (2015-CX1).
REFERENCES
- Baines C.P. How and when do myocytes die during ischemia and reperfusion: the late phase. J Cardiovasc Pharmacol Ther. 2011;16:239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- Dai Y., Wang Y.M., Zhang W.R., Liu X.F., Li X., Ding X.B., Guo H. The role of microRNA-1 and microRNA-206 in the proliferation and differentiation of bovine skeletal muscle satellite cells. In vitro Cell Dev Biol Animal. 2016;52:27–34. doi: 10.1007/s11626-015-9953-4. [DOI] [PubMed] [Google Scholar]
- Edwards M.G., Sarkar D., Klopp R., Morrow J.D., Weindruch R., Prolla T.A. Impairment of the transcriptional responses to oxidative stress in the heart of aged C57BL/6 mice. Ann N Y Acad Sci. 2004;1019:85–95. doi: 10.1196/annals.1297.017. [DOI] [PubMed] [Google Scholar]
- Eefting F., Rensing B., Wigman J., Pannekoek W.J., Liu W.M., Cramer M.J., Lips D.J., Doevendans P.A. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Engels B.M., Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25:6163–6169. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- Goren Y., Meiri E., Hogan C., Mitchell H., Lebanony D., Salman N., Schliamser J.E., Amir O. Relation of reduced expression of MiR-150 in platelets to atrial fibrillation in patients with chronic systolic heart failure. Am J Cardiol. 2014;113:976–981. doi: 10.1016/j.amjcard.2013.11.060. [DOI] [PubMed] [Google Scholar]
- Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M.C., Fornace A.J., Jr Genomic instability, centrosome amplification, cell cycle checkpoints and Gadd45a. Oncogene. 2002;21:6228–6233. doi: 10.1038/sj.onc.1205774. [DOI] [PubMed] [Google Scholar]
- Ke Z.P., Xu P., Shi Y., Gao A.M. MicroRNA-93 inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by targeting PTEN. Oncotarget. 2016;7:28796–28805. doi: 10.18632/oncotarget.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Radmark O., Kim S., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Limana F., Esposito G., D’Arcangelo D., Di Carlo A., Romani S., Melillo G., Mangoni A., Bertolami C., Pompilio G., Germani A., et al. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS One. 2011;6:e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Gregory R.I. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka H., Ide T., Matsushima S., Ikeuchi M., Kubota T., Sunagawa K., Kinugawa S., Tsutsui H. Targeted deletion of p53 prevents cardiac rupture after myocardial infarction in mice. Cardiovasc Res. 2006;70:457–465. doi: 10.1016/j.cardiores.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Moens A.L., Claeys M.J., Timmermans J.P., Vrints C.J. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Narula J., Hajjar R.J., Dec G.W. Apoptosis in the failing heart. Cardiol Clin. 1998;16:691–710. ix. doi: 10.1016/s0733-8651(05)70045-x. [DOI] [PubMed] [Google Scholar]
- Qin H., Chen G.X., Liang M.Y., Rong J., Yao J.P., Liu H., Wu Z.K. The altered expression profile of microRNAs in cardiopulmonary bypass canine models and the effects of mir-499 on myocardial ischemic reperfusion injury. J Transl Med. 2013;11:154. doi: 10.1186/1479-5876-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador J.M., Brown-Clay J.D., Fornace A.J., Jr Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv Exp Med Biol. 2013;793:1–19. doi: 10.1007/978-1-4614-8289-5_1. [DOI] [PubMed] [Google Scholar]
- Shan Z.X., Lin Q.X., Deng C.Y., Zhu J.N., Mai L.P., Liu J.L., Fu Y.H., Liu X.Y., Li Y.X., Zhang Y.Y., et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–3600. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Shan Z.X., Lin Q.X., Fu Y.H., Deng C.Y., Zhou Z.L., Zhu J.N., Liu X.Y., Zhang Y.Y., Li Y., Lin S.G., et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem Biophys Res Commun. 2009;381:597–601. doi: 10.1016/j.bbrc.2009.02.097. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- Tang Y., Zhang Y., Chen Y., Xiang Y., Xie Y. Role of the microRNA, miR-206, and its target PIK3C2alpha in endothelial progenitor cell function - potential link with coronary artery disease. FEBS J. 2015;282:3758–3772. doi: 10.1111/febs.13372. [DOI] [PubMed] [Google Scholar]
- Thind G.S., Agrawal P.R., Hirsh B., Saravolatz L., Chen-Scarabelli C., Narula J., Scarabelli T.M. Mechanisms of myocardial ischemia-reperfusion injury and the cytoprotective role of minocycline: scope and limitations. Future Cardiol. 2015;11:61–76. doi: 10.2217/fca.14.76. [DOI] [PubMed] [Google Scholar]
- Vairapandi M., Balliet A.G., Hoffman B., Liebermann D.A. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- Vaseva A.V., Moll U.M. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Ji Y., Cai S., Ding W. MiR-206 suppresses the progression of coronary artery disease by modulating vascular endothelial growth factor (VEGF) expression. Med Sci Monit. 2016;22:5011–5020. doi: 10.12659/MSM.898883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Guan Y., Yin Y., Duan J., Zhou D., Zhu Y., Quan W., Xi M., Wen A. Anti-inflammatory effect of protocatechuic aldehyde on myocardial ischemia/reperfusion injury in vivo and in vitro. Inflammation. 2013;36:592–602. doi: 10.1007/s10753-012-9581-z. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Matsumoto N., Kanazawa M., Fujita M., Takaoka M., Gariepy C.E., Yanagisawa M., Matsumura Y. Different contributions of endothelin-A and endothelin-B receptors in postischemic cardiac dysfunction and norepinephrine overflow in rat hearts. Circulation. 2005;111:302–309. doi: 10.1161/01.CIR.0000153351.86708.F7. [DOI] [PubMed] [Google Scholar]
- Yang Y., Del Re D.P., Nakano N., Sciarretta S., Zhai P., Park J., Sayed D., Shirakabe A., Matsushima S., Park Y., et al. miR-206 mediates YAP-induced cardiac hypertrophy and survival. Circ Res. 2015;117:891–904. doi: 10.1161/CIRCRESAHA.115.306624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Lu M., Wang P., Chen X. Trichostatin A ameliorates myocardial ischemia/reperfusion injury through inhibition of endoplasmic reticulum stress-induced apoptosis. Arch Med Res. 2012;43:190–196. doi: 10.1016/j.arcmed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Yu D., Li M., Tian Y., Liu J., Shang J. Luteolin inhibits ROS-activated MAPK pathway in myocardial ischemia/reperfusion injury. Life Sci. 2015;122:15–25. doi: 10.1016/j.lfs.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang W.P., Zong Q.F., Gao Q., Yu Y., Gu X.Y., Wang Y., Li Z.H., Ge M. Effects of endomorphin-1 postconditioning on myocardial ischemia/reperfusion injury and myocardial cell apoptosis in a rat model. Mol Med Rep. 2016;14:3992–3998. doi: 10.3892/mmr.2016.5695. [DOI] [PubMed] [Google Scholar]
- Zhou M., Liu Z., Zhao Y., Ding Y., Liu H., Xi Y., Xiong W., Li G., Lu J., Fodstad O., et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem. 2010;285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]