ABSTRACT
An important feature of Mycobacterium tuberculosis pathogenesis is the ability to control cell death in infected host cells, including inhibition of apoptosis and stimulation of necrosis. Recently an alternative form of programmed cell death, necroptosis, has been described where necrotic cell death is induced by apoptotic stimuli under conditions where apoptotic execution is inhibited. We show for the first time that M. tuberculosis and TNFα synergise to induce necroptosis in murine fibroblasts via RIPK1-dependent mechanisms and characterized by phosphorylation of Ser345 of the MLKL necroptosis death effector. However, in murine macrophages M. tuberculosis and TNFα induce non-necroptotic cell death that is RIPK1-dependent but independent of MLKL phosphorylation. Instead, M. tuberculosis-infected macrophages undergo RIPK3-dependent cell death which occurs both in the presence and absence of TNFα and involves the production of mitochondrial ROS. Immunocytochemical staining for MLKL phosphorylation further demonstrated the occurrence of necroptosis in vivo in murine M. tuberculosis granulomas. Phosphorylated-MLKL immunoreactivity was observed associated with the cytoplasm and nucleus of fusiform cells in M. tuberculosis lesions but not in proximal macrophages. Thus whereas pMLKL-driven necroptosis does not appear to be a feature of M. tuberculosis-infected macrophage cell death, it may contribute to TNFα-induced cytotoxicity of the lung stroma and therefore contribute to necrotic cavitation and bacterial dissemination.
KEYWORDS: fibroblast, macrophage, MLKL, Mycobacterium tuberculosis, necroptosis, RIPK1, RIPK3
Introduction
Mycobacterium tuberculosis is an intracellular pathogen that resides predominantly in macrophages but also in other cells including dendritic cells and non-professional phagocytes such as fibroblasts, adipocytes and endothelial cells.1,2,3 The bacterium has evolved sophisticated and robust systems to control the biology of its host cell; preserving its replicative niche, avoiding innate antimicrobial mechanisms and manipulating the generation of adaptive immunity.4,5 The fine control of inflammation is particularly important for M. tuberculosis because the bacterium must avoid stimulation of immunity that will limit its infection whilst maintaining the immune driven generation of a necrotic pulmonary granuloma, cavitation and subsequent respiratory transmission.
An important component of M. tuberculosis pathogenesis is the complex control over the mode and timing of host cell death. In general terms, macrophages infected with M. tuberculosis may undergo cell death by two mechanisms, apoptosis or necrosis, with drastically different outcomes for the host and bacterium. Several studies have demonstrated that apoptosis of infected macrophages results in killing of mycobacteria,6–10 probably by efferocytosis of mycobacteria-containing apoptotic bodies and subsequent lysosomal digestion or oxidative killing.11,12 Additionally, macrophage apoptosis stimulates protective T cell responses through the “detour” pathway of antigen presentation.13–15 In contrast, necrosis has been observed to facilitate release of viable bacteria from infected macrophages8,16 which may be taken up by phagocytes attracted by damage associated molecular patterns (DAMPs) released by the necrotic macrophage.17,18 This would allow further intracellular replication producing a cycle of host cell infection, necrosis and reinfection that may represent an important part of the generation of necrotic granuloma. Indeed, stimulation of necrosis is a hallmark of virulent mycobacterial strains16,19,20 and as such stimulation of necrosis is considered a virulence mechanism of M. tuberculosis.
In more specific terms, it has become apparent that M. tuberculosis is able to exert an exquisitely complex control over cell death of the host cell, by having the capacity to both induce and inhibit apoptosis and induce necrosis of the host cell. Apoptosis can be induced by the extrinsic (death receptor) or intrinsic (mitochondrial) pathways. M. tuberculosis is able to inhibit tumour necrosis factor alpha (TNFα)-mediated extrinsic apoptosis via a number of mechanisms including secretion of soluble TNF receptor 2 (sTNFR2),21 downregulation of pro-caspase-8 transcription,22 suppression of caspase-8 expression,23 and upregulation of caspase-8-inhibiting FLIP molecules transcription.22 However, inhibition of the extrinsic pathway occurs in the context of activation of the intrinsic mitochondrial pathway.23 During infection with avirulent mycobacterial strains such as H37Ra, mitochondrial outer membrane permeablisation and release of cytochrome C lead to host cell apoptosis.23 However virulent mycobacterial strains such as H37Rv induce irreversible mitochondrial inner membrane permeablisation, leading to mitochondrial permeability transition (MPT), causing further loss of mitochondrial integrity and function.23 This, plus further mechanisms inhibiting plasma membrane repair,24 leads to necrosis of the macrophage. Thus a model of macrophage infection has emerged where mycobacteria preserve themselves and their macrophage hosts by inhibition of apoptosis and then exit the cell to disseminate further via necrosis.
Necrosis of cells can be induced by a variety of cellular stresses and until recently was considered to be a disordered mode of death that did not involve intracellular signalling pathways. However, in the last decade, highly coordinated modes of programmed necrotic cell death have been described. Necroptosis is a pharmacologically tractable necrosis,25 that can be induced by death receptors including TNFR1,26,27 type I interferon,28 and recognition of pathogen-associated molecular patterns (PAMPS) by pattern recognition receptors including toll-like receptors TLR3, TLR4, and the cytosolic DNA-dependent activator or IFN regulatory factors DAI/ZBP1.29 Necroptosis occurs when cell death is induced by apoptotic stimuli under conditions where apoptotic execution is inhibited. In the case of TNFα-stimulated necroptosis, when TNFα signalling occurs in the presence of caspase inhibition (such as the pan caspase inhibitor zVAD.fmk30), the receptor interacting kinases RIPK1 and RIPK3 associate and become phosphorylated and the pseudokinase mixed lineage kinase domain-like protein (MLKL) is recruited and phosphorylated by pRIPK3.27,31,32 The resulting complex translocates to the nucleus and then to the cell membrane where oligomerized pMLKL has pore forming activity and causes necrotic cell lysis.33 Necroptosis can be inhibited using the RIPK1 inhibitor necrostatin-1 (Nec-1).34,35 RIPK1 also plays a role in cell survival by limiting capsase-8 and TNFR-induced apoptosis,36 as demonstrated by perinatal lethality in ripk1−/− mice.37 Additionally, RIPK1 in complex with RIPK3, FADD and caspase-8 can mediate apoptosis; as such RIPK1 dependence of cell death (such as cell death that can be inhibited by Nec-1) does not in itself confirm necroptosis as a mechanism.25
Necroptosis has been described in a number of pathological conditions with overt inflammatory signatures including Crohn's disease,38 and acts as a defence mechanism against some viral pathogens such as Vaccinia virus and murine cytomegalovirus.26,39,40 Accordingly, viruses have evolved mechanisms to inhibit necroptosis to counter this mechanism.40,41 Necroptosis has also been observed in bacterial infections including Salmonella and Listeria, where its induction was associated with loss of immune control and increased pathogen replication.28,42 More recently, programmed necrosis was reported in Mycobacterium marinum infection of leukotriene A4 hydrolase (LTA4H) mutant zebrafish, which express high levels of TNFα.43
TNFα is a pivotal cytokine in tuberculosis, being essential for protection but, paradoxically, at high levels also responsible for the generation of tissue necrosis, increased tissue pathology and enhanced bacterial growth.43–46 Given that M. tuberculosis is able to inhibit extrinsic apoptosis pathways in the context of high circulating levels of TNFα, our focus was drawn to the potential role of TNFα stimulated necroptosis in M. tuberculosis infection.
Much of what we know about M. tuberculosis host cell death has been gained from studies of macrophage infection. However, it has long been established that infection with M. tuberculosis sensitises fibroblasts to TNFα toxicity.47,48 Of further intrigue, infected fibroblasts are seen in the lungs of cadavers with latent M. tuberculosis infection but are not seen during active M. tuberculosis infection.2 An enhanced sensitivity of infected fibroblasts to TNFα has been postulated to account for this but no mechanistic detail of the mode of cell death has been elucidated.48
Given the prominent role of TNFα in tuberculosis and the capacity of M. tuberculosis to inhibit apoptosis in macrophages and sensitize fibroblasts the toxic effects of TNFα, we hypothesised that necroptosis may occur during M. tuberculosis infection and may represent an important mode of necrotic cell death. Thus, we investigated the occurrence of necroptosis in infected murine macrophage and fibroblast cells in the presence of TNFα. To definitively demonstrate the presence or absence of necroptosis, we determined the occurrence of phosphorylated MLKL in cells exposed to TNFα, and in vivo in murine granulomatous M. tuberculosis lesions.
Results
In order to investigate the presence of necroptosis in response to M. tuberculosis infection, we first compared the capability of murine fibroblasts and human and murine macrophages to undergo necroptosis induced by TNFα + zVAD treatment, as this capacity is not universal in eukaryotic cells.27 Monolayers of cells (plus control wells) were treated with TNFα (plus DMSO control), TNFα+zVAD, or TNFα+zVAD+Nec-1. After 20 hours, cell survival was determined by crystal violet assay. As seen in Fig. 1a-b, primary human monocyte-derived macrophages and U937 macrophages underwent cell death in response to TNFα+zVAD treatment, and this could be inhibited by the RIPK1 inhibitor Nec-1. However, THP-1 macrophages (Fig. 1c) were not sensitive to TNFα+zVAD treatment. In the murine system, L929 fibroblasts (a cell type well characterised in its ability to undergo necroptosis),27 and J774A.1 macrophages underwent cell death in response to TNFα+zVAD treatment, and cell death could be inhibited by Nec-1 (Fig. 1d-e). However, RAW 264.7 macrophages did not share the capacity to undergo cell death stimulated by TNFα+zVAD treatment (Fig. 1f).
Figure 1.
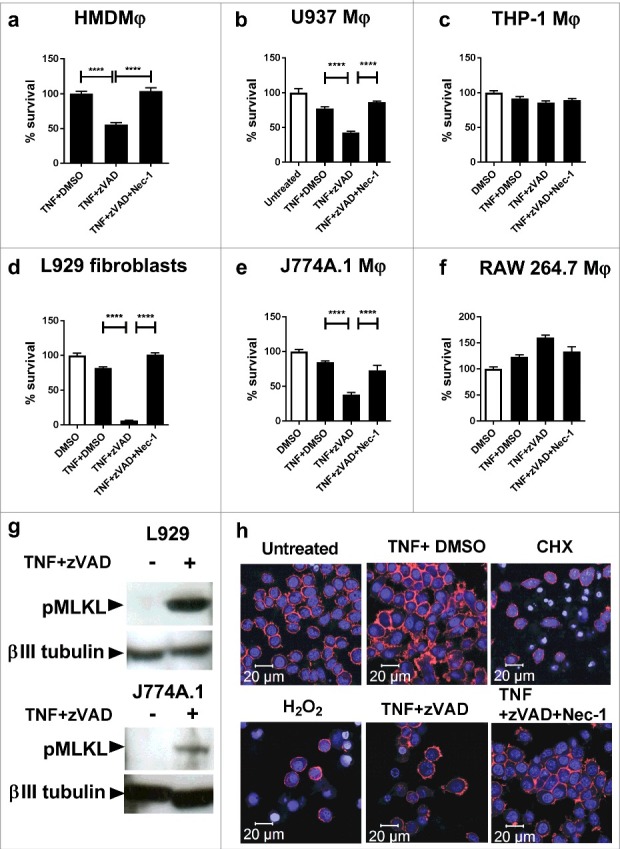
Necroptosis of macrophage cell lines, human MDMs and murine fibroblasts treated with TNFα+zVAD. (a) Human monocyte-derived macrophages (HMDMφ) were treated for 20 hours with 50ng/mL TNF+DMSO, TNF+30 µM zVAD and TNF+30 µM zVAD + 30 µM Nec-1, before measuring cell survival using a crystal violet assay (normalised to TNFα+DMSO treated control cells). Results are mean +/- SEM n = 4, and are representative of 3 independent experiments. (b) U937 macrophages were treated for 20 hours with 50ng/mL TNF, TNF+30 µM zVAD and TNF+30 µM zVAD + 30 µM Nec-1, before measuring cell survival using a crystal violet assay (normalised to untreated control cells). Results are mean +/- SEM n = 10, and are representative of at least 2 independent experiments. (c) THP-1 were treated for 20 hours with 50ng/mL TNF+DMSO, TNF+30 µM zVAD, and TNF + 30 µM zVAD + 30 µM Nec-1, before measuring cell survival using a crystal violet assay (normalised to DMSO treated control cells). Results are mean +/- SEM n = 10. (d) L929 fibroblasts, (e) J774A.1 macrophages and (f) RAW macrophages were treated for 20 hours with 10ng/ml TNF+ DMSO, TNF + 30 µM zVAD, and TNF + 30 µM zVAD + 30 µM Nec-1. Results are mean +/- SEM n = 10, are expressed as a percentage of DMSO treated controls, and representative of 2–3 independent experiments. Statistics are one way ANOVA with Tukey's post-test. ****p<0.0001. (g) Western blot of lysates of L929 fibroblasts and J774A.1 macrophages that had been treated with 10ng/ml TNFα and 25ng/ml TNFα respectively in the presence of 30 µM zVAD.fmk for 18 hours, developed with antibodies against MLKL phosphorylated at Ser345, or βIII tubulin as a loading control. Results are representative of 2–3 independent experiments. (h) J774A.1 macrophages were seeded on glass slide flasks and untreated or treated with cyclohexamide 2.5 µg/ml, 0.5mM H2O2, TNFα 25ng/ml, TNF + 30 µM zVAD, and TNF + 30 µM zVAD + 30 µM Nec-1. After 20 hours, cells were stained with Alexa-568-phalloidin (displayed as red) and counterstained with DRAQ5 (displayed as blue) before viewing by confocal microscopy. Results are representative of 2 independent experiments.
We focussed on characterising cell death in murine L929 fibroblasts and murine J774A.1 macrophages. As seen in Fig. 1g, cell death induced by TNFα+zVAD treatment in both L929 fibroblasts and J774A.1 macrophages was confirmed to be necroptosis by detection by Western blot of phosphorylation of MLKL at Ser345, which represents an essential step in the canonical effector mechanism of necroptotic death.49 We further sought to characterise the cell death characteristics of J774A.1 macrophages undergoing necroptosis. J774A.1 cells were induced to undergo apoptosis (by treatment with cyclohexamide), necrosis (by H2O2-treatment) and necroptosis (by TNFα+zVAD treatment) and examined by confocal microscopy. As seen in Fig. 1h, cells undergoing necroptosis undergo necrotic cell death that lacks the apoptotic hallmarks of nuclear condensation and fragmentation.
Having demonstrated that L929 fibroblasts and J774A.1 macrophages are able to undergo necroptosis, we next investigated the presence of necroptosis in M. tuberculosis infection of these cell types. L929 fibroblasts and J774A.1 macrophages were infected with M. tuberculosis in the presence and absence of TNFα and the RIPK1 inhibitor Nec-1. Cell survival was determined using a crystal violet assay. As seen in Fig. 2a and Fig. 2c, M. tuberculosis induced cell death in both L929 fibroblasts and J774A.1 macrophages in a dose-dependent manner. In the absence of TNFα this cell death was not inhibited by Nec-1 and thus was not dependent on RIPK1. Addition of TNFα to the cultures following infection induced an additional proportion of cell death that was inhibited by Nec-1, demonstrating RIPK1-dependent cell death in both cell lines in the combined presence of TNFα and M. tuberculosis infection. We next investigated the ability of M. tuberculosis and TNFα to induce phosphorylation of MLKL at Ser345 (pMLKL). The Western blot in Fig. 2b shows M. tuberculosis and TNFα synergise to induce necroptosis via phosphorylation of Ser345 of MLKL in L929 fibroblast cells. However, despite the capability of J774A.1 to undergo necroptosis, and the occurrence of RIPK1-dependent cell death in M. tuberculosis and TNFα treated macrophages, these dying cells did not undergo phosphorylation of MLKL (Fig. 2d). Thus, we conclude that M. tuberculosis does not induce classical necroptosis in this macrophage cell type.
Figure 2.
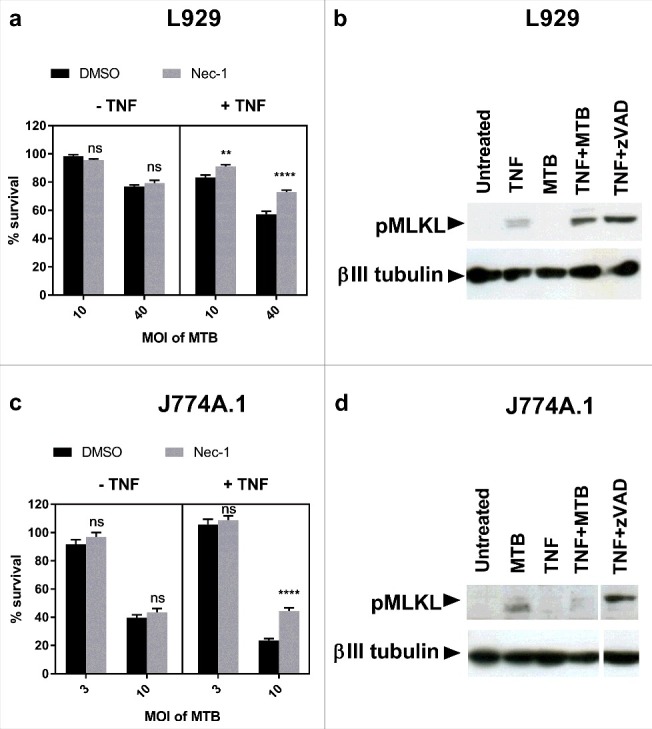
M. tuberculosis induces RIPK1-dependent cell death in the presence of excess TNFα in fibroblasts and macrophages, but only induces MLKL phosphorylation in fibroblasts. (a) L929 murine fibroblasts were infected with M. tuberculosis for 24 hours, then treated with DMSO or 30 µM Nec-1 in the presence or absence of 10ng/ml TNFα for 18 hours, before measuring cell survival using a crystal violet assay. Results are mean +/- SEM of n = 6 samples, and are expressed as a percentage of the uninfected control of each treatment. Statistics are two way ANOVA with Sidak post-test. Ns not significant; **p<0.01; ****p<0.0001. (b) Western blot of L929 fibroblasts infected with MOI 20 M. tuberculosis for 24 hours, then treated with TNFα for 24 hours, or with TNFα + 3 µM zVAD for 18 hours, developed with anti-pMLKL antibody and anti beta-III tubulin antibody. (c) J774A.1 murine macrophages were infected with M. tuberculosis for 3 hours, then treated with DMSO or 30 µM Nec-1 in the presence or absence of 25ng/ml TNFα for 48 hours. Results are mean +/- SEM of n = 10 samples, and are expressed as a percentage of the uninfected control of each treatment. Statistics are two way ANOVA with Sidak post-test. Ns not significant; ****p<0.0001. (d) Western blot of J774A.1 cells infected with MOI 10 M. tuberculosis for 3 hours and subsequently treated with 25ng/ml TNFα for 24 hours, or with TNF + 30 µM zVAD for 18 hours, developed with anti-pMLKL antibody and anti beta-III tubulin antibody. (a-d) All results are representative of at least 2 similar experiments.
We further sought to characterise the involvement of other effector molecules of the necroptotic pathway in macrophage cell death in response to M. tuberculosis infection in the presence and absence of TNFα. In the M. marinum/zebrafish model of tuberculosis, TNFα excess leads to RIPK1-RIPK3 dependent cell death mediated through phosphoglycerate mutase family member 5 (PGAM5) and mitochondrial reactive oxygen species (ROS) production.43 We therefore investigated the role of RIPK3 in J774A.1 macrophages by silencing the RIPK3 gene using sh-RNA (Fig. 3a-b). As seen in Fig. 3c, RIPK3-deficient macrophages were protected from cell death induced by M. tuberculosis, however this effect was independent of TNFα signalling. Furthermore, addition of the mitochondrial ROS inhibitor Necrox-2 (Fig. 3d) was similarly able to rescue a proportion of cell death in M. tuberculosis infected macrophages, and this effect was similarly independent of TNFα signalling. Thus although pMLKL-driven necroptosis through TNFα-signalling does not occur in murine macrophages, pharmacologically tractable programmed necrosis driven by RIPK3 and mitochondrial ROS does occur in this cell type in response to M. tuberculosis infection.
Figure 3.
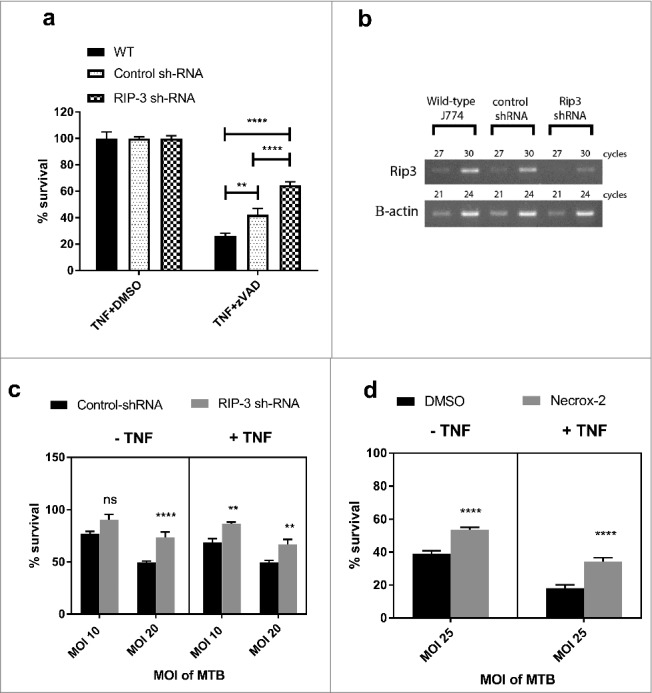
RIPK3 and mitochondrial ROS mediate M. tuberculosis-induced cell death both in the presence and absence of TNFα. (a) WT J774A.1 macrophages, RIP3K shRNA knockdown J774A.1 macrophages, and control shRNA J774A.1 macrophages were treated for 20 hours with DMSO control or 30 µM zVAD.fmk in the presence of 25ng/mL TNF, before measuring cell survival using a crystal violet assay. Results are mean +/- SEM of n = 10 samples and are expressed as a percentage of the TNFα+DMSO-treated control for each condition. Statistics are two way ANOVA with Sidak post-test. **p<0.01; ****p<0.0001. Results are representative of 3 independent experiments. (b) Knockdown of RIP3K mRNA was confirmed by RT-PCR, using murine RIP-3 and beta-actin primers (sc-61483-PR and sc-29192-PR, Santa Cruz). (c) RIP3K hRNA knockdown J774A.1 macrophages, and control shRNA J774A.1 macrophages were infected with M. tuberculosis for 3 hours, and subsequently incubated in the absence or presence of 25ng/ml TNF for 48 hours, before measuring cell survival using a crystal violet assay. Results are mean +/- SEM of n = 10 samples, and are expressed as a percentage of the uninfected control of each treatment. Statistics are two way ANOVA with Sidak post-test. *p>0.05; ***p<0.001; ****p<0.0001. Results are representative of 2 independent experiments (d) J774A.1 macrophages were infected with M. tuberculosis for 3 hours and incubated with Necrox-2 in the presence and absence of TNFα for 24 hours, before measuring cell survival using a crystal violet assay. Results are mean +/- SEM of n = 10 samples, and are expressed as a percentage of the uninfected control of each treatment. Statistics are one way ANOVA with Tukey's post-test. *p>0.05; ***p<0.001; ****p<0.0001.
We finally sought to investigate the presence of pMLKL-driven necroptosis in vivo in mice infected with M. tuberculosis. Granulomatous TB lesions were immunostained for pMLKL. As seen in Fig. 4a, granulomatous regions were heavily consolidated with abundant acid fast bacilli (Fig. 4a inset), morphologically identifiable foamy macrophages and areas of necrosis. pMLKL immunohistochemistry of the M. tuberculosis infected lungs demonstrated necroptosis specifically in cells in granulomatous regions with necrotic debris (Fig. 4c-d). Both nuclear and cytoplasmic staining was observed in these cells, consistent with the scenario that upon activation, pMLKL translocates first to the nucleus and then to the cytoplasm.33 It is not possible in our study to definitively determine the identity of pMLKL-positive cells but it was notable that obvious foamy macrophages were pMLKL negative even in areas where proximal cells were pMLKL-positive (Fig. 4d, white arrow). Additionally, pMLKL-positivity tended to occur in cells with a fusiform nuclear and cell body morphology which is consistent with non-professional phagocytes such as fibroblasts of the lung stroma.2
Figure 4.

Murine tuberculosis granulomas contain non-macrophage cells undergoing pMLKL driven necroptosis. Mice were infected with M. tuberculosis by intranasal challenge and lung pathology analysed at 21d.p.i. (A) H&E stain. Granulomatous inflammation within the lung with abundant foamy macrophages and small areas of necrosis (asterisk). Original magnification: 100X. Inset shows Ziehl-Neelsen staining revealing numerous acid fast bodies (AFBs) present within the lesion. Original magnification: 400X. (B) Non-immune 1 ͦ antibody isotype control staining for immunohistochemistry. Original magnification: 400X. (C) pMLKL immunohistochemistry. Positive staining within the cytoplasm of elongated cells within the granulomatous inflammation and necrosis. Original magnification: 400X. (D) pMLKL immunohistochemistry. Positive staining is observed predominantly in cells with fusiform-nuclei (black arrow) adjacent to non-immunoreactive foamy macrophages (white arrow) and necrotic cell debris. Original magnification: 400X.
Discussion
There is compelling evidence that M. tuberculosis exerts a finely balanced control over the mode of death of its parasitized host cell.19,50 A number of studies show that following a period of apoptosis inhibition, infected macrophages undergo a form of death that resembles necrosis. This is supported by the experimental observations that low multiplicities of infection with virulent M. tuberculosis inhibit macrophage apoptosis,51 whereas high multiplicities of infection result in necrosis.19,52,53 The association of this phenomenon with virulent mycobacterial strains19,20,23,54,55 has led to a postulated scenario that necrosis is a virulence mechanism which enables bacterial escape to infect new phagocytes or into the extracellular milieu, concurrent with the generation of inflammation to drive the development of a necrotic granuloma which is essential for transmission. As is the case for many virulence features of M. tuberculosis, there appears to be a plethora of mechanisms that contribute to the regulation/inhibition of apoptosis. These may include ligation of TLR2 receptors by bacterial ligands such as lipoarabinomannan (LAM) and the 19KDa lipoprotein,56,57 the activities of bacterial NuoG58 and SecA2,59 and upregulation of host anti-apoptotic proteins Mcl-160 and the Bcl-2 family member bfl-1/A1.61 However, there is a paucity of information regarding the mechanisms by which M. tuberculosis infected cells undergo necrosis, although individual molecular players are being discovered such as Rv2626c and PPE68; these genes contribute to induction of mitochondria-driven necrosis and enhance bacterial escape from the macrophage.62
There are likely a number of ways that M. tuberculosis causes cellular necrosis, but here we demonstrate for the first time that in a microenvironment of excess TNFα, M. tuberculosis-infected murine fibroblasts undergo necroptosis via RIP1K- and pMLKL-dependent mechanisms. Murine macrophages undergo RIPK1-dependent necrosis-like cell death, but do not undergo necroptosis because the scenario was not associated with phosphorylation of the MLKL death effector. We additionally demonstrate that necroptosis occurs in vivo in murine lung granulomas, where foamy macrophages lack pMLKL yet were spatially close to pMLKL-expressing cells including those with fusiform nuclei resembling fibroblasts.2
Necroptosis has been implicated as a mechanism of cell death in response to a range of micro-organisms including murine cytomegalovirus, Vaccinia virus, Salmonella typhimurium, and Mycobacterium marinum.43,63 However, direct comparison between these studies is complicated by varying definitions of “necroptosis”, as not all studies demonstrate definitive phosphorylation of MLKL. In the M. marinum/zebrafish model of tuberculosis, TNFα excess leads to RIPK1-RIPK3 dependent cell death, involving PGAM5 and mitochondrial ROS production.43 Although use of the MLKL inhibitor necrosulfonamide suggested a role for MLKL in programmed cell death in this scenario, definitive phosphorylation of MLKL was not demonstrated. Furthermore, recent phylogenetic analysis has shown that the members of the necroptotic signalling pathway are not well conserved through the animal kingdom; notably, MLKL is not present in zebrafish, suggesting that the M. marinum/zebrafish model does not fully replicate the programmed necrosis pathways of M. tuberculosis/macrophage infections when investigating pMLKL-driven necroptosis.64 Of further importance, not all cell lines are susceptible to necroptosis induced by TNFα and zVAD, which has been shown in some cell types to correlate with RIPK3 expression.27 In our hands, human primary macrophages, human U937 cells, murine J774A.1 cells and murine L929 cells were sensitive to TNFα+zVAD treatment, whereas THP-1 macrophages and RAW macrophages were not. Furthermore, the ability of J774A.1 macrophages and L929 fibroblasts to undergo pMLKL- dependent necroptosis was confirmed by Western blot, demonstrating their suitability for studying necroptosis. As necrotic pathways were the focus of this study, high MOIs (10-40) were used to infect macrophages and fibroblasts; cell death was observed to occur in a dose-dependent manner. Infection of macrophages with low MOI (MOI 3) was not able to stimulate RIPK1-dependent cell death in the presence of TNFα. It would be interesting to determine whether necroptosis occurs at low multiplicities of infection (when inhibition of apoptosis occurs) or whether it is a necrotic death phenotype that is exclusively dependent on a high multiplicity of infection.
During the preparation of this manuscript, Zhao et. al. published an elegant study demonstrating a key role of RIPK3 in inducing necrosis in M. tuberculosis-infected macrophages.65 They demonstrate that during infection of macrophages with virulent M. tuberculosis, a complex of RIPK1/RIPK3/pro-caspase-8 translocates to the mitochondria. Due to the presence of RIPK3 and Bcl-xL at the mitochondrial membrane, pro-caspase-8 remains in its inactive zymogen form; BAK/BAX activation is not initiated and this results in an inhibition of intrinsic apoptosis. However, RIPK3 is able to stimulate ROS-dependent necrosis by enhancing the binding of hexokinase II to the voltage dependent anion channel (VDAC) on the mitochondrial membrane, and by triggering cyclophilin-D (CypD)-dependent formation of the MPT pore via interaction of VDAC and mitochondrial adenine nucleotide translocator (ANT). Both mechanisms are required for increased ROS formation and necrosis, and deficiency in RIPK3 resulted in enhanced survival of macrophages infected with M. tuberculosis. Interestingly, although MLKL-expression is increased in macrophages by M. tuberculosis infection, and MLKL-knockdown inhibited cell death in M. tuberculosis-infected macrophages, evidence of MLKL phosphorylation was not directly provided by this study. Our data complement and extend these findings. We demonstrate RIPK3 and mitochondrial ROS-dependent cell death occurs in macrophages both in the presence and absence of TNFα, but that de facto phosphorylation of MLKL does not occur in this cell type in response to M. tuberculosis infection, either in vitro or in vivo in murine tuberculosis granulomas. Furthermore, RIPK3-deficiency protected macrophages against M. tuberculosis-induced cell death. However, we demonstrate that pMLKL-driven necroptosis does occur in fibroblasts in vitro in the presence of TNFα, and that pMLKL-driven necroptosis can be detected in vivo in murine TB lung granulomas, predominantly in non-macrophage cell types resembling fibroblasts.
M. tuberculosis infection of fibroblasts presents a particularly intriguing conundrum. Fibroblasts are recruited to TB granulomas where they are involved in tissue remodelling, and M. tuberculosis is able to replicate in fibroblasts in vitro.47,48 In situ PCR has demonstrated M. tuberculosis-infected fibroblasts in latently infected individuals, suggesting that fibroblasts and other non-professional APCs could contain a reservoir of bacteria.2 However, infected fibroblasts are not seen in active M. tuberculosis infection. This has previously been attributed to the toxic effects of TNFα in active M. tuberculosis infection, where immune competent patients have high levels of circulating TNFα and pyresis.47,48 Our data demonstrate that the synergistic effects of M. tuberculosis infection and TNFα can cause necroptosis in fibroblasts, implicating necroptosis as the mechanism for enhanced toxicity of TNFα in M. tuberculosis-infected fibroblasts and potentially as the reason why M. tuberculosis infected fibroblasts are not frequently observed in active tuberculosis. Furthermore, as proliferation of fibroblasts and a fibrotic response has been shown to be critical to the encapsulation of the granuloma and control of tuberculosis infection, this raises the possibility that the induction of necroptosis in fibroblasts by M. tuberculosis and TNFα may provide an escape route for the pathogen from the encapsulated granuloma and therefore aid bacterial dissemination and transmission.66
Our data support a strategy to pharmacologically target programmed necrosis in active M. tuberculosis lesions.25 Active tuberculosis is characterised by necrotic lung damage, which is both detrimental to the host and aids release of viable bacteria and their transmission. Targeting programmed necrosis in macrophages or the necroptosis pathway in fibroblasts may protect these cells from the toxic effects of M. tuberculosis and TNFα, preventing necrotic tissue damage, inhibiting cavitation and augmenting participation in tissue remodelling.
Materials and methods
Bacterial culture
M. tuberculosis GC1237 and H37Rv was grown at 37°C in Middlebrook 7H9 broth containing 10% albumin/dextrose/catalase (ADC) plus 0.1% Tween-80 or on Middlebrook 7H11 medium containing 0.2% glycerol and 10% oleic acid/ADC (OADC) enrichment (Becton Dickinson). For macrophage infections, bacteria were grown to late log phase (an OD600nm of 0.8-1.2) in 7H9 broth as described above, washed 1x in PBS-Tween-80 0.05% and once in PBS before resuspension in RPMI 1640 complete medium. OD600nm was used to estimate bacterial numbers, with OD600nm = 1 = 1 × 108 cfu/ml.
Macrophage and fibroblast cell culture
J774A.1 murine macrophage-like cells, L929 murine fibroblasts, THP-1 human monocytic cells and U937 human monocytic cells were cultured in RPMI 1640 complete medium containing 10% heat inactivated fetal calf serum (FCS) and 5 mM L-glutamine. RAW264.7 murine macrophage-like cells were grown in DMEM complete medium containing 10% FCS and 5 mM L-glutamine. J774A.1, L929 and RAW264.7 cells were seeded in 96-well plates and grown overnight at 37°C in 5% CO2 before mycobacterial infections and treatments to induce cell death. THP-1 and U937 monocytes were differentiated to macrophages using 25ng/ml PMA for 72 hours, then washed and rested for 24 hours before treatments to induce cell death.
Human PBMCs were isolated from component donation blood cones (NHS Blood and Transplant Service) by density centrifugation using Ficoll-Histopaque. The PBMC fraction was harvested and monocytes isolated with CD14 MicroBeads (Miltenyi Biotec). Monocytes were differentiated to macrophages in complete RPMI supplemented with 1% sodium pyruvate and 1% penicillin/streptomycin in 24 or 96 well plates with 20ng/mL of macrophage colony-stimulating factor (M-CSF; Miltenyi Biotec). Fresh medium with cytokines was added at day 3 and macrophages were used for further experiments after 6 days.
Cell Survival Assays
Cells in 96 well plates were treated with TNFα (0-25ng/ml; Miltentyi Biotec), zVAD-fmk (30 µM, Promega), necrostatin (30 µM, Enzo) or Necrox-2 (30 µM Enzo) as indicated. For mycobacterial infections, cells were infected with GC1237 for the times indicated in figure legends, and the bacteria removed by gently washing once with warm PBS-1% FCS before replacing with complete medium containing the treatments as indicated. Plates were then washed 2x with PBS, fixed for 24 hours with 4% paraformaldehyde, washed with PBS and stained with crystal violet as previously described.19
Confocal microscopy for cell morphology
J774A.1 cells were seeded in slide flasks, and the following day treated with TNF 25ng/ml, zVAD 30μM, Nec-1 30μM, 0.5mM H2O2, and 2.5μg/ml cyclohexamide. After 20h, cells were washed twice with PBS and fixed with 4% paraformaldehyde. Cells were washed and permeablised with 0.1% Triton-X100 for 3–5 minutes at room temperature, before staining with Alexa568-phalloidin (Molecular Probes) and DRAQ5 (Biostatus), mounting and viewing with a Zeiss LSM510META confocal microscope.
Western blot
Following induction of cell death or mycobacterial infection, cells were lysed with RIPA buffer containing protease inhibitor cocktail, 2mM PMSF and 1mM sodium orthovanadate. M. tuberculosis-infected lysates were passed twice through 0.22 µm spin filters (Corning Costar Spin X) before processing under biosafety level 1 conditions. Protein concentration was measured using a BCA kit (Pierce) and 20 µg protein was subjected to reducing SDS-PAGE on 10% Bis-Tris gels (Novex, Invitrogen) and transferred to a 0.2 µm pore PVDF membrane. Membranes were blocked with 10% BSA in tris-buffered saline (TBS) for 1 hour at room temperature, rinsed with 0.1% Tween-20 (TBST), and incubated overnight at 4°C with primary antibodies specific for MLKL phosphorylated at Ser345 (EPR9515(2)) at a dilution of 1:2000, or anti beta III tubulin antibody (EP1331Y; Abcam) at a dilution of 1:10,000. Blots were rinsed thrice with TBST for 10 minutes, and incubated with the secondary reagent goat anti-rabbit IgG-peroxidase at a dilution of 1:6000 for 90 minutes at room temperature. Membranes were developed using Clarity ECL Western Blot Substrate (BioRad) and exposure to autoradiography film.
RIPK3 shRNA knockdown cell lines
J774A.1 macrophages were transduced with lentiviral particles expressing shRNA of RIP3K (sc-61483-V) or control shRNA particles (sc-108080) according to the manufacturer's instructions (Santa Cruz). Transduced cells were selected with 1.25 µg/mL puromycin, and single cell colonies obtained by limiting dilution. Macrophages were washed and plated without puromycin selection for 24 hours prior to their use in assays for necroptosis or infection with M. tuberculosis. Knockdown of RIP3K mRNA was confirmed by RT-PCR, using murine RIP-3 and beta-actin primers (sc-61483-PR and sc-29192-PR, Santa Cruz).
Mouse infections and immunohistochemistry
Animal experiments were performed in accordance with the Animals (Scientific Procedures) Act 1986. Female Balb/c mice were infected intranasally with approximately 2 × 102 cfu of M. tuberculosis H37Rv (sample mean 2.13 × 102), and sacrificed 21 days post infection. Excised lung tissue was fixed with 4% paraformaldehyde for 24 hours and embedded in paraffin wax. 4 µm tissue sections were dewaxed, rehydrated, endogenous peroxidase activity blocked by incubation with 3% H2O2 in methanol and epitopes demasked with proteinase K. Samples were incubated with 1:50 pMLKL antibody in TBS; sequential sections were run with rabbit IgG as an isotype control. Samples were incubated with biotinylated horse anti-rabbit IgG and antibody binding was amplified using avidin-biotin-peroxidase conjugate. Samples were developed with NovaRed substrate (Vector Laboratories), and counter stained with Mayer's haematoxylin. Acid fast bacilli in lung sections were visualised using Ziehl-Neelsen staining.
Statistical analysis
One way ANOVA with Tukey's post-hoc tests, and two way ANOVA with Sidak post hoc test were performed using GraphPad Prism v6 software.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by The Wellcome Trust [WT090242MA].
References
- 1.Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immunity. 1996;64:1400–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez-Pando R, Jeyanathan M, Mengistu G, Aguilar D, Orozco H, Harboe M, Rook GA, Bjune G. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet. 2000;356:2133–8. doi: 10.1016/S0140-6736(00)03493-0. PMID:11191539 [DOI] [PubMed] [Google Scholar]
- 3.Mariotti S, Sargentini V, Pardini M, Giannoni F, De Spirito M, Gagliardi MC, Greco E, Teloni R, Fraziano M, Nisini R. Mycobacterium tuberculosis may escape helper T cell recognition by infecting human fibroblasts. Hum Immunol. 2013;74:722–9. doi: 10.1016/j.humimm.2013.02.005. PMID:23459076 [DOI] [PubMed] [Google Scholar]
- 4.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol. 2010;8:296–307. doi: 10.1038/nrmicro2321. PMID:20234378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–68. doi: 10.1111/j.1600-065X.2010.00984.x. PMID:21349098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–44. doi: 10.1016/S1074-7613(00)80364-7. PMID:9324363 [DOI] [PubMed] [Google Scholar]
- 7.Lopez M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 2003;170:2409–16. doi: 10.4049/jimmunol.170.5.2409. PMID:12594264 [DOI] [PubMed] [Google Scholar]
- 8.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–509. doi: 10.1084/jem.180.4.1499. PMID:7931080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998; 160:5448–54. PMID:9605147 [PubMed] [Google Scholar]
- 10.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, et al.. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. PMID:11222859 [DOI] [PubMed] [Google Scholar]
- 11.Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, et al.. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. PMID:22980326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe. 2012;12:301–12. doi: 10.1016/j.chom.2012.07.009. PMID:22980327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 2003; 9:1039–46. doi: 10.1038/nm906. PMID:12872166 [DOI] [PubMed] [Google Scholar]
- 14.Winau F, Kaufmann SH, Schaible UE. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 2004;6:599–607. doi: 10.1111/j.1462-5822.2004.00408.x. PMID:15186397 [DOI] [PubMed] [Google Scholar]
- 15.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–17. doi: 10.1016/j.immuni.2005.12.001. PMID:16413927 [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Repasy T, Papavinasasundaram K, Sassetti C, Kornfeld H. Mycobacterium tuberculosis induces an atypical cell death mode to escape from infected macrophages. PloS One. 2011;6:e18367. doi: 10.1371/journal.pone.0018367. PMID:21483832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37. doi: 10.1038/nri2873. PMID:21088683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P.. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–64. doi: 10.1016/j.it.2011.01.005. PMID:21334975 [DOI] [PubMed] [Google Scholar]
- 19.Butler RE, Brodin P, Jang J, Jang MS, Robertson BD, Gicquel B, Stewart GR. The balance of apoptotic and necrotic cell death in Mycobacterium tuberculosis infected macrophages is not dependent on bacterial virulence. PloS One. 2012;7:e47573. doi: 10.1371/journal.pone.0047573. PMID:23118880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukocyte Biol. 2006;79:80–6. doi: 10.1189/jlb.0505250. PMID:16275894 [DOI] [PubMed] [Google Scholar]
- 21.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol. 1998;161:2636–41. PMID:9725266 [PubMed] [Google Scholar]
- 22.Abebe M, Kim L, Rook G, Aseffa A, Wassie L, Zewdie M, Zumla A, Engers H, Andersen P, Doherty TM. Modulation of cell death by M. tuberculosis as a strategy for pathogen survival. Clin Dev Immunol. 2011;2011:678570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006;176:3707–16. doi: 10.4049/jimmunol.176.6.3707. PMID:16517739 [DOI] [PubMed] [Google Scholar]
- 24.Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, Fortune S, Behar SM, Remold HG. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. PMID:19561612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–66. doi: 10.1038/nrd.2015.6. PMID:26775689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. PMID:19524513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. PMID:19524512 [DOI] [PubMed] [Google Scholar]
- 28.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–62. doi: 10.1038/ni.2397. PMID:22922364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. PMID:24019532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFalpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differentiation. 2011;18:26–37. doi: 10.1038/cdd.2010.72. PMID:20539307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al.. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. PMID:22265413 [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–7. doi: 10.1073/pnas.1200012109. PMID:22421439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon S, Bogdanov K, Kovalenko A, Wallach D. Necroptosis is preceded by nuclear translocation of the signaling proteins that induce it. Cell death Differentiation. 2016;23:253–60. doi: 10.1038/cdd.2015.92. PMID:26184911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al.. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. PMID:18408713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. PMID:16408008 [DOI] [PubMed] [Google Scholar]
- 36.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, et al.. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. doi: 10.1016/j.cell.2014.04.018. PMID:24813850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/S1074-7613(00)80535-X. PMID:9529147 [DOI] [PubMed] [Google Scholar]
- 38.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al.. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–9. doi: 10.1038/nature10400. PMID:21921917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–21. doi: 10.1074/jbc.M305633200. PMID:14532286 [DOI] [PubMed] [Google Scholar]
- 40.Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–70. doi: 10.1074/jbc.C800051200. PMID:18442983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–13. doi: 10.1016/j.chom.2010.03.006. PMID:20413098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McComb S, Cheung HH, Korneluk RG, Wang S, Krishnan L, Sad S. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differentiation. 2012;19:1791–801. doi: 10.1038/cdd.2012.59. PMID:22576661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–34. doi: 10.1016/j.cell.2013.03.022. PMID:23582643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasindran SJ, Torrelles JB. Mycobacterium Tuberculosis Infection and Inflammation: what is Beneficial for the Host and for the Bacterium? Front Microbiol. 2011;2:2. doi: 10.3389/fmicb.2011.00002. PMID:21687401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, et al.. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–46. doi: 10.1016/j.cell.2011.12.023. PMID:22304914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobin DM, Vary JC Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, et al.. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–30. doi: 10.1016/j.cell.2010.02.013. PMID:20211140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filley EA, Bull HA, Dowd PM, Rook GA. The effect of Mycobacterium tuberculosis on the susceptibility of human cells to the stimulatory and toxic effects of tumour necrosis factor. Immunology. 1992; 7:505–9 [PMC free article] [PubMed] [Google Scholar]
- 48.Filley EA, Rook GA. Effect of mycobacteria on sensitivity to the cytotoxic effects of tumor necrosis factor. Infect Immunity. 1991;59:2567–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, et al.. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differentiation. 2016;23:76–88. doi: 10.1038/cdd.2015.70. PMID:26024392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, Hartman M, Kornfeld H. Macrophage apoptosis in tuberculosis. Yonsei Medical J. 2009;50:1–11. doi: 10.3349/ymj.2009.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–20. doi: 10.4049/jimmunol.164.4.2016. PMID:10657653 [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176:4267–74. doi: 10.4049/jimmunol.176.7.4267. PMID:16547264 [DOI] [PubMed] [Google Scholar]
- 53.O'Sullivan MP, O'Leary S, Kelly DM, Keane J. A caspase-independent pathway mediates macrophage cell death in response to Mycobacterium tuberculosis infection. Infect Immunity. 2007;75:1984–93. doi: 10.1128/IAI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn H, Lee KS, Kim SY, Shin DM, Shin SJ, Jo EK, Park JK, Kim HJ. Induction of cell death in human macrophages by a highly virulent Korean Isolate of Mycobacterium tuberculosis and the virulent strain H37Rv. Scandinavian J Immunol. 2009;69:43–50. doi: 10.1111/j.1365-3083.2008.02188.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Jiang R, Takayama H, Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbiol Immunol. 2005;49:845–52. doi: 10.1111/j.1348-0421.2005.tb03673.x. PMID:16172539 [DOI] [PubMed] [Google Scholar]
- 56.Ciaramella A, Cavone A, Santucci MB, Garg SK, Sanarico N, Bocchino M, Galati D, Martino A, Auricchio G, D'Orazio M, et al.. Induction of apoptosis and release of interleukin-1 beta by cell wall-associated 19-kDa lipoprotein during the course of mycobacterial infection. J Infect Dis. 2004;190:1167–76. doi: 10.1086/423850. PMID:15319868 [DOI] [PubMed] [Google Scholar]
- 57.Loeuillet C, Martinon F, Perez C, Munoz M, Thome M, Meylan PR. Mycobacterium tuberculosis subverts innate immunity to evade specific effectors. J Immunol. 2006;177:6245–55. doi: 10.4049/jimmunol.177.9.6245. PMID:17056554 [DOI] [PubMed] [Google Scholar]
- 58.Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR Jr, Porcelli SA, Briken V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. PMID:17658950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, et al.. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117:2279–88. doi: 10.1172/JCI31947. PMID:17671656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol. 2003;170:430–7. doi: 10.4049/jimmunol.170.1.430. PMID:12496428 [DOI] [PubMed] [Google Scholar]
- 61.Dhiman R, Raje M, Majumdar S. Differential expression of NF-kappaB in mycobacteria infected THP-1 affects apoptosis. Biochimica Et Biophysica Acta. 2007;1770:649–58. doi: 10.1016/j.bbagen.2006.11.016. PMID:17204371 [DOI] [PubMed] [Google Scholar]
- 62.Danelishvili L, Everman J, Bermudez LE. Mycobacterium tuberculosis PPE68 and Rv2626c genes contribute to the host cell necrosis and bacterial escape from macrophages. Virulence. 2016;7:23–32. doi: 10.1080/21505594.2015.1102832. PMID:26605666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. PMID:24565922 [DOI] [PubMed] [Google Scholar]
- 64.Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJ, Vandenabeele P. An evolutionary perspective on the necroptotic pathway. Trends Cell Biol. 2016;26:721–32. doi: 10.1016/j.tcb.2016.06.004. PMID:27368376 [DOI] [PubMed] [Google Scholar]
- 65.Zhao X, Khan N, Gan H, Tzelepis F, Nishimura T, Park SY, Divangahi M, Remold HG. Bcl-xL mediates RIPK3-dependent necrosis in M. tuberculosis-infected macrophages. Mucosal Immunol 2017. doi: 10.1038/mi.2017.12. PMID:28401933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gil O, Diaz I, Vilaplana C, Tapia G, Diaz J, Fort M, Cáceres N, Pinto S, Caylà J, Corner L, et al.. Granuloma encapsulation is a key factor for containing tuberculosis infection in minipigs. PloS One. 2010;5:e10030. doi: 10.1371/journal.pone.0010030. PMID:20386605 [DOI] [PMC free article] [PubMed] [Google Scholar]


