Abstract
Long term exposure to trace metals in various media is of great concern for people living in known pollution sources, such as mining and industrial activities. Health risk assessment and human hair analysis can provide important information for local environmental management. Information on distribution characteristics of trace metals in soil, water, sediment, air, local crops, and human hair from a typical mining area in southern China was collected. Results show there exists severely trace metal contamination in soil, sediment, and air. Arsenic and Pb contents in the local children’s hair are higher than the upper reference values, and the accumulation of residents’ hair trace metals shows great correlation with the ingestion and inhalation pathways. Arsenic contributes 52.27% and 58.51% to the total non-cancer risk of adults and children, respectively. The cancer risk of Cd in adults and children are 4.66 and 3.22 times higher than the safe level, respectively. Ingestion exposure pathway of trace metals largely contributes to the total non-cancer and cancer effect. The metals As, Cd, and Pb are major risk sources and pollutants that should be given priority for management, and ingestion pathway exposure to trace metals through soil and crops should be controlled.
Keywords: trace metals, multi-media, hair, risk assessment, exposure assessment
1. Introduction
Like many other developing countries, such as Congo, Chile, and Vietnam, China has been suffering from serious pollution caused by trace metals [1,2,3,4,5]. Rapid development of industrialization and urbanization in these countries has accelerated the release of trace elements from traffic, fuel components, and other non-specific sources [6,7,8]. Additionally, industrial and mining activities are one of the most important trace metal pollution sources in the environment [9,10,11]. After releasing into the environment, trace metals can accumulate in different environmental medias in many chemical forms [12] and damage human health through various absorption pathways, such as the soil-food chain, inhalation, and oral intake [13,14,15]. Even at low concentrations, these contaminations are toxic to human health, especially to children’s health. Therefore, trace metals exposure has been attracted great attentions [16,17,18,19,20,21].
Human hair has been considered as a suitable indicator for estimating long-term metal exposure in China [22,23] and other countries [24,25,26]. The comprehensive analysis of the content of trace elements in human hair, not only reflects the overall characteristics of regional geochemistry, but also can reflect the metabolic status and health level of life elements in different people. Although there are many researches about the trace metals levels of exposed residents’ hair, studies on the influencing factors about the accumulation of metals in hair are relatively lacking.
Serious systemic health problems can develop as a result of excessive dietary accumulation of trace metals in the human body [17]. The hazard quotient (HQ), formalized by the U.S. EPA (Environmental Protection Agency), has been widely utilized to evaluate the potential health risks that are associated with long-term exposure to metals in various media, including soil, drinking water, ambient air, and food [27]. There are many human health risk assessment of trace metals through various exposure pathways [16,28,29], but the reports about different media and systematic multi-pathways analysis are rare. Limited work has been reported on the correlation between trace metals exposure pathway and residents’ hair.
In this study, a farmland closing to the Dabaoshan mining area, Southern China is selected as a case to assess the potential health risks posed by trace metal exposure to multiple media and the accumulation of trace metals in hair of local dwellers. The study site has experienced substantial uncontrolled development over the past 30 years, with the expansion of mining and industrial activities. Large amounts of metals were released through by sewage, atmospheric deposition, or solid wastes, resulting in serious metal pollution. The specific objectives of this study are: (1) to explore the distribution of trace metal in different environmental media under long term mining and industrial activities; (2) to study the concentrations of trace metals in the hair of residents and influencing factors; and, (3) to determine the potential health risk of trace metals via the multiple routes of ingestion, inhalation, dermal exposure, and diet from the soil-crop-water-air system.
2. Materials and Methods
2.1. Study Area
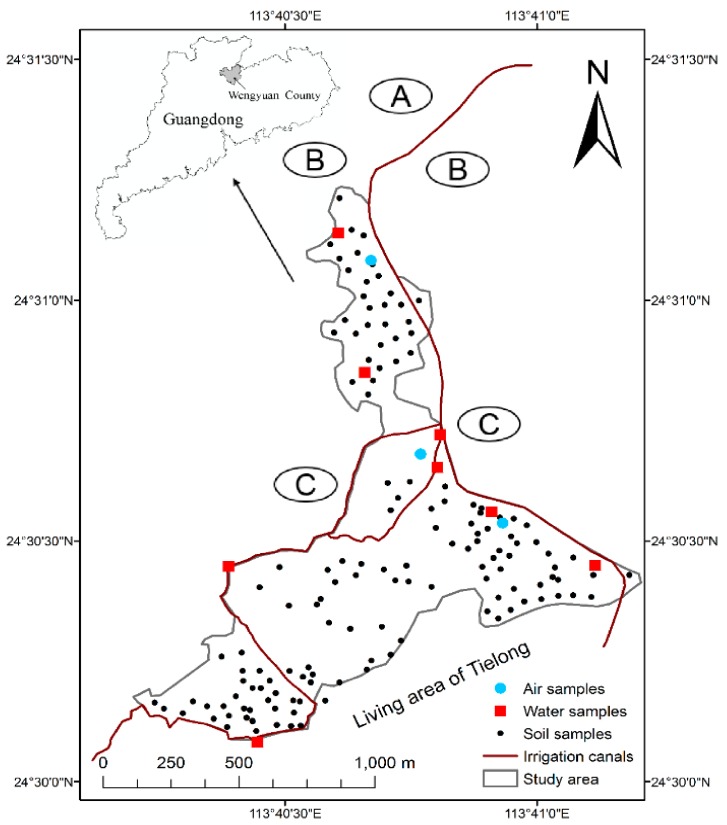
The study locates in Tielong town, Shaoguan city, Guangdong province (24°5′ N, 113°68′ E), covering a total area of 96.5 km2 and having a total population of 5962 inhabitants (Figure 1). There were a non-ferrous metals company (A) and mining company (B) built on the north of the Tielong town, companies has been operating since 1980s, but are now closed, large amounts of tailings were produced during mining in the Zn-Cu poly-metallic deposit. A cement plant (C) is located in the middle of the town. The township has two irrigation canals, one of the canals flows down the mine and was once used for agricultural irrigation.
Figure 1.
Location of study area and sampling sites.
2.2. Samples Collection and Analysis
Various media samples, including 145 surface soil samples (0–20 cm), 24 surface water samples, 6 sediment samples, and 12 air deposits were collected to obtain the mean and ranges of trace metal concentrations in the study area.
Each soil sample was the mixture of nine subsamples around the designated location. The soil samples were air-dried, picked for residues/stones, and sieved twice using a 2- and 0.149-mm mesh. Of the sieved soil sample, 0.25 g was digested in a mixture of HCl-HNO3-HClO4-HF, until the solution was translucent or reduced to about 2–3 mL. The final solution was diluted to 50 mL, using double-distilled water (ddH2O).
Atmospheric depositions were collected from three sampling sites in the study area. A total 12 samples of air samples were collected monthly with membrane filter, by using an air sampler with a larger volume (20 cm × 38.5 cm). Samples were digested with an HCl-HNO3-HF-HClO4 solution for the metal concentrations.
A total of 24 irrigation water samples were collected in 1 L acid-washed polyethylene bottles from eight sites at random to represent the entire area. Microwave digestion of water samples for metal analysis was done by measuring 50 mL into 5 mL HNO3.
Six different types of plant species were harvested from the study area. The edible portions of the plants were harvested for trace metal analysis. The six species plants were cabbage (n = 12), oil wheat (n = 6), tomato (n = 10), radish (n = 15), corn (n = 14), and pepper (n = 3). Plant samples were washed by using ddH2O and were dried in a hot air oven at 75 °C for 24 h. The dried residues were mashed into fine particles before microwave digestion, 0.2 g of mashed samples were digested with 8 mL of HNO3 and H2O2 with 3:1 ratio.
Hair samples were collected from 76 farmers in Tielong town who settled in the town, with a residence time of at least 10 years (except children). Farmers with a history of trace metal exposure from other sources rather than the study area were not included in the study, the hair was selected after the natural growth of the occipital without heat dyed. Hair samples from human subjects were collected after informing farmers of the purpose of the research and subsequently soliciting consent from farmers. Hair samples were washed with neutral detergent and three times with ddH2O and put in a drying furnace at 70 °C over night. After cooling, the samples were caught in small pieces of 2 mm length and 0.1 g of hair samples was weighted, mixed with 6 mL HNO3 and 1 mL HClO4.
The concentrations of As, Cd, Cr, Cu, Ni, Pb, Zn in the samples were analyzed by inductive coupled plasma mass spectrometer (ICP-MS) (7500a, Agilent Technologies Inc., Santa Clara, CA, USA). All of the experiments were conducted within strict experiment conditions to eliminate/minimize possible contamination and interference. Standard reference samples (including GSS-5, GSB-26, and GSH-1) and blank samples were used for quality control and recovery analysis. Recovery rates for metal contents were controlled between 80% and 120%.
2.3. Daily Intake (DI) of Trace Metals
The daily intake (DI) of trace metals depended on both the trace metal concentration in crops and the amount of consumption of crops [30]. The DI for human were determined by:
| (1) |
where (mg·kg−1, on dry weigh basis) is the concentration of trace metals in crops. represents conversion factor, 0.085 is used to convert dry weight to fresh weight. (kg·(person·day)−1) represents the average daily consumption of crops, the average value for adults and children are considered 0.345 and 0.232 kg·(person·day)−1 [30,31]. The trace metal intakes are compared with the provisional tolerable daily intake (PTDI) [32,33].
2.4. Risk Assessment
Currently, there is no agreed limit for acceptable maximum carcinogenic and non-carcinogenic risk levels in China, we therefore employed the U.S. EPA model and their threshold values in order to assess the potential human health risk that is posed by trace metal pollution in the study [34]. Six exposure routes are considered, including: (1) direct ingestion of soil particles; (2) dermal contact with soil particles; (3) diet through the food chain; (4) inhalation of soil particles from the air; (5) oral intake from groundwater; and (6) dermal intake from groundwater [17].
The calculation for the average daily dose of contaminants via various exposure pathways are as follows [35,36]:
| (2) |
| (3) |
| (4) |
where is the metal concentration in the samples, is the ingestion rate, is the inhalation rate, is the particle emission factor, is the surface area of skin exposed to pollutants, is the skin adherence factor, is the dermal absorption factor, is the exposure frequency, is the exposure duration, is the body weight, and AT is the average time for non-carcinogens or carcinogens, is the units conversion factor.
Both the carcinogenic and non-carcinogenic risk for all of the metals through ingestion, inhalation, dermal, and diet exposure pathways were calculated. The non-carcinogenic risk from individual trace metals can be expressed as the hazard quotient (HQ).
| (5) |
where the non-cancer HQ is the ratio of exposure to hazardous substances, and is the estimated maximum permissible risk that is imposed on humans through daily exposure. Experiencing adverse health effects is unlikely when HQ ≤ 1, whereas potential non-carcinogenic effects can occur when HQ > 1.
Moreover, the HQ calculated for each metal is summarized to assess the overall potential non-carcinogenic effects posed by more than one trace metal. If multiple pathways are available, a total exposure hazard index (HI) can be utilized to communicate non-cancer risks through different pathways:
| (6) |
HI values > 1 shows that there is a chance that non-carcinogenic risk may occur; and when HI ≤ 1 the reverse applies.
Cancer risk (CR) and total cancer risk (TCR) can be evaluated from:
| (7) |
| (8) |
where cancer risk represents the probability of an individual lifetime health risk from carcinogens; SF is the slope factor of hazardous substances. The acceptable of tolerable risk for regulatory purposes is within the range of 10−6–10−4. In accordance with Agency for Research on Cancer, As and Cd are treated as having a potential carcinogen effect.
Human health risk from trace metals in multi-media are related to total metal concentrations, ingestion rate, and the bioavailability. In reality, trace metals in multi-media cannot be absorbed by humans to much of an extent. So in our study, total metal concentrations in multi-media will overestimate the residents’ potential risks.
2.5. Statistical Analysis
Descriptive statistics were calculated by using the SPSS 20.0 software (IBM corporation, Armonk, NY, USA) and Excel 2013 (Microsoft corporation, Redmond, WA, USA). The data were displayed using the parameters of the minimum value, maximum value, mean value, the median, and standard deviation. Statistical analysis including Pearson correlation analysis, two significant levels at 5% and 1% were used in the statistics.
3. Results and Discussion
3.1. Trace Metals in Various Media and Local Crops
The trace metal concentrations in different media are presented in Table 1. The concentrations of seven metals in soil were much higher than the background values in Guangdong province. According to the Chinese Soil Environmental Quality Standard (GB15618-2008), mean concentrations of As (54.65 mg·kg−1), Cd (2.38 mg·kg−1), Pb (172.76 mg·kg−1), and Zn (332.53 mg·kg−1) were 2.73 times, 3.96 times, 3.46 times, and 1.11 times higher than the Grade II values, respectively. The mean concentrations of Cu (40.98 mg·kg−1), Ni (39.20 mg·kg−1), Cr (87.54 mg·kg−1) were blow the Grade II criteria (threshold value of soil contamination). Notably, even the minimum concentration of Cd (0.68 mg·kg−1) in the soils was higher than the Grade II values, indicating the severely contamination degree of Cd in the study area.
Table 1.
Trace metal concentrations in different media in the study area.
| Media | As | Cd | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|
| Mean ± Std. Deviation (Minimum, Maximum) | |||||||
| Soils (mg·kg−1) | 54.65 ± 29.12 | 2.38 ± 2.22 | 87.54 ± 10.20 | 40.98 ± 12.67 | 39.20 ± 6.02 | 172.76 ± 106.76 | 332.53 ± 114.01 |
| (11.43, 175.73) | (0.68, 16.06) | (57.41, 114.75) | (20.05, 89.36) | (21.33, 62.03) | (89.84, 1258.29) | (118.11, 999.83) | |
| Crops (mg·kg−1) | 0.11 ± 0.11 | 0.30 ± 0.28 | 0.29 ± 0.29 | 4.35 ± 2.26 | 0.30 ± 0.45 | 0.42 ± 0.71 | 13.65 ± 6.90 |
| (0.01, 0.34) | (0.06, 1.27) | (0.01, 0.94) | (0.91,10.32) | (0.00, 2.79) | (0.01,3.82) | (1.37, 29.41) | |
| Surface sediments (mg·kg−1) | 50.17 ± 25.96 | 14.83 ± 9.81 | 62.93 ± 8.82 | 102.23 ± 49.32 | 26.8 ± 6.92 | 247.77 ± 115.18 | 712.62 ± 244.19 |
| (23.64, 87.18) | (2.17, 30.65) | (55.37, 75.85) | (39.22, 171.62) | (18.12, 35.78) | (101.32, 395.58) | (345.18, 986.58) | |
| Surface water (μg·L−1) | 2.09 ± 1.22 | 2.25 ± 4.31 | 2.94 ± 1.20 | 5.37 ± 4.37 | 0.36 ± 0.21 | 6.25 ± 14.60 | 63.75 ± 87.27 |
| (0.79, 5.34) | (0.16, 15.62) | (0.45, 5.25) | (1.75, 19.26) | (0.17, 0.91) | (0.52, 68.76) | (9.79, 329.40) | |
| Air (μg·(m2·d)−1) | 10.52 ± 4.74 | 0.40 ± 0.28 | 7.38 ± 6.31 | 11.27 ± 13.48 | 1.45 ± 1.11 | 30.85 ± 27.59 | 111.70 ± 118.11 |
| (5.02, 16.87) | (0.05, 0.72) | (1.58, 18.39) | (1.20, 38.12) | (0.25, 3.37) | (5.75, 82.92) | (14.18, 331.79) | |
Six different species vegetable were collected in the field. When compared with the threshold values issued by Chinese Ministry of Health (GB2762-2012), except for the Pb contents in oil wheat (2.05 mg·kg−1), trace metal contents of vegetable (including leafy vegetable) were all under the food safety limits, which suggests that most vegetables have no potential risk in view of product quality. The DIs of trace metals for adults and children through food consumption are presented in Table 2. The DIs of trace metals in crops were in the following order: Zn > Cu > Ni > Cd > Pb > Cr > As. Intake of trace metals was much lower than the PTDI value. But, because staple food (rice, wheat) was not considered in this study, the results of DI would be limited.
Table 2.
Dietary intake of trace metals (μg·day−1) for adults and children.
| Daily Intake of Metals | As | Cd | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|
| Adults | 3.23 | 8.80 | 8.50 | 127.56 | 8.80 | 12.32 | 400.29 |
| Children | 2.17 | 5.92 | 5.72 | 85.78 | 5.92 | 8.28 | 269.18 |
| PTDI | 580 | 70 | 500 | 6500 | 1200 | 200 | 33000 |
The trace metal concentrations of surface water in the study area were lower than the standards for drinking water quality (GB5749-2006). However, the concentrations of As (50.17 mg·kg−1), Cd (14.83 mg·kg−1), Pb (247.77 mg·kg−1), and Zn (712.62 mg·kg−1) in sediments were much higher than the Chinese Soil Environmental Quality Standard (GB15618-2008). The high concentrations of trace meals in sediments may be because of the deposits of suspended particulates from the surface water, indicating that the water was polluted by the historical mining activities.
Trace metal concentrations in air deposition were higher than the average concentration of China level [37] and other regions [37,38]. Particularly, Pb and Zn concentrations in air deposition were much higher than those of other trace metals, suggesting that the accumulation of these two metals in soil may be mainly through fallout. There are three sampling locations set up in the north, middle and south of the study area, respectively. The results of ANOVA test showed that the interaction of sampling locations and months had no significant effect on the deposition rates by analyzing the trace metal content of each air sample during the four months. Monthly variations in deposition rates of trace metals were similar at different sampling locations, suggesting that trace metals deposition was mainly governed by local anthropogenic activities.
3.2. Trace Metal Concentrations in Human Hair
The bioaccumulation of trace metals in human hair is rather a complex process, factors that influence bioaccumulation include: nourishment, chemical forms of the metal and their binding site, age, sex, genetic inheritance, and environmental quality. In this study, the hair samples are classified into two groups of age (children (≤15 years) and adults (>15 years)).
The results of trace metals in the hair of inhabitants in different age ranges are represented in Table 3. The children group had much higher average concentrations of As (1.13 mg·kg−1), Cd (0.20 mg·kg−1), and Pb (10.88 mg·kg−1), about 1.59, 1.46, and 2.13 times higher than the adults group, respectively. The results indicate that children are sensitive to trace metal exposure, contaminated soil and dust may be ingested either directly or indirectly as a result of hand-to-mouth activity of children. The mean concentration of Zn (176.42 mg·kg−1) in group of adults was significantly higher than children. Food consumption was found to be an important source of endogenous deposition of trace metals to human hair. Although food requirements of adults are more than children, the need for trace elements of adults generally are less than children. Indicating that the change of growth rate may not parallel the rate of deposition of trace elements into hair [39].
Table 3.
Trace metals concentration of different age groups in human hair (mg·kg−1).
| Age Group | Statistical Analysis | As | Cd | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| Children | Mean | 1.13 | 0.19 | 0.17 | 11.43 | 1.21 | 10.88 | 120.70 |
| SD | 0.64 | 0.07 | 0.06 | 2.35 | 0.36 | 3.28 | 29.62 | |
| Adults | Mean | 0.71 | 0.13 | 0.21 | 11.37 | 1.21 | 5.118 | 162.997 |
| SD | 0.36 | 0.06 | 0.13 | 2.65 | 0.526 | 3.020 | 51.803 | |
| Total | Mean | 0.77 | 0.14 | 0.20 | 11.38 | 1.21 | 5.86 | 169.46 |
| SD | 0.43 | 0.06 | 0.13 | 2.60 | 0.51 | 3.56 | 57.42 |
SD: standard deviation.
In comparison with other regions in China and other countries [40,41,42], the levels of Pb and As in the hair of inhabitants were relatively high (Table 4). The mean concentration of Cu in hair samples was higher than the average level of other regions in China, but much lower than the mean level of Canada and USA inhabitants. When compared with the standard for hair’s trace metals of various Chinese populations, which were reported by the Trace Element Research Council of China (TERCC) [43], the average concentrations of trace metals in adults were between in the reference ranges. But, in children, the levels of As (1.13 mg·kg−1) and Pb (10.88 mg·kg−1) were higher than the upper limit value that was set by TERCC, indicating that children’s health has been affected by the trace metal contamination in the study area. Blood lead poisoning of local children were also reported in 2014 (local webpage). Therefore, it is necessary to control the Pb and As discharge of local mines and enterprises, and improve the risk perception of residents (especially children) on the prevention of trace metal contamination.
Table 4.
Comparison of mean hair trace metal levels (mg·kg−1, dry weight) from the present study with those reported for other regions of the world.
| Country/Region | As | Cd | Cr | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|
| Canada [40] | 0.016 | 0.503 | 0.35 | 63.1 | 0.26 | 5.38 | 248 | |
| USA [40] | 0.019 | 0.97 | 0.234 | 108 | 1.01 | 5.35 | 124 | |
| Japan [40] | 0.053 | 0.28 | 0.23 | 10.7 | 2.7 | 3.62 | 114 | |
| Guangdong in China (Adults) [41] | 0.77 | 0.052 | 0.34 | NR | 0.44 | 5.56 | NR | |
| Ningbo in China [42] | 0.282 | 0.209 | 1.16 | 10.7 | 0.812 | 2.98 | NR | |
| Normal reference value by TERCC [43] | Adults | 1.0 | 0.6 | 0.30–1.20 | 8.0–20.0 | NR | 10 | 120–210 |
| Children | 1.0 | 0.5 | 0.18–0.72 | 8.0–16.0 | NR | 10 | 90–170 | |
NR: not recommended. TERCC: Trace Element Research Council of China.
3.3. Health Risk Assessment
3.3.1. Daily Exposure Levels
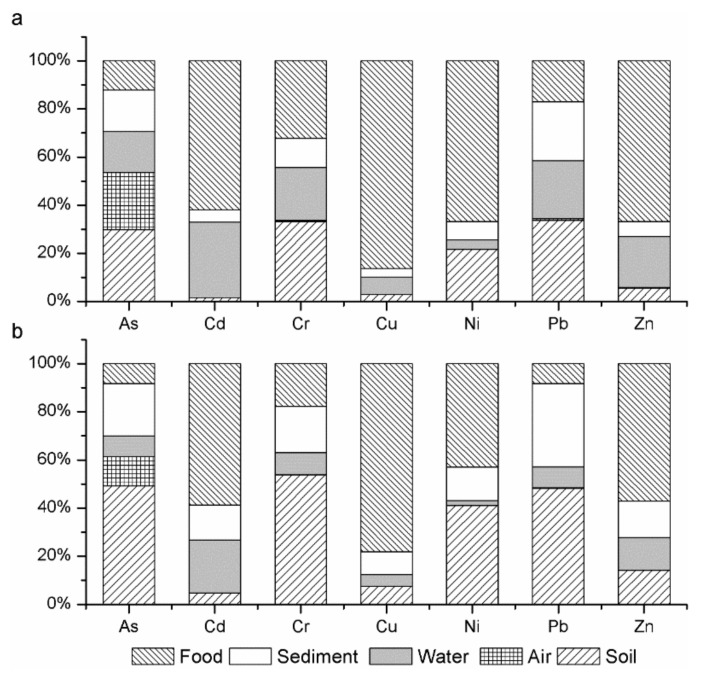
Figure 2 showed the contribution of each exposure route to estimated ADD values of adults and children in the study area. For adults, food ingestion accounted for the majority of total ADD of Cd (61.84%), Cu (86.30%), Ni (66.83%), and Zn (66.84%), respectively. The intakes of the As and Pb in soil played the most important role of the total ADD, which accounted for 29.92% and 33.71%, respectively. For Cr, the ingestion pathway through food (32.23%) contributed nearly as much as the three pathway through soil (33.26%). For children, food ingestion contributed a major portion of the total ADD of Cd, Cu, and Zn, which accounted for 58.72%, 78.20%, and 57.09%, respectively. Soil plays the most important factor for As (49.27%), Cr (53.84%), and Pb (48.23%) intake. Soil provided 41.08% of the ADD of the Ni values nearly as much as food (42.97%). By contrast, air contributed less than 5% of ADD for adults and children, except for As. Thus, soil ingestion and food consumption are considered as the important pathways for trace metal exposure.
Figure 2.
The contribution of Multi-media to the average daily exposure dose. (a) Each media’s contribution to the average daily dose of the local adults; (b) Each media’s contribution to the average daily dose of the local children.
Furthermore, the correlation between the trace metal concentrations in hair and the average daily dose of trace metals via different exposure pathways was analyzed (Table 5). Hair trace metal accumulation were highly correlated with ingestion and inhalation pathways for both adults and children, indicating that human exposure to trace metals occurs predominately through ingestion and inhalation.
Table 5.
Correlation coefficients between the concentrations of trace metals in hair and average daily dose of trace metals via different pathways.
| Age Group | Ingest-Soil | Inhale-Soil | Dermal-Soil | Inhale-Air | Dermal-Air | Ingest-Water | Ingest-Sediment | Dermal-Sediment | Ingest-Crop |
|---|---|---|---|---|---|---|---|---|---|
| Adults | 0.855 ** | 0.884 ** | −0.015 | 0.959 ** | −0.011 | 0.998 ** | 0.958 ** | 0.251 | 0.970 ** |
| Children | 0.903 ** | 0.905 ** | −0.024 | 0.969 ** | −0.021 | 0.999 ** | 0.972 ** | 0.245 | 0.970 ** |
Levels of significance: **: p < 0.01.
3.3.2. Non-Carcinogenic Risks of Different Media
Table 6 summarized the non-carcinogenic risk assessment results for adults. The HI of trace metals decreased in the order of As > Cd > Pb > Cr > Cu > Zn > Ni. With regard to the pathways, the highest non-carcinogenic risk was from food ingestion, followed by the soil ingestion, water ingestion, and dermal contact with air. The adults’ HI of each metal was lower than 1, indicating that the potential health risk of individual metals was low. The majority of adults’ non-cancer risks was contributed by As (52.27%). Therefore, for the non-carcinogenic risks of adults, As should be received more attentions.
Table 6.
Non-carcinogenic risk of trace metals for adults.
| Type | Pathway | As | Cd | Cr | Cu | Ni | Pb | Zn | Total |
|---|---|---|---|---|---|---|---|---|---|
| Soil | Ingestion | 2.82 × 10−1 | 3.68 × 10−3 | 4.51 × 10−2 | 1.58 × 10−3 | 3.03 × 10−3 | 7.63 × 10−2 | 1.71 × 10−3 | 4.13 × 10−1 |
| Inhalation | 3.05 × 10−5 | 3.97 × 10−7 | 5.05 × 10−4 | 1.71 × 10−7 | 3.28 × 10−7 | 8.25 × 10−6 | 1.85 × 10−7 | 5.44 × 10−4 | |
| Dermal contact | 2.37 × 10−2 | 4.12 × 10−3 | 2.53 × 10−2 | 5.92 × 10−5 | 1.26 × 10−4 | 5.76 × 10−3 | 9.60 × 10−4 | 6.00 × 10−2 | |
| Air | Inhalation | 1.01 × 10−4 | 6.37 × 10−7 | 4.58 × 10−4 | 4.96 × 10−7 | 1.29 × 10−7 | 1.54 × 10−5 | 5.72 × 10−7 | 5.76 × 10−4 |
| Dermal contact | 7.45 × 10−2 | 6.26 × 10−3 | 2.17 × 10−2 | 1.62 × 10−4 | 4.68 × 10−5 | 1.02 × 10−2 | 2.81 × 10−3 | 1.16 × 10−1 | |
| Water | Ingestion | 2.15 × 10−1 | 6.96 × 10−2 | 3.03 × 10−2 | 4.15 × 10−3 | 5.56 × 10−4 | 5.52 × 10−2 | 6.57 × 10−3 | 3.82 × 10−1 |
| Sediment | Ingestion | 1.29 × 10−1 | 1.15 × 10−2 | 1.62 × 10−2 | 1.98 × 10−3 | 1.04 × 10−3 | 5.47 × 10−2 | 1.84 × 10−3 | 2.17 × 10−1 |
| Dermal contact | 2.17 × 10−2 | 2.57 × 10−2 | 1.82 × 10−2 | 1.48 × 10−4 | 8.60 × 10−5 | 8.25 × 10−3 | 2.06 × 10−3 | 7.61 × 10−2 | |
| Food | Ingestion | 1.53 × 10−1 | 1.38 × 10−1 | 4.42 × 10−2 | 4.93 × 10−2 | 9.46 × 10−3 | 3.91 × 10−2 | 2.06 × 10−2 | 4.53 × 10−1 |
| HI | 8.99 × 10−1 | 2.59 × 10−1 | 2.02 × 10−1 | 5.74 × 10−2 | 1.43 × 10−2 | 2.50 × 10−1 | 3.66 × 10−2 | 1.72 | |
HI: hazard index.
The results of non-carcinogenic risks of trace metals for local children through different exposure routes are shown in Table 7. Among the metals, As and Pb presented highest potential health risks, with the HI value being greater than 1. The children’s HQ of trace metals by soil ingestion (3.22), sediment ingestion (1.69) and crop ingestion (1.19) were higher than 1. Children had higher HQ via non-dietary ingestion of soil than adults because of more outdoor-playing times and hand-to-mouth activities (mouthing and chewing) [44,45]. According to other surveys, children accompany their parents to the farmland and are often exposed to the contaminated environment without any protective measures, which causes them easily be exposed to soil trace metals [46]. Therefore, Risk control should be taken about ingestion pathway to reduce the trace metal exposure level of children.
Table 7.
The non-carcinogenic risk of trace metals for children.
| Type | Pathway | As | Cd | Cr | Cu | Ni | Pb | Zn | Total |
|---|---|---|---|---|---|---|---|---|---|
| Soil | Ingestion | 2.20 | 2.87 × 10−2 | 3.52 × 10−1 | 1.24 × 10−2 | 2.36 × 10−2 | 5.95 × 10−1 | 1.34 × 10−2 | 3.22 |
| Inhalation | 6.16 × 10−5 | 8.04 × 10−7 | 1.02 × 10−3 | 3.47 × 10−7 | 6.63 × 10−7 | 1.67 × 10−5 | 3.75 × 10−7 | 1.10 × 10−3 | |
| Dermal contact | 4.61 × 10−2 | 8.03 × 10−3 | 4.93 × 10−2 | 1.15 × 10−4 | 2.45 × 10−4 | 1.12 × 10−2 | 1.87 × 10−3 | 1.17 × 10−1 | |
| Air | Inhalation | 2.21 × 10−4 | 1.39 × 10−6 | 1.00 × 10−3 | 1.08 × 10−6 | 2.82 × 10−7 | 3.36 × 10−5 | 1.25 × 10−6 | 1.26 × 10−3 |
| Dermal contact | 1.45 × 10−1 | 1.22 × 10−2 | 4.24 × 10−2 | 3.17 × 10−4 | 9.13 × 10−5 | 1.98 × 10−2 | 5.48 × 10−3 | 2.25 × 10−1 | |
| Water | Ingestion | 4.20 × 10−1 | 1.36 × 10−1 | 5.91 × 10−2 | 8.09 × 10−3 | 1.08 × 10−3 | 1.08 × 10−1 | 1.28 × 10−2 | 7.44× 10−1 |
| Sediment | Ingestion | 1.01 | 8.95 × 10−2 | 1.27 × 10−1 | 1.54 × 10−2 | 8.08 × 10−3 | 4.27 × 10−1 | 1.43 × 10−2 | 1.69 |
| Dermal contact | 1.09 × 10−2 | 1.28 × 10−2 | 9.08 × 10−3 | 7.38 × 10−5 | 4.30 × 10−5 | 4.13 × 10−3 | 1.03 × 10−3 | 3.81 × 10−2 | |
| Crop | Ingestion | 4.00 × 10−1 | 3.62 × 10−1 | 1.16 × 10−1 | 1.29 × 10−1 | 2.48 × 10−2 | 1.03 × 10−1 | 5.41 × 10−2 | 1.19 |
| HI | 4.23 | 6.48 × 10−1 | 7.56 × 10−1 | 1.66 × 10−1 | 5.80 × 10−2 | 1.27 | 1.03 × 10−1 | 7.23 | |
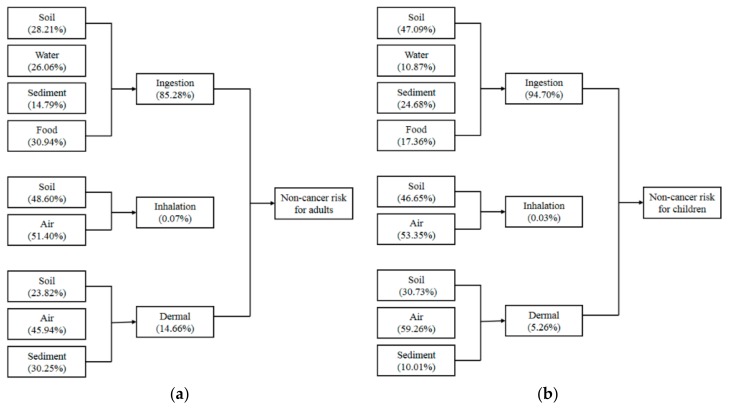
Figure 3 showed the contribution of each pathway to the health risk of local adults and children based on the average HQ values. Ingestion exposure of these trace metals largely contributed to the non-cancer effect, accounting for 85.28% and 94.70% to the total non-cancer risk of adults and children, respectively. Dermal contact was the second primary exposure route of all metals to the adults and children, which accounted for 14.66% and 5.26%, respectively. The effect of inhalation on human health can be ignored due to the low portion of the total non-cancer risks.
Figure 3.
Multi-pathway analysis of hazard quotient (HQ). (a) Each pathway’s contribution to the total HQ of the local adults; (b) Each pathway’s contribution to the total HQ of the local children.
3.3.3. Carcinogenic Risks of Different Media
Of the seven investigated elements, only As and Cd were considered highly carcinogenic. The proportion of different exposure routes, as indicated from the potential cancer risk assessments for adults and children, were asymmetrical (Table 8). The risk levels through inhalation from soil and air 10−11 to 10−10, much lower than the safe level, indicating that the cancer risk of inhalation can be ignored. Crops ingestion was the dominating exposure route to adults and children, which accounted for 47.30% and 40.16% of the total cancer risk, respectively. The cancer risk of Cd via water and crops ingestion for adults were 1.46 × 10−4 and 2.88 × 10−4, and the risk of Cd via crops ingestion for children was 1.89 × 10−4, were higher than the maximum safe value 10−4. The total cancer risk values of Cd for adults (4.66 × 10−4) and children (3.22 × 10−4) were much higher than those of As. Therefore, Cd appears to be the main pollutant source to produce cancer between the two elements. The cancer risk of As through ingestion and dermal contact pathways were all within the acceptable range of 10−6 to 10−4, existing some potential risk. Therefore, the most important step is to control the Cd pollution sources to prevent the further pollution, this would provide a powerful balance between environmental protection, crops safety improvement, reducing health risks, and massive savings of cost.
Table 8.
Carcinogenic risk of trace metals for adults and children.
| Age Group | Type | Pathway | As | Cd | Total |
|---|---|---|---|---|---|
| Adults | Soil | Ingest | 4.35 × 10−5 | 7.69 × 10−6 | 5.12 × 10−5 |
| Inhale | 1.35 × 10−11 | 5.18 × 10−11 | 6.52 × 10−11 | ||
| Dermal | 1.46 × 10−5 | 8.61 × 10−8 | 1.47 × 10−5 | ||
| Air | Inhale | 4.47 × 10−11 | 8.30 × 10−11 | 1.28 × 10−10 | |
| Dermal | 4.59 × 10−5 | 1.31 × 10−7 | 4.61 × 10−5 | ||
| Water | Ingest | 3.32 × 10−5 | 1.46 × 10−4 | 1.79 × 10−4 | |
| Sediment | Ingest | 2.00 × 10−5 | 2.40 × 10−5 | 4.39 × 10−5 | |
| Dermal | 1.34 × 10−5 | 5.37 × 10−7 | 1.39 × 10−5 | ||
| Crop | Ingest | 2.35 × 10−5 | 2.88 × 10−4 | 3.12 × 10−4 | |
| TCR | 1.94 × 10−4 | 4.66 × 10−4 | 6.60 × 10−4 | ||
| Children | Soil | Ingest | 8.48 × 10−5 | 1.50 × 10−5 | 9.97 × 10−5 |
| Inhale | 6.82 × 10−12 | 2.62 × 10−11 | 3.30 × 10−11 | ||
| Dermal | 7.12 × 10−6 | 4.20 × 10−8 | 7.16 × 10−6 | ||
| Air | Inhale | 2.44 × 10−11 | 4.54 × 10−11 | 6.99 × 10−11 | |
| Dermal | 2.24 × 10−5 | 6.38 × 10−8 | 2.25 × 10−5 | ||
| Water | Ingest | 1.62 × 10−5 | 7.09 × 10−5 | 8.71 × 10−5 | |
| Sediment | Ingest | 3.89 × 10−5 | 4.68 × 10−5 | 8.57 × 10−5 | |
| Dermal | 1.68 × 10−6 | 6.72 × 10−8 | 1.74 × 10−6 | ||
| Crop | Ingest | 1.54 × 10−5 | 1.89 × 10−4 | 2.04 × 10−4 | |
| TCR | 1.86 × 10−4 | 3.22 × 10−4 | 5.08 × 10−4 | ||
TCR: total cancer risk.
4. Conclusions
We investigated the distribution of trace metals in different environmental media and residents’ hair in a typical rural area affected by long term mining and industrial activities, and determined the potential health risk encountered through different exposure pathways. The main findings are:
-
(1)
After long term mining and industrial activities, the area had been seriously polluted by trace metals, especially, As, Cd, and Pb, mainly through air deposition (for Pb) or irrigation (for As and Cd).
-
(2)
Human hair is a suitable indicator for study the long-term metal exposure. Hair analysis results indicated that local resident, especially children, had been seriously affected by the trace metal contamination in the study area, and As and Pb concentrations in residents’ hair were relatively high.
-
(3)
It also lead to high potential health risk. As and Pb presented highest potential non-carcinogenic health risks to children, with a HI value greater than 1, and the cancer risk of Cd exposure were higher than the maximum safe value 10−4.
-
(4)
Risk control should be taken to reduce the ingestion exposure level of children, which was the dominated contribution to the non-cancer effect. Soil remediation should be conducted to reduce food and soil ingestion exposure.
By combining the accumulation of trace metals in residents’ hair with daily metal exposure value, we systematically analyzed the human health risk of trace metal through different pathways in multi-medias. The findings in our study suggest that local government should give priority to the soil remediation, and increase the risk perception of local children to reduce the trace metals exposure by hands to mouth activities. Further studies are needed in order to more accurately assess the trace metal intakes from soil and crops by local children.
Acknowledgments
We gratefully acknowledge financial support provided by the National Natural Science Foundation of China (Grant No. 41401588).
Supplementary Materials
Author Contributions
The author Wushuang Xie wrote the main manuscript text, developed the risk assessment framework and prepared the figures. Chi Peng provided many suggestions on the sampling strategy and helped the data collection. Hongtao Wang provided the metal data. Weiping Chen helped the manuscript writing and organization. In addition, Weiping Chen provided financial supporting for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen R., de Sherbinin A., Ye C., Shi G. China’s soil pollution: Farms on the frontline. Science. 2014;344:691. doi: 10.1126/science.344.6185.691-a. [DOI] [PubMed] [Google Scholar]
- 2.Pourret O., Lange B., Bonhoure J., Colinet G., Decrée S., Mahy G., Séleck M., Shutcha M., Faucon M.-P. Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo) Appl. Geochem. 2016;64:43–55. doi: 10.1016/j.apgeochem.2015.07.012. [DOI] [Google Scholar]
- 3.Tume P., González E., King R.W., Cuitiño L., Roca N., Bech J. Distinguishing between natural and anthropogenic sources for potentially toxic elements in urban soils of Talcahuano, Chile. J. Soils Sediments. 2017 doi: 10.1007/s11368-017-1750-0. [DOI] [Google Scholar]
- 4.Kikuchi T., Furuichi T., Hai H.T., Tanaka S. Assessment of heavy metal pollution in river water of Hanoi, Vietnam using multivariate analyses. Bull. Environ. Contam. Toxicol. 2009;83:575–582. doi: 10.1007/s00128-009-9815-4. [DOI] [PubMed] [Google Scholar]
- 5.Banza C.L.N., Nawrot T.S., Haufroid V., Decrée S., De Putter T., Smolders E., Kabyla B.I., Luboya O.N., Ilunga A.N., Mutombo A.M., et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009;109:745–752. doi: 10.1016/j.envres.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Guo L., Zhao W., Gu X., Zhao X., Chen J., Cheng S. Risk assessment and source identification of 17 metals and metalloids on soils from the half-century old tungsten mining areas in Lianhuashan, Southern China. Int. J. Environ. Res. Public Health. 2017;14:1475. doi: 10.3390/ijerph14121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J., Feng K., Pei Z., Meng F., Sun J. Multivariate analysis combined with GIS to source identification of heavy metals in soils around an abandoned industrial area, Eastern China. Ecotoxicology. 2016;25:380–388. doi: 10.1007/s10646-015-1596-4. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman C.L.S., Zereini F., Püttmann W. Metal and metalloid accumulation in cultivated urban soils: A medium-term study of trends in Toronto, Canada. Sci. Total Environ. 2015;538:564–572. doi: 10.1016/j.scitotenv.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 9.Gray J.E., Crock J.G., Lasorsa B.K. Mercury methylation at mercury mines in the Humboldt River Basin, Nevada, USA. Geochem. Explor. Environ. Anal. 2002;2:143–149. doi: 10.1144/1467-787302-017. [DOI] [Google Scholar]
- 10.Kuo C.C., Weaver V., Fadrowski J.J., Lin Y.S., Guallar E., Navas-Acien A. Arsenic exposure, hyperuricemia, and gout in U.S. adults. Environ. Int. 2015;76:32–40. doi: 10.1016/j.envint.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Li G.H., Feng X., Qiu G., Bi X., Li Z., Zhang C., Wang D., Shang L., Guo Y. Environmental mercury contamination of an artisanal zinc smelting area in Weining County, Guizhou, China. Environ. Pollut. 2008;154:21–31. doi: 10.1016/j.envpol.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Cullen W.R., Reimer K.J. Arsenic speciation in the environment. Chem. Rev. 1989;89:713–764. doi: 10.1021/cr00094a002. [DOI] [Google Scholar]
- 13.Lu Y., Yin W., Huang L., Zhang G. Assessment of bioaccessibility and exposure risk of arsenic and lead in urban soils of Guangzhou City, China. Environ. Geochem. Health. 2011;33:93–102. doi: 10.1007/s10653-010-9324-8. [DOI] [PubMed] [Google Scholar]
- 14.Komárek M., Ettler V., Chrastný V., Mihaljevic M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008;34:562–577. doi: 10.1016/j.envint.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Park R.M., Bena J.F., Stayner L., Smith R.J., Gibb H.J., Lees P.S.J. Hexavalent chromium and lung cancer in the chromate industry: A quantitative risk assessment. Risk Anal. 2004;24:1099–1108. doi: 10.1111/j.0272-4332.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 16.Man Y.B., Sun X.L., Zhao Y.G., Lopez B.N., Chung S.S., Wu S.C., Cheung K.C., Wong M.H. Health risk assessment of abandoned agricultural soils based on heavy metal contents in Hong Kong, the world’s most populated city. Environ. Int. 2010;36:570–576. doi: 10.1016/j.envint.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Song Q., Tang Y., Li W., Xu J., Wu J., Wang F., Brookes P. Human health risk assessment of heavy metals in soil-vegetable system: A multi-medium analysis. Sci. Total Environ. 2013;463–464:530–540. doi: 10.1016/j.scitotenv.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 18.Li Z.Y., Ma Z.W., Kuijp T.J.V.D., Yuan Z.W., Huang L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014;468–469:843–853. doi: 10.1016/j.scitotenv.2013.08.090. [DOI] [PubMed] [Google Scholar]
- 19.Massaquoi L.D., Ma H., Liu X.H., Han P.Y., Zuo S.M., Hua Z.X., Liu D.W. Heavy metal accumulation in soils, plants, and hair samples: An assessment of heavy metal exposure risks from the consumption of vegetables grown on soils previously irrigated with wastewater. Environ. Sci. Pollut. Res. Int. 2015;22:18456–18468. doi: 10.1007/s11356-015-5131-1. [DOI] [PubMed] [Google Scholar]
- 20.Vimercati L., Baldassarre A., Gatti M.F., Gagliardi T., Serinelli M., De M.L., Caputi A., Dirodi A.A., Galise I., Cuccaro F. Non-occupational exposure to heavy metals of the residents of an industrial area and biomonitoring. Environ. Monit. Assess. 2016;188:673. doi: 10.1007/s10661-016-5693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai H.Y., Hseu Z.Y., Chen T.C., Chen B.C., Guo H.Y., Chen Z.S. Health risk-based assessment and management of heavy metals-contaminated soil sites in Taiwan. Int. J. Environ. Res. Public Health. 2010;7:3595–3614. doi: 10.3390/ijerph7103596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang M., Chen X., Shao D., Zhao Y., Wang W., Wong M. Risk assessment of arsenic and other metals via atmospheric particles, and effects of atmospheric exposure and other demographic factors on their accumulations in human scalp hair in urban area of Guangzhou, China. Ecotoxicol. Environ. Saf. 2014;102:84–92. doi: 10.1016/j.ecoenv.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Luo R.X., Zhuo X.Y., Ma D. Determination of 33 elements in scalp hair samples from inhabitants of a mountain village of Tonglu city, China. Ecotoxicol. Environ. Saf. 2014;104:215–219. doi: 10.1016/j.ecoenv.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Chojnacka K., Zielińska A., Górecka H., Dobrzański Z., Górecki H. Reference values for hair minerals of Polish students. Environ. Toxicol. Pharmacol. 2010;29:314–319. doi: 10.1016/j.etap.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Dongarrà G., Lombardo M., Tamburo E., Varrica D., Cibella F., Cuttitta G. Concentration and reference interval of trace elements in human hair from students living in Palermo, Sicily (Italy) Environ. Toxicol. Pharmacol. 2011;32:27–34. doi: 10.1016/j.etap.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Skalny A.V., Skalnaya M.G., Tinkov A.A., Serebryansky E.P., Demidov V.A., Lobanova Y.N., Grabeklis A.R., Berezkina E.S., Gryazeva I.V., Skalny A.A. Reference values of hair toxic trace elements content in occupationally non-exposed Russian population. Environ. Toxicol. Pharmacol. 2015;40:18–21. doi: 10.1016/j.etap.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Bian B., Zhou L.J., Li L., Lv L., Fan Y.M. Risk assessment of heavy metals in air, water, vegetables, grains, and related soils irrigated with biogas slurry in Taihu Basin, China. Environ. Sci. Pollut. Res. 2015;22:7794–7807. doi: 10.1007/s11356-015-4292-2. [DOI] [PubMed] [Google Scholar]
- 28.Baastrup R., Sørensen M., Balstrøm T., Frederiksen K., Larsen C.L., Tjønneland A., Overvad K., Raaschou-Nielsen O. Arsenic in drinking-water and risk for cancer in Denmark. Environ. Health Perspect. 2008;116:231–237. doi: 10.1289/ehp.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mari M., Nadal M., Schuhmacher M., Domingo J. Exposure to heavy metals and PCDD/Fs by the population living in the vicinity of a hazardous waste landfill in Catalonia, Spain: Health risk assessment. Environ. Int. 2009;35:1034–1039. doi: 10.1016/j.envint.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y.C., Qiao M., Liu Y.X., Zhu Y.G. Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. J. Environ. Sci. 2012;24:690–698. doi: 10.1016/S1001-0742(11)60833-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang X.L., Sato T., Xing B.S., Tao S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005;350:28–37. doi: 10.1016/j.scitotenv.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO) Joint FAO/WHO Food Standards Rogramme Codex Committee on Contaminants in Foods. Codex Alimentarius Commission; Rio de Janeiro, Brazil: 1993. [Google Scholar]
- 33.Chen T.B., Song B., Zheng Y.M., Huang Z.C., Lei M., Liao X.Y. A survey of lead concentrations in vegetables and soils in Beijing and their health risks. Sci. Agric. Sin. 2006:1589–1597. (In Chinese) [Google Scholar]
- 34.Peng C., Cai Y.M., Wang T.Y., Xiao R.B., Chen W.P. Regional probabilistic risk assessment of heavy metals in different environmental media and land uses: An urbanization-affected drinking water supply area. Sci. Rep. 2016;6:37084. doi: 10.1038/srep37084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Environmental Protection Agency (U.S. EPA) Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual—Part E, Supplemental Guidance for Demal Risk Assessment. Office of Superfund Remediation and Technology Innovation; Washington, DC, USA: 2004. [Google Scholar]
- 36.US. Environmental Protection Agency (U.S. EPA) Exposure Factors Handbook: 2011 Edition. U.S. Environmental Protection Agency; Washington, DC, USA: 2011. Final Report. [Google Scholar]
- 37.Duan J.C., Tan J. Atmospheric heavy metals and arsenic in China: Situation, sources and control policies. Atmos. Environ. 2013;74:93–101. doi: 10.1016/j.atmosenv.2013.03.031. [DOI] [Google Scholar]
- 38.Tan J.H., Duan J.C., Ma Y.L., Yang F.M., Cheng Y., He K.B., Yu Y.C., Wang J. Source of atmospheric heavy metals in winter in Foshan, China. Sci. Total Environ. 2014;493:262–270. doi: 10.1016/j.scitotenv.2014.05.147. [DOI] [PubMed] [Google Scholar]
- 39.Rivai I. Heavy metals in human hair related to age groups and automotive pollution levels of Bandarlampung city, Indonesia. Bull. Environ. Contam. Toxicol. 2001;66:443–448. doi: 10.1007/s001280026. [DOI] [PubMed] [Google Scholar]
- 40.Takagi Y., Matsuda S., Imai S., Ohmori Y., Masuda T., Vinson J.A., Mehra M., Puri B., Kaniewski A. Trace elements in human hair: An international comparison. Bull. Environ. Contam. Toxicol. 1986;36:793–800. doi: 10.1007/BF01623585. [DOI] [PubMed] [Google Scholar]
- 41.Qin J.F., Li J.X., Lou M.T. Establishing principle and method of normal reference value of elements in human hair and blood. Guangdong Weiliang Yuansu Kexue. 2005;12:2–11. (In Chinese) [Google Scholar]
- 42.Wang T., Fu J.J., Wang Y.W., Liao C.Y., Tao Y.Q., Jiang G.B. Use of scalp hair as indicator of human exposure to heavy metals in an electronic waste recycling area. Environ. Pollut. 2009;157:2445–2451. doi: 10.1016/j.envpol.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Ni S.Q., Li R.P., Wang A.J. Heavy metal content in scalp hair of the inhabitants near Dexing Copper Mine, Jiangxi Province, China. Sci. China Earth Sci. 2011;54:780–788. doi: 10.1007/s11430-011-4194-1. [DOI] [Google Scholar]
- 44.Moya J., Bearer C.F., Etzel R.A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics. 2004;113:996–1006. [PubMed] [Google Scholar]
- 45.Tao X.Q., Shen D.S., Shentu J.L., Long Y.Y., Feng Y.J., Shen C.C. Bioaccessibility and health risk of heavy metals in ash from the incineration of different e-waste residues. Environ. Sci. Pollut. Res. 2015;22:3558–3569. doi: 10.1007/s11356-014-3562-8. [DOI] [PubMed] [Google Scholar]
- 46.Leung A.O., Duzgorenaydin N.S., Cheung K.C., Wong M.H. Heavy metals concentrations of surface dust from e-waste recycling and its human health implications in southeast China. Environ. Sci. Technol. 2008;42:2674–2680. doi: 10.1021/es071873x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.