Abstract
We report a case of a 26-year-old man with no significant medical history, who presented with fatigue, pruritus, jaundice, dark urine and clay colored stool for one month. He had been taking methyl-1-etiochoenolol-epietiocholanolone, an androgenic anabolic steroid (AAS). He was initially found to have a total bilirubin (Tbili) of 6 mg/dL. He discontinued the AAS but the patients’ symptoms worsened and Tbili increased to 36 mg/d. This prompted inpatient management of his drug-induced liver injury (DILI). Molecular adsorbent recirculating system (MARS) is an extracorporeal liver support system that replaces the detoxification function of the liver. The patient was initiated on a 4-day trial of MARS therapy. Over the course of his therapy, he clinically improved and his Tbili decreased to 20.7 mg/dL. At follow-up, his symptoms resolved and Tbili was 3.3 mg/dl. This case demonstrates the efficacy of MARS in treating severe cholestatic DILI refractory to standard medical therapy.
INTRODUCTION
Acute liver injury (ALI) is associated with high mortality. Drug-induced liver injury (DILI) is responsible for ~50% of cases of ALI in the USA [1, 2]. Extracorporeal liver assist devices can help achieve hemodynamic stability while patients with ALI await liver transplant, with the possibility of hepatic regeneration and recovery. Molecular Adsorbent Recirculating System (MARS) is an extracorporeal liver support system that replaces the detoxification function of the liver using an albumin-enriched dialysate to aid in the elimination of albumin-bound and water-soluble toxins [3].
CASE REPORT
A 26-year-old Caucasian male with no significant past medical history presented with fatigue, pruritus, jaundice, dark urine and clay colored stools for 1 month. The patient had been taking methyl-1-etiochoenolol-epietiocholanolone, an androgenic anabolic steroid (AAS).
Initially, at an outpatient clinic he was found to have a total bilirubin (Tbili) of 6 mg/dl. He was advised to discontinue the AAS. He discontinued the AAS but the patients’ symptoms worsened. Repeat blood work 2 weeks later showed a Tbili of 32.5 mg/d, which prompted inpatient management.
The patient denied use of other hepatotoxic drugs, herbal supplements, tobacco, illicit drugs or alcohol. He had a similar episode 5 years ago; he had been taking a similar AAS and developed mild jaundice, which resolved spontaneously once he discontinued the AAS.
On exam, his vital signs were normal. He was extensively jaundiced, had a normal mental status without asterixis, and normal cardio-respiratory and abdominal exam without ascites or hepatomegaly. Liver biochemistries revealed aspartate aminotransferase (AST) 67 U/l, alanine transaminase (ALT) 106 U/L, alkaline phosphatase 166 U/l, Tbili 36 mg/dl, and international normalized ratio (INR) 0.95. Complete blood cell count (CBC) and Renal Function Panel (RFP) were normal. An extensive infectious, autoimmune, and metabolic work was negative. His abdominal ultrasound was normal.
At this stage, he was initiated on a 4-day trial of MARS therapy due to refractory hyperbilirubinemia after standard medical therapy (SMT). By day 3 of MARS therapy his pruritus, fatigue, hemoglobinuria and jaundice improved. Liver biochemistries at that time revealed AST 95 U/l, ALT 147 U/l, alkaline phosphatase 166 U/l, Tbili 24.3 mg/dl and INR 1.01. CBC and RFP remained normal.
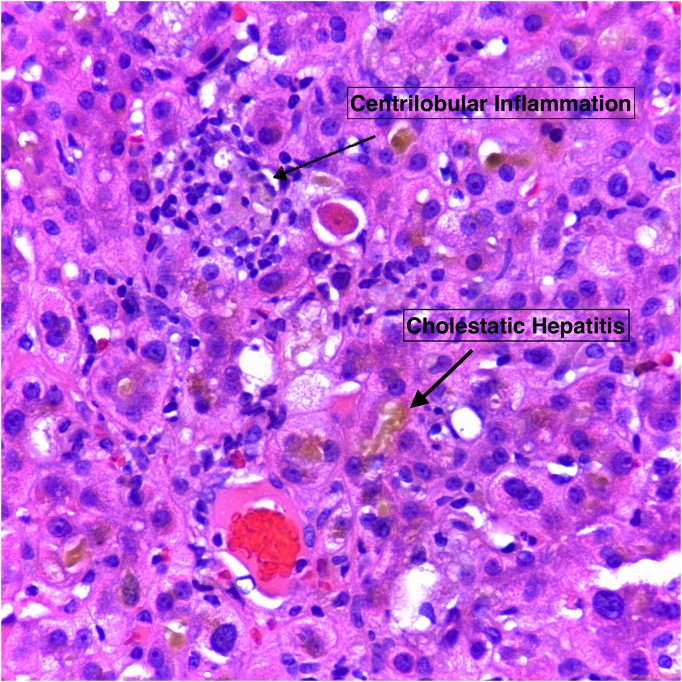
Over the course of his therapy, he remained hemodynamically stable, clinically improved and his Tbili decreased to 20.7 mg/dl. A percutaneous liver biopsy was performed showing severe cholestasis with bile duct injury/inflammation and hepatocyte injury without steatosis (Fig. 1), consistent with DILI. Patient was discharge with outpatient follow-up.
Figure 1:
Centrizonal parenchyma demonstrating severe cholestasis with formation of cholestatic rosettes, mild lymphocytic inflammation, reactive changes in hepatocytes, and an acidophil body (apoptotic hepatocyte). Hematoxylin and eosin stain, 400x overall magnification.
At 2 months follow-up he ceased taking AAS and noted a resolution of his fatigue, jaundice, pruritus, and abnormal stool and urine color. Labs showed AST 56, ALT 104 and Tbili 3.3 mg/dl.
DISCUSSION
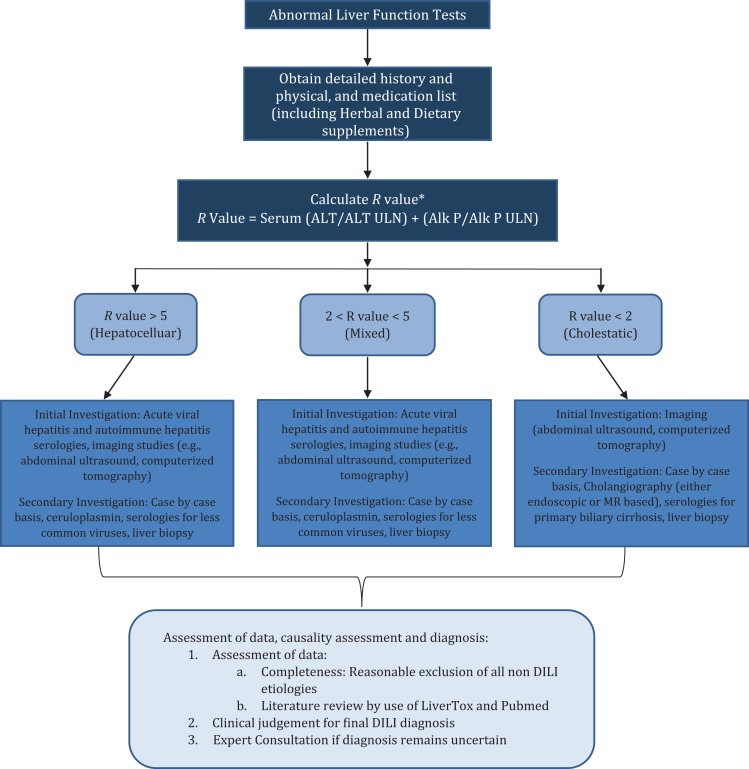
DILI presents a challenging diagnosis for many physicians as its presentation can vary anywhere between acute fulminant hepatic failure to mild hepatitis. The American College of Gastroenterology created guidelines to help clinicians with the diagnosis of DILI (Fig. 2) [4]. DILI is a diagnosis of exclusion, in which proper history and correlating the use of the drug in question to the onset of the symptoms and lab abnormalities is key [5].
Figure 2:
Flow chart for the diagnosis of DILI [4].
DILI can be due to numerous medications and/or drugs, both prescription and over the counter with >1000 estimated medications and supplements being implicated [6, 7]. DILI accounts for ~10% of all cases of acute hepatitis [8], and it is the most common cause of ALI in the USA [1, 9]. In this case, the patients’ previous history of similar symptoms after using AAS with use of another AAS, and hyperbilirubinemia pointed towards the diagnosis of DILI, with a percutaneous liver biopsy confirming the diagnosis.
AAS have grown tremendously in popularity especially amongst athletes and bodybuilders. Robles et al. reports a 3-fold increase in the number of AAS induced DILI cases over 1 decade, a total growth from 1 to 8% between 2000 and 2013 [10, 11]. Consequentially, this upsurge of AAS use has not only led to increasing cases of DILI, but also to multi-organ side effects including cardiac dysfunction, neuropsychiatric disorders, and renal impairment (Table 1) [5, 10–14]. AAS DILI presents predominantly as cholestasis, but hepatocellular damage or both patterns can be seen [5]. It is postulated that the mechanism of cholestasis in AAS DILI is secondary to impaired excretion of bile rather than direct injury to the hepatocytes [11, 12].
Table 1:
Side effects of anabolic steroids by organ system
| Organ Systems | DISEASES |
|---|---|
| LIVER | Hepatitis, cholestatic hepatitis, Peliosis hepatis, hepatic adenoma, Hepatocellular carcinoma, Focal nodular hyperplasia |
| CARDIAC | Left ventricular hypertrophy, hypertension, myocardial infarction, arrhythmias |
| RENAL | Focal segmental glomerulosclerosis, cholemic nephrosis |
| NEUROPSYCHIATRIC | Depression, anxiety, addiction potential |
Thus far, there has been no remedy for DILI other than SMT, which includes withdrawal of the offending agent and supportive care [12, 15]. Growing interest in MARS has led us to explore its use in DILI caused by AAS. In addition to water-soluble substances that are removed by renal replacement therapy, MARS has the added benefit of removing albumin-bound toxins from the patient’s serum (including bilirubin). This ability of MARS to remove albumin-bound toxins is facilitated by the use of an albumin dialysate and a dialysis membrane with the appropriate pore size. Due to this property of extracting albumin-bound toxins MARS addresses many of the consequences of DILI such as pruritus, cholestatic hepatitis, bile acid nephropathy and HE [15].
Though there is a paucity of clinical trials for the use of MARS in AAS DILI, there are prior case reports on this topic. Diaz et al. outlines a case series, which demonstrates successful therapy with MARS in four patients, all of whom consumed an AAS, and developed severe cholestatic jaundice refractory to SMT; MARS helped expedite their recovery [12]. Avegail et al. demonstrates the efficacy of MARS in patients with severe bile acid nephropathy and other forms of renal dysfunction more commonly seen with AAS DILI [9, 10]. Other case reports and series have reported an improvement in both symptoms (including jaundice, pruritus and confusion) and biochemical parameters after 3–4 sessions of MARS therapy.
In regards to acute liver failure (ALF) the use of MARS has not been fully established. MARS is a useful tool that has been shown to stabilize clinical conditions in ALF patients awaiting transplant, and facilitate hepatic regeneration. Several clinical trials have concluded MARS as a reasonable tool in ALF patients’ by reducing bilirubin levels, improving hemodynamic status, renal dysfunction and hepatic encephalopathy [16–18]. A multicenter, randomized, controlled trial involving patients with acute liver failure showed no survival benefit [18]. Although survival benefit has not been proven, it has a the potential to benefit specific patient subgroups.
CONCLUSION
MARS has been reported to stabilize clinical parameters in patients with DILI and DILI associated ALI patients, with a goal to bridge the patient to spontaneous recovery or liver transplant. In the setting of cholestatic liver injury, MARS may be of particular benefit given its ability to extract bilirubin, which is an albumin-bound toxin. This case demonstrates the efficacy of MARS in treating severe cholestatic DILI due to AAS that was refractory to standard medical therapy.
CONFLICT OF INTEREST STATEMENT
Informed consent was obtained for publication of this case report. This case report in part has been previously presented as an abstract poster at American College of Gastroenterology 2015 Annual Scientific Meeting. It has thus been expanded and enriched from the abstract version.
AUTHORS' ROLES
J.E. and R.A. drafted, reviewed and edited the manuscript. R.S. reviewed and edited the manuscript. R.A. is the article guarantor.
REFERENCES
- 1. Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. . Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947–54. [DOI] [PubMed] [Google Scholar]
- 2. Reuben A, Koch DG, Lee WM. Acute Liver Failure Study Group Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010;6:2065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saliba F. The Molecular Adsorbent Recirculating System (MARS®) in the intensive care unit: a rescue therapy for patients with hepatic failure. Crit Care 2006;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Fontana RJ. Practice Parameters Committee of the American College of Gastroenterology ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. [DOI] [PubMed] [Google Scholar]
- 5. Neuberger J. Editorial: showing due DILI-gence—the lessons from anabolic steroids. Aliment Pharmacol Ther 2015;41:321–3. [DOI] [PubMed] [Google Scholar]
- 6. Stirnimann G, Kessebohm K, Lauterburg B. Liver injury caused by drugs: an update. Swiss Med Wkly 2010;140:w13080. [DOI] [PubMed] [Google Scholar]
- 7. Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther 2012;37:3–17. [DOI] [PubMed] [Google Scholar]
- 8. Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis 2000;4:73–96. [DOI] [PubMed] [Google Scholar]
- 9. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. . Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364–72. [DOI] [PubMed] [Google Scholar]
- 10. Flores A. Severe cholestasis and bile acid nephropathy from anabolic steroids successfully treated with plasmapheresis. ACG Case Rep J 2016;3:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robles-Diaz M, Gonzalez-Jimenez A, Medina-Caliz I, Stephens C, García-Cortes M, García-Muñoz B, et al. . Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment Pharmacol Ther 2013;41:116–25. [DOI] [PubMed] [Google Scholar]
- 12. Diaz FC, Saez-Gonzalez E, Benlloch S, Álvarez-Sotomayor D, Conde I, Polo B, et al. . Albumin dialysis with MARS for the treatment of anabolic steroid-induced cholestasis. Ann Hepatol 2016;15:939–43. [DOI] [PubMed] [Google Scholar]
- 13. Ryan AJ. Anabolic steroids are fool’s gold. Fed Proc 1981;40:2682–6. [PubMed] [Google Scholar]
- 14. Anand JS, Chodorowski Z, Hajduk A, Waldman W. Cholestasis induced by parabolan successfully treated with the molecular adsorbent recirculating system. ASAIO J 2006;52:117–8. [DOI] [PubMed] [Google Scholar]
- 15. Tan HK. Molecular adsorbent recirculating system. Ann Acad Med Singapore 2004;33:329–35. [PubMed] [Google Scholar]
- 16. Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, et al. . Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 2013;57:1153–62. [DOI] [PubMed] [Google Scholar]
- 17. Hassanein TI, Tofteng F, Brown RS, McGuire B, Lynch P, Mehta R, et al. . Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology 2007;46:1853–62. [DOI] [PubMed] [Google Scholar]
- 18. Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, et al. . Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med 2013;159:522–31. [DOI] [PubMed] [Google Scholar]