Abstract
Background
Neonatal herpes is a potentially devastating infection that results from acquisition of herpes simplex virus (HSV) type 1 or 2 from the maternal genital tract at the time of vaginal delivery. Current guidelines recommend (1) cesarean delivery if maternal genital HSV lesions are present at the time of labor and (2) antiviral suppressive therapy for women with known genital herpes to decrease HSV shedding from the genital tract at the time of vaginal delivery. However, most neonatal infections occur in infants born to women without a history of genital HSV, making current prevention efforts ineffective for this group. Although routine serologic HSV testing of women during pregnancy could identify women at higher risk of intrapartum viral shedding, it is uncertain how this knowledge might impact intrapartum management, and a potential concern is a higher rate of cesarean sections among women known to be HSV-2 seropositive.
Methods
To assess the effects of prenatal HSV-2 antibody testing, history of genital herpes, and use of suppressive antiviral medication on the intrapartum management of women, we investigated the frequency of invasive obstetric procedures and cesarean deliveries. We conducted a retrospective cohort study of pregnant women delivering at the University of Washington Medical center in Seattle, Washington. We defined the exposure of interest as HSV-2 antibody positivity or known history of genital herpes noted in prenatal records. The primary outcome was intrapartum procedures including fetal scalp electrode, artificial rupture of membranes, intrauterine pressure catheter, or operative vaginal delivery (vacuum or forceps). The secondary outcome was incidence of cesarean birth. Univariate and multivariable logistic regressions were performed.
Results
From a total of 449 women included in the analysis, 97 (21.6%) were HSV-2 seropositive or had a history of genital herpes (HSV-2/GH). Herpes simplex virus-2/GH women not using suppressive antiviral therapy were less likely to undergo intrapartum procedures than women without HSV-2/GH (odds ratio [OR], 0.49; 95% confidence interval [CI], 0.25–0.95; P = .036), but this relationship was attenuated after adjustment for potential confounders (adjusted OR, 0.69; 95% CI, 0.34–1.41; P = .31). There was no difference in intrapartum procedures for women on suppressive therapy versus women without HSV-2/GH (OR, 1.17; 95% CI, 0.66–2.07; P = .60). Similar proportions of cesarean sections were performed within each group of women: 25% without history of HSV-2/GH, 30% on suppressive treatment, and 28.1% without suppressive treatment (global, P = .73).
Conclusions
In this single-site study, provider awareness of genital herpes infection either by HSV serotesting or history was associated with fewer invasive obstetric procedures shown to be associated with neonatal herpes, but it was not associated with an increased rate of cesarean birth.
Keywords: cesarean section, genital herpes, herpes simplex virus-2, pregnancy, suppressive therapy
Neonatal herpes is a rare but potentially devastating infection of infants that usually results from infection with herpes simplex virus (HSV) type 1 or 2 in the maternal genital tract at the time of vaginal birth. The incidence of neonatal herpes ranges from 8 to 60 cases per 100000 live births in the United States [1–6]. To date, prevention efforts have focused on avoiding neonatal exposure to HSV in genital secretions of infected pregnant women by performing cesarean delivery in women with genital lesions at the time of labor. However, most cases of neonatal herpes occur among infants born to women with asymptomatic genital HSV, especially those who become infected late in pregnancy [1, 7–9]. Thus, the strategy of cesarean delivery for women with lesions in labor fails to address the majority of cases [1]. Intrapartum procedures, such as operative vaginal delivery or use of fetal scalp electrodes, can disrupt the fetal skin and increase the risk of HSV exposure in the genital tract, thus increasing the risk of neonatal herpes infection [1, 10]. Membrane rupture also contributes to risk [11]. Avoiding intrapartum procedures in women with antibodies to HSV-2 could decrease risk of neonatal herpes.
Herpes simplex virus antibody testing identifies women with HSV infection, and maternal HSV antibody testing may be helpful in identifying neonates at risk of intrapartum HSV exposure. However, routine prenatal serology testing for HSV is not currently recommended due to lack of cost-effective screening strategies and lack of evidence that antiviral treatment in asymptomatic HSV-2-seropositive women will decrease neonatal herpes infection [12]. Maternal HSV antibody status, in addition to clinical history, could inform provider decision making at delivery and decrease the use of intrapartum procedures associated with increased neonatal herpes risk in asymptomatic women with genital HSV infection. However, a concern about such a screening strategy is that it may increase the rate of cesarean deliveries among HSV-2-seropositive women [13, 14].
Herpes simplex virus antibody testing has been done routinely as part of prenatal care at the University of Washington Medical Center (UWMC). To assess the effect of provider knowledge regarding HSV status either by serology or history on intrapartum management, we compared the frequency of invasive intrapartum procedures and of cesarean births among pregnant women with either HSV-2 antibody or a clinical history of herpes to those in women with no evidence of genital HSV infection.
METHODS
Subjects and Setting
We conducted a retrospective cohort study of 750 consecutive deliveries performed in 2006 at the UWMC, a quaternary care referral center serving the Pacific Northwest that performs approximately 2000 deliveries per year. We reviewed charts using a standardized data collection form and abstracted data on HSV antibody result, clinical history of genital herpes, demographics, use of intrapartum procedures, final method of delivery, and underlying conditions and complications of pregnancy or delivery. A subset of charts was audited to confirm accuracy of data collection. We included women with any HSV antibody result or genital herpes history. Women with active genital lesions suspicious for HSV during labor were excluded. Women with nonvertex fetal presentation were excluded because this was assumed, a priori, to be very strongly associated with cesarean delivery. Women undergoing scheduled cesarean births were also excluded from the analysis, because they are not at risk for intrapartum procedures, and the impact of genital HSV infection status on provider decisions during labor cannot be evaluated.
We extracted demographic characteristics of the women and other aspects of the medical and obstetric history. History of genital herpes was ascertained from the clinical history interview obtained during prenatal care, and HSV Western blot analysis was obtained with other prenatal serologies. Other variables included parity, prematurity, gestational number, failure to progress in labor, group B streptococcus culture, induction of labor, placental abruption, fetal distress, high-risk pregnancy, and other sexually transmitted diseases (STDs). Prematurity was defined as delivery before 37 weeks. High-risk pregnancy was defined by the presence of any one of several maternal and fetal factors. Maternal factors included asthma, diabetes (including gestational), hypertension (including pregnancy-induced), pre-eclampsia, renal disease, and cardiac disease. Fetal factors included oligohydraminios, intrauterine growth restriction, and congenital anomalies. Specific STDs assessed included any history of syphilis, gonorrhea, chlamydia, trichomoniasis, or human papilloma virus diagnosis during pregnancy. The University of Washington Institutional Review Board approved the study.
Exposures and Outcomes
We defined the exposure of interest as HSV-2 antibody positivity or known history of genital herpes (HSV-2/GH) noted in the prenatal and delivery records. Herpes simplex virus serology was determined using the University of Washington Western Blot [15]. We further categorized the exposure based on use of suppressive antiviral therapy (acyclovir or valacyclovir) for HSV at the end of pregnancy, because we assumed that this could modify any effect of HSV-2/GH status on provider behavior and thus on the outcomes of interest. Because orolabial infection with HSV-1 is common in the general population, we grouped women with only HSV-1 antibody among those without any HSV antibody. The primary outcome, intrapartum procedures use, was a composite of the following: use of fetal scalp electrode, artificial rupture of membranes, intrauterine pressure catheter, or vaginal operative (vacuum or forceps) delivery. The secondary outcome was cesarean delivery.
Statistical Analysis
We used the χ2 test to compare proportions and the t test to compare means. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression and performed univariate analyses of the associations between HSV-2/GH with and without suppressive therapy and the primary and secondary outcomes. We also explored associations between other covariates and each outcome that may confound the association of HSV-2/GH and the outcomes. Two-sided P values ≤.05 were considered statistically significant. For each outcome, we constructed an initial multivariable logistic regression model incorporating the exposure of interest, HSV-2/GH, and covariates that showed possible associations (P ≤ .1) with that outcome in univariate analysis. The final multivariable models were prepared by backwards elimination from each model of covariates lacking a strong association with the outcome (P > .05). The statistical analysis was carried out using Stata versions 9 and 10 (StataCorp, College Station, TX).
Study Power
We assumed that approximately 80% of reviewed charts would have available data on the exposure of interest, because some participants may not have undergone HSV antibody testing. From preliminary data, we also estimated a 14% overall prevalence of the exposure, HSV-2/GH, in the study population. Although the risk of undergoing several of the intrapartum procedures is less than 10%, on an individual basis, in a given delivery, the risk of a composite outcome of undergoing any intrapartum procedure is approximately 40% at our center. For an outcome with a 40% probability, we had 80% power to detect ORs less than 0.47 or greater than 1.96.
RESULTS
Of the 750 charts reviewed, 606 women had known HSV-2/GH status. Of the 144 women missing such HSV-2/GH information, only 39.7% had received prenatal care at UWMC, with many presenting late in pregnancy or during labor, whereas 91.7% of women with known HSV-2/GH had received prenatal care at UWMC. After excluding women with active genital lesions (n = 2), women with nonvertex fetal presentations (n = 55), and women undergoing scheduled cesarean birth (n = 100), 449 women were included in the analysis (Figure 1A). Among women who underwent scheduled cesarean births, the indication was prior cesarean delivery in 76.3%; indications in the remaining 23.7% were largely a mix of fetal anomalies, macrosomia, and maternal anatomic abnormalities. Herpes simplex virus was listed as a secondary indication for scheduled cesarean delivery in 2 of these women: one who had a primary indication of history of fetal shoulder dystocia in a prior delivery and another who underwent a planned repeat cesarean delivery. Because there was a primary indication for scheduled cesarean birth unrelated to HSV in both of these cases, it was assumed that both women would have had scheduled cesarean delivery regardless of HSV status and the exclusions were judged to be valid.
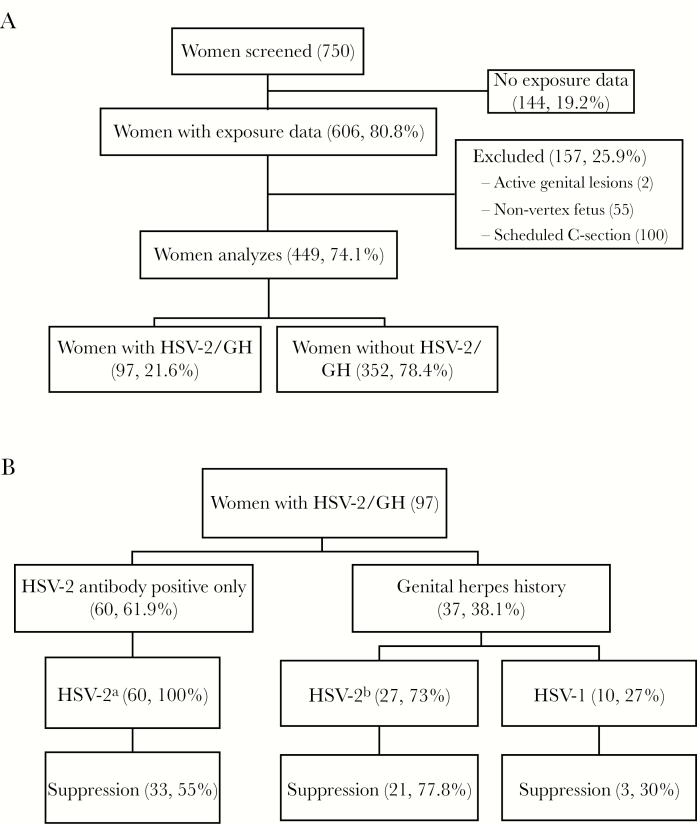
Figure 1.
(A) shows a flow diagram of study participant selection. Charts were reviewed from 750 women with consecutive deliveries at University of Washington Medical Center in 2006, 606 of which had exposure data. After excluding women with active genital lesions, nonvertex fetal presentation, and scheduled cesarean births, 449 women were included in the analysis. Of these, 97 (21.6%) had herpes simplex virus-2 antibody positive or with a history of genital herpes (HSV-2/GH). (B) shows the distribution of HSV-2 antibody positivity and clinical history of genital herpes in the 97 women with HSV-2/GH. Women with a clinical history are further divided into those who were HSV-2 antibody positive and those positive for antibodies to HSV-1 only. The distribution of the 57 women (58.8%) on suppressive anti-HSV therapy is also shown. aHSV-1 antibodies detected in 39 (65%); bHSV-1 antibodies detected in 14 (51.9%). C-section, cesarean section.
Of the 449 women included in the analysis, the mean age was 30.2 years (Table 1). Ninety-seven (21.6%) women had HSV-2 antibody or a clinical history of genital herpes. Of 60 HSV-2-seropositive women without a clinical history of genital herpes, 33 (55%) received suppressive antiviral therapy at the end of pregnancy based on provider preference. Of 37 women with a clinical history of genital herpes, 24 (65%) received suppressive antiviral therapy. Overall, among women with HSV-2 or GH, 40 (41%) were not receiving suppressive antiviral therapy (Figure 1B). African American women were as likely as white women to receive suppressive therapy (59.2% vs 60%). The 352 women who were neither HSV-2 seropositive nor with a clinical history of GH were used as the comparison group.
Table 1.
Demographic and Clinical Factors of Women Included in Analysisa
| Factor | HSV-2/GH | Total | P c | |
|---|---|---|---|---|
| Yes, n (%)b | No, n (%)b | |||
| All subjects | 97 (21.6) | 352 (78.4) | 449 | |
| Antiviral suppression | 57 (58.8) | |||
| No antiviral suppression | 40 (41.2) | |||
| Mean age (n = 448) | 31.1 | 30.0 | 30.2 | .126d |
| Race (n = 440) | <.001 | |||
| African American | 27 (28.1) | 34 (9.9) | 61 (13.9) | |
| Asian | 10 (10.4) | 60 (17.4) | 70 (15.9) | |
| White | 55 (57.3) | 237 (68.9) | 292 (66.4) | |
| Biracial/other | 4 (4.2) | 13 (3.8) | 17 (3.9) | |
| Hispanic ethnicity (n = 434) | 7 (7.5) | 37 (10.9) | 44 (10.1) | .347 |
| Non-Hispanic | 86 (92.5) | 304 (89.2) | 390 (89.9) | |
| Married (n = 446) | 53 (55.2) | 272 (77.7) | 325 (72.9) | <.0001 |
| Not married | 43 (44.8) | 78 (22.3) | 121 (27.1) | |
| Multiparous | 55 (56.7) | 163 (46.3) | 218 (48.6) | .070 |
| Primiparous | 41 (43.3) | 189 (53.7) | 231 (51.5) | |
| Premature | 25 (25.8) | 65 (18.5) | 90 (20.0) | .111 |
| Not premature | 72 (74.2) | 287 (81.5) | 359 (80.0) | |
| Multiple gestation (n = 442) | 7 (7.4) | 17 (4.9) | 24 (5.4) | .347 |
| Singleton | 88 (92.6) | 330 (95.1) | 418 (94.6) | |
| High-risk conditione | 58 (59.8) | 181 (51.4) | 239 (53.2) | .143 |
| No high-risk condition | 39 (40.2) | 171 (48.6) | 210 (46.8) | |
| HSV-1 antibody positive (n = 441) | 62 (66.7) | 223 (64.1) | 285 (64.6) | .643 |
| HSV-1 antibody negative | 31 (33.3) | 125 (35.9) | 156 (35.4) | |
| Any intrapartum procedure | 51 (52.6) | 203 (57.7) | 254 (56.6) | .370 |
| No intrapartum procedure | 46 (47.4) | 149 (42.3) | 195 (43.4) | |
| Intrapartum Procedure by Type | ||||
| Fetal scalp electrodes | 10 (10.3) | 51 (14.5) | 61 (13.6) | |
| AROM | 35 (36.1) | 144 (40.9) | 179 (39.9) | |
| IUPC | 27 (27.8) | 89 (25.3) | 116 (25.8) | |
| Vacuum extraction | 4 (4.1) | 11 (3.1) | 15 (3.3) | |
| Forceps extraction | 4 (4.1) | 6 (1.7) | 10 (2.2) | |
| Cesarean section | 28 (28.9) | 88 (25.0) | 116 (25.8) | .441 |
| Vaginal delivery | 69 (71.1) | 264 (75.0) | 333 (74.2) | |
Abbreviations: AROM, artificial rupture of membranes; HSV, herpes simplex virus; HSV-2/GH, HSV-2 antibody positive or clinical history of genital herpes; IUPC, intrauterine pressure catheter.
N = 449 except as noted.
Column percentage as a subset of the total for each covariate presented; percentage may not sum to 100% due to rounding.
Calculated with χ2 test except as noted.
Two-sided t test.
Asthma, diabetes, hypertension, pre-eclampsia, renal or cardiac disease, intrauterine growth restriction, oligohydramnios, congenital anomaly.
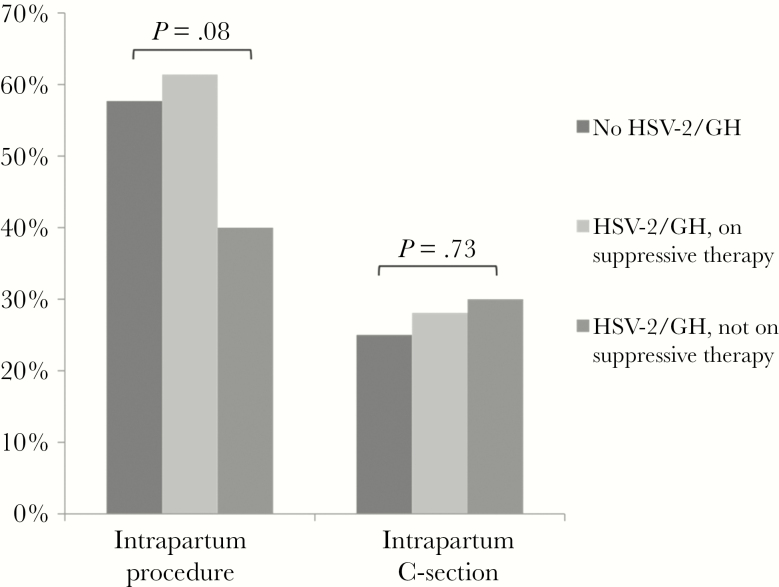
Intrapartum procedures were performed in 16 (40.0%) of 40 women with HSV-2/GH who were not receiving antiviral therapy (Figure 2), 35 (61.4%) of 57 women with HSV-2/GH using antiviral suppression, and 203 (57.7%) of 352 women without HSV-2/GH (global P = .08) (Figure 2). Comparable proportions of women in each group underwent unplanned cesarean births: 12 of 40 women (30.0%) with unsuppressed HSV-2/Gt; .73).
Figure 2.
Proportions of women undergoing intrapartum procedures and cesarean delivery, by risk group, are shown. Risk groups include asymptomatic women with and without herpes simplex virus (HSV)-2 antibody positivity or a clinical history of genital herpes (HSV-2/GH). Women with HSV-2/GH are further stratified by whether or not they were receiving suppressive antiviral therapy at delivery. Global P values for differences between groups are shown. C-section, cesarean section.
In univariate regression analysis, women with HSV-2/GH who were not on suppressive antiviral therapy were less likely to undergo intrapartum procedures than women without HSV-2/GH (OR, 0.49; 95% CI, 0.25–0.95; P = .036). We did not detect a difference in the rate of intrapartum procedures for women with HSV-2/GH on suppressive therapy compared with women without HSV-2/GH (OR, 1.17; 95% CI, 0.66–2.07; P = .60). Women ages 39 and older were much less likely to undergo intrapartum procedures than women 20 years and younger (OR, 0.11; 95% CI, 0.04–0.35; P ≤ .001). Intrapartum procedures were used less frequently in multiparous women than in primiparous women (OR, 0.62; 95% CI, 0.42–0.90; P = .01) and in women with multiple gestation pregnancies compared with women with singleton pregnancies (OR, 0.23; 95% CI, 0.09–0.60; P = .003). Prematurity was associated with a decreased likelihood of intrapartum procedures (OR, 0.46; 95% CI, 0.29–0.73; P = .001), but failure to progress in labor was associated with an increased likelihood (OR, 2.22; 95% CI, 1.21–4.08; P = .01). Compared with white woman, African American women were as likely (OR, 1.02; 95% CI, 0.59–1.77; P = .95) and Asian women were more likely (OR, 1.88; 95% CI, 1.09–3.26; P = .02) to undergo intrapartum procedures. Seventeen women categorized as biracial or of other racial backgrounds were examined as a single category; 16 of these women underwent an intrapartum procedure (OR, 14.74; 95% CI, 1.93–112.58; P = .01). Covariates examined for associations with intrapartum procedures that were not significant on univariate analysis included Hispanic ethnicity, marital status, high-risk pregnancy, obesity, infection with another STD during pregnancy, group B streptococcus culture-positive, fetal distress, placental abruption, and induction of labor.
On multivariate analysis, after adjusting for age, race, prematurity, and failure to progress in labor, the association between HSV-2/GH status and intrapartum procedures in women was attenuated and no longer statistically significant. Women with unsuppressed HSV-2/GH had somewhat lower risk of intrapartum procedures compared with women without HSV-2/GH (OR, 0.69; 95% CI, 0.34–1.41; P = .31). In contrast, women with suppressed HSV-2/GH had somewhat higher risk of intrapartum procedures compared with women without HSV-2/GH (OR, 1.41; 95% CI, 0.77–2.72; P = .26) (Table 2). To examine the effect of race on the model, the analysis was repeated excluding the “biracial/other” category and again excluding the entire race covariate, but these had only a minor impact on the adjusted ORs.
Table 2.
Predictors of Invasive Obstetric Procedurea Use at Delivery
| N = 449b | Invasive Procedure | OR (95% CI) | P | aOR (95% CI) | P | |
|---|---|---|---|---|---|---|
| Yes, n (%)c | No, n (%)c | |||||
| HSV-2/GH Status | ||||||
| Uninfected | 149 (76.4) | 203 (79.9) | Ref | .08d | Ref | .25d |
| Antiviral suppression | 35 (13.8) | 22 (11.3) | 1.17 (0.66–2.07) | .60 | 1.45 (0.77–2.72) | .26 |
| No antiviral suppression | 16 (6.3) | 24 (12.3) | 0.49 (0.25–0.95) | .04 | 0.69 (0.34–1.41) | .31 |
| Age (Years) | ||||||
| ≤20 | 24 (9.5) | 8 (4.1) | Ref | <.001d | Ref | .01d |
| 21 to 38 | 222 (87.4) | 163 (83.6) | 0.45 (0.20–1.04) | .06 | 0.51 (0.21–1.25) | .14 |
| ≥39 | 8 (3.2) | 24 (12.3) | 0.11 (0.04–0.35) | <.001 | 0.15 (0.05–0.50) | .002 |
| Race (n = 440) | ||||||
| White | 152 (61.5) | 140 (72.5) | Ref | .01d | Ref | .03d |
| Asian | 47 (19) | 23 (11.9) | 1.88 (1.09–3.26) | .02 | 1.84 (1.04–3.25) | .04 |
| African American | 32 (13) | 29 (15) | 1.02 (0.59–1.77) | .95 | 1.17 (0.64–2.14) | .62 |
| Biracial/Other | 16 (6.5) | 1 (0.5) | 14.74 (1.93–112.6) | .01 | 11.35 (1.46–88.5) | .02 |
| Hispanic (n = 434) | 25 (10.2) | 19 (10.1) | 1.02 (0.54–1.91) | .96 | ||
| Married (n = 446) | 186 (73.8) | 139 (71.7) | 1.11 (0.73–1.70) | .61 | ||
| Multiparous | 110 (43.3) | 108 (55.4) | 0.62 (0.422–0.90) | .01 | 0.65 (0.42–0.99) | .05 |
| High-riske | 134 (52.8) | 105 (53.9) | 0.96 (0.66–1.39) | .82 | ||
| Obesity | 30 (11.8) | 24 (12.3) | 0.95 (0.54–1.69) | .87 | ||
| Multiple gest. (n = 442) | 6 (2.4) | 18 (9.5) | 0.23 (0.09–0.60) | .003 | ||
| Other STDf | 21 (8.3) | 23 (11.8) | 0.67 (0.36–1.26) | .22 | ||
| GBS positive (n = 421) | 69 (28.8) | 57 (31.5) | 0.88 (0.58–1.34) | .54 | ||
| Premature | 37 (14.6) | 53 (27.2) | 0.46 (0.29–0.73) | .001 | 0.43 (0.26–0.72) | .001 |
| Failure to progress | 42 (16.5) | 16 (8.2) | 2.22 (1.21–4.08) | .01 | 1.98 (1.03–3.82) | .04 |
| Fetal distress | 22 (8.7) | 25 (12.8) | 0.65 (0.35–1.18) | .16 | ||
| Placental abruption | 2 (0.8) | 5 (2.6) | 0.30 (0.06–1.57) | .16 | ||
| Induction of labor | 58 (22.8) | 42 (21.5) | 1.08 (0.69–1.69) | .74 | ||
Abbreviations: aOR, odds ratio adjusted for age, race, multiparity, prematurity, failure to progress in labor; CI, confidence interval; GBS, group B streptococcus culture; gest., gestation; HSV-2/GH, herpes simplex virus type 2 seropositive or clinical history of genital herpes; OR, odds ratio; Ref, reference category; STD, sexually transmitted disease.
Fetal scalp electrode, artificial rupture of membranes, intrauterine pressure catheter, vacuum extraction or forceps extraction.
Except where noted otherwise.
Percentages may not sum to 100% because of rounding.
Global P value for all categories.
Asthma, diabetes, hypertension, pre-eclampsia, renal or cardiac disease, intrauterine growth restriction, oligohydramnios, congenital anomaly.
Syphilis, gonorrhea, chlamydia, trichomoniasis, or human papillomavirus diagnosed during pregnancy.
Potential predictors of cesarean births were also explored. On univariate analysis, we did not detect any increase in likelihood of cesarean delivery for women with unsuppressed (OR, 1.29; 95% CI, 0.63–2.64; P = .49) or suppressed HSV-2/GH (OR, 1.17; 95% CI, 0.63–2.19; P = .62) relative to women without HSV-2/GH. Multiparity was associated with a decreased likelihood of undergoing cesarean births (OR, 0.53; 95% CI, 0.35–0.82; P = .004), and high-risk pregnancy was associated with an increased likelihood (OR, 1.89; 95% CI, 1.22–2.92; P = .004). Multiple gestation (OR, 4.51; 95% CI, 1.94–10.47; P < .001), prematurity (OR, 1.81; 95% CI, 1.10–2.97; P = .02), and chorioamnionitis (OR, 3.38; 95% CI, 1.58–7.25; P = .002) were all associated with an increased likelihood of cesarean delivery. Covariates not found to have a significant association with cesarean birth on univariate analysis included age, race, Hispanic ethnicity, marital status, obesity, other STDs during pregnancy, positive group B streptococcus culture, preterm rupture of membranes, placental abruption, and induction of labor.
After adjusting for parity, high-risk pregnancy, and multiple gestation pregnancy in a multivariate model, we did not detect a significant increase in the likelihood of cesarean delivery in women with HSV-2/GH. This was true regardless of whether they were receiving (OR, 1.21; 95% CI, 0.63–2.33; P = .57) or not receiving suppressive antiviral therapy (OR, 1.13; 95% CI, 0.51–2.50; P = .76) (Table 3).
Table 3.
Predictors of Cesarean Section Use at Delivery
| N = 449a | Unplanned Cesarean Birth | OR (95% CI)b | P | aOR (95% CI)b | P | |
|---|---|---|---|---|---|---|
| Yes, n (%)c | No, n (%)c | |||||
| HSV-2/GH status | ||||||
| Uninfected | 88 (75.9) | 264 (79.3) | Ref | .73d | Ref | .83d |
| Antiviral suppression | 16 (13.8) | 41 (12.3) | 1.17 (0.63–2.19) | .62 | 1.21(0.63–2.33) | .57 |
| No antiviral suppression | 12 (10.3) | 28 (8.4) | 1.29 (0.63–2.64) | .49 | 1.13 (0.51–2.50) | .76 |
| Multiparous | 43 (37.1) | 175 (52.6) | 0.53 (0.35–0.82) | .004 | 0.51 (0.32–0.80) | .003 |
| High-riske | 75 (64.7) | 164 (49.3) | 1.89 (1.22–2.92) | .004 | 1.95 (1.23–3.08) | .003 |
| Multiple gest. (n = 442) | 14 (12.4) | 10 (3.0) | 4.51 (1.94–10.47) | <.001 | 4.56 (1.92–10.81) | .001 |
| Premature | 32 (27.6) | 58 (17.4) | 1.81 (1.10–2.97) | .02 | ||
| Chorioamnionitis | 15 (12.9) | 14 (4.2) | 3.38 (1.58–7.25) | .002 | ||
Abbreviations: aOR, odds ratio adjusted for multiparity, high-risk pregnancy, multiple gestation; CI, confidence interval; gest., gestation; HSV-2/GH, herpes simplex virus type 2 seropositive or clinical history of genital herpes; OR, odds ratio; Ref, reference category.
Except where otherwise noted.
Age, race, Hispanic ethnicity, marital status, obesity, other sexually transmitted disease during pregnancy (syphilis, gonorrhea, chlamydia, trichomoniasis, or human papillomavirus), group B streptococcus culture positive, preterm rupture of membranes, placental abruption, and induction of labor were not associated with increased risk of intrapartum cesarean birth.
Percentages may not sum to 100% because of rounding.
Global P value for all categories.
Asthma, diabetes, hypertension, pre-eclampsia, renal or cardiac disease, intrauterine growth restriction, oligohydramnios, congenital anomaly.
DISCUSSION
To our knowledge, this is the first study to evaluate the effect of prenatal HSV antibody screening programs on management of labor. We found that the rate of intrapartum invasive procedures was lower among asymptomatic pregnant women with antibodies to HSV-2 or a clinical history of genital herpes who were not receiving suppressive antiviral therapy compared with women without HSV-2 antibodies or a history of genital herpes, although the relationship was not significant after adjustment for potential confounders. Furthermore, women with antibodies to HSV-2 or a clinical history of genital herpes who received suppressive therapy were at similar risk for intrapartum procedures compared with women without HSV-2 antibodies or a clinical history of genital herpes.
Shedding of HSV in the maternal genital tract during birth poses substantial risk of neonatal herpes, but prolonged amniotic membrane disruption, vacuum-assisted delivery, and fetal scalp monitors have also been shown to increase the risk of neonatal HSV [1, 10, 11]. Collectively, the relevant literature strongly suggests that any intrapartum instrumentation that can breach the infant skin should be avoided in the setting of possible maternal mucosal HSV shedding. Current practice guidelines suggest using suppressive antiviral therapy for women with a history of recurrent genital HSV lesions in pregnancy, which reduces recurrence of genital herpes, viral shedding, and rate of cesarean delivery use, but evidence that this strategy prevents neonatal herpes is lacking [12, 16–21]. Furthermore, in the last 2 decades, a period in which this approach has been increasingly popular, there has been no change in the incidence of neonatal herpes in the United States [1–6]. This is consistent with the observation in nonpregnant women with genital HSV infection that antiviral therapy reduces but does not eliminate subclinical HSV shedding from genital mucosa [22, 23]. A recent series reported 8 cases of neonatal herpes in infants whose mothers received antiviral suppression at the end of pregnancy [24]. Thus, management of women with genital herpes with antiviral therapy does not eliminate the risk of neonatal herpes. The findings from our study suggest that, at our institution, providers modify their use of intrapartum procedures that increase risk of neonatal herpes in asymptomatic HSV-2 infected women with known, unsuppressed genital HSV infection, but that women who are receiving suppressive antiviral therapy are considered to be at low risk and are managed similarly to women without genital HSV infection. These findings indicate that improved provider education about the risks of intrapartum procedures and breakthrough neonatal herpes despite suppressive therapy is warranted.
We also investigated the impact of prenatal HSV testing on the use of cesarean births. Cesarean delivery is recommended for women who have active genital herpes lesions at term [12]. However, one potential consequence of prenatal HSV testing is increased use of medically unwarranted cesarean delivery in women with asymptomatic genital HSV infection [13, 14]. In this study, we did not observe such an increase in cesarean births in our cohort of women who had undergone prenatal HSV testing, showing that this concern is unfounded. Pregnant women with HSV-2 antibodies or a clinical history of genital herpes, but without active lesions, underwent cesarean births with similar frequency as women without such history, and antiviral suppressive therapy did not affect this outcome.
This study offers evidence that routine prenatal HSV testing does not result in harm by increasing cesarean delivery and that such testing may benefit women who are identified as HSV-2 antibody positive. Specifically, our findings suggest that providers may be more wary of use of intrapartum procedures that could increase the risk of HSV transmission to neonates delivered by asymptomatic women with known genital HSV infection. Although most obstetricians believe neonatal herpes merits systematic prevention strategies, few report performing regular prenatal HSV antibody testing outside of academic settings [25]. In addition to cesarean delivery use, costs and psychosocial burdens of prenatal HSV testing have been put forth as concerns [13, 14, 26]. Cost-effectiveness models of neonatal herpes prevention strategies that include prenatal HSV-2 antibody testing are conflicting, although the most recent models are more favorable [26–30]. With regard to the psychosocial burden, in a recent systematic review of studies exploring the impact of HSV-2 antibody testing in asymptomatic persons, most participants testing positive did not suffer sustained emotional harm [31]. In pregnancy, the motivation to be tested may be higher because there is a desire to protect the fetus. Of note, studies assessing the acceptability of such programs suggest that pregnant women are amenable to prenatal HSV testing [32–34]. More targeted prenatal HSV screening would likely miss a substantial proportion of cases [2]. However, routine prenatal screening alone is unlikely to impact the incidence of neonatal herpes, because the greatest risk occurs in women who acquire genital herpes late in pregnancy and lack detectable HSV antibodies at delivery [1]. Further strategies need to be developed to identify women who are infected near the time of delivery and are asymptomatic.
Our study has several limitations. Observational studies are subject to the effects of unmeasured confounding, and a randomized trial of prenatal HSV testing would be necessary to address this problem. The size of the study population also limited our approach to the analysis in several ways. We could not assess neonatal herpes as an outcome. In addition, to increase statistical power, we combined all women with evidence of genital HSV infection regardless of prior symptom history, as long as they were asymptomatic at delivery. An important limitation in our analysis is the lack of unified management of HSV-2, likely due to physicians practice and women’s preference. It is possible that providers treated women with a clinical history of genital herpes differently from HSV-2 antibody positive women without any such history. Thus, a potential bias for antiviral treatment in patients with clinical and serological diagnosis of genital herpes could have extended to the use of obstetric procedures and cesarean delivery use, although we are not clear whether these would be positively or negatively associated. Because our data come from a teaching hospital, the delivering physician is not always the same physician that provides prenatal care. Therefore, examining associations between antiviral therapy and intrapartum procedures may not be helpful.
We also lacked the power to assess the effect of the exposure on individual intrapartum procedures. Combining these outcomes may have masked differing associations between the exposure and individual procedure use. There are also inherent limitations to using chart data to assess provider behavior, and provider decision making was not measured directly, and some interventions may not be recorded in the chart. Finally, this study was conducted at an academic medical center with strong institutional knowledge of neonatal herpes disease and risk factors. The impact of prenatal HSV testing programs, in the absence of clear guidelines for management of asymptomatic women with genital HSV infection, could differ substantially by setting. In our population, suppressive antiviral therapy was administered to some HSV-2 antibody positive women without a clinical history of genital herpes, which is not currently recommended [12].
CONCLUSIONS
Improved interventions are needed to reduce neonatal herpes. In our study, identification of women with HSV-2 infection with a routine prenatal antibody testing program appeared to reduce the use of procedures known to increase neonatal herpes risk in women not on antiviral suppression without an increase in cesarean sections.
Acknowledgments
Financial support. This work was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant numbers P01 AI030731 and K24 AI071113).
Potential conflicts of interest. A. S. M. has been a consultant to Immune Design Corporation and AiCuris. A. W. has received research funding from Genocea and Vical, has been a consultant to Aicuris and GSK, and received paid travel from Admedus. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brown ZA, Wald A, Morrow RA et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003; 289:203–9. [DOI] [PubMed] [Google Scholar]
- 2. Mark KE, Kim HN, Wald A et al. Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate?Am J Obstet Gynecol 2006; 194:408–14. [DOI] [PubMed] [Google Scholar]
- 3. Whitley R, Davis EA, Suppapanya N. Incidence of neonatal herpes simplex virus infections in a managed-care population. Sex Transm Dis 2007; 34:704–8. [DOI] [PubMed] [Google Scholar]
- 4. Morris SR, Bauer HM, Samuel MC et al. Neonatal herpes morbidity and mortality in California, 1995–2003. Sex Transm Dis 2008; 35:14–8. [PubMed] [Google Scholar]
- 5. Handel S, Klingler EJ, Washburn K et al. Population-based surveillance for neonatal herpes in New York City, April 2006-September 2010. Sex Transm Dis 2011; 38:705–11. [DOI] [PubMed] [Google Scholar]
- 6. Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics 2011; 127:e1–8. [DOI] [PubMed] [Google Scholar]
- 7. Stone KM, Brooks CA, Guinan ME, Alexander ER. National surveillance for neonatal herpes simplex virus infections. Sex Transm Dis 1989; 16:152–6. [DOI] [PubMed] [Google Scholar]
- 8. Brown ZA, Selke S, Zeh J et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997; 337:509–15. [DOI] [PubMed] [Google Scholar]
- 9. James SH, Kimberlin DW. Neonatal herpes simplex virus infection. Infect Dis Clin North Am 2015; 29:391–400. [DOI] [PubMed] [Google Scholar]
- 10. Kohelet D, Katz N, Sadan O, Somekh E. Herpes simplex virus infection after vacuum-assisted vaginally delivered infants of asymptomatic mothers. J Perinatol 2004; 24:147–9. [DOI] [PubMed] [Google Scholar]
- 11. Nahmias AJ, Josey WE, Naib ZM et al. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol 1971; 110:825–37. [DOI] [PubMed] [Google Scholar]
- 12. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. No. 82 June 2007. Management of herpes in pregnancy. Obstet Gynecol 2007; 109:1489–98. Available at: https://www.ncbi.nlm.nih.gov/pubmed/?term=Clinical+management+guidelines+for+obstetrician-gynecologists.+No.+82+June+2007.+Management+of+herpes+in+pregnancy. Accessed 8 February 2017. [DOI] [PubMed] [Google Scholar]
- 13. Arvin AM. Debate: the argument against. Should all pregnant women be offered type-specific serological screening for HSV infection?Herpes 2002; 9:48–50. Availalble at: https://www.ncbi.nlm.nih.gov/pubmed/12106512. Accessed 8 February 2017. [PubMed] [Google Scholar]
- 14. Tita AT, Grobman WA, Rouse DJ. Antenatal herpes serologic screening: an appraisal of the evidence. Obstet Gynecol 2006; 108:1247–53. [DOI] [PubMed] [Google Scholar]
- 15. Ashley RL, Militoni J, Lee F et al. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–7. Available at: http://jcm.asm.org/content/26/4/662.long. Accessed 10 February 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott LL, Sanchez PJ, Jackson GL et al. Acyclovir suppression to prevent cesarean delivery after first-episode genital herpes. Obstet Gynecol 1996; 87:69–73. [DOI] [PubMed] [Google Scholar]
- 17. Scott LL, Hollier LM, McIntire D et al. Acyclovir suppression to prevent recurrent genital herpes at delivery. Infect Dis Obstet Gynecol 2002; 10:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watts DH, Brown ZA, Money D et al. A double-blind, randomized, placebo-controlled trial of acyclovir in late pregnancy for the reduction of herpes simplex virus shedding and cesarean delivery. Am J Obstet Gynecol 2003; 188:836–43. [DOI] [PubMed] [Google Scholar]
- 19. Sheffield JS, Hill JB, Hollier LM et al. Valacyclovir prophylaxis to prevent recurrent herpes at delivery: a randomized clinical trial. Obstet Gynecol 2006; 108:141–7. [DOI] [PubMed] [Google Scholar]
- 20. Andrews WW, Kimberlin DF, Whitley R et al. Valacyclovir therapy to reduce recurrent genital herpes in pregnant women. Am J Obstet Gynecol 2006; 194:774–81. [DOI] [PubMed] [Google Scholar]
- 21. Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev 2008; CD004946. [DOI] [PubMed] [Google Scholar]
- 22. Wald A, Corey L, Cone R et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest 1997; 99:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta R, Wald A, Krantz E et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis 2004; 190:1374–81. [DOI] [PubMed] [Google Scholar]
- 24. Pinninti SG, Angara R, Feja KN et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr 2012; 161:134–8.e1–3. [DOI] [PubMed] [Google Scholar]
- 25. Gardella C, Barnes J, Magaret AS et al. Prenatal herpes simplex virus serologic screening beliefs and practices among obstetricians. Obstet Gynecol 2007; 110:1364–70. [DOI] [PubMed] [Google Scholar]
- 26. Rouse DJ, Stringer JS. An appraisal of screening for maternal type-specific herpes simplex virus antibodies to prevent neonatal herpes. Am J Obstet Gynecol 2000; 183:400–6. [DOI] [PubMed] [Google Scholar]
- 27. Barnabas RV, Carabin H, Garnett GP. The potential role of suppressive therapy for sex partners in the prevention of neonatal herpes: a health economic analysis. Sex Transm Infect 2002; 78:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker D, Brown Z, Hollier LM et al. Cost-effectiveness of herpes simplex virus type 2 serologic testing and antiviral therapy in pregnancy. Am J Obstet Gynecol 2004; 191:2074–84. [DOI] [PubMed] [Google Scholar]
- 29. Thung SF, Grobman WA. The cost-effectiveness of routine antenatal screening for maternal herpes simplex virus-1 and -2 antibodies. Am J Obstet Gynecol 2005; 192:483–8. [DOI] [PubMed] [Google Scholar]
- 30. Tuite AR, McCabe CJ, Ku J, Fisman DN. Projected cost-savings with herpes simplex virus screening in pregnancy: towards a new screening paradigm. Sex Transm Infect 2011; 87:141–8. [DOI] [PubMed] [Google Scholar]
- 31. Ross K, Johnston C, Wald A. Herpes simplex virus type 2 serological testing and psychosocial harm: a systematic review. Sex Transm Infect 2011; 87:594–600. [DOI] [PubMed] [Google Scholar]
- 32. Vonau B, Low-Beer N, Barton SE, Smith JR. Antenatal serum screening for genital herpes: a study of knowledge and attitudes of women at a central London hospital. Br J Obstet Gynaecol 1997; 104:347–9. [DOI] [PubMed] [Google Scholar]
- 33. Edmiston N, O’Sullivan M, Charters D et al. Study of knowledge of genital herpes infection and attitudes to testing for genital herpes among antenatal clinic attendees. Aust N Z J Obstet Gynaecol 2003; 43:351–3. [DOI] [PubMed] [Google Scholar]
- 34. Baker DA, Pressley A, Meek L et al. HSV serologic testing for pregnant women: willingness to be tested and factors affecting testing. Infect Dis Obstet Gynecol 2011; 2011:874820. [DOI] [PMC free article] [PubMed] [Google Scholar]