Abstract
Background
Recent mumps outbreaks among 2-dose measles mumps rubella (MMR) vaccine recipients have raised questions regarding the potential benefits of a third dose of vaccine (MMR3). If MMR3 provides a sustained elevation in mumps antibody, it may be beneficial for certain at-risk groups or as an outbreak control measure.
Methods
Sera were collected immediately prior to MMR3 and at 1 month and 1 year post-MMR3 from 656 healthy adults aged 18–28 years in a nonoutbreak setting. Immunoglobulin G (IgG) was measured by enzyme-linked immunosorbent assay (ELISA) using whole mumps virus (commercial ELISA), hemagglutinin (HN; major neutralizing target), and nucleoprotein (NP; immunodominant) antigens. ELISA measurements were compared with in vitro plaque reduction neutralization (PRN) titers, and baseline antibody was compared with post-MMR3 levels.
Results
There were modest but statistically significant (P < .05) increases in mumps antibody at 1 month post-MMR3 by all 3 ELISA methods and by PRN titer. At 1 year post-MMR3, mumps antibody declined toward baseline but remained elevated (P < .05). The correlation between PRN titers and ELISA measurements was poor (r2 = .49), although sera with the highest amount of HN IgG also had the highest PRN titers.
Conclusions
Individuals with the lowest baseline PRN titers had the largest increase in frequency of samples that became positive for HN and NP by ELISA. A third dose of MMR may benefit certain individuals with a low level of mumps virus–neutralizing antibody, especially in the context of an outbreak or other high-risk setting. Additionally, poor correlation among serologic tests does not allow effective prediction of PRN titer by ELISA.
Keywords: mumps, third-dose measles mumps rubella (MMR) vaccine, mumps immunogenicity, vaccine preventable disease, immunization, nucleoprotein, hemagglutinin
Primary infection with mumps virus typically causes swelling of the parotid salivary glands (parotitis) and may lead to serious complications such as meningitis, orchitis, and hearing loss. Although once considered common among school-aged children, mumps disease incidence dropped >99% in the United States after the introduction of a routine 2-dose measles mumps rubella (MMR) vaccination policy in 1989 for improved measles control among school-aged children (current age recommendations for MMR vaccination are 12–18 months and 4–6 years). Mumps incidence hit an average low of 0.1 cases per 100000 persons in the United States from 2001 to 2005, compared with well over 100 cases per 100000 persons in the prevaccine era [1]. In the past 10 years, however, sporadic mumps outbreaks have occurred among highly vaccinated populations in the United States [2–10] and globally [11–16]. Mumps outbreaks in vaccinated populations have usually occurred in close-contact settings such as college campuses, boarding schools, and summer camps that promote frequent high-intensity exposures. Spread of the disease beyond these settings into the broader communities has been generally limited.
Although existing evidence does not otherwise support the routine application of a third dose of MMR vaccine (MMR3) for prevention or control of mumps outbreaks, further investigation is needed to determine if and when it may be of benefit. There are no parameters of the immune response that are known to reliably assure protection from symptomatic wild-type mumps virus infection, although neutralizing antibody is assumed to be essential. Recently, neutralizing mumps antibody titers were measured by in vitro plaque reduction neutralization (PRN) from a large cohort of individuals who received MMR3 in a nonoutbreak setting [17]. There was a modest rise in PRN titers 1 month post-MMR3, but titers returned to near baseline by 1 year post-MMR3. The main objective of this study was to determine if there is a sustained elevation of hemagglutinin-neuraminidase (HN)- and nucleoprotein (NP)-specific antibody following MMR3 that was not otherwise reflected by PRN titers, as there may be important protective roles in vivo for antibody that is non-neutralizing in vitro [18, 19]. The HN and NP antigens were selected for this analysis because HN is a major target for neutralizing antibody and because NP is an immunodominant antigen [20, 21]. A second objective of this study was to determine if the degree of correlation between PRN titer and enzyme-linked immunosorbent assay (ELISA) measurements is sufficient to enable prediction of PRN titer based on ELISA methods, which are faster and more convenient to perform.
MATERIALS AND METHODS
Study Population and Study Design
The study population and exclusion criteria have been previously described in detail [17, 22]. Briefly, the study population included healthy young adults aged 18–28 years who received care from the Marshfield Clinic (Marshfield, WI) or who participated in a longitudinal study examining immune response to a second dose of MMR vaccine at Marshfield Clinic. Of 656 subjects who received MMR3, 610 provided 3 serum samples (immediately before administration of MMR3 at baseline, and 1 month and 1 year post-MMR3) with sufficient volume available for inclusion in this analysis. The study was approved by the institutional review boards of the Marshfield Clinic Research Foundation and the Centers for Disease Control and Prevention (CDC). Informed consent was obtained from all participants.
Mumps Immunoglobulin G ELISAs
Detection of immunoglobulin G (IgG) serum antibody to mumps virus and specific viral antigens was done by 3 methods. First, a commercially available test for mumps IgG (mumps IgG ELISA II; catalog 425900CE; Wampole Laboratories, Inc.) was used according to the manufacturer’s instructions, as previously described [21]. This test uses whole mumps virus as antigen (Enders strain), and the manufacturer reports 96.6% sensitivity and 90.4% specificity. The cutoff points for the Wampole ELISA (based on index standard ratio [ISR] values) were seronegative, ISR ≤0.90; indeterminate, ISR 0.91–1.09; and seropositive, ISR ≥1.10. Although end point titers were not determined, this test has been reported to be linear across a wide dilution range [7]. ELISAs to detect IgG specific for the mumps virus HN and NP were developed in-house for research use and have been previously described [21]. The NP ELISA has a 97.3% sensitivity and 97.3% specificity (ISR cutoff > 0.360). The HN ELISA has an 86.5% sensitivity and 97.3% specificity (ISR cutoff > 0.306). Values for the ELISA data were calculated and reported as ISRs, as previously described [21].
In Vitro Plaque Reduction Neutralization Tests
The in vitro PRN method and titers for this study population were previously reported [17]. Briefly, heat-inactivated sera were serially diluted 2-fold from 1:4 to 1:128, followed by addition of the Jeryl Lynn vaccine virus, resulting in a final serum dilution range of 1:8 to 1:256. Virus/serum mixtures (containing approximately 40 plaque-forming units of virus) were incubated for 1 hour and then transferred to Vero cell monolayers and covered with 2% methylcelluose. After 5 days of incubation, the monolayers were stained with neutral red and plaques were counted. The neutralizing antibody titer was calculated as the serum dilution capable of reducing the mean number of virus plaques by ≥50% compared with the mean number of plaques in virus control wells. Sera not reaching a 50% end point were retested in assays using a higher dilution series. For analysis purposes, we considered PRN titers <1:8 as seronegative, titers between 1:8 and 1:<16 (ie, within a single dilution factor of the limit of detection) to be low seropositive, and titers 1:≥16 to be high seropositive.
Data Analysis
GraphPad Prism software (version 5.04) was used for statistical analysis, linear regression, and graphing of the data. One-way analysis of variance (ANOVA; repeated measures) was used to compare baseline with 1 month and 1 year post-MMR3 ELISA measurements to determine if the average antibody level was significantly increased by MMR3 (Figure 1). Statistical significance was assigned to P values <.05 for all analyses. Values for Pearson r (correlation coefficient) and r2 (coefficient of determination) were used to compare baseline and 1 year post-MMR3 ELISA results to determine if the antibody level of sera from each individual was significantly changed from baseline (Figures 2 and 3).
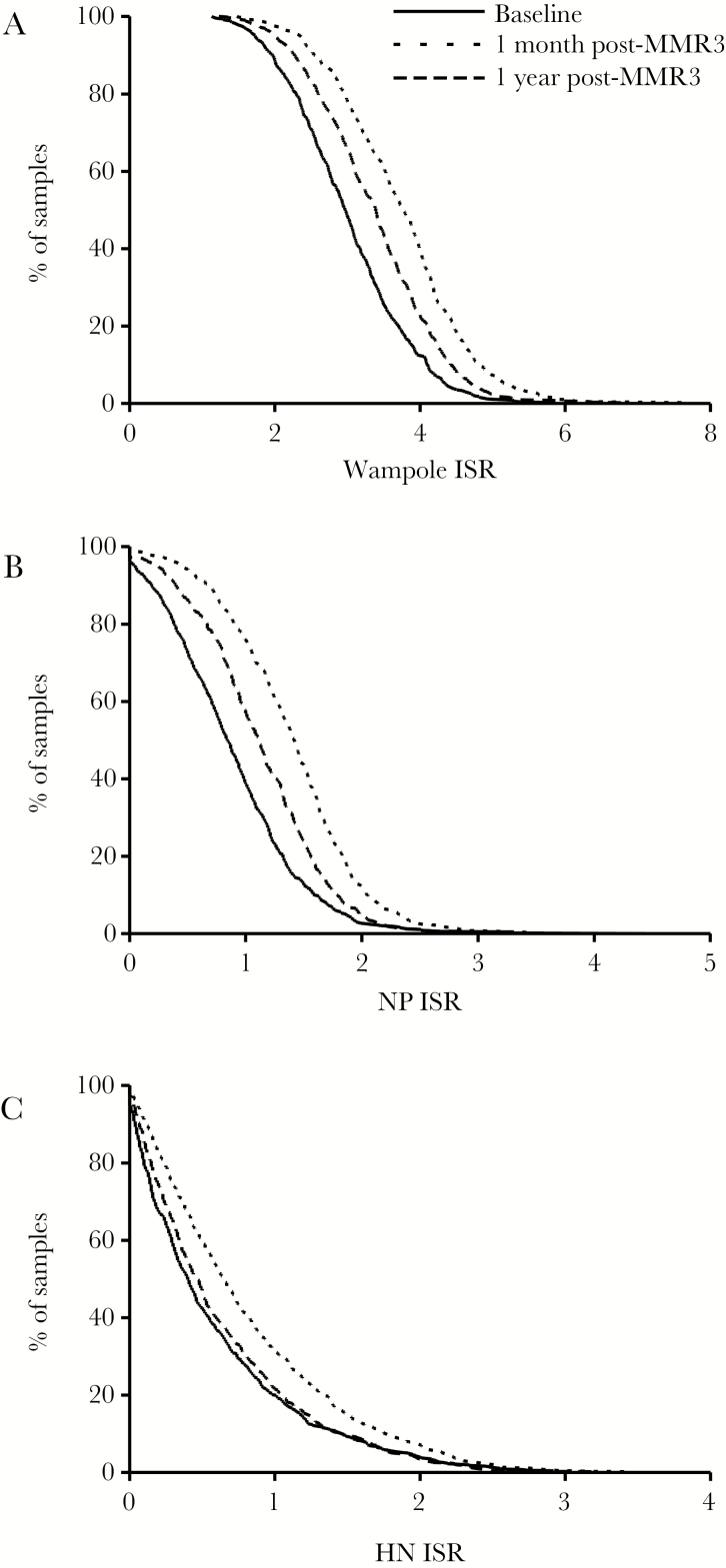
Figure 1.
Reverse cumulative distribution curves by percentage of subjects at baseline, 1 month, and 1 year following a third dose of measles mumps rubella vaccine. (A) Commercial enzyme-linked immunosorbent assay (whole-virus antigen), (B) nucleoprotein-specific IgG, and (C) hemagglutinin-specific IgG. Abbreviations: HN, hemagglutinin-neuraminidase; ISR, index standard ratio; NP, nucleoprotein.
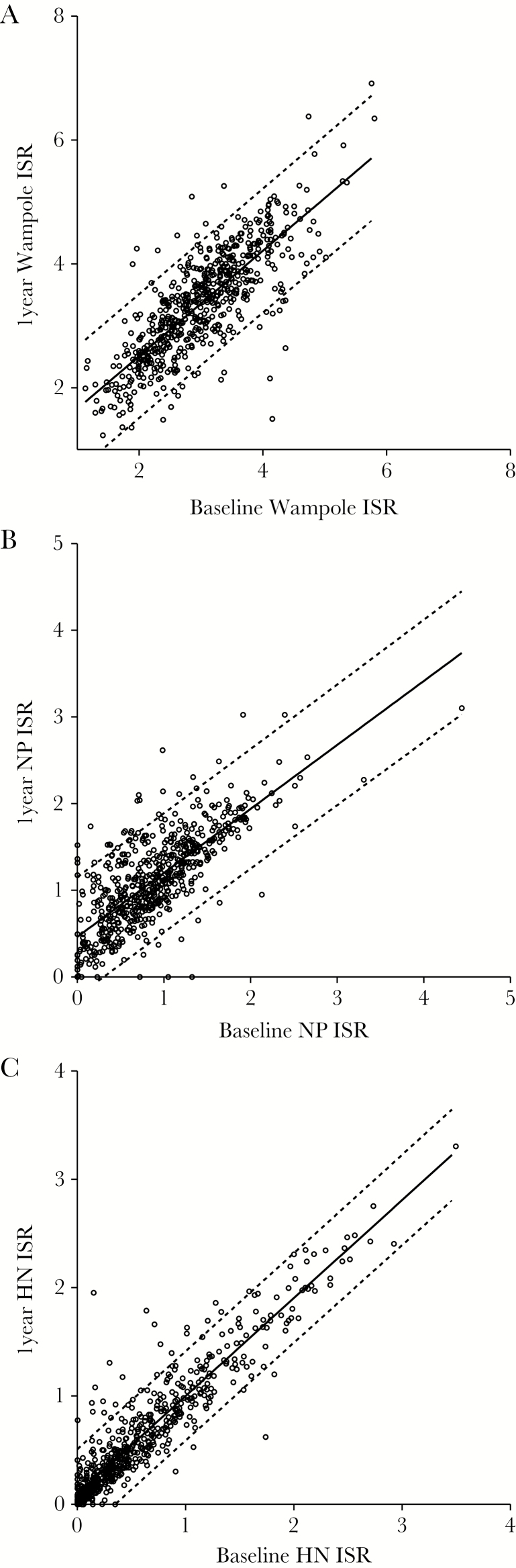
Figure 2.
Correlation of Immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) measurements of mumps serum samples collected at baseline and 1 year after the third dose of the measles mumps rubella vaccine. (A) Commercial ELISA (Pearson r = .8022; P < .0001; r2 = .6432), (B) nucleoprotein-specific IgG (Pearson r = .7592; P < .0001; r2 = .5763), (C) hemagglutinin-specific IgG (Pearson r = .9302; P < .0001; r2 = .8653). The solid line represents the linear regression, and the dotted lines indicate the 95% confidence interval. Abbreviations: HN, hemagglutinin-neuraminidase; ISR, index standard ratio; NP, nucleoprotein.
Figure 3.

Correlations among serologic tests. (A) Commercial enzyme-linked immunosorbent assay (ELISA) vs plaque reduction neutralization (PRN; Pearson r = .2881; r2 = .083), (B) nucleoprotein (NP) vs PRN (Pearson r = .3289; r2 = .108), (C) hemagglutinin (HN) vs PRN (Pearson r = .7002; r2 = .490), (D) NP vs commercial ELISA (Pearson r = .6478; r2 = .420), (E) HN vs commercial ELISA (Pearson r = .3970; r2 = .158), (F) HN vs NP (Pearson r = .4895; r2 = .240). Abbreviations: HN, hemagglutinin-neuraminidase; ISR, index standard ratio; NP, nucleoprotein; PRN, plaque reduction neutralization.
Baseline PRN titers were used to rank the sera into quartiles, and the mean ELISA ISR values were calculated for sera in each quartile to determine if MMR3 had a greater effect on HN and NP antibody levels in individuals with low PRN titer (Table 1). The baseline values were compared with the 1-year time point values for each ELISA using a paired 2-tailed t test.
Table 1.
Mumps IgG ELISA Means Ranked by in Vitro Plaque Neutralization Titer Quartile
| Means | % of Samples | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Quartile | PRNT | Whole Virus | HN | NP | HN+ | NP+ |
| Baseline | 4 | 554.1 | 3.442 | 1.278 | 1.247 | 93.9 | 93.9 |
| 3 | 152.8 | 3.111 | 0.626 | 0.949 | 79.3 | 89.0 | |
| 2 | 75.6 | 2.811 | 0.340 | 0.779 | 49.4 | 81.7 | |
| 1 | 26.3 | 2.620 | 0.119 | 0.577 | 9.8 | 62.8 | |
| 1 mo post-MMR3 | 4 | 714.5 | 4.087 | 1.516 | 1.671 | 97.5 | 98.8 |
| 3 | 199.0 | 3.696 | 0.827 | 1.335 | 88.4 | 97.6 | |
| 2 | 128.9 | 3.626 | 0.591 | 1.344 | 72.0 | 95.1 | |
| 1 | 66.1 | 3.531 | 0.304 | 1.206 | 37.8 | 94.5 | |
| 1 y post-MMR3 | 4 | 607.3 | 3.688 | 1.270 | 1.365 | 94.2 | 96.8 |
| 3 | 172.1 | 3.376 | 0.652 | 1.122 | 84.6 | 93.6 | |
| 2 | 100.4 | 3.272 | 0.415 | 1.061 | 59.3 | 91.3 | |
| 1 | 42.2 | 3.099 | 0.173 | 0.899 | 16.6 | 83.4 | |
Baseline mumps PRN titers were used to rank sera into quartiles. Ranges of geometric mean titer for baseline quartiles were: Q1 (3.3–49.7), Q2 (50.0–106.9), Q3 (107.0–212.7), Q4 (213.8–3019.6). Paired samples from sera in each baseline quartile are represented in matching quartiles indicated at 1 month and 1 year following administration of the third dose of measles mumps rubella vaccine. Mean ELISA index standard ratio values and the percentage of sera that were positive for hemagglutinin or nucleoprotein IgG in each quartile are indicated.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HN, hemagglutinin-neuraminidase; IgG, immunoglobulin G; ISR, index standard ratio; MMR3, third dose of measles mumps rubella vaccine; NP, nucleoprotein; PRN, plaque reduction neutralization titers.
RESULTS
At baseline, all individuals were seropositive for IgG to whole mumps virus antigen by the commercial ELISA, including 5 individuals who were previously reported to have a PRN titer <1:8 and 38 individuals who had a baseline PRN titer between 1:8 and 1:16 [17]. By comparison, only 9.8% of sera in the lowest baseline PRN quartile (range, <8–49.7) were positive for HN IgG, and 62.8% were positive for NP IgG. The mean ISR values for the baseline, 1 month, and 1 year post-MMR3 samples were 3.000, 3.736, and 3.360, respectively, for the commercial ELISA, indicating an overall increase in mumps antibody following MMR3 that partially diminished toward baseline by 1 year (Figure 1A). The results from the NP-specific IgG ELISA were similar to the commercial ELISA (Figure 1B). The mean ISR values at baseline, 1 month, and 1 year post-MMR3 were 0.8818, 1.385, and 1.113 respectively, indicating a transient increase in NP-specific IgG that partially declined by 1 year. By comparison, the mean HN-ISR values for baseline, 1 month, and 1 year post-MMR3 were 0.5911, 0.8066, and 0.6298, respectively. Although there was a slight increase in HN-specific IgG at 1 month, the levels returned to almost baseline by 1 year (Figure 1C). The increases in antibody observed by each method at 1 month and 1 year were modestly higher than baseline, but were statistically significant increases (P < .0001).
Correlations between the baseline and 1 year post-MMR3 IgG measurements for paired sera are shown in Figure 2 for each ELISA. The levels of IgG measured at baseline generally correlated with the 1-year measurements: commercial ELISA (Pearson r = .8022; r2 = .6432) (Figure 2A), NP (Pearson r = .7592; r2 = .5763) (Figure 2B), HN (Pearson r = .9302; r2 = .8653) (Figure 2C). The 2-tailed P values were <.0001 for each case.
Baseline PRN titers were used to rank the sera into quartiles, and the mean ELISA ISR values were calculated for sera in each quartile (Table 1). With the exception of the fourth quartile HN-specific IgG measurements, there were statistically significant increases in all other values at 1 year post-MMR3 compared with baseline for each ELISA and for the PRN titers (P < .05). The percentages of samples that were positive for HN and NP IgG are also listed in Table 1. The lowest PRN quartile (mean PRN titer, 26.3) also had the lowest percentages of HN- and NP-specific IgG-positive sera at each time point (9.8% and 62.8%, respectively). Sera in the bottom 50% of baseline PRN titers showed the most dramatic increases in antibody at 1 month post-MMR3. However, the PRN titers and the fraction of sera that were positive for HN and NP IgG returned to near baseline at 1 year. In contrast, sera in the top 50% of PRN titers at baseline showed little change in antibody at the 1-month and 1-year time points.
Overall, the correlations among the serologic testing methods were poor (Figure 3). The HN ELISA correlated best with PRN titers (Pearson r = .700; r2 = .490). However, there were many samples with little HN IgG that had high PRN titer. The NP ELISA correlated best with the commercial ELISA (Pearson r = .648; r2 = .420).
DISCUSSION
We measured changes in mumps IgG with antigen-specific and whole-virus ELISAs to determine if a third dose of MMR vaccine might sustain elevated levels of mumps antibody 1 month and 1 year after vaccination that were not otherwise revealed by the minimal changes in neutralization titers, as previously reported [17]. Following MMR3, we observed correlations between individual baseline ELISA measurements and the 1-year time points for each assay (Figure 2). Overall, individuals who had a low baseline antibody (regardless of test method) also had the lowest measurements at 1 month and 1 year post-MMR3 (Table 1). However, individuals in the lowest 2 quartiles of PRN titer also had the largest increases in the frequency of individuals who were HN and NP IgG positive at 1 year post-MMR3 (9.8%–16.6% for HN; 62.8%–83.4% for NP). There was little change in the fraction of sera that were positive for HN and NP IgG following MMR3 among individuals who had the highest baseline PRN titers. Overall, the commercial, HN-, and NP-specific ELISA methods did not reveal substantial changes in antibody apart from previously reported PRN titer results.
The specific correlations between the PRN and ELISA assays were poor. In general, sera with the lowest PRN titers also had the lowest levels of IgG according to the commercial ELISA and according to the NP-specific ELISA. Although sera with high levels of HN antibody also had high PRN titer, not all high PRN titer sera had detectable HN antibody. As a result, individuals with low neutralizing titer cannot be reliably identified by these ELISA methods, unless they are completely seronegative. At baseline, all individuals were seropositive for IgG to whole mumps virus antigen by the commercial ELISA, including 5 individuals who were previously reported to have a negative PRN titer, whereas only 9.8% and 62.8% of sera in the lowest baseline PRN quartile were positive for HN and NP IgG, respectively. These observations may be explained by a combination of factors. First, additional viral antigens are likely present in the commercial test, which uses antigen prepared from infected cells. This likely enables detection of IgG-specific for additional viral proteins such as the fusion protein (also a neutralizing target) and could explain why some samples that were negative for HN and NP IgG had high PRN titer. Second, the antigen-specific ELISA results were corrected for background signal by subtracting reactivity to negative control antigen (prepared from untransfected cells in parallel with the HN and NP antigen preparations). In contrast, there was no negative control antigen provided in the commercial test, and some sera may have background reactivity with host cell proteins (negative control serum was included).
In agreement with our previous report [17], our additional data from the antigen-specific and whole-virus ELISA tests suggest that there is a set point for the overall antibody response to mumps that is minimally affected over the long term by MMR3 in the majority of individuals. The greatest benefit of MMR3 may be for those individuals with very low neutralizing antibody titer in the context of an outbreak, but our data do not otherwise support the routine application of MMR3 as a method to increase overall long-term population immunity to mumps.
Acknowledgment
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Food and Drug Administration.
References
- 1. Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine 2009; 27:6186–95. [DOI] [PubMed] [Google Scholar]
- 2. Mumps outbreak at a summer camp--New York, 2005. MMWR Morb Mortal Wkly Rep 2006; 55:175–177. [PubMed] [Google Scholar]
- 3. Update: multistate outbreak of mumps--United States, January 1-May 2, 2006. MMWR Morb Mortal Wkly Rep 2006; 55:559–563. [PubMed] [Google Scholar]
- 4. Marin M, Quinlisk P, Shimabukuro T et al. . Mumps vaccination coverage and vaccine effectiveness in a large outbreak among college students–Iowa, 2006. Vaccine 2008; 26:3601–7. [DOI] [PubMed] [Google Scholar]
- 5. Cortese MM, Jordan HT, Curns AT et al. . Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis 2008; 46:1172–80. [DOI] [PubMed] [Google Scholar]
- 6. Mumps outbreak - New York, New Jersey, Quebec, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1270–1274. [PubMed] [Google Scholar]
- 7. Rota JS, Turner JC, Yost-Daljev MK et al. . Investigation of a mumps outbreak among university students with two measles-mumps-rubella (MMR) vaccinations, Virginia, September-December 2006. J Med Virol 2009; 81:1819–25. [DOI] [PubMed] [Google Scholar]
- 8. Barskey AE, Schulte C, Rosen JB et al. . Mumps outbreak in Orthodox Jewish communities in the United States. N Engl J Med 2012; 367:1704–13. [DOI] [PubMed] [Google Scholar]
- 9. Nelson GE, Aguon A, Valencia E et al. . Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles-mumps-rubella vaccine for outbreak control–Guam 2009 to 2010. Pediatr Infect Dis J 2013; 32:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. 2012. Mumps outbreak on a university campus - California, 2011. MMWR Morb Mortal Wkly Rep 61:986–989. [PubMed] [Google Scholar]
- 11. Whelan J, van Binnendijk R, Greenland K et al. . Ongoing mumps outbreak in a student population with high vaccination coverage, Netherlands, 2010. Euro Surveill 2010; 15. [DOI] [PubMed] [Google Scholar]
- 12. Aasheim ET, Inns T, Trindall A et al. . Outbreak of mumps in a school setting, United Kingdom, 2013. Hum Vaccin Immunother 2014; 10:2446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gobet A, Mayet A, Journaux L et al. . Mumps among highly vaccinated people: investigation of an outbreak in a French Military Parachuting Unit, 2013. J Infect 2014; 68:101–2. [DOI] [PubMed] [Google Scholar]
- 14. Vareil MO, Rouibi G, Kassab S et al. . Epidemic of complicated mumps in previously vaccinated young adults in the South-West of France. Med Mal Infect 2014; 44:502–8. [DOI] [PubMed] [Google Scholar]
- 15. Del Valle A, García AA, Barrón BL. Detection of mumps virus genotype H in two previously vaccinated patients from Mexico City. Arch Virol 2016; 161:1639–44. [DOI] [PubMed] [Google Scholar]
- 16. Bangor-Jones RD, Dowse GK, Giele CM et al. . A prolonged mumps outbreak among highly vaccinated Aboriginal people in the Kimberley region of Western Australia. Med J Aust 2009; 191:398–401. [DOI] [PubMed] [Google Scholar]
- 17. Fiebelkorn AP, Coleman LA, Belongia EA et al. . Mumps antibody response in young adults after a third dose of measles-mumps-rubella vaccine. Open Forum Infect Dis 2014; 1:ofu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henry Dunand CJ, Leon PE, Huang M et al. . Both neutralizing and non-neutralizing human H7N9 influenza vaccine-induced monoclonal antibodies confer protection. Cell Host Microbe 2016; 19:800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmaljohn AL. Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts. Curr HIV Res 2013; 11:345–53. [DOI] [PubMed] [Google Scholar]
- 20. Matsubara K, Iwata S, Nakayama T. Antibodies against mumps virus component proteins. J Infect Chemother 2012; 18:466–71. [DOI] [PubMed] [Google Scholar]
- 21. Latner DR, McGrew M, Williams NJ et al. . Estimates of mumps seroprevalence may be influenced by antibody specificity and serologic method. Clin Vaccine Immunol 2014; 21:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiebelkorn AP, Coleman LA, Belongia EA et al. . Measles virus neutralizing antibody response, cell-mediated immunity, and immunoglobulin g antibody avidity before and after receipt of a third dose of measles, mumps, and rubella vaccine in young adults. J Infect Dis 2016; 213:1115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]