Abstract
In response to a predator attack, many Aphidinae species release an alarm pheromone, which induces dispersal behavior in other individuals within the colony. The major component of this pheromone is the sesquiterpene (E)-β-farnesene (Eβf), but variations occur between aphid species. In the present work, we collected, identified, and quantified the alarm pheromone of Aphis craccivora Koch (Hemiptera: Aphididae), before quantifying the escape behavior induced in the neighboring individuals. We compared the semiochemistry and associated behavior of alarm signaling with two other aphid species: Myzus persicae (Sulzer) (Hemiptera: Aphididae) and Aphis fabae Scopoli (Hemiptera: Aphididae). Eβf was the only volatile found for each species. M. persicae produces a higher quantity of Eβf (8.39 ± 1.19 ng per individual) than A. craccivora (6.02 ± 0.82 ng per individual) and A. fabae (2.04 ± 0.33 ng per individual). Following exposure to natural doses of synthetic Eβf (50 ng and 500 ng), A. craccivora respond more strongly than the two other Aphidinae species with 78% of the individuals initiated alarm behavior for 500 ng of Eβf.
Keywords: aphid, (E)-β-farnesene, escape, behavior
(E)-β-Farnesene (Eβf) is the major component of aphid volatile organic compounds and is used as an alarm pheromone in numerous species (Francis et al. 2005). This alarm signal is secreted by two cornicles in the presence of danger and induces a dispersal response in the surrounding individuals (Nault et al. 1973). Eβf emissions may be affected by the size and composition of aphid colonies (Vandermoten et al. 2012). The induced response may range from falling, walking, or even hiding (Montgomery and Nault 1977).
The majority of Aphidinae species were shown to produce Eβf as unique alarm pheromone constituent, with some exception of species producing Eβf with additional terpenes or even producing no Eβf at all: e.g., Therioaphis maculate Buckton (Hemiptera: Aphididae) produces (–)-germacrene A only (Bowers et al. 1977). Other species such as Euceraphis punctipennis Zetterstedt (Hemiptera: Aphididae) and Drepanosiphum platanoides Schrank (Hemiptera: Aphididae) also do not release any Eβf (Francis et al. 2005).
Aphis craccivora Koch (Hemiptera: Aphididae) is a cosmopolitan and polyphagous species causing damages to Fabaceae and many other plant families (Blackman and Eastop 2007). To the best of our knowledge, the alarm pheromone of A. craccivora has not been elucidated yet. The aim of this study is to collect, identify, and quantify the alarm pheromone of A. craccivora. We aim to compare the composition and quantification to two other well-studied aphid species, Aphis fabae Scopoli (Hemiptera: Aphididae) and Myzus persicae (Sulzer) (Hemiptera: Aphididae), which are known to produce Eβf as only component of alarm pheromone. Finally, we want to compare the behavioral response of A. craccivora toward its alarm pheromone to the behavioral response observed in these two other aphid species.
Materials and Methods
Aphid and Plant Rearing
A. craccivora were collected from Libreville in Gabon (0° 27ʹ30.46ʺ N; 9° 25ʹ6.30ʺ E) on Amaranthus hybridus L. (Amaranthaceae) and were subsequently reared in Belgium under laboratory conditions (22 ± 2°C, 50–70% RH, and 16:8 [L:D] h) on A. hybridus. A. fabae and M. persicae were collected on Vicia faba L. (Fabaceae) in Belgium and reared under identical laboratory conditions on A. hybridus. Plants were grown in 9 × 8-cm plastic pots containing a general-purpose potting soil (La plaine chassart, Wagnelée, Belgium).
Chemicals
Synthetic Eβf was synthesized from farnesol (Tanaka et al. 1975) and used for the behavioral assays and chromatographic analyses diluted in n-hexane (> 97% purity; VWR International, Leuven, Belgium). The purity of Eβf (> 98% purity) was confirmed with gas chromatography (GC). N-butylbenzene (> 99% purity; Sigma-Aldrich, Saint-Louis, MO) was used as an internal standard (IS) for quantification purposes.
Sampling and Identification of Aphid Volatile Organic Compounds
In order to collect aphid volatiles, a dynamic collection system was set up, in which aphids were grinded according to the extraction system described by Fischer and Lognay (2012) with some minor modifications. One hundred aphids (fourth instar larvae or apterae adults) of each species were used to improve the detection of minor compounds, if any. They were placed in a ‘Y-shaped’ glass tube. A magnetic stir bar (45 mm) was then introduced. The tube was held upon a magnetic stir plate in order to activate the stir bar and to efficiently crush the aphids. An adsorbent cartridge containing 60 mg of Tenax TA (Gerstel, Mülheim an der Ruhr, Germany) was placed at the output of the collection system. A charcoal filtered air with a flow rate of 100 ml/min was then blown within the system thank to a suction pump (Gilian , West Caldwell, NJ). Once the system was set up, the stir plate was turned on during 15 min. The experiment was conducted in a laboratory room under constant temperature (23 ± 1°C). To avoid any contamination, all the cartridges were cleaned before sampling at the conditioning temperature and time based on the recommendation of the manufacturer. The experiment was repeated at least five times for each species.
The cartridges were analyzed using a gas chromatograph (7890A; Agilent Technologies, Palo Alto, CA) coupled with a mass spectrometer (5975C; Agilent Technologies) as described previously in Delory et al. (2016). One micro liter of IS solution (dosed with 5 ng/µl of n-butylbenzene) was added on each cartridge before desorption. The trapped volatile organic compounds (VOCs) were thermally desorbed from Tenax TA traps using a thermal desorption unit (TDU) (Gerstel, Mülheim an der Ruhr, Germany) running in splitless mode for 10 min at 280°C . During thermal desorption, VOCs were cryo-cooled in a CIS/PTV inlet (Gerstel) at −100°C with liquid nitrogen. At the end of the desorption process, aphid VOCs were injected for 1.5 min in solvent vent mode inside a polar GC column (HP-5MS, 30-m length × 0.250-mm ID × 0.25-µm film thickness; Agilent Technologies) by heating the CIS/PTV inlet to 300°C for 5 min at a rate of 12 °C/s. The GC oven had the following temperature program: 40°C for 2 min, ramp 10°C/ min to 280°C held for 5 min. The MS was used in electron ionization mode (70 eV) with a gain factor of 1.5 and operated synchronously in SCAN and SIM modes. In SCAN mode, the MS scanned m/z ratios from 35 to 400 amu with a threshold value and a sampling rate set at 100 were quantified in SIM mode based on the IS (m/z 69 and 100) response. For each ion, the dwell time was set at 100 ms. The source and the quadrupole temperature were set at 230 and 150°C, respectively. Aphid VOCs were identified by interpretation of MS fragmentation patterns and injection of pure chemicals.
Quantification of Eβf
Five individuals (fourth instar larvae or young apterae adults) of each species were gently removed from plants with a soft hair brush and were weighed with a precision balance (precision: 0.01 mg; Kern 120-ABT, Kern, Germany) prior being placed as a pool in a threaded glass tube containing 200-µl n-hexane. The solvent solution was previously dosed with 5 ng/µl of n-butylbenzene as IS (Boullis et al. 2017). A stir bar was placed inside the glass tube, which was then sealed and disposed onto a magnetic stir bar, switched on for 30 min. In total, 100 µl of supernatant was then collected and stored at −80°C before GC analyses. A positive control was also realized: 3 µl of Eβf solution (dosed with 50 ng/µl) was added to a threaded glass tube in similar conditions as described earlier. This procedure was repeated five times for each aphid species.
The analysis was performed using a gas chromatograph with flame ionization detection at 290°C (Trace GC Ultra—Thermo Scientific , Interscience, Belgium). Aliquots (1 µl) were injected on a splitless injector maintained at 270°C (Splitless time of 1 min). The column (Optima 5MS; 30-m length × 0.25-mm ID × 0.25-µm thickness; Macherey Nagel-Düren, Germany). The oven temperature program was as follow: initial temperature at 40°C for 2 min to 280°C at a constant rate of 10°C/min with a final hold of 5 min to 280°C. Helium was the carrier gas (constant flow 1 ml/min). The quantification of Eβf was performed by comparing the ratio of peak area (Eβf/IS) obtained in our samples with those from the calibration curve edited in Boullis et al. (2017) accomplished with the same molecules and the same GC method.
Behavioral Response to Eβf
Fifteen fourth instar or young apterous adult aphids of each species were confined in a clip cage (2 cm of diameter) on the underside of an A. hybridus leaf. After 24 h, the leaves were cut from plant and the clip cage was removed. Leaves were put upside down on a flat cork support and the number of nonsteressed aphids was recorded. A circle piece of filter paper (Whatmann #1; 5 mm of diameter) was placed 2 cm from the end of an entomological pin. In total, 5 µl of a solution of Eβf diluted in n-hexane (10 or 100 ng/µl) was added on the filter paper 30 s before the pin was placed through the leaf. The quantities of Eβf used in these behavioral tests were determined; thanks to preliminary experiments done with our different aphid clones. The behavioral responses of the different aphid species were considered as nonsignificant when the Eβf dose was lower than 50 ng. Moreover, this quantity represents a natural dose of Eβf (Vosteen et al. 2016), as those emitted by an adult aphid Acyrthosiphon pisum Harris (Hemiptera: Aphididae) under attack (Boullis et al. 2017). For the second dose of Eβf, we decided to use a 10-fold increased quantity to obtain a significant difference between treatments. The filter paper was maintained at 1 cm up to the leaf in the middle of the aphid colony. The number of aphids initiating movement was determined during 5 min. We considered that aphids were disturbed by the volatiles not only when they left the leaf but also when they wriggled (squirmed) on the spot without moving. During squirming, aphids twist their body from side to side, without leaving the leaf; the stylet is still inserted in the leaf tissue, but the aphid does not have the kicking behavior as often observed during the attack of aphid predator. To confirm that aphids did not respond to solvent, control experiments were also realized by depositing 5 µl of pure n-hexane. The experiment was repeated 10 times per species and Eβf concentration.
Statistical Analysis
All statistical analyses were performed with R 3.3.1 software (R Core Team 2016). Comparisons of alarm pheromone quantities in aphids from different species and weight were assessed using two-way analysis of variance (ANOVA). In addition, Tukey’s post hoc test was conducted for the comparisons between species. Before this parametric test, normality of the residuals and homoscedasticity were checked using Shapiro-Wilk and Bartlett tests, respectively (P > 0.05). The proportion of aphids initiating movement was modeled with an analysis of covariance (ANCOVA), using the colony size as a covariate, and aphid species, and amount of Eβf designed as fixed effects. By using orthogonal contrasts, ANCOVA assumptions, namely 1) residuals normality, 2) homoscedasticity, 3) independence of covariant and independent variables, 4) homogeneity of regression slopes, and 5) linear relationship between the dependent variables and the covariate, were checked prior to statistical analysis. We then performed a model simplification (deletion tests) to eliminate unnecessary parameters using a step function. As the Akaike information criterion was better without the covariate, colony size was eliminated from the model and the ANCOVA replaced by a two-way ANOVA. Before this analysis, percentage data were arcsine-transformed to stabilize variance. To explore differences among treatment groups of fixed effects, post hoc Tukey’s tests were implemented.
Results
Identification and Quantification of Aphid Volatile Compounds
For each of the three aphid species, Eβf was the only volatile chemical identified from crushed individuals. The quantity of Eβf was determined by using the following equation of the calibration curve: y = 0.9113x + 0.0022 (y: ratio of peak area Eβf/IS; x: quantity of Eβf present in the sample – R2 = 0.9983). The mean amount of Eβf and mean aphid weight were variable and differed between species, as illustrated in Table 1. Significant differences were found in Eβf quantities between the three aphid species (F2,9 = 19.6505; P < 0.001).
Table 1.
Quantification of (E)-β-farnesene (Eβf) in aphids reared on A. hybridus (n = 5 for each aphid species)
| Datum | A. craccivora | A. fabae | M. persicae |
|---|---|---|---|
| Individual aphid weight (µg) | 131.60 ± 18.20a | 174.40 ± 16.60a | 112.40 ± 15.01a |
| Eβf per mg aphid (ng) | 48.63 ± 13.51a | 12.02 ± 4.24b | 75.37 ± 18.64a |
| Eβf per aphid (ng) | 6.20 ± 0.82a | 2.04 ± 0.33b | 8.39 ± 1.19a |
Means within rows sharing the same lowercase letter do not differ significantly at P = 0.05.
Behavioral Responses to Eβf
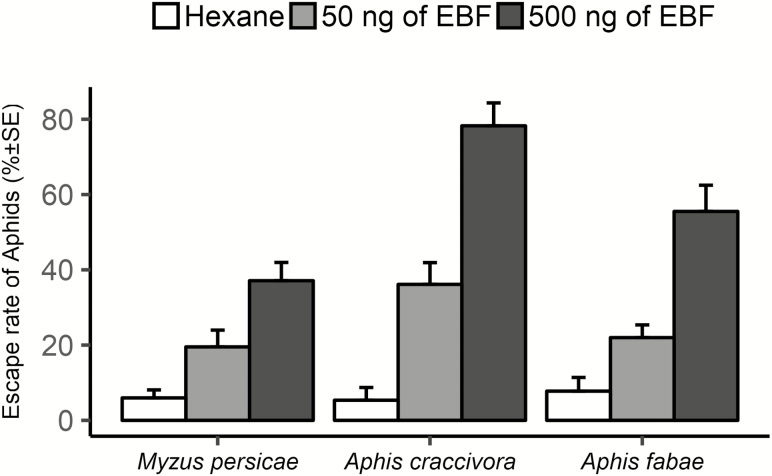
During the tests with Eβf solution, the majority of the responding aphids moved during the first minute. When only solvent was presented in aphid colonies, a nonsignificant escape response was observed (Fig. 1). The escape rate was significantly different between the different Eβf treatments, without considering the aphid species (Table 2). Without considering the Eβf dose, a significant difference was only observed between A. craccivora and M. persicae (Table 2). When the lower dosage of Eβf is presented (i.e., 50 ng), no significant differences were observed between the three species. However, when only the higher dose of Eβf is considered, A. craccivora has a significantly higher escape rate, but only when compared to M. persicae (Turkey’s tests: A. craccivora versus M. persicae: P < 0.001; A. craccivora versus A. fabae: P = 0.190).
Fig. 1.
Effect of (E)-β-farnesene (Eβf) on escape response of different aphid species (n = 10 for each modality).
Table 2.
Multiple comparisons of means between EβF quantities and aphid species on escape response (n = 10 for each modality)
| Comparisons | Estimate | P-value |
|---|---|---|
| 500 ng of EβF—50 ng of EβF | 0.4092 | <0.001*** |
| Hexane 50 ng of EβF | -0.3196 | <0.001*** |
| Hexane 500 ng of EβF | -0.7288 | <0.001*** |
| A. fabae—A. craccivora | -0.1170 | 0.12411 |
| M. persicae—A. craccivora | -0.2372 | <0.001*** |
| M. persicae—A. fabae | -0.1205 | 0.10967 |
Discussion
In the present study, we showed that Eβf is the only volatile compound found in A. craccivora, which is in accordance to previous report on various other Aphidinae species (Francis et al. 2005). We also confirmed that M. persicae and A. fabae produce Eβf as only volatile, even when these generalist aphid species are reared on Amaranth. We also confirmed that in A. craccivora, aphid weight is not related to Eβf content. A. craccivora apterous adults have a mean weight of 131.6 ± 18.20 µg, similar to those of a cotton aphid [Aphis gossypii Glover (Hemiptera: Aphididae)], but A. craccivora produce far more Eβf than A. gossypii, with 6.2 ± 0.82 and of 0.7 ± 0.1 ng of Eβf per aphid, respectively (Byers 2005). We also showed here that A. craccivora produces a similar amount of Eβf than M. persicae, representing three times more Eβf than A. fabae (<3 ng/individual).
Following Eβf exposure, a dispersal behavior from neighboring individuals is observed in all species. However, the observed difference in behavioral response between species is dependent on the Eβf dose exposed. With low dosage of alarm pheromone (i.e., 50 ng), a similar dispersion occurs in all three species, involving less than 40% of the individuals. A higher dose (i.e., 500 ng) induces 80% of escape response in A. craccivora, while the other two aphid species remain under 55% of escape behavior, suggesting a greater response of A. craccivora to the common aphid alarm signal. But, it is difficult to compare the escape response between different aphid species from laboratory strains, because the rearing conditions can drastically reduce their sensitivity to Eβf (Thieme and Dixon 2015). We can speculate that some aphid species may be more disposable to reduce their reaction to alarm pheromone than others. Moreover, we observed that A craccivora start escaping directly after Eβf exposure (<5 s), while longer exposure was needed to induce alarm behavior in M. persicae (about 30 s) and A. fabae (about 60 s). This difference of behavior could be explained by aphid physiology or even by the synthetic nature of the stimulus. When aphids perceive the alarm signal, they stopped feeding and generally move away from the signal by walking away or falling down (Pickett et al. 1992). Here, leaves with aphid colonies were cut off from the plants and placed flat upside down on the support, thus the falling behavior of insects could not be considered. However, as myrmecophilous aphids, A. fabae individuals are less responsive to alarm signal and more likely to walk or to ‘waggle’ instead of falling down compared to other species (Nault et al. 1976; Montgomery and Nault 1977).
In conclusion, this study showed that Eβf is the only volatile terpene emitted by these three aphid species; it is also the first time that Eβf was described as an alarm pheromone in A. craccivora. M. persicae individuals contain greater amounts of this molecule among the species tested, whereas no relation between Eβf quantities and aphid weight was highlighted. Because the control of A. craccivora is dependent on conventional insecticides, these results show that Eβf has a potential in the mobility of infesting aphids. Complementary researches could be carried to improve the way to apply this semiochemical on aphid populations according to ecological conditions.
Acknowledgments
We are grateful to Prof. Lognay Georges and Professor Fauconnier Marie-Laure (Gembloux Agro-Bio Tech, University of Liege) for the access to laboratories. Trisman Danny and Michels Franck (General and organic chemistry, Gembloux Agro-Bio Tech, University of Liege) for experiments help and Fabre Anguilet Edgar (Institut de Recherches Agronomiques et Forestières, Gabon) for statistical analysis help.
References
- Blackman R. L. and Eastop V. F.. 2007. Taxonomic issues, pp. 1–29. Invan Emden H. F., Harrington R (eds.), Aphids as crop pests. CABI, Wallingford, United Kingdom. [Google Scholar]
- Boullis A., Fassotte B., Sarles L., Lognay G., Heuskin S., Vanderplanck M., Bartram S., Haubruge E., Francis F., and Verheggen F. J.. 2017. Elevated carbon dioxide concentration reduces alarm signaling in aphids. J. Chem. Ecol. 43: 164–171. [DOI] [PubMed] [Google Scholar]
- Bowers W. S., Nishino C., Montgomery M. E., Nault L. R., and Nielson M. W.. 1977. Sesquiterpene progenitor, germacrene A: an alarm pheromone in aphids. Science 196: 680–681. [DOI] [PubMed] [Google Scholar]
- Byers J. A. 2005. A cost of alarm pheromone production in cotton aphids, Aphis gossypii. Naturwissenschaften 92: 69–72. [DOI] [PubMed] [Google Scholar]
- Delory B. M., Delaplace P., du Jardin P., and Fauconnier M. L.. 2016. Barley (Hordeum distichon L.) roots synthesise volatile aldehydes with a strong age-dependent pattern and release (E)-non-2-enal and (E,Z)-nona-2,6-dienal after mechanical injury. Plant Physiol. Biochem. 104: 134–145. [DOI] [PubMed] [Google Scholar]
- Fischer C. Y. and Lognay G. C.. 2012. Simple and automatic closed grinding and extraction system. J. Chem. Educ. 89: 1611–1612. [Google Scholar]
- Francis F., Vandermoten S., Verheggen F., Lognay G., and Haubruge E.. 2005. Is the E-b-farnesene the only volatile terpenoid in aphids?Jen. 129: 6–11. [Google Scholar]
- Montgomery M. E. and Nault L. R.. 1977. Comparative response of aphids to the alarm pheromone, (E)-β-farnesene. Entomol. Exp. Appl. 22: 236–242. [Google Scholar]
- Nault L. R., Edwards L. J., and Styer W. E.. 1973. Aphid alarm pheromones: secretion and reception. Environ. Entomol. 2: 101–105. [Google Scholar]
- Nault L. R., Montgomery M. E., and Bowers W. S.. 1976. Ant-aphid association: role of aphid alarm pheromone. Science 192: 1349–1351. [DOI] [PubMed] [Google Scholar]
- Pickett J. A., Wadhams L. J., Woodcock C. M., and Hardie J.. 1992. The chemical ecology of aphids. Annu. Rev. Entomol. 37: 67–90. [Google Scholar]
- R Core Team 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Tanaka S., Yasuda A., Yamamoto H., and Nozaki H.. 1975. General method for the synthesis of 1,3-dienes. Simple syntheses of beta- and trans-alpha-farnescene from farnesol. J. Am. Chem. Soc. 97: 3252–3254. [Google Scholar]
- Thieme T. and Dixon A. F. G.. 2015. Is the response of aphids to alarm pheromone stable?J. Appl. Entomol. 139: 741–746. [Google Scholar]
- Vandermoten S., Mescher M. C., Francis F., Haubruge E., and Verheggen F. J.. 2012. Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem. Mol. Biol. 42: 155–163. [DOI] [PubMed] [Google Scholar]
- Vosteen I., Weisser W. W., and Kunert G.. 2016. Is there any evidence that aphid alarm pheromones work as prey and host finding kairomones for natural enemies?Ecol. Entomol. 41: 1–12. [Google Scholar]