Abstract
Background
The Strategic Timing of AntiRetroviral Treatment (START) trial demonstrated that immediate (at CD4+ >500 cells/µL) vs deferred (to CD4+ <350 cells/µL or AIDS) antiretroviral therapy (ART) initiation reduced risk for AIDS and serious non-AIDS (SNA). We investigated associations of inflammation, coagulation, and vascular injury biomarkers with AIDS, SNA or death, and the effect of immediate ART initiation.
Methods
Biomarkers were measured from stored plasma prior to randomization and at month 8. Associations of baseline biomarkers with event risk were estimated with Cox regression, pooled across groups, adjusted for age, gender, and treatment group, and stratified by region. Mean changes over 8 months were estimated and compared between the immediate and deferred ART arms using analysis of covariance models, adjusted for levels at entry.
Results
Baseline biomarker levels were available for 4299 START participants (92%). Mean follow-up was 3.2 years. Higher levels of IL-6 and D-dimer were the only biomarkers associated with risk for AIDS, SNA or death, as well as the individual components of SNA and AIDS events (HRs ranged 1.37–1.41 per 2-fold higher level), even after adjustment for baseline CD4+ count, HIV RNA level, and other biomarkers. At month 8, biomarker levels were lower in the immediate arm by 12%–21%.
Conclusions
These data, combined with evidence from prior biomarker studies, demonstrate that IL-6 and D-dimer consistently predict clinical risk across a broad spectrum of CD4 counts for those both ART-naïve and treated. Research is needed to identify disease-modifying treatments that target inflammation beyond the effects of ART.
Keywords: coagulation, comorbidities, end-organ disease, HIV disease, inflammation risk
Effective antiretroviral therapy (ART) has prolonged survival and shifted the morbidity spectrum for HIV+ persons away from AIDS and toward serious non-AIDS (SNA)-defining conditions, including cardiovascular disease, cancer, and other end-organ diseases [1, 2]. A key factor contributing to excess SNA event risk among HIV+ patients includes persistent abnormalities in systemic inflammation and coagulation [3–8]. Based on data from earlier large randomized trials, we and others have demonstrated that plasma measures of inflammation and/or coagulation activation predict risk for cardiovascular disease (CVD) [6, 9, 10], cancer [7], and all-cause mortality [3, 11–13], along with the composite of SNA [8, 14]. These data include patients with advanced HIV disease and/or those with low nadir CD4+ counts. It remains unclear whether ongoing inflammation is associated with clinical event risk specifically among HIV+ patients with recent diagnosis and preserved immunity (ie, CD4+ counts >500 cells/µL).
Inflammation may improve with ART, but biomarker levels continue to be abnormal among ART-treated patients with viral suppression when compared with uninfected controls [4, 15, 16]. Early HIV diagnosis and early initiation of ART is now the goal for management of HIV infection in much of the world. Importantly, prior data characterizing the effect of ART on levels of inflammatory biomarkers typically involve nonrandomized comparisons, lack of a comparison group not receiving ART, and/or participants with low CD4+ counts [15, 17–21].
The International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) Strategic Timing of AntiRetroviral Treatment (START) trial randomized 4684 ART-naïve HIV+ persons with CD4+ counts >500 cells/µL to initiate ART immediately after randomization, vs deferring ART until a CD4+ count of <350 cells/µL or progression to AIDS. In START, immediate ART initiation led to a 57% reduction in a composite outcome of AIDS and SNA nonfatal events or deaths; reductions in the risk of AIDS and SNA events were 72% and 39%, respectively [22]. START represents a global HIV+ population, and 68% of the primary end points in START occurred among participants with CD4+ counts >500 cells/µL [22]. The START trial provides a unique opportunity to study associations between biomarkers of inflammation, coagulation, and vascular injury with major clinical outcomes among patients with relative immune preservation.
METHODS
Study Design and Clinical Data Collection
The design and primary findings from the START trial have been described [22]. All participants provided written informed consent. We used specimens from study visits that occurred prior to randomization (defined as “baseline”) and at month 8, and included events that occurred through May 26, 2015, when the trial results were unblinded.
All clinical events and deaths in START were reviewed and adjudicated by an end point committee blinded to treatment assignment using pre-established criteria [22]. AIDS was defined as serious events within the 1993 expanded surveillance document of the Centers for Disease Control and Prevention, but also including Hodgkin lymphoma and excluding nonfatal Herpes simplex virus infection and esophageal candidiasis (excluded because of their lesser severity). SNA-related events consisted of any of the following conditions: cardiovascular disease (CVD; myocardial infarction, stroke, or coronary revascularization) or death from CVD; end-stage renal disease (initiation of dialysis or renal transplantation) or death from renal disease; liver disease (decompensated) or death from liver disease; non-AIDS-defining cancer (except for basal cell or squamous cell skin cancer) or death from cancer; and any other death not attributable to AIDS (see the Appendix of the primary START manuscript) [22].
Plasma Biomarkers
Using plasma collected at baseline and the month 8 visit, which was stored centrally at –70°C, 7 plasma biomarkers were measured that reflected systemic inflammation (IL-6, high-sensitivity C-reactive protein [hsCRP] and serum amyloid A [SAA]), vascular inflammation (soluble intercellular adhesion molecule-1 [sICAM] and vascular adhesion molecule-1 [sVCAM]), immune activation (interleukin-27 [IL-27]), and coagulation activation (D-dimer). IL-6 was measured using high-sensitivity enzyme-linked immunosorbent assay (R&D Systems), D-dimer using the VIDAS system (BioMerieux), and IL-27 using immunoassay (MesoScale). A multiplex platform was used to measure hsCRP, SAA, sICAM, and sVCAM (Vascular Injury II Panel, MesoScale). All samples were analyzed by researchers blinded to treatment group.
Statistical Analyses
Biomarkers were analyzed either on the log2 scale or using rank-based methods because biomarker distributions were right-skewed, and close to normal after log transformation; also, model assumptions were usually better satisfied for log-transformed biomarkers compared with no transformation.
Associations of baseline biomarker levels (log2 scale) with event risk (serious AIDS, SNA, all-cause mortality, and their composite) were estimated with Cox proportional hazards regression models, pooled across treatment groups, separately for each biomarker. We pooled the treatment groups because tests for heterogeneity showed that the associations between biomarkers and the risk of the primary outcome were similar in the immediate and the deferred ART groups for all 7 biomarkers. In addition, the homogeneity of the associations between biomarkers and clinical events was assessed across age and gender. The Cox models were adjusted for age, gender, and treatment group and stratified by region. Additional adjustment considered baseline HIV RNA level and CD4+ count given the importance for clinical risk. Biomarker associations with events were also assessed in models that contained all 7 biomarkers. The proportional hazards assumption was examined with expanded Cox models that included an interaction term for the biomarker and log failure time. Kaplan-Meier estimates of the cumulative proportion of participants with clinical events were computed in 2 ways, (1) pooled over treatment groups, by quartiles of baseline D-dimer and IL-6 levels, and (2) separately for the immediate and deferred groups, by whether the baseline biomarker level was above or below the median. In all analyses, follow-up was censored on May 26, 2015, or the date of last study contact.
Spearman’s rank correlation coefficients were computed between baseline levels of biomarkers, as well as between baseline factors (age, gender, CD4+ and CD8+ cell count, plasma HIV RNA level, body mass index [BMI], smoking status, and time since HIV diagnosis) and baseline biomarker levels.
Mean changes in biomarker levels (log2 scale) from baseline to the month 8 visit were compared between the immediate and deferred ART arms by intent to treat using analysis of covariance (ANCOVA) models, adjusted for baseline biomarker levels. Mean changes were back-transformed and presented as percent change on the original biomarker scale. Similar ANCOVA models were used for subgroup analyses by CD4+ and CD8+ cell counts and HIV RNA at baseline; homogeneity of the immediate vs deferred treatment difference across subgroup variables was assessed by testing for interaction between the treatment group indicator and the subgroup variable.
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute Inc., Cary NC), and R software, version 3.3 (R Foundation for Statistical Computing, Vienna, Austria). P values ≤0.05 were considered significant.
RESULTS
Participant Characteristics and Biomarker Levels at Baseline (Prior to Randomization)
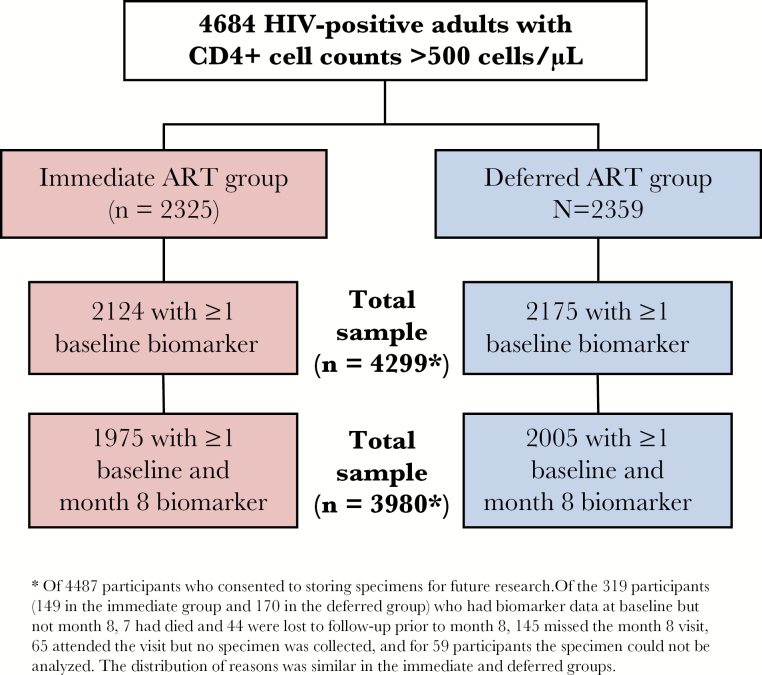
The START trial enrolled 4684 HIV+ participants from 215 sites in 35 countries, and 4487 (96%) consented to store plasma. Among the 4299 (92% of START) participants with available stored plasma samples who had baseline biomarker determinations for at least 1 of the 7 biomarkers (Figure 1), the median age was 36 years; 27% were female (Table 1). Self-reported race/ethnicity reflects global enrollment from the United States (11%), South America and Mexico (24%), Europe and Israel (40%), Australia (2%%), Africa (21%), and Asia (8%). The median CD4+ count and HIV viral load at entry were 651 cells/µL and 12911 copies/mL, respectively; the median time since HIV diagnosis was 1.0 year (interquartile range [IQR], 0.4–3.0 years). The mean follow-up time was 3.2 years.
Figure 1.
Flow diagram for availability of biomarker data among START participants. *Of 4487 participants who consented to storing specimens for future research. Of the 319 participants (149 in the immediate group and 170 in the deferred group) who had biomarker data at baseline but not month 8, 7 had died and 44 were lost to follow-up prior to month 8, 145 missed the month 8 visit, 65 attended the visit but no specimen was collected, and for 59 participants the specimen could not be analyzed. The distribution of reasons was similar in the immediate and deferred groups.
Table 1.
Baseline Characteristics in START, Overall and According to Primary Event Status (n = 4299)
| Median [IQR], n (%) | P Valueb | |||
|---|---|---|---|---|
| Overall | Primary Eventa | No Primary Eventa | ||
| Participants | 4299 | 129 | 4170 | |
| Demographics | ||||
| Age, y | 36 [29–44] | 44 [34–51] | 36 [29–44] | <0.001 |
| Female gender | 1141 (26.5) | 27 (20.9) | 1114 (26.7) | 0.14 |
| Race | 0.042 | |||
| Black | 1301 (30.3) | 41 (31.8) | 1260 (30.2) | |
| White | 1938 (45.1) | 68 (52.7) | 1870 (44.8) | |
| Other | 1060 (24.7) | 20 (15.5) | 1040 (24.9) | |
| Medical history | ||||
| Duration of HIV diagnosis, y | 1.0 [0.4–3.0] | 1.2 [0.5–3.6] | 1.0 [0.4–3.0] | 0.34 |
| CD4,c cells/µL | 651 [584–765] | 632 [584–742] | 651 [584–765] | 0.26 |
| CD8+, cells/µL | 1040 [775–1399] | 1081 [799–1505] | 1040 [774–1395] | 0.12 |
| CD4:CD8 ratio | 0.66 [0.48–0.89] | 0.61 [0.42–0.82] | 0.66 [0.48–0.89] | 0.031 |
| HIV RNA, copies/mL | 12 911 [3120–43 730] | 22 726 [7485–63 956] | 12 647 [3057–42 889] | 0.001 |
| Hepatitis B/C | 276 (6.5) | 10 (7.8) | 266 (6.5) | 0.54 |
| Prior CVDd | 25 (0.6) | 3 (2.3) | 22 (0.5) | 0.04 |
| BMI, kg/m2 | 24.5 [22.1–27.8] | 24.6 [22.5–27.7] | 24.5 [22.1–27.8] | 0.66 |
| Current smoking | 1398 (32.5) | 57 (44.2) | 1341 (32.2) | 0.004 |
| Biomarkers | ||||
| D-Dimer, μg/mL | 0.33 [0.23–0.50] | 0.40 [0.29–0.66] | 0.32 [0.22–0.49] | <0.001 |
| IL-6, pg/mL | 1.39 [0.97–2.12] | 1.65 [1.26–2.57] | 1.38 [0.96–2.11] | <0.001 |
| hsCRP, μg/mL | 1.71 [0.74–4.01] | 1.89 [0.93–4.54] | 1.69 [0.73–3.97] | 0.07 |
| SAA, mg/L | 4.52 [2.53–8.75] | 5.68 [3.15–9.77] | 4.47 [2.51–8.74] | 0.014 |
| IL-27, pg/mL | 251 [126–520] | 261 [118–506] | 251 [126–521] | 0.97 |
| sICAM, μg/mL | 541 [423–693] | 622 [502–751] | 538 [422–691] | <0.001 |
| sVCAM, μg/mL | 722 [62–922] | 808 [28–987] | 720 [61–919] | 0.004 |
Abbreviations: BMI = body mass index; CVD = cardiovascular disease; hsCRP = high-sensitivity C-reactive protein; IL = interleukin; IQR: interquartile range; MI = myocardial infarction; SAA = Serum Amyloid A; sICAM = soluble intercellular adhesion molecule; SNA = serious non-AIDS events, sVCAM = soluble vascular cellular adhesion.
Primary event refers to the composite study end point of AIDS, SNA, or death.
P value comparing those with a primary event with those without a primary event. Medians were compared using Wilcoxon rank-sum tests and proportions compared using chi-square tests. The Fisher exact test was used for comparison of prior CVD risk.
Average of screening and baseline values.
MI, coronary revascularization, or stroke.
Baseline biomarker levels were moderately correlated with one another (r ≥ 0.2; P < 0.0001) for all combinations except for any biomarkers with IL-27, and correlations of sVCAM with D-dimer and IL-6, or sICAM with D-dimer. The strongest correlations were between sICAM and sVCAM (r = 0.75), and hsCRP with SAA (r = 0.68) or IL-6 (r = 0.51). At baseline, higher levels of all 7 biomarkers were associated with higher HIV RNA level and CD8+ counts, but not lower CD4+ counts (Supplemental Figure A).
ART Use and CD4+ Cell Counts
All 4299 participants were naïve to ART at baseline (as specified by START eligibility criteria). ART was used for 94% and 29% of follow-up time in the immediate and deferred ART groups, respectively. Through all follow-up, the average CD4+ count increased in the immediate group by 173 cells/µL (95% confidence interval [CI], 164–183) and decreased in the deferred group by 20 cells/µL (95% CI, 10–29), for a difference of 193 cells/µL (95% CI, 184–203) between groups.
Among the 3980 participants for whom baseline to month 8 biomarker changes were assessed, 1941 (98%) participants in the immediate ART group and 163 (8%) in the deferred group had initiated ART by 8 months. ART had been used for 91% and 4% of time between baseline and month 8 among the immediate and deferred ART groups, respectively. By month 8, the average CD4+ cell count had increased from baseline in the immediate group by 91 cells/µL and decreased by 76 cells/µL in the deferred group. Among the immediate group participants who initiated therapy by month 8, ART included tenofovir disoproxil fumarate (TDF) for 89%, efavirenz (EFV) for 73%, a ritonavir-boosted protease inhibitor (PI) for 18%, and an integrase strand transfer inhibitor (INSTI) for 4%.
Associations of Baseline Biomarker Levels With Clinical Event Risk
Primary events (AIDS, SNA, or death) were experienced by 129 participants, 40 in the immediate and 89 in the deferred ART groups; 57 experienced fatal or nonfatal AIDS events (23 tuberculosis [TB]) and 74 SNA events or non-AIDS deaths; 50 experienced cancer (AIDS or non-AIDS; 12 Kaposi’s sarcoma) and 24 CVD. Participants with, compared with without, a primary event had higher median biomarker levels at baseline (Table 1).
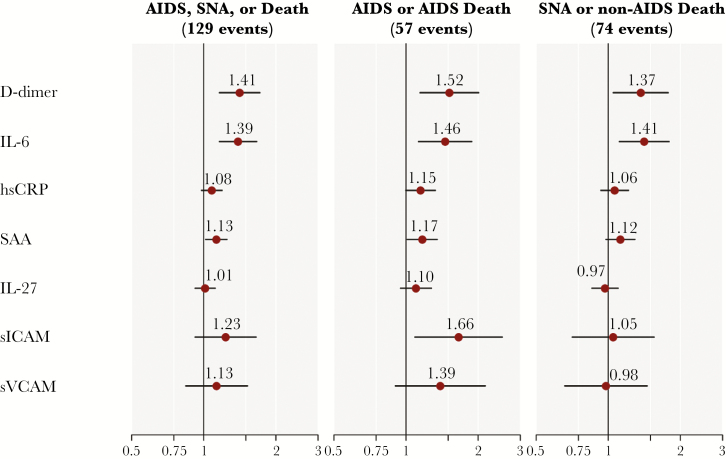
Figure 2 presents event risk over follow-up as predicted by baseline biomarker levels. Higher levels of D-dimer and IL-6 were associated with increased risk for the primary composite of AIDS, SNA, or death, as well as the individual components of SNA or non-AIDS death and AIDS (fatal or nonfatal). Risk for AIDS events was also associated with higher SAA and sICAM levels, and hsCRP approached significance (P = .053). D-dimer and IL-6 associations with AIDS, SNA, or death persisted after additional adjustment for baseline CD4+ count and HIV RNA level (HR, 1.36 per 2× higher D-dimer; 95% CI, 1.11–1.66; HR, 1.35 per 2× higher IL-6; 95% CI, 1.12–1.62). When considering models that included baseline levels of all 7 biomarkers jointly, along with age, gender, and treatment group, risk for AIDS, SNA, or death was significantly associated with IL-6 (HR, 1.34 per 2× higher IL-6 levels; 95% CI, 1.08–1.67) and D-dimer (HR, 1.32 per 2× higher D-dimer levels; 95% CI, 1.07–1.63), but none of the other biomarkers.
Figure 2.
Hazard ratios (HRs) with 95% confidence intervals (CIs) for the risk of clinical events associated with 2× higher baseline biomarker levels in START (n = 4299). HRs were estimated in separate proportional hazards models, pooled across treatment groups, adjusted for age, gender, and treatment group, and stratified by geographic location. HRs are per 2× higher biomarker(s). Biomarker associations with event risk were homogeneous across treatment groups, except for sVCAM with AIDS (higher HR in the deferred group) and sICAM with SNA (higher HR in the immediate group). Abbreviations: IL = interleukin; hsCRP = high-sensitivity C-reactive protein; SAA = serum amyloid A; sICAM = soluble intercellular adhesion molecule; sVCAM = soluble vascular cellular adhesion molecule.
Figure 3A presents Kaplan-Meier estimates for the cumulative percentage of participants experiencing AIDS and SNA events by quartiles of the baseline levels of IL-6 or D-dimer. The associations for IL-6 and D-dimer were stronger for events that occurred more proximal to the biomarker measurements (P values for interaction with log follow-up time were 0.008 for D-dimer and 0.016 for IL-6). This change in event rates over time was more pronounced in the deferred arm for IL-6 (P = 0.005 for interaction with log time in the deferred arm, P = 0.70 in the immediate arm), but the relationship was consistent across both treatment arms for D-dimer.
Figure 3.
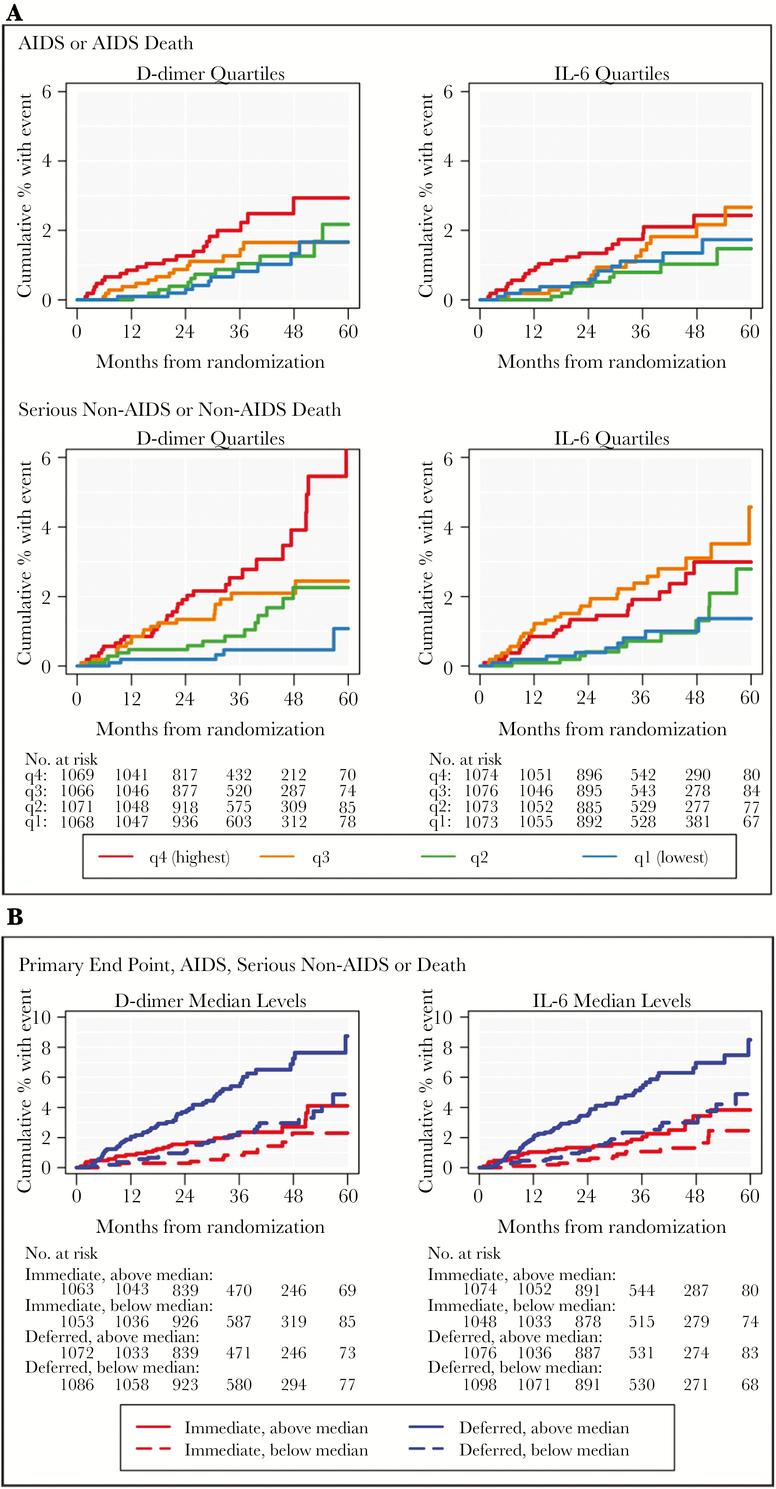
Kaplan-Meier (KM) estimates for the cumulative percentage of participants experiencing AIDS, serious non-AIDS, or death in START (4299). (A) Kaplan-Meier estimates for the cumulative percentage of participants experiencing an AIDS or AIDS-death (top row) and serious non-AIDS or non-AIDS death (bottom row) pooled over study groups by quartiles of D-dimer and IL-6 at baseline (first quartile = blue; second quartile = green; third quartile = orange; fourth quartile = red). The number at risk at each year of follow-up is presented below the x-axis. (B) Kaplan-Meier estimates for the cumulative percentage of participants experiencing the composite outcome of AIDS, serious non-AIDS or death in the immediate (red lines) and deferred (blue lines) ART groups in START, separately by whether baseline level of D-dimer and IL-6 were above (solid lines) or below (dashed lines) their median at baseline.
Associations of IL-6 and D-dimer with the risk of AIDS, SNA, or death were similar across treatment groups (P = 0.96 and P = 0.97, respectively, for interaction). This is illustrated in Figure 3B, which presents the cumulative percentage of participants experiencing the primary composite within the immediate and deferred ART groups in START, separately for those with above- or below-median IL-6 and D-dimer levels at baseline. This figure shows that the relative difference in event risk between high and low biomarker levels is similar for both the immediate and deferred groups, even though the event rate for the deferred group is higher than the event rate for the immediate group for both biomarkers. For example, in the immediate group, the cumulative event rates at month 36 were 2.2% and 0.8% for those whose baseline D-dimer was above or below the median, respectively (a ratio of 2.75); corresponding rates for the deferred group were 5.4% and 2.3% (a ratio of 2.35) (Figure 3B).
Changes in Biomarkers From Baseline to Month 8 With Immediate vs Deferred ART
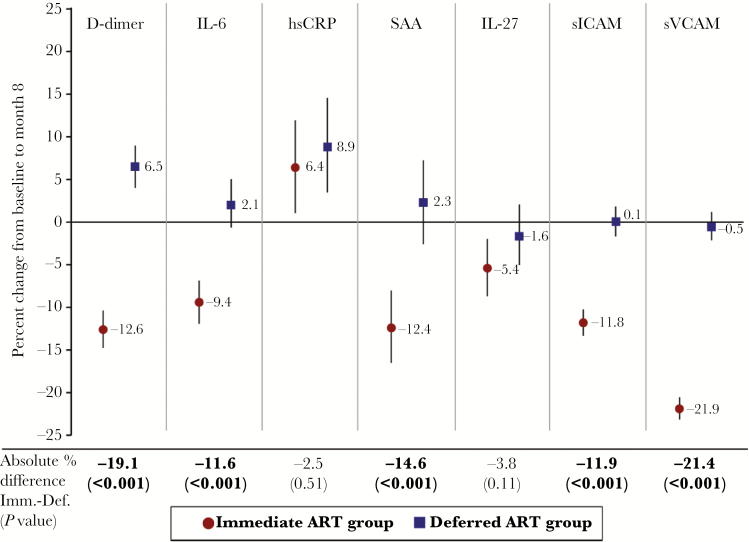
In the immediate group (ART initiated at baseline), levels of IL-6, D-dimer, SAA, IL-27, sICAM, and sVCAM declined between baseline and month 8 (Figure 4). D-dimer levels increased by month 8 in the deferred ART group, and hsCRP increased in both the immediate and the deferred groups. Levels of D-dimer, IL-6, SAA, sICAM, and sVCAM declined from baseline to month 8 by 12%–21% more in the immediate group compared with the deferred group (P < .001 for each biomarker) (Figure 4). These differences persisted, with slightly larger magnitudes, when excluding deferred group participants who started ART prior to month 8 (data not shown). The ART-associated declines in biomarker levels at 8 months (eg, 19.1% for D-dimer and 11.6% for IL-6) are modest in absolute terms (Supplementary Table A); median D-dimer levels within the immediate ART group were 0.32 μg/mL at baseline and 0.28 μg/mL at month 8, and corresponding values for IL-6 were 1.41 pg/mL and 1.27 pg/mL.
Figure 4.
Biomarker changes from baseline to month 8 with immediate vs deferred art initiation in START (n = 3980). The percentage change (95% CI) from baseline to month 8 for each of the 7 biomarkers, adjusted for baseline level, is plotted for both the immediate (red circles) and deferred (blue squares) ART groups. The absolute difference between immediate and deferred groups for the percentage change from baseline to month 8 is presented below the x-axis (with P-values) for each of the biomarkers. Abbreviations: CI = confidence interval; hsCRP = high-sensitivity C-reactive protein; IL = interleukin; sICAM = soluble intercellular adhesion molecule, sVCAM = soluble vascular cellular adhesion molecule.
We carried out subgroup analyses to determine whether the immediate vs deferred treatment difference in biomarker changes was homogeneous across baseline levels of CD4+, CD8+, and HIV RNA (Supplemental Tables B, C, D). For all biomarkers, the treatment difference varied across HIV RNA levels (except for IL-27) and CD8+ count (except for SAA), and participants with higher HIV RNA levels at baseline demonstrated the greatest ART-associated declines in the immediate group and the greatest increases in the deferred group (except for IL-27) over 8 months. Specifically, among persons with HIV RNA levels ≥30 000 copies/mL at entry, D-dimer levels declined by 21% in the immediate group, but actually increased by 8% in the deferred group; corresponding changes for IL-6 levels were a 14% decline and a 12% increase, respectively.
Discussion
In a large randomized clinical trial (START) of HIV+ patients who were early in the course of disease and had preserved immunity, we report the associations of biomarker levels of systemic inflammation, coagulation, and vascular injury with risk for clinical events, and the effect of immediate vs deferred ART initiation on these same biomarkers. Greater systemic inflammation (ie, IL-6 levels) and coagulation activity (ie, D-dimer levels) at entry in START were associated with increased risk for AIDS events, SNA events, and the composite of AIDS, SNA, or death. The detrimental effect of inflammation and/or coagulation activation was present in both the immediate and deferred ART groups in START (ie, were independent of the timing of ART initiation), and demonstrated similar associations for AIDS and SNA events when examined separately. In randomized comparisons of immediate and deferred ART initiation, immediate treatment led to significant reductions over 8 months in D-dimer, IL-6, SAA, sICAM, and sVCAM levels, both in absolute terms and relative to deferral of ART.
It is well established that HIV infection is associated with elevations in systemic inflammatory markers, cytokines, and circulating vascular adhesion molecules [4, 23, 24]. Consistent with this, we report median levels of IL-6 (1.39 pg/mL) and D-dimer (0.33 μg/mL) at entry in START that are higher than levels previously reported among populations of HIV-negative participants (1.23 pg/mL and 0.22 μg/mL, respectively, in the Multi-Ethnic Study of Atherosclerosis; median age, 60 years) [4]. In other studies, ART treatment has also been shown to decrease levels of D-dimer, IL-6, and sVCAM [15, 17, 24–26], though the degree of decrease may vary by ART regimen [17] and ART-induced viral suppression may not fully restore levels of these biomarkers to those of age-adjusted controls [4, 15, 23, 24]. Specifically, when compared with levels among uninfected individuals, IL-6 and D-dimer remain approximately 40%–75% higher among HIV+ patients on ART with viral suppression [4, 27]. We show that immediate ART initiation reduced levels of D-dimer, IL-6, SAA, sICAM, and sVCAM, even when started early in the course of HIV disease, before a clinically significant decline in CD4+ count has occurred. When compared with the effect of ART initiation (vs deferral) among a subset of participants in the SMART trial who were not using ART at entry (prior to initiating ART: median CD4+ count, 447 cells/mm3; HIV RNA, 39 810 copies/mL), we report here a smaller reduction of D-dimer levels in START (31% vs 19%, respectively), but a similar reduction for IL-6 levels (13% vs 12%, respectively, though ART-related IL-6 changes did not reach significance in the smaller subset of SMART) [15]. Finally, we reported a paradoxical increase in hsCRP levels with ART treatment, but this lack of a decline (or an increase) has been previously described and may be the result of improved hepatocyte synthetic function (ie, improved hsCRP release), which was previously impaired in the context of untreated HIV infection [15, 17, 28–31].
Our findings on clinical risk prediction add to recent literature that has reshaped our current understanding of the mechanisms contributing to morbidity and mortality during HIV disease in the current era. Notably, measures of systemic inflammation are consistently associated with risk for SNA events and the development of individual end-organ diseases [6, 7, 32–35]. Recent data have also suggested that the independent contribution of CD4+ counts to CVD and other SNA event risk may be more modest, or not apparent, at higher CD4+ counts [22, 36]. This may explain why we did not detect a significant association with clinical risk for IL-27, which is produced by antigen-presenting cells and is important in T-cell immune responses. We have previously reported that among 3766 ART-treated and virologically suppressed HIV+ patients, risk for SNA events or death was associated with elevations in IL-6 and D-dimer (HR, 1.45 for IL-6 and 1.28 D-dimer level, per 2× higher biomarker level, respectively) [8]. Even though the START study population included only untreated participants with higher CD4+ counts at entry, we estimate similar HRs for SNA event risk (HR, 1.41 for IL-6 and 1.37 for D-dimer). We also show that the adverse effects from systemic inflammation, in terms of clinical risk over the long term, are present for HIV+ patients whether they start ART very early or later in their disease course (ie, both treatment groups in START). Additional follow-up is required in START to accurately estimate biomarker associations with clinical event risk after viral suppression is achieved among this population treated at higher CD4+ counts, especially given our initial observation that biomarker associations were stronger for early vs later events in START.
Important questions remain regarding the underlying pathogenesis driving chronic inflammation and coagulation activity among HIV+ patients, both before and after ART initiation. Hypothesized mechanisms have been previously reviewed [37, 38] and may involve factors related to persistence of HIV and related immune activation, incomplete immune recovery, loss of regulatory function, transcription of defective proviruses [39], reduced control of copathogens (eg, cytomegalovirus), injury to mucosal surfaces with translocation of microbial antigens, toxicity from ART, and host and lifestyle factors. We show that even among HIV+ patients who start ART very early in the course of disease, there is a subset of ART-treated patients with greater clinical risk; such patients might benefit from reducing ongoing inflammation whatever the underlying mechanisms. A central focus and priority of current HIV research is to identify anti-inflammatory strategies and determine if that will translate into reductions in clinical risk for 1 or more SNA conditions [38]. Importantly, any candidate adjunct treatment intervention would need to establish safety (or entail minimal risk) and not compromise ART effectiveness, given the excellent outcomes now experienced among HIV-positive patients treated early with ART.
Strengths of this study include the randomized design, large sample size, globally diverse study population, inclusion of HIV+ patients with preserved immunity initiating ART early (reflective of current goals for clinical practice), and the standardized ascertainment and adjudication of clinical events. Limitations include the lack of additional laboratory measures, the lack of functional measures that more directly reflect potential disease mechanisms, and the use of a single follow-up time point at 8 months that precluded determination of whether immediate vs deferred ART differences in biomarker levels persisted after more prolonged viral suppression (eg, >2 years after ART initiation). Longer follow-up of START will help clarify whether the full effect of more sustained virologic suppression over years ultimately reverses the harmful effects of ART deferral (reflected in the higher levels of inflammation and coagulation biomarkers), when compared with immediate ART treatment at higher CD4+ counts. The relatively low number of events in START overall, and in the immediate ART group in particular, precluded reliable estimates of (a) event risk among any individual end-organ disease condition or focused composite, (b) event risk after viral suppression was achieved and biomarkers reached their new set points, and (c) the interaction between biomarkers and treatment group or follow-up time. Finally, participants in clinical trials may have greater adherence and health-seeking behaviors, which may impact the generalizability of results.
In START, among a global population of HIV+ persons with preserved immunity, immediate ART initiation reduced biomarker levels of systemic inflammation, coagulation, and vascular injury compared with ART deferral. Differences in biomarkers between groups were the result of reductions after ART initiation, concurrent with increases among participants who deferred ART. Associations between IL-6 and D-dimer levels and risk for AIDS, SNA, and death were present for both treatment groups in START. When combined with data from prior studies [3, 6, 8, 10, 32, 40, 41], higher levels of IL-6 and D-dimer consistently predict increased clinical risk across a broad spectrum of CD4+ counts for HIV+ patients, both ART-naïve and ART-treated. Cumulatively, these observations support the need for research to better understand the mechanisms driving ongoing inflammation and coagulation abnormalities during effectively treated HIV disease, and to identify disease-modifying treatments or lifestyle interventions that reduce inflammation beyond the effects of ART.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to specifically thank the participants in the START trial. See N Engl J Med 2015;373:795–807 for the complete list of START investigators.
Financial support The START study (NCT00867048) is registered at clinicaltrials.gov. The START study is primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers UM1-AI068641 and UMN1-AI120197, with additional support from the National Institutes of Health Clinical Center, National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (France), National Health and Medical Research Council (Australia), National Research Foundation (Denmark), Bundesministerium für Bildung und Forschung (Germany), European AIDS Treatment Network, Medical Research Council (United Kingdom), National Institute for Health Research, National Health Service (United Kingdom), and University of Minnesota.
Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
Disclosures The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A.P. received fees for speaking at 2 meetings sponsored by Gilead Sciences, for consulting for GSK Biologicals, and for attendance at an advisory board meeting from AbbVie. The remaining authors declare no relevant financial interests.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Miller CJ, Baker JV, Bormann AM et al. ; INSIGHT SMART Study Group; ESPRIT Study Group. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 2014; 9:e95061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mocroft A, Reiss P, Gasiorowski J et al. ; EuroSIDA Study Group. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr 2010; 55:262–70. [DOI] [PubMed] [Google Scholar]
- 3. Kuller LH, Tracy R, Belloso W et al. ; INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neuhaus J, Jacobs DR Jr, Baker JV et al. . Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandler NG, Wand H, Roque A et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duprez DA, Neuhaus J, Kuller LH et al. ; INSIGHT SMART Study Group. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borges ÁH, Silverberg MJ, Wentworth D et al. ; INSIGHT SMART; ESPRIT; SILCAAT Study Groups. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grund B, Baker JV, Deeks SG et al. . INSIGHT SMART/ESPRIT/SILCAAT Study Group. Relevance of Interleukin-6 and D-Dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016; 11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ford ES, Greenwald JH, Richterman AG et al. . Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010; 24:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tien PC, Choi AI, Zolopa AR et al. . Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010; 55:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ledwaba L, Tavel JA, Khabo P et al. . Project Phidisa Biomarkers Team. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012; 7:e24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wada NI, Bream JH, Martínez-Maza O et al. . Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis 2016; 63:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenorio AR, Zheng Y, Bosch RJ et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker JV, Neuhaus J, Duprez D et al. . INSIGHT SMART Study Group. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr 2011; 56:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wada NI, Jacobson LP, Margolick JB et al. . The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McComsey GA, Kitch D, Daar ES et al. . Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS 2012; 26:1371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hileman CO, Kinley B, Scharen-Guivel V et al. . Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keating SM, Golub ET, Nowicki M et al. . Women’s Interagency HIV Study. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS 2011; 25:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kristoffersen US, Kofoed K, Kronborg G et al. . Reduction in circulating markers of endothelial dysfunction in HIV-infected patients during antiretroviral therapy. HIV Med 2009; 10:79–87. [DOI] [PubMed] [Google Scholar]
- 21. van Vonderen MG, Hassink EA, van Agtmael MA et al. . Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy. J Infect Dis 2009; 199:1186–94. [DOI] [PubMed] [Google Scholar]
- 22. INSIGHT Start Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gattegno L, Bentata-Peyssare M, Gronowski S et al. . Elevated concentrations of circulating intercellular adhesion molecule 1 (ICAM-1) and of vascular cell adhesion molecule 1 (VCAM-1) in HIV-1 infection. Cell Adhes Commun 1995; 3:179–85. [DOI] [PubMed] [Google Scholar]
- 24. Calza L, Pocaterra D, Pavoni M et al. . Plasma levels of VCAM-1, ICAM-1, E-Selectin, and P-Selectin in 99 HIV-positive patients versus 51 HIV-negative healthy controls. J Acquir Immune Defic Syndr 2009; 50:430–2. [DOI] [PubMed] [Google Scholar]
- 25. Wolf K, Tsakiris DA, Weber R et al. . Swiss HIV Cohort Study. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis 2002; 185:456–62. [DOI] [PubMed] [Google Scholar]
- 26. Hamlyn E, Stöhr W, Cooper DA et al. . SPARTAC Investigators. The effect of short-course antiretroviral therapy initiated in primary HIV-1 infection on interleukin-6 and D-dimer levels. AIDS 2015; 29:1355–61. [DOI] [PubMed] [Google Scholar]
- 27. Freiberg MS, Bebu I, Tracy R et al. . Infectious Disease Clinical Research Program HIV Working Group. D-dimer levels before HIV seroconversion remain elevated even after viral suppression and are associated with an increased risk of non-AIDS events. PLoS One 2016; 11:e0152588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palella FJ Jr, Gange SJ, Benning L et al. . Inflammatory biomarkers and abacavir use in the women’s interagency HIV study and the multicenter AIDS cohort study. AIDS 2010; 24:1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mendeni M, Focà E, Gotti D et al. . Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis 2011; 52:1164–73. [DOI] [PubMed] [Google Scholar]
- 30. Baker JV, Brummel-Ziedins K, Neuhaus J et al. . INSIGHT SMART Study Team. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc 2013; 2:e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sales PC, Williams BR, Silva AM. Regulation of double-stranded RNA dependent protein kinase expression and attenuation of protein synthesis induced by bacterial toll-like receptors agonists in the absence of interferon. J Interferon Cytokine Res 2012; 32:495–504. [DOI] [PubMed] [Google Scholar]
- 32. Tenorio AR, Zheng Y, Bosch RJ et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Subramanian S, Tawakol A, Burdo TH et al. . Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burdo TH, Lo J, Abbara S et al. . Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker JV, Hullsiek KH, Singh A et al. . CDC SUN Study Investigators. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014; 28:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sabin CA, Ryom L, De Wit S et al. . Associations between immune depression and cardiovascular events in HIV infection. AIDS 2013; 27:2735–48. [DOI] [PubMed] [Google Scholar]
- 37. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imamichi H, Dewar RL, Adelsberger JW et al. . Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borges ÁH, O’Connor JL, Phillips AN et al. . INSIGHT SMART Study and ESPRIT Groups. Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J Infect Dis 2016; 214:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boulware DR, Hullsiek KH, Puronen CE et al. . INSIGHT Study Group. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.