Abstract
This study investigates in vitro targets related to diabetes in 30 herbal extracts from Peru, for the first time, using α-glucosidase, aldose reductase (AR) inhibitory assays and 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) scavenging assays. Among the 30 herbal extracts, Hypericum laricifolium Juss. (HL) was the herb which showed more than 50% inhibition in all assays, presenting 97.2 ± 2.0%, 56.9 ± 5.6%, 81.9 ± 2.5%, and 58.8 ± 4.6% inhibition for the α-glucosidase, AR, DPPH, and ABTS assays, respectively. Finally, six bioactive compounds, namely, protocatechuic acid, chlorogenic acid, caffeic acid, kaempferol 3-O-glucuronide, quercetin, and kaempferol were identified in HL by offline high-performance liquid chromatography (HPLC). Quercetin exhibited the strongest inhibition in all enzyme assays and the strongest antioxidant activity. The results suggest that HL shows great potential for the complementary treatment of diabetes and its complications.
Keywords: Peruvian herbal extracts, α-glucosidase, aldose reductase, screening, ultrafiltration, Hypericum laricifolium Juss.
1. Introduction
According to the World Health Organization (WHO), the prevalence of diabetes continues to increase at an alarming rate. Globally, an estimated 346 million people are living with diabetes, figures that are predicted to have a severe impact on human health by 2025 [1]. Diabetes mellitus (DM) is a group of chronic metabolic diseases characterized by chronic hyperglycemia. This condition is caused by the reduction of insulin secretion and/or insulin resistance and is considered as the primary factor for the pathogenesis of long-term diabetic complications [2]. Thus, diabetes is associated with the long-term damage, dysfunction and failure of various organs, leading to a series of complications caused by the disruption of carbohydrate, protein and fat metabolism, and these complications include nephropathy, neuropathy, retinopathy, atherosclerosis, skin complications, and cardiac dysfunction [1,3]. Therapy for DM relies on several approaches, many of which comprise drug targets for type 2 diabetes. Moreover, various efforts have been made to obtain other effective and safe enzyme inhibitors from plant extracts to control diabetes [4]. There are different targets related to diabetes and its complications such as α-glucosidase, aldose reductase (AR), and free radicals. α-Glucosidase (EC 3.2.1.20) is an important enzyme that catalyzes the final step of carbohydrate digestion. The inhibition of this enzyme can delay the digestion and absorption of dietary carbohydrates and consequently suppress postprandial hyperglycemia [4,5]. AR (EC 1.1.1.21) is the first enzyme in the polyol pathway. The high blood glucose levels characteristic of DM cause a significant flux of glucose through the polyol pathway in tissues such as kidney, nerve, and retina tissue [6]. Consequently, the accumulation of sorbitol generates osmotic stress and can activate AR, resulting in various diabetic complications [7]. Oxidative stress causes an imbalance between the free-radical-generating and free-radical-scavenging capacities. This imbalance is mainly responsible for the auto-oxidation of glucose in DM and its complications. The increased free radical production and reduced antioxidant defense may partially mediate the initiation and progression of diabetes-associated complications [8]. Thus, α-glucosidase and AR inhibitors and strong antioxidants may be useful tools to decrease postprandial blood glucose and insulin levels in patients with type 2 diabetes, prevent the polyol pathway, and ameliorate oxidative stress, respectively [4,9].
Research over the past two centuries has led to the development of a significant number of pharmaceuticals derived from plants from different regions of the world such as the South American rainforests [10]. In Peru, various types of plants are produced and consumed on a large scale. However, literature and information on the antidiabetic activity of these plants (especially on α-glucosidase and AR inhibition), which may lead to the development of new antidiabetic agents, is limited. Thus, this study investigates the efficacy of 30 herbal extracts from Peru for α-glucosidase and AR inhibitors and antioxidants. Hypericum laricifolium Juss. (HL) is a species of Hypericum (Clusiaceae) that is widely distributed in high altitude tropical regions, particularly in South America. In Peru, it is called “Chinchango, Abrecaminos, Hierba de la fortuna”, while in Ecuador it is called “Matikillkana, Romerillo, Hierba de San Juan” and has been used as folk medicine [11]. Previous reports have revealed the presence of various xanthones [12], phenolic acids, flavonoids, triterpenoids [13], and acylphloroglucinol derivatives in HL [14].
Traditional methods comprising isolation, fractionation, purification, and structure elucidation have been widely used to discover new bioactive compounds with antioxidants, α-glucosidase, and AR inhibitory activities. However, these traditional methods are time-consuming, labor intensive, and of low efficiency due to the loss of compound activity during isolation and purification [15]. Thus, it is necessary to establish effective and rapid methods, such as various offline high-performance liquid chromatography (HPLC) assays, to identify active compounds from mixtures. These include offline α-glucosidase ultrafiltration-HPLC, offline AR ultrafiltration-HPLC, offline 2,2-diphenyl-1-picrylhydrazyl (DPPH)-HPLC and offline 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)-HPLC assays.
To the best of our knowledge, no screening method has been applied to herbal extracts related to diabetes and no affinity reports based on offline HPLC assay have been reported for HL to date. Thus, this study uses innovative screening methods for 30 herbal extracts from Peru related to diabetes and subsequently, an ultrafiltration method and offline DPPH-HPLC and ABTS-HPLC assays to screen active compounds for HL.
2. Results and Discussion
2.1. Evaluation of α-Glucosidase and Aldose Reductase (AR) Inhibition and Antioxidant Activity of Peruvian Plants
In this study, an assortment of plant parts including leaves, aerial parts, seeds, and roots were used in the extraction of 30 plants obtained from a popular market in Lima. α-Glucosidase and AR inhibition and the antioxidant activity of the 30 Peruvian herbal extracts were investigated (Table 1). Acarbose, quercetin, l-ascorbic acid, and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) were used as positive controls. Of the 30 herbal extracts assayed, only 23 crude extracts exhibited inhibition at 500 μg/mL in the α-glucosidase inhibition assay. Among these extracts, 9 presented relatively high α-glucosidase inhibition (>50%). Desmodium molliculum (Kunth) A.P. De Candolle (P80), Geranium dielsianum R. Knuth (A35), and HL (A10) showed significantly (p < 0.05) higher α-glucosidase inhibition (≥80%) than those of others. Even though the α-glucosidase inhibition of HL was lower than P80 and A35, they are not significantly different. In the AR inhibition assay, only 23 crude extracts exhibited inhibition, with Otholobium mexicanum (L.f.) J.W. Grimes (A3), HL, Flaveria bidentis (L.) Kuntze (A31), Tessaria integrifolia Ruiz & Pav. (A17), Gentianella tristicha (Gilg) J.S. Pringle (P7), and Argyrochosma nivea (Poir.) Windham (A2), presenting significantly (p < 0.05) high AR inhibition (>50%) at 10 μg/mL. The crude extracts were assessed by DPPH and ABTS assays for their antioxidant capacities. A total of 13 extracts, namely A10, A35, Phyllanthus niruri L. (P5), Clinopodium brevicalyx (Epling) Harley & A. Granda (P11), Schkuhria pinnata (Lam.) Kuntze ex Thell. (P14), Cheilanthes pilosa Goldm. (P17), Eucalyptus globolus Labill. (P39), Equisetum giganteum L. (P55), Chuquiraga spinosa Less. (P77), Tiquilia Paronychioides (Phil.) Rich. (P36), P80, Gentianella tristicha (Gilg) J.S. Pringle (P7) and Peumus boldus Molina (P40) presented relatively high antioxidant capacities (>50%) in both the DPPH assay at 143 μg/mL and the ABTS assay at 33 μg/mL, whereas only 19 crude extracts exhibited high antioxidant capacities (>50%) in the DPPH assay. HL (DPPH = 81.9%, ABTS = 58.8%), P36 (DPPH = 95.7%, ABTS = 97.1%), A35 (DPPH = 96.8%, ABTS = 83.9%), P5 (DPPH = 97%, ABTS = 99.6%), P11 (DPPH = 95.8%, ABTS = 94.1%), P14 (DPPH = 67.1%, ABTS = 71.4%), P39 (DPPH = 77.5%, ABTS = 99.5%), P40 (DPPH = 86.2%, ABTS = 95.7%) and P80 (DPPH = 89.9%, ABTS = 73.6%) showed significantly (p < 0.05) higher antioxidant activities than others. Even though the antioxidant capacity of HL was lower than those of A35, P5, P36, P80, P11, and P40 these latter samples displayed low inhibitory activity in the AR assay (<35%). These results suggested that HL was the most active sample in all assays and that HL should be further investigated.
Table 1.
Ethnobotanical data related to diabetes, treatment, complications, symptoms, obesity, infection, inflammation and α-glucosidase, aldose reductase (AR) inhibitory activities, and antioxidants of Peruvian herbal extracts.
| No. | Voucher | Scientific Name | Common Name | Family | Used Part 1 | References
[13,17,19,20,21,22,23,24,25,26,27,28] 2 |
Yield (%) 3 | Inhibition (%) 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Glucosidase (500 μg/mL) 5 | AR (10 μg/mL) 6 |
Antioxidants | |||||||||
| DPPH (143 μg/mL) 7 |
ABTS (33 μg/mL) 8 |
||||||||||
| 1 | A5 | Annona muricata L. | Hoja de huanabana, Graviola | ANNONACEAE | L | Hypertension [ 24], Inflammation [20,24] | 19.5 | 26.3 ± 1.7 c | 0.8 ± 0.8 a | 64.2 ± 0.9 e,f,g,h,i | 31.5 ± 2.9 f,g |
| 2 | A32 | Acanthoxanthium spinosu (L.) Fourr. | Juan Alonso | ASTERACEAE | A | Diabetes [ 25], Inflammation [20] | 8.4 | 3.2 ± 2.7 a | 22.4 ± 1.2 e,f | 11.0 ± 3.5 a,b | 2.1 ± 1.8 a |
| 3 | A16 | Ambrosia arborescens Mill. | Marco | ASTERACEAE | L | Inflammation [ 25] | 12.7 | 18.5 ± 1.9 c | 31.8 ± 6.1 f,g | 3.5 ± 0.7 a,b | 6.1 ± 1.8 a,b |
| 4 | P78 | Baccharis genistelloides (Lam.) Pers. | Karqueja | ASTERACEAE | A | Diabetes [ 20,21], Inflammation [20], Burn fat [20], Cholesterol [20,21] | 19.7 | 6.2 ± 3.4 a,b | NA | 47.0 ± 9.2 c,d | 27.9 ± 1.1 e,f,g |
| 5 | P77 | Chuquiraga spinosa Less. | Huamanpinta | ASTERACEAE | A | Inflammation [ 25], Kidneys [25] | 28.7 | NA 9 | 12.2 ± 0.7 c,d | 70.3 ± 11.0 g,h,i,j | 50.3 ± 3.1 h |
| 6 | A31 | Flaveria Bidentis (L.) Kuntze | Mata gusano | ASTERACEAE | L | Inflammation [ 26] | 7.9 | 17.3 ± 8.4 b,c | 86.4 ± 1.9 k | 5.3 ± 10.8 a,b | 8.0 ± 2.1 a,b |
| 7 | P14 | Schkuhria pinnata (Lam.) Kuntze ex Thell. | Canchalagua | ASTERACEAE | A | Diabetes [ 20,21], Inflammation [20,21] | 2.4 | 0.1 ± 2.7 a | NA | 67.1 ± 3.3 f,g,h,i | 71.4 ± 8.4 j |
| 8 | A6 | Smallanthus sonchifolius (Poepp.) H. Rob. | Hojas de yacon | ASTERACEAE | L | Diabetes [ 20,21,22,23], Inflammation [20], Kidneys [20], Cholesterol [20] | 5.3 | 5.5 ± 1.4 a,b | 31.9 ± 9.1 g | NA | 5.5 ± 3.0 a,b |
| 9 | P49 | Taraxacum officinale F.H. Wigg. | Diente de leon | ASTERACEAE | A, F | Inflammation [ 20] | 4.8 | NA | NA | 41.9 ± 10.8 c | 15.7 ± 1.6 b,c,d |
| 10 | A17 | Tessaria integrifolia Ruiz & Pav. | Pajaro bobo | ASTERACEAE | L | Kidneys [ 20], Liver [20], Inflammation [20] | 18.4 | 41.6 ± 1.1 d | 79.9 ± 0.1 k | 52.2 ± 3.9 c,d,e | 31.1 ± 3.3 f,g |
| 11 | P79 | Cordia lutea Lam. | Flor de overo | BORAGINACEAE | F | Inflammation [ 20,21], Liver [20,21] | 12.3 | NA | 3.6 ± 1.3 a,b | 60.2 ± 2.1 d,e,f,g,h | 36.6 ± 1.7 g |
| 12 | P36 | Tiquilia Paronychioides (Phil.) Rich. | Flor de arena | BORAGINACEAE | A | Inflammation [ 20] | 18.5 | 2.9 ± 1.1 a | 26.0 ± 0.1 f,g | 95.7 ± 0.8 m,n | 97.1 ± 0.4 m |
| 13 | A24 | Sambucus peruviana H. B. K. | Sauco | CAPRIFOLIAEAE | L | Kidneys [ 20,23], Inflammation [20] | 8.1 | 47.4 ± 4.8 d | 47.2 ± 0.9 h | 53.3 ± 1.5 c,d,e,f | 33.8 ± 6.1 g |
| 14 | A10 | Hypericum laricifolium Juss. | Hierba de la fortuna | CLUSIACEAAE | L | Inflammation [ 13,28], Infections, Musculoskeletal, Bone pain [19] | 15.9 | 97.2 ± 2.0 h | 56.9 ± 5.6 i | 81.9 ± 2.5 j,k,l,m | 58.8 ± 4.6 h,i |
| 15 | P55 | Equisetum giganteum L. | Cola de caballo | EQUISETACEAE | A | Kidneys [ 20,21], Inflammation [20,21] | 11.2 | 77.8 ± 5.9 g | 0.7 ± 0.0 a | 73.9 ± 8.9 h,i,j,k | 51.8 ± 0.3 h |
| 16 | P5 | Phyllanthus niruri L. | Chanca piedra | EUPHORBIACEAE | L | Diabetes [ 24], Kidneys [20], Liver [20], Inflammation [20] | 12.9 | 39.9 ± 1.4 d | 25.3 ± 3.7 f,g | 97.0 ± 0.1 n | 99.6 ± 0.1 m |
| 17 | P80 | Desmodium molliculum (Kunth) A.P. De Candolle | Manayupa | FABACEAE | L | Kidneys [ 20,21], Inflammation [20,21] | 23.8 | 97.9 ± 9.1 h | 10.9 ± 0.5 b,c,d | 89.9 ± 4.2 l,m,n | 73.6 ± 1.3 j,k |
| 18 | A3 | Otholobium mexicanum (L. f.) J.W. Grimes | Culen negro | FABACEAE | A | Diabetes [ 21] | 6.1 | NA | 50.4 ± 0.5 h,i | 4.5 ± 5.7 a,b | 9.6 ± 1.9 a,b,c |
| 19 | A4 | Otholobium pubescens (Poir.) J.W. Grimes | Culen blanco | FABACEAE | A | Diabetes [ 21] | 19.6 | NA | 8.8 ± 0.5 a,b,c,d | 6.1 ± 0.5 a,b,c,d | 27.4 ± 1.5 e,f,g |
| 20 | A49 | Vicia Faba | Haba | FABACEAE | Fr | Renal disorders [ 22] | 7.6 | 0.1 ± 2.7 a | NA | 4.2 ± 2.0 a,b | 13.0 ± 3.0 b,c,d |
| 21 | P7 | Gentianella tristicha (Gilg) J.S. Pringle | Hercampure | GENTIANACEAE | A | Diabetes [ 17], Cholesterol [26] | 30.3 | NA | 68.1 ± 4.2 j | 53.1 ± 0.6 cdef | 66.5 ± 4.5 i,j |
| 22 | A35 | Geranium dielsianum R. Knuth | Pasuchaca | GERANIACEAE | L | Diabetes [ 17,24] | 6.3 | 97.7 ± 1.1 h | 15.6 ± 0.2 d,e | 96.8 ± 0.3 n | 83.9 ± 4.1 k,l |
| 23 | P11 | Clinopodium brevicalyx (Epling) Harley & A. Granda | Inka muña | LAMIACEAE | L | Inflammation [ 26] | 26.8 | 1.8 ± 5.5 a | 31.9 ± 0.7 g | 95.8 ± 0.2 m,n | 94.1 ± 2.7 l,m |
| 24 | P4 | Salvia hispanica L. | Chia | LAMIACEAE | S | Diabetes [ 27], Obesity [27] | 3.5 | NA | NA | 17.3 ± 3.0 b | 21.7 ± 1.8 d,e,f |
| 25 | P40 | Peumus boldus Molina | Boldo | MONIMIACEAE | L | Inflammation [ 20], Kidneys [20], Liver [20] | 32.5 | 75.2 ± 3.0 g | 7.6 ± 0.9 a,b,c,d | 86.2 ± 3.9 k,l,m,n | 95.7 ± 0.5 m |
| 26 | P39 | Eucalyptus globolus Labill. | Eucalipto | MYRTACEAE | L | Burn fat [ 20] | 18.0 | 62.3 ± 0.9 e,f | 11.2 ± 0.8 b,c,d | 77.5 ± 0 i,j,k,l | 99.5 ± 0.3 m |
| 27 | A2 | Argyrochosma nivea (Poir.) Windham | Cuti - Cuti hembra blanca | PTERIDACEAE | A | Diabetes [ 17] | 2.3 | 50.5 ± 3.2 d,e | 59.1 ± 0.3 i,j | 71.2 ± 0.7 g,h,i,j | 34.7 ± 7.3 g |
| 28 | P17 | Cheilanthes pilosa Goldm. | Cuti Cuti | PTERIDACEAE | A | Diabetes [ 26], Liver [26] | 14.3 | 72.4 ± 0.6 f,g | 9.6 ± 0.9 b,c,d | 58.9 ± 4.8 d,e,f,g | 56.4 ± 4.3 h,i |
| 29 | A1 | Cheilanthes pruinata Kaulf. | Cuti - Cuti marron macho | PTERIDACEAE | A | Diabetes [ 17] | 30.3 | 73.4 ± 2.9 f,g | 41.6 ± 1.1 h | 53.2 ± 0.3 c,d,e,f | 31.1 ± 4.1 f,g |
| 30 | A19 | Buddleja Americana L. | Flor blanca | SCROPHULARIA CEAE |
F | Inflammation [ 26] | 8.5 | 16.1 ± 5.6 b,c | 3.7 ± 0.1 a,b,c | 2.4 ± 1.0 a | 19.6 ± 1.2 c,d,e |
1 L, leaf; A, arial part; F, flowers; Fr, fruits; S, seed. 2 Information on medicinal uses and previous publications, [13,17,19,20,21,22,23,24,25,26,27,28]. 3 Yield (%) was calculated as weight of air-dried crushed plant material with respect to the starting material. 4 Inhibition (%) was provided as the mean ± standard deviation. a–n Different letters in the same column indicate significant differences, p < 0.05. 5 α-Glucosidase inhibitory activities. Positive control, acarbose (54.2 ± 3.6% Inhibition). 6 AR inhibitory activities. Positive control, quercetin (88.1 ± 1.6% Inhibition). 7 Antioxidant activity of DPPH radical scavenging. Positive control, l-ascorbic acid (98.9 ± 0.6% Inhibition). 8 Antioxidant activity of ABTS radical scavenging. Positive control, trolox (97.2 ± 2.0% Inhibition). 9 NA is not active. AR: Aldose reductase; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
Previous studies on the effect of methanol (MeOH) extracts of Geranium dielsianum R. Knuth (A35) on DM in the Peruvian Andes have shown a suppression in blood glucose elevation that has been attributed to inhibitory activity on α-glucosidase [16]. Moreover, some Geranium species are sold as antidiabetics in markets in Lima, Peru [17]. Another study reported Eucalyptus globolus L. (P39) was found to exhibit antioxidant activity against DPPH, ABTS, and B-carotene free radicals (75.6%, 81.60%, and 60.40%, respectively, at 50 μg/mL [18]. In Argentina, the branches and stems from Equisetum giganteum L. (P55) exhibited a great antioxidant activity [19]. There are no reports on the AR activity of these samples.
2.2. Effect of Hypericum laricifolium Juss. (HL) on α-Glucosidase and Aldose Reductase (AR) Inhibition and Antioxidant Activity
In our previous study, we demonstrated that HL, which is cultivated in various places in Peru at an altitude ranging between 2000 and 4500 m above sea level, exhibits tyrosinase activity [26]. In Peru, one study reported good effects on antiproliferative activities on human hepatocellular carcinoma Hep3B cells [21]. Previous studies have also reported that HL comprises caffeic acid, esters of long-chain aliphatic alcohols, sterols, triterpenoids, benzoic and cinnamic acid derivatives, flavonols, and flavonol glycosides. Moreover, quercetin and caffeic acid displayed anti-inflammatory activity through the inhibition of cyclooxygenase-1 and cyclooxygenase-2 [13,14]. In other studies, ethanol extracts from the leaves of HL exhibited inhibition against Candida albicans [29].
No information on α-glucosidase and AR in HL have been reported to date, but some publications mentioned that species of same genera (Hypericum) have been used in folk medicine for DM, such as the popular specie called Hypericum perforatum (St. John’s wort) [30,31]. Hypericum perforatum L., the most well-known of these species, whose main components were determined as flavonoid derivates, quercetin, kaempferol, isoquercetin, and rutin [29,30,32].
Following the results discussed in Section 2.1 and Section 2.2, we decided to further investigate the effect of HL on α-glucosidase, AR, and antioxidant activity by comparing the differences in α-glucosidase, AR, and antioxidant activity in nonpolar (methylene chloride (MC)) and polar (70% MeOH) solvent extracts at concentrations of 500 μg/mL for the α-glucosidase assay, 10 μg/mL for the AR assay, and 143 μg/mL and 33 μg/mL for the DPPH and ABTS assays, respectively. As illustrated in Table 2, the MC extract displayed low inhibition for all assays and thus, the IC50 values could not be determined. The 70% MeOH extract demonstrated good inhibition of α-glucosidase activity, with an inhibition rate of 92.36% at 500 μg/mL (IC50 = 56.6 μg/mL). These values were significantly (p < 0.05) higher than those observed for acarbose (55.82%; IC50 = 367.4 μg/mL). In the AR inhibition assay, the MeOH extract inhibited AR by 64.51% at 10 μg/mL (IC50 = 3.3 μg/mL), a value significantly (p < 0.05) lower than that observed for quercetin (83.7%; IC50 = 1.3 μg/mL). For the antioxidant capacities, HL inhibited DPPH radical by 93% at a concentration of 143 μg/mL (IC50 = 42.5 μg/mL) and ABTS radicals by 78.9% at 33 μg/mL (IC50 = 14.4 μg/mL). L-Ascorbic acid and trolox displayed IC50 values of 17.6 and 4.6 μg/mL in the DPPH and ABTS assays, respectively; these values indicated higher activity than those observed for the HL MeOH extract. However, even with these results, HL can still be considered as having a significant inhibitory effect on DPPH and ABTS free radicals. Following these results, a 70% MeOH extract was selected to determine the constituents responsible for antidiabetic activity. A study by Levent et al. [30] investigating MeOH and ethyl acetate extracts from Hypericum perforatum L. revealed that at a concentration of 200 μg/mL, the MeOH extract exhibited higher DPPH antioxidant activity than the ethyl acetate extract. This demonstrated that the MeOH extract was more effective in reducing DPPH radicals.
Table 2.
Inhibitory activities and IC50 values (µg/mL) of Hypericum laricifolium Juss. (HL) non-polar and polar extracts against α-glucosidase, aldose reductase (AR), and antioxidants.
| Extracts | α-Glucosidase 1 | AR 2 | DPPH 3 | ABTS 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (μg/mL) 5 | Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | |
| MC | 11.65 ± 2.1 * | - | 28.9 ± 7.4 * | - | 17.5 ± 0.3 * | - | 14.4 ± 1.3 * | - |
| 70% MeOH | 92.36 ± 1.1 * | 56.6 ± 2.5 | 64.51 ± 1.3 * | 3.3 ± 52 | 93.0 ± 0.1 * | 42.5 ± 0.6 | 78.9 ± 0.6 * | 14.4 ± 1.3 |
| Acarbose | 55.82 ± 2.3 | 367.4 ± 2.1 | - | - | - | - | - | - |
| Quercetin | - | - | 83.7 ± 2.6 | 1.3 ± 3.6 | - | - | - | - |
| L-Ascorbic | - | - | - | - | 99.1 ± 0 | 17.6 ± 0.1 | - | - |
| Trolox | - | - | - | - | - | - | 100 ± 0.1 | 4.6 ± 0.1 |
1 The inhibition rates (%) were calculated at a concentration of 500 μg/mL. 2 The inhibition rates (%) were calculated at a concentration of 10 μg/mL. 3 The inhibition rates (%) were calculated at a concentration of 143 μg/mL except for that of l-ascorbic acid that was calculated at a concentration of 71 μg/mL. 4 The inhibition rates (%) were calculated at a concentration of 33 μg/mL except for that of trolox that was calculated at a concentration of 17 μg/mL. 5 IC50 is defined as the half-maximal inhibitory concentration and data is presented as means ± SD (n = 3). * The asterisk indicates a significant difference compared to positive control group (p < 0.05). MC: Methylene chloride; MeOH: Methanol.
2.3. Identification of the Major Bioactive Components in Hypericum laricifolium Juss. (HL) by Offline High-Performance Liquid Chromatography (HPLC)
The concept of offline HPLC-based activity profiling has been proposed and implemented for the effective screening of bioactive compounds in natural product extracts [33]. Recently, many offline HPLC-based assays have been introduced and developed to provide new and rapid analytical methods and many studies have reported the successful application of ultrafiltration-HPLC, DPPH-HPLC, and ABTS-HPLC to identify bioactive components from complex extracts.
2.3.1. Identification of the Bioactive Compounds in HL by α-Glucosidase Ultrafiltration Combined with HPLC
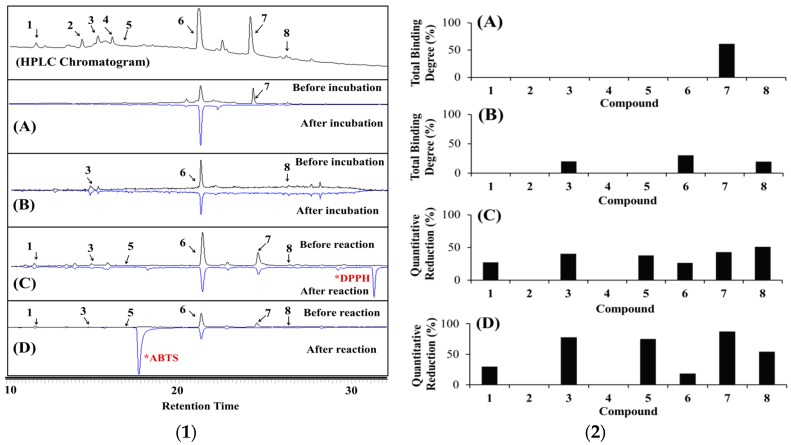
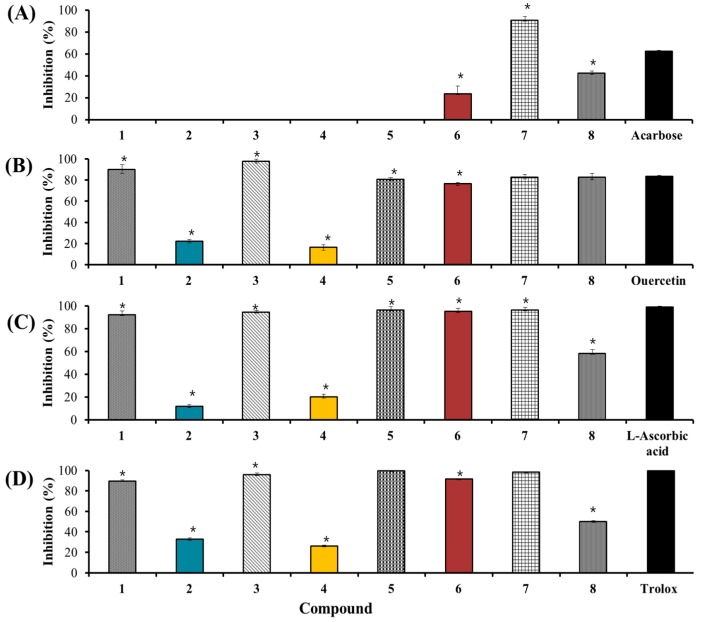
An ultrafiltration-HPLC assay was performed in this study to identify the potential α-glucosidase inhibitors in HL. The ultrafiltration-HPLC assay is an affinity-based screening method for bioactive compounds in a complex matrix whereby the ligands bind to the enzyme and are separated from unbound small molecules by the ultrafiltration membrane [34]. Thus, examination of the HPLC pattern of the decreased peaks indicates which ligands would be suitable as inhibitors. This pattern represents the ligands that bind to the enzyme, with a higher binding degree indicating compounds with higher binding affinities. Figure 1(1A,2A) illustrates that compound 7 exhibited a decrease in the peak area with a total binding degree (TBD) of 61.57% when the α-glucosidase ultrafiltration-HPLC system was applied, indicating that this compound is a strong α-glucosidase inhibitor. This is confirmed by its percentage inhibition (91%) and IC50 (15.9 μM) values (Figure 2 and Table 3, respectively). Compounds 1–6 and 8 were inactive.
Figure 1.
(1) HPLC chromatogram of crude Hypericum laricifolium Juss. (HL) 70% MeOH extract at 210 nm and offline HPLC of (A) HL based on offline α-glucosidase UF-HPLC. (B) HL based on offline HRAR UF-HPLC. Antioxidants determined by (C) offline DPPH-HPLC and (D) offline ABTS-HPLC. (2) Total Binding Degree (%) and Quantitative Reduction (%) of (A) offline α-glucosidase UF-HPLC, (B) offline HRAR UF-HPLC, (C) offline DPPH-HPLC and (D) offline ABTS-HPLC. * DPPH peak; * ABTS peak. HPLC: High-Performance Liquid Chromatography; UF: Ultrafiltration; HRAR: Human recombinant aldose reductase; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); MeOH: Methanol.
Figure 2.
Inhibitory effects of the compounds isolated from Hypericum laricifolium Juss.: (A) α-glucosidase inhibitory activity; (B) aldose reductase (AR) inhibitory activity; (C) 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity; (D) 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity. Acarbose was used as positive control for the α-glucosidase assay, quercetin as positive control for the aldose reductase assay, l-ascorbic acid as positive control for the DPPH assay, and trolox as positive control for the ABTS assay. * The asterisk indicates a significant difference compared to positive control group (p < 0.05).
Table 3.
Inhibition and IC50 values (µM) for tyrosinase, α-glucosidase, and aldose reductase (AR) and antioxidant activities of isolated compounds from Hypericum laricifolium Juss.
| Compounds | α-glucosidase 1 | AR 2 | Antioxidants | |||||
|---|---|---|---|---|---|---|---|---|
| DPPH 3 | ABTS 4 | |||||||
| Conc. (μg/mL) | IC50 (μM) 5 | Conc. (μg/mL) | IC50 (μM) | Conc. (μg/mL) | IC50 (μM) | Conc. (μg/mL) | IC50 (μM) | |
| Protocatechuic acid (1) | 50 | NA 6 | 10 | 16.9 ± 1.9 e | 143 | 263.4 ± 0.2 e | 17 | 9.7 ± 0.9 c |
| p-Hydroxybenzoic acid (2) | 50 | NA | 10 | NA | 143 | NA | 33 | NA |
| Chlorogenic acid (3) | 50 | NA | 10 | 0.23 ± 1.8 a | 143 | 123.1 ± 2.9 d | 33 | 23.1 ± 3.5 f |
| Vanilic acid (4) | 50 | NA | 10 | NA | 143 | NA | 33 | NA |
| Caffeic acid (5) | 50 | NA | 10 | 28.3 ± 0.9 f | 143 | 47.2 ± 0.1 b | 17 | 7.2 ± 1.1 b |
| Kaempferol 3-O-glucuronide (6) | 50 | NA | 10 | 6.0 ± 1.1 c | 143 | 58.8 ± 2.1 c | 17 | 11.0 ± 0.2 d |
| Quercetin (7) | 50 | 15.9 ± 1.2 a | 10 | 2.5 ± 0.8 b | 71 | 0.33 ± 1.4 a | 17 | 0.33 ± 0.6 a |
| Kaempferol (8) | 50 | NA | 10 | 9.7 ± 0.5 d | 143 | 326.4 ± 3.3 f | 33 | 191.8 ± 1.1 g |
| Acarbose | 500 | 439.9 ± 8.9 b | - | - | - | - | - | - |
| Quercetin | - | - | 10 | 2.4 ± 1.2 b | - | - | - | - |
| l-Ascorbic | - | - | - | - | 71 | 0.57 ± 0.4 a | - | - |
| Trolox | - | - | - | - | - | - | 17 | 18.8 ± 0.5 e |
1 The inhibition rates (%) were calculated at a concentration of 50 μg/mL except that for that of acarbose that was calculated at a concentration of 500 μg/mL. 2 The inhibition rates (%) were calculated at a concentration of 10 μg/mL. 3 The inhibition rates (%) were calculated at a concentration of 143 μg/mL except for that of l-ascorbic acid that was calculated at a concentration of 71 μg/mL. 4 The inhibition rates (%) were calculated under the concentration of 17 μg/mL. 5 IC50 value is defined as the half-maximal inhibitory concentration and data is presented as means ± SD (n = 3). a–g Different letters in the same column indicate significant differences, p < 0.05. 6 NA is not active.
2.3.2. Identification of the Bioactive Compounds in HL by Aldose Reductase (AR) and Human Recombinant AR (HRAR) Ultrafiltration Combined with HPLC
For the HRAR ultrafiltration-HPLC assay (Figure 1(1B,2B)), three compounds exhibited decreases in peak areas: compound 3 displayed a decrease in peak area with a TBD of ~20%; compound 6 a decrease with a TBD of 30%, and compound 8 a decrease with a TBD < 20%. These values indicate that compounds 3 and 6 were the most potent AR inhibitors. Subsequently, an AR inhibitory assay was performed to confirm their activity. The IC50 values of compounds 1, 3, 5, 6, 7, and 8 toward AR were 16.9 μM, 0.23 μM, 28.3 μM, 6.0 μM, 2.5 μM, and 9.7 μM, respectively, while compounds 2 and 4 were inactive (Figure 2 and Table 3). These values suggest that both compound 3 (0.23 μM) and compound 7 (2.5 μM) are strong AR inhibitors. The disappearance of some peaks after incubation was attributed to the low concentration of some components. However, in the case of quercetin, this was attributed to its solubility, binding affinities, and binding sites [34].
2.3.3. Identification of Antioxidants Using Offline 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)-(HPLC) and 2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS)-(HPLC)
A DPPH-HPLC/ABTS-HPLC approach was employed to identify the potential antioxidants in HL. In this simple and rapid approach, the extract was spiked with DPPH/ABTS. The peak areas of the antioxidants decreased or disappeared from the HPLC chromatogram after spiking with DPPH/ABTS, while almost no changes in peak areas were observed for components that did not exhibit any antioxidant activities. Thus, the more significant the reduction in peak area, the higher the antioxidant activity of the component. In the present study, the peaks from the DPPH-HPLC analysis of compounds 1, 3, 5, 6, 7, and 8 decreased (Figure 1(1C,2C)) after the reaction, indicating that these compounds are antioxidants. The peak area reduction was quantitatively analyzed with % quantitative reduction (QR) values ranging between 20% and 50%. Moreover, compounds 7 and 8 displayed greater quantitative reductions (42.8% and 51% respectively), suggesting that these two compounds are more potent antioxidants than compounds 1, 3, 5, and 6. The IC50 values of compounds 1, 3, 5, 6, 7, and 8 in the DPPH assay were determined as 263.4 μM, 123.1 μM, 47.2 μM, 58.8 μM, 0.3 μM, and 326.4 μM, respectively, whereas compounds 2 and 4 were inactive (Figure 1 and Table 3).
For the identification of antioxidants using ABTS-HPLC (Figure 1(1D,2D)), the ABTS-HPLC peaks of compounds 1, 3, 5, 6, 7, and 8 decreased after the reaction, confirming the inhibition capacity of these compounds. Compound 7 presented a quantitative reduction percentage > 80%. This was followed by compounds 3 (77%), 5 (76%), and 8 (54%). The IC50 values of compounds 1, 3, 5, 6, 7, and 8 in the ABTS assay were determined as 9.7 μM, 23.1 μM, 7.2 μM, 11.0 μM, 0.3 μM, and 191.8 μM, respectively; compound 7 exhibited the strongest activity while compounds 2 and 4 were inactive. From these observed results, we concluded that the % QR values in offline ABTS-HPLC were stronger than the corresponding values in offline DPPH-HPLC. This was attributed to the faster reaction kinetics and greater response to antioxidants of the ABTS assay that increased its sensitivity towards antioxidant activity [35]. Kaempferol and quercetin displayed higher quantitative reduction percentages in both offline DPPH-HPLC and ABTS-HPLC analyses.
Some studies have reported that members of the genus Hypericum, especially H. perforatum L., exhibit antioxidant effects as a result of their flavonoid and phenolic acid compositions [36]. Moreover, it has been reported that chlorogenic acid is the most active compound that contributes to the DPPH radical scavenging activity of H. perforatum L. [37]. In our study, chlorogenic acid also displayed antioxidant activity for DPPH and ABTS radicals, but was not the strongest antioxidant, probably due to its low concentration. On the other hand, it associated the antioxidant activity and α-glucosidase inhibitory properties of two species of Hypericum, namely H. humifusum and H. perfoliatum, with hypericin and hyperforin [38].
3. Materials and Methods
3.1. Materials
Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate (NADPH), 1Mmp-nitrophenyl-α-d-glucopyranoside, DL-glyceraldehyde dimer, DPPH, trolox, ABTS diammonium salt, aminoguanidine, acarbose, quercetin, and l-ascorbic acid were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Human recombinant AR (HRAR) was obtained from Wako Pure Chemical Industries Co. (Osaka, Japan). Sephadex LH-20 was obtaimed from GE Healthcare Co. (Uppsala, Sweden). All organic solvents were obtained from J.T. Baker Co. (Phillipsburg, NJ, USA). All other reagents were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ultrapure water, used for dilutions and HPLC analysis, was obtained using a Milli-Q laboratory water purification system with a resistivity over 18.2 MΩ cm (Millipore Co., Bedford, MA, USA).
3.2. Collection of Plant Material
The plant materials used in this study, 30 dried Peruvian plants, were purchased from Mercado Aviacion in Lima, Peru (October 2015). The samples were identified using available literature in English and Spanish and verified by Paul H. Gonzales Arce from the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru. The voucher specimens were deposited at the Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, Korea. Plant names were verified on www.theplantlist.org in July 2015. Information on species name, family, plant organ and traditional uses is given in Table 1.
3.3. Preparation of Extracts and Isolation of Plant Samples
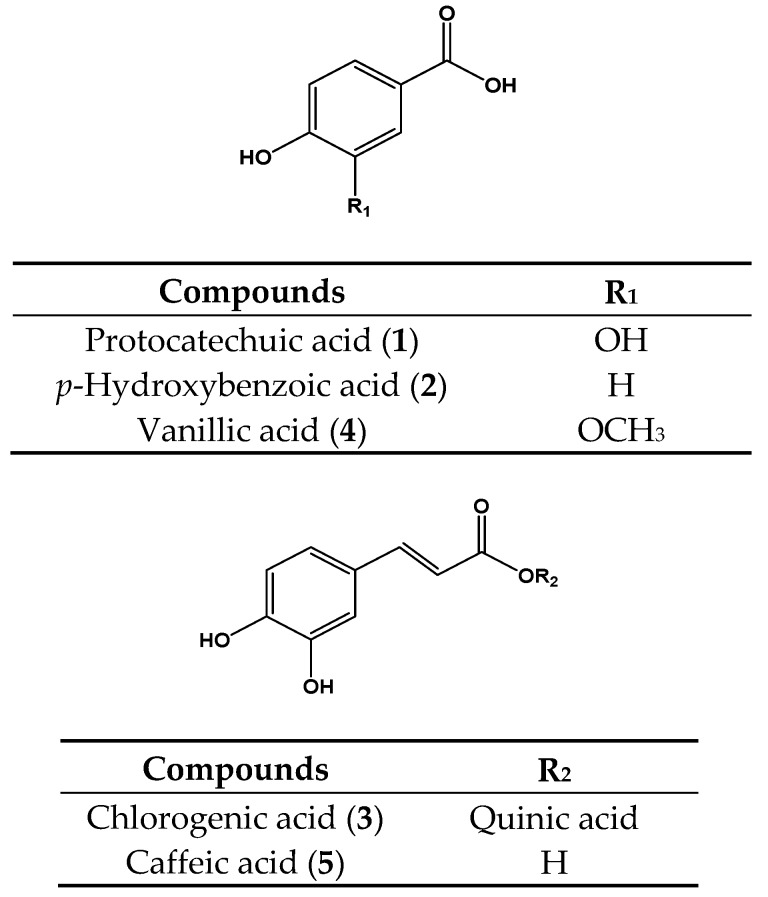
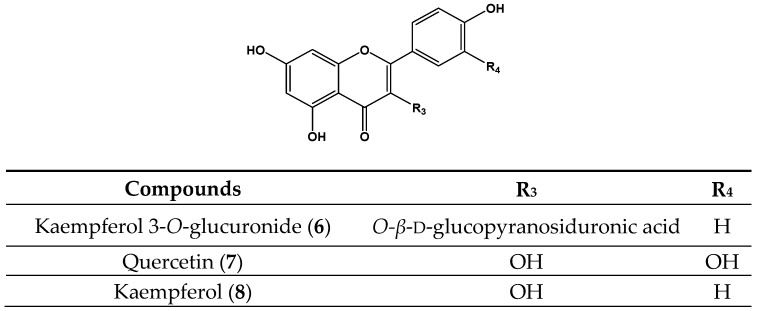
The 30 dried Peruvian plants (50 g) were pulverized and extracted by maceration at room temperature with 70% MeOH for 72 h. The yield for each extract, after solvent removal, was determined and expressed as a percentage of the dry weight of the plant parts used such as leaves, root, seed, fruit and aerial part. The major compounds of the HL 70% MeOH extract were isolated by column chromatography Sephadex LH-20 column [26]. In total, eight major compounds were identified: protocatechuic acid (1), p-hydroxybenzoic acid (2), chlorogenic acid (3), vanillic acid (4), caffeic acid (5), kaempferol 3-O-glucuronide (6), quercetin (7), and kaempferol (8) (Figure 3).
Figure 3.
Chemical structures of the constituents isolated from Hypericum laricifolium Juss.
3.4. Evaluation of the α-Glucosidase Inhibitory Assay
α-Glucosidase inhibitory activity was determined using a previously reported method with slight modifications [39]. The initial concentration of the enzyme solution was 0.015 mg/mL in 0.1 M phosphate buffer (pH 6.9) and the initial concentration of the substrate solution was 1 mM in the same phosphate buffer. The enzyme solution was mixed in a clear 96-well microplate (flat bottom) and the reaction was initiated by the addition of the substrate to the solution. The plates were incubated at 37 °C for 10 min. Enzyme inhibition was determined from the absorbance of 4-nitrophenol (product) at 405 nm, measured with a microplate reader (EL800 Universal Microplate reader, BioTek instruments, Winooski, VT, USA). Acarbose was used as positive control. The percentage inhibition was calculated as follows: % inhibition α-glucosidase = [1 − (As − Ab/Ac − Ab)] × 100%, where Ac is the absorbance of the solution, buffer, enzyme, substrate (190 µL), and dimethyl sulfoxide (DMSO) (10 µL); Ab is the absorbance of the buffer solution (190 µL) with DMSO (10 µL); and As is the absorbance of the solution, buffer, enzyme, and substrate (190 µL) with the sample solution (10 µL). The results were also expressed as IC50 values (the concentration of sample required for 50% inhibition of enzyme activity).
3.5. Animal Care
All animal experiment procedures were conducted in accordance with the guidelines and approval of the Institutional Animal Care and Use Committees (IACUC) of Hallym University (Code: Hallym-2016-95; Date: 13 May 2016). The rat lenses (RL) were prepared as follows: the RL were removed from the eyes from 10-week-old Sprague-Dawley rats (weight = 250–280 g) and frozen until required. The rats were housed in a standardized laboratory environment with a 12 h light/12 h dark cycle at constant room temperature (22 ± 1 °C) and humidity (6 ± 5%) before experimentation. All animals had free access to water and food.
3.6. Evaluation of the Rat Lens Aldose Reductase (RLAR) Assay
The AR inhibitory activities were evaluated using RLAR. Prior to analysis, the lenses were homogenized in 0.1 M phosphate buffer saline (pH 6.2) and subsequently centrifuged at 10,000× g for 30 min at 4 °C. The rat lens aldose reductase (RLAR)-containing supernatant was then collected. A substrate comprising 531 μL 0.1 M potassium buffer (pH 7.0), 90 μL NADPH solution (1.6 mM in potassium buffer), 90 μL RLAR homogenate (6.5 U/mg), 90 μL ammonium sulfate solution (4 M in potassium buffer), and 90 μL DL-glyceraldehyde (25 mM in potassium buffer) was placed into a 1.0-mL cuvette and mixed with 9 μL sample (1 mg/mL dissolved in DMSO). RLAR activity was assessed spectrophotometrically by measuring the decrease in NADPH absorption at 340 nm over a 4 min period. Quercetin was used as positive control. The percentage inhibition was calculated as follows: % inhibition RLAR = [1 − (ΔAs − ΔAb/ΔAc − ΔAb)] × 100%, where ΔAs is the reduction of absorbance of the reaction mixture ΔAc is the same, but without the sample; and ΔAb is the reduction of absorbance of the reaction mixture without the RLAR homogenate. The results were also expressed as IC50 values (the concentration of sample required for 50% inhibition of enzyme activity).
3.7. Evaluation of the DPPH Assay
The DPPH free radical scavenging activity was determined as previously described with slight modifications [34]. Briefly, 180 µL of DPPH solution (0.32 mM in methanol) was mixed with 30 µL of each sample at different concentrations (1.0–5.0 mg/mL in methanol). After 15 min incubation in the darkroom, the absorbance change at 570 nm was measured on a microplate reader (EL800 Universal Microplate reader, BioTek Instruments, Winooski, VT, USA). L-Ascorbic acid was used as positive control. The DPPH radical scavenging activity was calculated as % inhibition using the formula: % DPPH inhibition = [1 − (As − Ab/Ac − Ab)] × 100%, where Ac is the absorbance of the DPPH solution (180 µL) with methanol (30 µL); Ab is the absorbance of distilled water (180 µL) with methanol (30 µL); and As is the absorbance of DPPH solution (180 µL) with sample solution (30 µL). The results were also expressed as IC50 values (the concentration of sample required for inhibition of 50% DPPH radicals).
3.8. Evaluation of the ABTS Assay
The ABTS free radical scavenging activity was determined using ABTS radical cations (ABTS+) with slight modifications [34]. Briefly, potassium persulfate (3.5 mM) and ABTS diammonium salt (2.0 mM) were mixed, diluted in distilled water, and subsequently allowed to stand in the dark at room temperature for 24 h before use, to allow free radical generation. To determine the scavenging activity, the absorbance was recorded at 750 nm using an EL-800 Universal microplate reader (BioTek Instrument Inc., Winooski, VT, USA) after incubating the sample solutions (10 µL, 1 mg/mL, 0.5 mg/mL in methanol) with ABTS solution (290 µL) for 10 min in a 96-well plate. Trolox was used as a positive control. The ABTS radical scavenging activity was expressed as % inhibition using the following formula: % ABTS inhibition = [1 − (As − Ab/Ac − Ab)] × 100%, where Ac is the absorbance of the ABTS solution (290 µL) with methanol (10 µL); Ab is the absorbance of distilled water (290 µL) with methanol (10 µL); and As is the absorbance of the ABTS solution (290 µL) with the sample solution (10 µL). The results were also expressed as IC50 values (the concentration of sample required for the inhibition of 50% ABTS radicals).
3.9. Determination of the α-Glucosidase Ultrafiltration-HPLC Assay
The α-glucosidase present in the HL was profiled using a newly developed α-glucosidase ultrafiltration assay. Specifically, the HL 30 μL sample (final concentration = 0.1 mg/mL) was incubated with α-glucosidase (3.9 μM) in PBS (300 μL, pH 7.0) at 37 °C for 30 min. The incubated mixture was then filtered by centrifugation at 5167× g for 30 min at room temperature using a Microcon YM-10 centrifugal filter unit (Millipore, Bedford, MA, USA). The filtrate was subsequently analyzed by HPLC on a Dionex system (Dionex, Sunnyvale, CA, USA) consisting of a P850 pump, an ASI-100 automated sample injector, a Synergi Hydro-RP 80A column (150 mm × 4.6 mm, 4 μm; Phenomenex, Torrance, CA, USA) maintained at 30 °C, and a UVD170S detector. The injection volume was 10 μL and the flow rate of the mobile phase was 0.7 mL/min. The mobile phase compositions were as follows: 0.1% trifluoroacetic acid (A-line) and methanol (B-line). The gradient elution system was modified as follows: 0–35 min, starting with 5% B-line, programmed to reach 40% B-line at 15 min using a linear gradient, followed by 100% B-line from 15–35 min. The detector monitored the eluent at a wavelength of 254 nm. The sample incubated without α-glucosidase was used as control. The relative binding affinity of the inhibitors in the extract sample toward α-glucosidase was defined as the “total binding degree (TBD)”, calculated from the equation: TBD (%) = (A − B)/A × 100%, where A and B are the HPLC peak areas of a compound interacting without and with α-glucosidase, respectively.
3.10. Determination of the HRAR Ultrafiltration-HPLC Assay
The AR Inhibitors (ARIs) present in HL were profiled using a newly developed AR ultrafiltration assay. Specifically, the HL sample (final concentration = 0.1 mg/mL) was incubated with 0.6 M ammonium sulfate and 3.9 μM HRAR (Human recombinant AR) in a total volume of 300 μL at 37 °C for 30 min. The incubated mixture was then filtered by centrifugation at 5167× g for 30 min at room temperature using a Microcon YM-10 centrifugal filter unit. The filtrate was subsequently analyzed by HPLC (Section 3.9). The sample incubated without HRAR was used as control.
3.11. Evaluation of Offline DPPH-HPLC Analysis
The DPPH radical assay was modified by an offline DPPH-HPLC assay [40]. Briefly, 180 μL DPPH solution (0.32 mM in MeOH) was prepared and mixed with 30 μL 70% MeOH extract of HL (5 mg/mL in MeOH). After incubation (15 min) in the dark room, the mixture was filtered through a 0.45-μm membrane and subsequently analyzed by HPLC (Section 3.9). The sample incubated with methanol was used as control. The decreases in peak area were expressed as the % quantitative reduction (% QR), calculated from the equation: % QR = (A − B)/A × 100%, where A and B are the HPLC peak areas of the compound incubated without and with the DPPH methanol solution, respectively.
3.12. Evaluation of Offline ABTS-HPLC Analysis
Offline ABTS-HPLC was investigated by modifying a previously reported protocol [41]. Briefly, 10 μL of 70% methanol extract from HL (20 mg/mL) was mixed with 140 μL ABTS solution that was prepared one day in advance. The mixture was then incubated at room temperature for 10 min and subsequently filtered through a 0.45 μm filter. The filtrate was analyzed by HPLC (Section 3.9). The methanol extract of HL (20 mg/mL in MeOH was used as control. The detector monitored the eluent at a wavelength of 300 nm and the % QR was calculated as described in Section 3.11.
3.13. Statistical Analysis
The mean values were calculated based on at least three independent replicates (n = 3) and the results are expressed as mean ± standard deviations (SD). All data were statistically analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test and Dunnet’s test using the SPSS software package, IBM SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
4. Conclusions
In this study, α-glucosidase, AR, and antioxidant test screening of 30 Peruvian herbal extracts illustrated that HL exhibited the greatest activity in all assays. Consequently, compounds such as quercetin, present in HL were identified by α-glucosidase ultrafiltration-HPLC, while chlorogenic acid, kaempferol 3-O-glucuronide, and kaempferol were identified by AR ultrafiltration-HPLC. Protocatechuic acid, chlorogenic acid, caffeic acid, kaempferol 3-O-glucuronide, quercetin, and kaempferol were identified as antioxidants by off-line DPPH and ABTS-HPLC. The results suggest that HL shows great potential for the complementary treatment of diabetes and its complications.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01059199) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116020-3).
Abbreviations
| AR | Aldose reductase |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DMSO | Dimethyl sulfoxide |
| HL | Hypericum laricifolium Juss. |
| MeOH | Methanol |
| RLAR | Rat lens aldose reductase |
| HRAR | Human recombinant aldose reductase |
| HPLC | High-performance liquid chromatography |
| TBD | Total binding degree |
| QR | Quantitative reduction |
Author Contributions
Yanymee N. Guillen Quispe conducted the experiments on the extracts and compounds, analyzed data, and wrote the manuscript. Seung Hwan Hwang contributed towards the isolation of compounds and the elucidation of their structures. Zhiqiang Wang and Soon Sung Lim revised the manuscript. Guanglei Zuo carried the experiment of Aldose reductase enzyme on compounds. All the authors read and approved the final manuscript and all their names are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization . Diabetes Fact Sheet No. 312. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:62–69. [Google Scholar]
- 3.Yokozawa T., Kim H.Y., Cho E.J. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2002;50:5485–5489. doi: 10.1021/jf020168z. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo X., Liang J., Zhang Y., Zhao H., Guo Y., Shi S. Separation and purification of α-glucosidase inhibitors from Polygonatum odoratum by stepwise high-speed counter-current chromatography combined with Sephadex LH-20 chromatography target-guided by ultrafiltration-HPLC screening. J. Chromatogr. B. 2015;985:149–154. doi: 10.1016/j.jchromb.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Niwa T., Doi U., Osawa T. Inhibitory Activity of Corn-Derived Bisamide Compounds Against α-Glucosidase. J. Agric. Food Chem. 2003;51:90–94. doi: 10.1021/jf020758x. [DOI] [PubMed] [Google Scholar]
- 6.Kato A., Minoshima Y., Yamamato J., Adachi I., Watson A.A., Nash R.J. Protective effects of dietary chamomile tea on diabetic complications. J. Agric. Food Chem. 2008;56:8206–8211. doi: 10.1021/jf8014365. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.K., Lee Y.S., Kim S.H., Bae Y.S., Lim S.S. Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol. Pharm. Bull. 2011;34:160–163. doi: 10.1248/bpb.34.160. [DOI] [PubMed] [Google Scholar]
- 8.Murugan P., Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79:1720–1728. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Chang Y.X., Ge A., Sineeporn D., Li J., Bai Y., Liu J., He J., Yang X., Song L.J., Zhang B.L., et al. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 2015;164:210–222. doi: 10.1016/j.jep.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Hajdu Z. Ph.D. Thesis. University of Szeged; Szeged, Hungary: Nov, 2014. An Ethnopharmacological Survey Conducted in the Bolivian Amazon, and Identification of N-Alkylamides and Lignans from Lepidium meyenii and Heliopsis helianthoides var. Scabra with Effects on the Central Nervous System. [Google Scholar]
- 11.Acosta Solis M.A. Plantas Medicinales del Ecuador. Abya Yala; Quito, Ecuador: 1992. p. 127. [Google Scholar]
- 12.Ramírez-Gonzáilez I., Amaro-Luis J.M., Bahsas A. Xanthones from aerial parts of Hypericum laricifolium Juss. Nat. Prod. Commun. 2013;8:1731–1732. [PubMed] [Google Scholar]
- 13.El-Seedi H.R., Ringbom T., Torssell K., Bohlin L. Constituents of Hypericum laricifolium and their cyclooxygenase (COX) enzyme activities. Chem. Pharm. Bull. 2003;51:1439–1440. doi: 10.1248/cpb.51.1439. [DOI] [PubMed] [Google Scholar]
- 14.Ccana-Ccapatinta G.V., Von Poser G.L. Acylphoroglucinol derivates from Hypericum laricifolium Juss. Phytochem. Rev. 2015;12:63–66. [Google Scholar]
- 15.Robards K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A. 2003;1000:657–691. doi: 10.1016/S0021-9673(03)00058-X. [DOI] [PubMed] [Google Scholar]
- 16.Karato M., Yamaguchi K., Takei S., Kino T., Yazawa K. Inhibitory effects of pasuchaca (Geranium dielsianum) extract on α-glucosidase in mouse. Biosci. Biotechnol. Biochem. 2006;70:1482–1484. doi: 10.1271/bbb.50420. [DOI] [PubMed] [Google Scholar]
- 17.Bussmann R.W., Paniagua-Zambrana N., Rivas Chamorro M., Molina Moreira N., Cuadros Negri M.R., Olivera J. Perfil in the market-classification and dosage of species used as anti-diabetics in Lima, Peru. J. Ethnobiol. Ethnomed. 2013;9:1–37. doi: 10.1186/1746-4269-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Moein N.M., Mahmoud E.A., Shalaby E.A. Antioxidant Mechanism of Active Ingredients Separated from Eucalyptus globulus. Org. Chem. Curr. Res. 2012;1:2. doi: 10.4172/2161-0401.1000106. [DOI] [Google Scholar]
- 19.Ricco R.A., Agudelo I., Garces M., Evelson P., Wagner M.L., Gurni A.A. Polifenoles y actividad antioxidante en Equisetum giganteum L. (Equisetaceae) Bol. Latinoam. Caribe Plantas Med. Aromat. 2011;10:325–332. [Google Scholar]
- 20.Bussmann R.W., Sharon D. Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006;2:1–47. doi: 10.1186/1746-4269-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carraz M., Lavergne C., Jullian V., Wright M., Gairin J.E., Gonzales de la Cruz M., Bourdy G. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015;166:185–199. doi: 10.1016/j.jep.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 22.Chirinos R., Pedreschi R., Rogez H., Larondelle Y., Campos D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crop. Prod. 2013;47:145–152. doi: 10.1016/j.indcrop.2013.02.025. [DOI] [Google Scholar]
- 23.Neto C.C., Owens C.W., Langfield R.D., Comeau A.B., St. Onge J., Vaisberg A.J., Hammond G.B. Antibacterial activity of some Peruvian medicinal plants from the Callejon de Huaylas. J. Ethnopharmacol. 2002;79:133–138. doi: 10.1016/S0378-8741(01)00398-1. [DOI] [PubMed] [Google Scholar]
- 24.Berlowski A., Zawada K., Wawer I., Paradowska K. Antioxidant Properties of Medicinal Plants from Peru. Food Nutr. Sci. 2013;4:71–74. doi: 10.4236/fns.2013.48A009. [DOI] [Google Scholar]
- 25.De la Cruz H., Vilcapoma G., Zevallos P.A. Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. J. Ethnopharmacol. 2007;111:284–294. doi: 10.1016/j.jep.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Guillen Quispe Y.N., Hwang S.H., Wang Z., Lim S.S. Screening of Peruvian plants for Tyrosinase Inhibitory Properties: Identification of Tyrosinase Inhibitors in Hypericum laricifolium Juss. Molecules. 2017;22:402. doi: 10.3390/molecules22030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuksan V., Jenkins A.L., Brissette C., Choleva L., Jovanovski E., Gibbs A.L., Bazinet R.P., Au-Yeung F., Zurbau A., Ho H.V., et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017;27:138–146. doi: 10.1016/j.numecd.2016.11.124. [DOI] [PubMed] [Google Scholar]
- 28.Llanga Guaman B.G. Ph.D. Thesis. Tecnologias y Ciencias de la Ingenieria; Riobamba, Ecuador: Jul 8, 2014. Determinacion de la Actividad Antioxidante de los Extractos de Quishuar (Buddleja incana), Aliso (Alnus acuminate) y Romerillo (Hypericum laricifolium) Localizadas en 3 Zonas Geograficas Diferentes; pp. 20–21. (In Spanish) [Google Scholar]
- 29.Crockett S., Eberhardt M., Kunert O., Schuhly W. Hypericum species in the Paramos of Central and South America: A special focus upon H. irazuense Kuntze ex N. Robson. Phytochem. Rev. 2010;9:255–269. doi: 10.1007/s11101-009-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levent Altun M., Seever Yilmaz B., Erdogan L., Saltan Citoglu G. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L. (St John’s wort) Ind. Crops Prod. 2013;43:87–92. doi: 10.1016/j.indcrop.2012.07.017. [DOI] [Google Scholar]
- 31.Galeotti N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017:136–146. doi: 10.1016/j.jep.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Roja J., Buitrago A., Rojas L.B., Morales A. Chemical composition of Hypericum laricifolium Juss. Essential oil Collected from Merida—Venezuela. Med. Aromat. Plants. 2013;2:1–3. [Google Scholar]
- 33.Cies´la L., Moaddel R. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat. Prod. Rep. 2016;33:1131–1145. doi: 10.1039/C6NP00016A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z., Hwang S.H., Guillen Quispe Y.N., Gonzales Arce P.H., Lim S.S. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. 2017;231:222–230. doi: 10.1016/j.foodchem.2017.03.107. [DOI] [PubMed] [Google Scholar]
- 35.Lee K.J., Oh Y.C., Cho W.K., Ma J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC assay. Based Complement. Altern. Med. 2015:165457. doi: 10.1155/2015/165457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orcic D.Z., Mimica-Dukić N.M., Francišković M.M., Petrović S.S., Jovin E.D. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011;5:34–42. doi: 10.1186/1752-153X-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gioti E.M., Fiamegos Y.C., Skalcos D.C., Stalikas C.D. Antioxidant activity and bioactive components of the arterial parts of Hypericum perforatum L. from Epirus, Greece. Food Chem. 2009;117:398–404. doi: 10.1016/j.foodchem.2009.04.016. [DOI] [Google Scholar]
- 38.Béjaoui A., Salem I.B., Rokbeni N., M’rabet Y., Boussaid M., Boulila A. Bioactive compounds from Hypericum humifusum and Hypericum perfoliatum: Inhibition potential of polyphenols with acetylcholinesterase and key enzymes linked to type-2 diabetes. Pharm. Biol. 2017;55:906–911. doi: 10.1080/13880209.2016.1270973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S.J., Kim J.K., Jang J.M., Shin K.H., Lim S.S. Rapid identification of the α-glucosidase inhibitory compounds from Thunberg’s Geranium (Geranium thunbergii Sieb. et Zucc.) Food Sci. Biotechnol. 2012;2:987–996. doi: 10.1007/s10068-012-0129-7. [DOI] [Google Scholar]
- 40.Dai X., Huang Q., Zhou B., Gong Z., Liu Z., Shi S. Preparative isolation and purification of seven main antioxidants from Eucommia ulmodies Oliv. (Duzhong) leaves using HSCCC guided by DPPH-HPLC experiment. Food Chem. 2013;139:563–570. doi: 10.1016/j.foodchem.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.B., Hwang S.H., Wang Z., Yu J.M., Lim S.S. Rapid Identification and Isolation of Rat Lens Aldose Reducatse and Antioxidant in Maackia amurensis. BioMed Res. Int. 2017:4941825. doi: 10.1155/2017/4941825. [DOI] [PMC free article] [PubMed] [Google Scholar]