Abstract
The influence of climatic factors, e.g., low temperature, on the phytochemical composition and bioactivity of the arctic plant Dracocephalum palmatum Steph. ax Willd. (palmate dragonhead), a traditional food and medical herb of Northern Siberia, was investigated. D. palmatum seedlings were grown in a greenhouse experiment at normal (20 °C, NT) and low (1 °C, LT) temperature levels and five groups of components that were lipophilic and hydrophilic in nature were characterized. The analyses indicated that D. palmatum under NT demonstrates high content of photosynthetic pigments, specific fatty acid (FA) profile with domination of saturated FA (53.3%) and the essential oil with trans-pinocamphone as a main component (37.9%). Phenolic compounds were identified using a combination of high performance liquid chromatography with diode array detection and electrospray ionization mass-spectrometric detection (HPLC-DAD-ESI-MS) techniques, as well as free carbohydrates and water soluble polysaccharides. For the first time, it was established that the cold acclimation of D. palmatum seedlings resulted in various changes in physiological and biochemical parameters such as membrane permeability, photosynthetic potential, membrane fluidity, leaf surface secretory function, reactive oxygen species–antioxidant balance, osmoregulator content and cell wall polymers. In brief, results showed that the adaptive strategy of D. palmatum under LT was realized on the accumulation of membrane or surface components with more fluid properties (unsaturated FA and essential oils), antioxidants (phenolic compounds and enzymes), osmoprotectants (free sugars) and cell wall components (polysaccharides). In addition, the occurrence of unusual flavonoids including two new isomeric malonyl esters of eriodictyol-7-O-glucoside was found in LT samples. Data thus obtained allow improving our understanding of ecophysiological mechanisms of cold adaptation of arctic plants.
Keywords: Dracocephalum palmatum, fatty acids, essential oil, phenolic compounds, carbohydrates, low-temperature cultivation, antioxidant activity, gas chromatography-mass spectrometry (GC-MS), high performance liquid chromatography with diode array detection and electrospray ionization mass-spectrometric detection (HPLC-DAD-ESI-MS)
1. Introduction
Environmental stress is one of the most important factors limiting the productivity of the majority of economically important crops [1]. Extreme growth conditions can damage the plant habitat, their inner ultrastructure and biochemical composition, causing dramatic changes incompatible with the functions of living organisms [2]. To elevate their vitality, plants have developed the remarkable ability to adapt to severe environmental conditions. These specific potentials have allowed the use of plants as crops even in areas with extremely uncomfortable climate conditions [3].
The territories of the Arctic floristic region of Siberia and the Republic of Sakha (Yakutia) belong to an area of high-risk farming due to unfavorable environmental conditions complicating the implementation of agronomic measures. In the period of maximum growth (vegetation) of agricultural plants (May–September), short-term drops of night temperatures are possible from +2 (July) to −10 °C (May and September) [4]. To understand agronomical events in regions with similar weather conditions, it is necessary to use specific cultures that are particularly resistant to the effects of extreme abiotic factors, including temperature. Dracocephalum palmatum Steph. ex Willd. (syn. D. schelechowii Turcz. ex Ledeb., Ruyschiana palmata (Steph. ex Willd.) House, palmate dragonhead) is an example of a widespread species in the Lamiaceae family in the northern part of Siberia (Aldansky, Indigirsky and Kolymsky floral regions) in the permafrost territory and within the Arctic Circle in the range of the Arctic floristic region with a high ecological adaptability [5]. Increased resistance to the effects of chilling temperatures and short-term frosts characterizes this plant species, and it can therefore be defined as a cold-tolerant plant [6].
The herb D. palmatum has economic value as a medicinal plant and in the food industry as a spice and tea component; therefore, D. palmatum is cultivated in the territory of the modern Republic of Sakha (Yakutia) [7]. Early investigations have shown that D. palmatum is characterized by the presence of various groups of natural compounds, including phenylpropanoids, flavonoids, coumarins and triterpenes [8,9]. The pronounced antioxidant activity of D. palmatum extracts due to the presence of flavone glycosides of luteolin and apigenin explains its application in medical practice as a hepatoprotective remedy [8]. It should be noted that the use of plant species of the genus Dracocephalum in human households is common occurrence. D. moldavica L. is the most famous Dracocephalum species, which is the object of large-tonnage production as an essential oil source and medicinal plant [10]. Moreover, the traditional medicinal plants D. heterophyllum Benth and D. tanguticum Maxim. are widely used in the medical systems of China and Tibet for the treatment of asthma, bronchitis, gastropathies and hepatitis [11]. In this regard, the application of the local plant species D. palmatum as a food and medicinal preparation in the territory of the north of Siberia is quite justified.
Previous research studies have shown the influence of different types of abiotic stress factors on the essential oil content of Dracocephalum plants. Alaei et al. reported that high levels of salinity may have resulted in a two-fold increase in the amount of essential oil in D. moldavica herb [12]. Studies have also demonstrated that stress levels due to rising water resulted in the decrease of essential oil amounts [13]. It is generally accepted that essential oils are the principal components of Dracocephalum plants, similar to D. heterophyllum [14], D. moldavica [15], D. kotchii [16] and others [11]. However, it should not be forgotten that there are many important natural components that are lipophilic (fatty acids and terpenoids) and hydrophilic in nature (phenolics and carbohydrates) that were identified in the Dracocephalum genus [11]. There is a complete lack of scientific information about the effect of cold stress on chemical components of non-“essential oil”-nature in Dracocephalum species, making it impossible to understand the fundaments of its adaptive process. Possession of the composite data of both lipophilic and hydrophilic compounds variation in Dracocephalum species will be useful for creation of an effective strategy for maximizing the production of secondary metabolites.
The present study was designed to understand the physiological mechanism of cold tolerance of D. palmatum. The chemical responses of D. palmatum seedlings were estimated by analyzing the phytochemical profile of plants cultivated under low (LT) and normal temperature (NT). The following lipophilic groups of compounds were chosen as markers of lipidome homeostasis: chlorophylls and carotenoids due to their role as important and critical biomolecules in photosynthesis with function of light absorbance and light energy transformation; fatty acids as components responsible for the liquid properties of plant cell membranes; and essential oils as a group of structurally variable components with functions closely related to leaf ontogeny and possible role as plant growth regulators. Due to the strong relationship between cold stress level and production of reactive oxygen level in plant cells, the composition of antioxidative components as phenolics was also analyzed. Upon further investigation, particular attention was paid to the profile of the osmoprotectant components (free sugars) and cell wall polymers (polysaccharides). Some physiological parameters such as electrolyte leakage, malondialdehyde concentration and activity levels of superoxide dismutase and catalase in plant tissue were also determined. This research allows improving the theoretical knowledge in the field of ecophysiological adaptation of D. palmatum and helps to estimate the usefulness of cold-temperature cultivation of the arctic plants.
2. Results and Discussion
2.1. Phenotypic Changes, Electrolyte Leakage, Photosynthetic Pigment Content and Parameters of Photosynthesis of D. palmatum during Low-Temperature (LT) Cultivation
We investigated the phenotypic response of D. palmatum to low-temperature (LT) stress. Seedlings were cultivated in growth chambers with photoperiod of 16 h light/8 h dark installed under normal temperature conditions (20 °C; NT) for two months. One group of seedlings remained under normal conditions and another group was transferred and subjected to LT (1 °C) for 20 days. The level of LT exposure was close to cold-down periods affected by D. palmatum plants in his natural habitat. The time-frame was chosen to avoid the visible plant damages caused by long-term LT application. To evaluate whether period can be applied successfully, 10–30 days LT impact was used. As a result, it was demonstrated that the duration of LT (1 °C) should not exceed 20 days.
After the 20-day period, the seedlings grown in 20 °C were at a height where the plant stems reach an average of 100 mm. During the cold treatment at 1 °C, the seedlings were stockier, with the height of the plant stems reaching an average of 80 mm. A significant increase in the yield of fresh herb was not observed, but leaves of the LT seedlings were a saturated green color and the areas of young leaves were not different from the leaves of the group with moderate temperature conditions.
To evaluate the extent of cell damage caused by cold stress in LT seedlings, electrolyte leakage was measured (Table 1). The D. palmatum plants after 20 days of LT exposure presented 1.44-fold higher electrolyte leakage than normal temperature plants, which suggests that the membrane is likely to be impaired in these seedlings subjected to cold stress [2].
Table 1.
Electrolyte leakage (percent of total electrolytes ± SD), photosynthetic pigments content (µg/g of fresh leaf weight (FW) ± standard deviation (SD)), carbon assimilation rate (µM CO2/m2·s ± SD) and effective quantum yield of PSII (Fv/Fm; ±SD) in D. palmatum leaves cultivated under normal (20 °C) and low (1 °C) temperatures. 1
| Parameter | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| Electrolyte leakage, % of total electrolytes | 18.2 ± 0.9 a | 26.7 ± 1.4 a |
| Chlorophyll a content (Chla), µg/g FW | 273.45 ± 9.29 a | 406.19 ± 13.40 a |
| Chlorophyll b content (Chlb), µg/g FW | 75.85 ± 2.50 a | 141.21 ± 4.79 a |
| Total chlorophylls content (ΣChl), µg/g FW | 349.30 | 547.40 |
| Chla/Chlb | 3.61 | 2.88 |
| Pheophytin a content, µg/g FW | 6.01 ± 0.16 a | 7.06 ± 0.14 a |
| Pheophytin b content, µg/g FW | 4.57 ± 0.10 a | 5.64 ± 0.11 a |
| Total pheophytins content, µg/g FW | 10.58 | 12.70 |
| Carotenoids content (Car), µg/g DW | 36.86 ± 1.07 a | 53.31 ± 1.55 a |
| ΣChl/Car | 9.48 | 10.27 |
| Carbon assimilation rate, µM CO2/m2·s | 8.3 ± 0.8 b | 5.8 ± 0.4 b |
| Fv/Fm | 0.62 ± 0.04 b | 0.54 ± 0.03 b |
1 Averages ± standard deviations were obtained from three (a) or ten (b) different experiments.
The photosynthetic pigments from the leaves of the three groups were measured (chlorophylls, pheophytins, and carotenoids). Total chlorophylls and carotenoid concentration of the LT group was significantly higher (547.40 and 53.31 µg/g FW, respectively) than in NT group (349.30 and 36.86 µg/g FW, respectively), indicating that the photosynthetic pigment levels might contribute to the difference in the photosynthetic capacity among the temperature conditions [17,18]. In contrast, total content of pheophytins in LT plants remained almost unchanged. The extent of pheophytin accumulation under LT stress can play a key role as a measure of chlorophyll damage, which, in this case, is not observed. The ratio of chlorophyll a and b (Chla/Chlb) in NT group was 3.61 and reduced by 20.2% under LT conditions. By contrast, the ratio of total chlorophyll content ant total carotene content (ΣChl/Car) in D. palmatum plants under LT stress was 10.27, which was 8.3% higher than in NT plants. These changes showed that the LT treatment caused the reorientation of chlorophyll synthesis also with respect to total carotene level. It is also an indicator of a shift in content of light-harvesting complex 2 of a leaf photosystem caused by the impact of the stress factors [19].
The value of carbon assimilation rate in LT plants was lower (5.8 µM CO2/m2·s) than in plant cultivated at normal temperature (8.3 µM CO2/m2·s). In addition, the effective quantum yield of PSII (Fv/Fm) in plants exposed to LT was slightly lower than the value of NT plants, demonstrating inhibition of photosynthetic processes under cold temperature. A possible explanation given by us for the differences of photosynthesis rate and chlorophyll content in LT plants was the ability of D. palmatum to accumulate chlorophylls while low rate of its cold-induced degradation.
The known data demonstrate that, at the seedling stage, the impact of chilling stress increases the concentration of the accessory pigments (chlorophyll b and carotenoids) when compared to chlorophyll a, most likely to increase the photon capture [20]. Moreover, in the case of cold-tolerant lines of crops, more chlorophylls accumulate under cold stress than cold sensitive lines of crops [21]. The results showed that D. palmatum adapted to the environmental temperature and can be grown and perform better under low-temperature conditions.
2.2. Changes of the Fatty Acids of D. palmatum during LT-Cultivation
The influence of temperature on the composition of fatty acids in D. palmatum was investigated. The total lipid fraction yield of D. palmatum grown under NT was 1.24% (of dry plant weight; DW) and the fatty acid composition was characterized by high amounts of palmitic acid (27.9%), linolenic acids (14.6%), linoleic acid (14.1%) and oleic acid (11.3%) (Table 2). Concerning the amount of fatty acids in other species of the Dracocephalum genus, the fatty acid composition of D. kotschyi oil is composed of a high amount of polyunsaturated fatty acids and contains linolenic acid (61.2%) as the predominant fatty acid followed by oleic (18.1%) and linoleic (13.5%) acids [22]. The amount of linolenic acid in D. moldavica oil was 59.4% [23]. These results are in accordance with previous data regarding the Lamiaceous plant fatty acid profile where the authors demonstrated the close ratio of the aforementioned acids in herbs [24,25].
Table 2.
Fatty acids (FA) composition of D. palmatum herb under different temperatures of cultivation (percentage of total FA content).
| Compound | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| Pelargonic acid (9:0) | 0.1 | Tr. |
| Capric acid (10:0) | 0.2 | Tr. |
| Lauric acid (12:0) | 5.7 | 3.2 |
| Tridecylic acid (13:0) | 1.4 | 0.5 |
| Myristic acid (14:0) | 2.7 | 1.7 |
| Pentadecylic acid (15:0) | 0.4 | 0.3 |
| Palmitic acid (16:0) | 27.9 | 11.2 |
| Palmitoleic acid (16:1) | 3.8 | 6.9 |
| Margaric acid (17:0) | 1.8 | 1.4 |
| Stearic acid (18:0) | 8.0 | 5.2 |
| Oleic acid (18:1 ω-9) | 11.3 | 18.3 |
| Linoleic acid (18:2 ω-6) | 14.1 | 19.3 |
| α-Linolenic acid (18:3 ω-3) | 12.5 | 18.2 |
| γ-Linolenic acid (18:3 ω-6) | 2.1 | 7.4 |
| Arachidic acid (20:0) | 4.5 | 0.5 |
| Gondoic acid (20:1 ω-9) | 2.0 | 3.7 |
| Behenic acid (22:0) | 0.5 | Tr. |
| Erucic acid (22:1 ω-9) | 0.7 | 1.2 |
| Lignoceric acid (24:0) | 0.1 | Tr. |
| Total | 99.8 | 99.0 |
| Saturated FA | 53.3 | 24.0 |
| Unsaturated FA | 46.5 | 75.0 |
| Monounsaturated FA | 17.8 | 30.1 |
| Polyunsaturated FA | 28.7 | 44.9 |
Tr., traces (<0.1%).
The effect of LT environments on D. palmatum resulted in the increase of the total lipid fraction yield to 3.22% DW and the decrease in the relative amount of saturated acids compared with normal temperature conditions (specifically, the amounts of tridecylic, palmitic and arachidic acids were reduced 2.8, 2.5 and 9 times, respectively) and an increase in the amount of unsaturated fatty acids (γ-linoleic, gondoic and erucic acids increased 3.5, 1.9 and 1.7 times, respectively). The influence of LT conditions on the D. palmatum caused a shift in the saturated/unsaturated ratio compared with cultivation in NT (24.0/75.0 vs. 53.3/46.5). Saturated palmitic acid was the predominant fatty acid in D. palmatum cultivated in NT conditions (27.9%), while polyunsaturated linoleic acid dominated in samples that grew under LT conditions (19.3%).
Fatty acids play an important role in the protection of cell membrane against negative effects of long-tern cold exposure [26]. The dominant effect of LT on the lipid composition of cell membranes is the rising level of unsaturated components (e.g., fatty acids) which makes membrane more fluid [27]. This is confirmed by the fact of high content of unsaturated fatty acids (75%) in D. palmatum herb cultivated under cold temperature.
2.3. Changes of the Essential Oil Profile of D. palmatum during LT-Cultivation
The samples of D. palmatum herbal essential oil (EO) were isolated by hydrodistillation and the composition was determined for the first time after gas chromatography-mass spectrometry (GC/MS) analysis. EO are greenish liquids characterized by specific smells caused by the presence of odoriferous components. Thirty-eight compounds were identified in three samples of EO from D. palmatum cultivated under different temperatures, including aliphatic compounds, simple phenols, monoterpenes and sesquiterpenes (Table 3).
Table 3.
Essential oil (EO) composition (percentage of total component content) of D. palmatum herb under different temperatures of cultivation.
| Compound | RI | MI a | Temperature (°C) | |
|---|---|---|---|---|
| 20 | 1 | |||
| Aliphatic compounds | ||||
| Isoamyl acetate | 875 | i, ii, iii | 0.6 | 0.5 |
| Subtotal | 0.6 | 0.5 | ||
| Simple phenols | ||||
| p-Cymene | 1024 | i, ii, iii | 1.7 | 1.8 |
| p-Cymene-8-ol | 1186 | i, ii | 1.9 | 2.0 |
| p-Cumenol | 1222 | i, ii, iii | 0.4 | 0.4 |
| m-Cumenol | 1225 | i, ii | 0.1 | 0.1 |
| Cuminaldehyde | 1241 | i, ii, iii | 1.2 | 1.2 |
| Subtotal | 5.3 | 5.5 | ||
| Monoterpenes | ||||
| α-Thujene | 926 | i, ii | 0.6 | 0.4 |
| α-Pinene | 932 | i, ii, iii | 1.1 | 1.2 |
| Camphene | 947 | i, ii, iii | 0.1 | 0.2 |
| Sabinene | 973 | i, ii, iii | 2.5 | 2.0 |
| β-Pinene | 975 | i, ii, iii | 8.6 | 9.0 |
| β-Myrcene | 991 | i, ii, iii | 0.6 | 0.2 |
| Pseudolimonene | 1003 | i, ii | 0.3 | 0.2 |
| β-Phellandrene | 1027 | i, ii, iii | 4.8 | 5.2 |
| Limonene | 1029 | i, ii, iii | 1.8 | 1.8 |
| 1,8-Cineol | 1031 | i, ii, iii | 5.5 | 5.8 |
| γ-Terpinene | 1058 | i, ii, iii | 0.2 | 0.3 |
| Linalool | 1100 | i, ii, iii | 1.2 | 1.4 |
| β-Pinone | 1105 | i, ii | 0.8 | 1.0 |
| trans-Pinocarveol | 1138 | i, ii, iii | 1.0 | 1.4 |
| trans-Pinocamphone | 1161 | i, ii | 37.9 | 40.7 |
| cis-Pinocamphone | 1175 | i, ii | 8.0 | 8.7 |
| cis-Pinocarveol | 1186 | i, ii | 0.2 | 0.5 |
| Terpinene-4-ol | 1177 | i, ii, iii | 0.5 | 0.4 |
| Myrtenol | 1197 | i, ii | 2.7 | 3.3 |
| Phellandral | 1276 | i, ii | 0.2 | 0.1 |
| Bornyl acetate | 1287 | i, ii, iii | 0.3 | 0.2 |
| trans-Pinocarvyl acetate | 1301 | i, ii | 1.8 | 2.0 |
| cis-Pinocarvyl acetate | 1315 | i, ii | 0.4 | 0.7 |
| Myrtenyl acetate | 1327 | i, ii, iii | 3.5 | 3.7 |
| p-Mentha-1,4-dien-7-ol | 1329 | i, ii | 0.4 | 0.1 |
| Subtotal | 85.0 | 90.8 | ||
| Sesquiterpenes | ||||
| β-Caryophyllene | 1420 | i, ii, iii | 1.0 | 0.4 |
| γ-Cadinene | 1518 | i, ii | 0.5 | Tr. |
| Germacrene B | 1560 | i, ii | 0.7 | 0.1 |
| Caryophyllene oxide | 1587 | i, ii, iii | 1.8 | 0.5 |
| Viridiflorol | 1594 | i, ii | 3.2 | 1.6 |
| α-Cadinol | 1659 | i, ii | 1.2 | 0.3 |
| Germacrone | 1696 | i, ii | 0.6 | 0.2 |
| Subtotal | 9.0 | 3.1 | ||
| Total | 99.9 | 99.9 | ||
a Methods of identification: i, retention index; ii, mass spectrum; iii, co-injection with authentic sample. Tr., traces (<0.1%).
The yield of the EO was 1.1% for the sample cultivated in moderate environments (20 °C) and 3.7% for D. palmatum grown in LT environments. There were no significant differences in the quantitative amounts of aliphatic compounds and simple phenols at both temperatures of cultivation. The predominance of bicyclic monoterpene trans-pinocamphone was noticed at all temperature conditions ranging from 37.9% to 40.7%. The effect of LT environments on D. palmatum EOs resulted in a slight increase in the amount of monoterpenes and a significant decrease of total sesquiterpenes compared with growth under normal temperature conditions. Thus, the amount of camphene, cis-pinocarveol and cis-pinocarvyl acetate increased 2, 2.5 and 1.8 times compared with samples grown at 20 °C. A decrease in the levels of α-tujene, β-myrcene, phellandral and p-mentha-1,4-dien-7-ol at 1.5, 3, 2 and 4 times, respectively, was noted.
The total amount of sesquiterpenes decreased 2.9 times compared with samples grown under normal temperature conditions. It can be concluded that temperature environmental conditions should be carefully considered in the cultivation of D. palmatum to get the desired concentrations of terpenoids.
The literature regarding the chemical composition of the EO of the Dracocephalum genus indicates the similarity of the volatile compounds. Most dominant compounds of the Dracocephalum genus related to monoterpenes. D. moldavica EO is the most studied. The main components were acyclic monoterpenes E-citral (30.4%), geranyl acetate (29.6%) and neral (22.1%) [28]. Other isomers of citral (geranial (63.4%), limonene (23.4%) and p-menth-1-en-9-ol (4.4%)) dominated the EOs from the aerial portion of D. subcapitatum [29]. The predominance of the acyclic monoterpenes perilla aldehyde and limonene was revealed in the EO from the aerial portions of D. surmondinum (54.3% and 30.1%, respectively) [30], D. multicaule (71.5% and 28.1%, respectively) [31] and D. polychaetum (63.4% and 22.1%, respectively) [32]. Other acyclic monoterpenes such as p-mentha-1,8-dien-10-al (39.2%), limonene (17.0%) and geranial (4.6%) prevailed in the EO from the aerial portion of D. foetidum [33]. The EO from the aerial portion of D. heterophyllum contained citronellol (74.9%), citronellyl formate (6.7%) and citronellal (6.7%) [34]. Volatile constituents of the aerial portion of D. peregrinum contained monocyclic monoterpene 1,8-cineol (18.5%), α-pinene (8.4%) and limonene (5.8%) [35]. The predominance of acyclic and bicyclic monoterpenes was noticed in the EO from the aerial portion of D. kotschyi, including neral (11%), α-citral (12%) and α-pinene (10%) [36]. D. aucherry was a species with a prevalence of EO from the flowering shoots that contained bicyclic monoterpene sabinene (55.2%), germacrone (9.9%) and α-thujene (5.5%) [37]. Another species with a predominance of bicyclic monoterpenes in the EO from aerial portion was D. wallichii (D. speciosum) (trans-pinocarvyl acetate (60.5%) and cis-pinocarvyl acetate (5.7%)) [38].
Temperature levels have been previously reported to influence the EO amount in several aromatic crops of the Lamiaceous group [39]. A decrease in temperature may favor the accumulation of various groups of volatile terpenoids in EO glands on the leaf surface [40]. In sage leaves (Salvia officinalis L.), the largest percentage of monoterpenes was observed in the cold period of cultivation (October) [41]. The same effect on monoterpene accumulation was reported for peppermint EO (Mentha piperita L.) isolated from the plants grown at LTs [42,43]. The data obtained indicated a similarity of the temperature-induced physiological responses previously detected in Lamiaceous plants and D. palmatum. In this way, the production of EO in D. palmatum is not only exerted in a development-specific fashion but is also highly susceptible to modulation through temperature regulation.
2.4. Changes of Phenolic Compounds of D. palmatum during LT-Cultivation
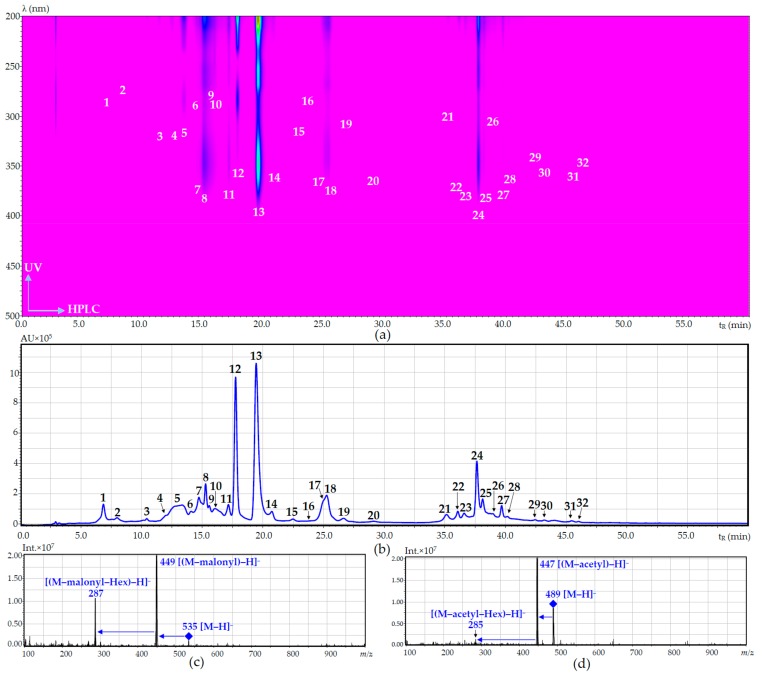
The methanol extracts of D. palmatum were analyzed by reversed phase high performance liquid chromatography with diode array detection and electrospray ionization mass-spectrometric detection (RP-HPLC-DAD-ESI-MS) in both negative and positive ionization modes. The HPLC with ultraviolet detection (HPLC-UV) map and HPLC-DAD chromatogram are shown in Figure 1, and chromatographic parameters, UV and ESI-MS data are in Table 4. By comparing the retention times (tR), UV and ESI-MS spectra with those of references substances and literature data, 32 components were identified in all plant samples (23 components in NT samples and 32 components in LT sample), including two simple phenolic glycosides (1 and 2), four caffeic acid derivatives or phenylpropanoids (3–5 and 19) and 26 flavonoids (6–18 and 20–32) as glycosides and aglycones.
Figure 1.
HPLC-UV map (a); and RP-HPLC-DAD chromatogram at 280 nm (b) of low-temperature sample of D. palmatum herb; ESI-MS spectra of unknown compounds 6 (eriodictyol-O-malonyl-hexoside) and 20 (luteolin-O-acetyl-hexoside) presented at (c,d), respectively. The numbers in the Figure 1a,b correspond to the compounds indicated in Table 4. AU, absorbance units; Int., signal intensity.
Table 4.
HPLC parameters, ultraviolet spectra data (UV) and electrospray ionization mass spectrometry (ESI-MS) data of components 1–32 from D. palmatum herb.
| No. | Compound | tR (min) | UV, λmax (nm) | ESI-MS (m/z) | Refs. Comp.a |
|---|---|---|---|---|---|
| 1 | O-Malonyl-arbutin | 6.79 | 280 | 381 [M + Na]+, 352 [M + H]+, 295 [M + Na]+ |
iii [45] |
| 2 | Arbutin | 7.89 | 280 | 295 [M + Na]+, 273 [M + H]+ | i |
| 3 | 5-O-Caffeoylquinic acid | 10.43 | 331 | 353 [M − H]−, 183 | i |
| 4 | 3-O-Caffeoylquinic acid | 12.03 | 331 | 353 [M − H]−, 183 | i |
| 5 | Caffeic acid | 13.15 | 323 | 179 [M − H]− | i |
| 6 | Eriodictyol-O-malonyl-hexoside (isomer) | 14.09 | 284 | 535 [M − H]−, 449 [(M − malonyl) − H]−, 287 [(M − malonyl − Hex) − H]− |
- |
| 7 | Luteolin-7,4′-di-O-rutinoside (dracopalmaside) | 14.87 | 253, 265, 345 | 905 [M − H]−, 447 [(M − Rha − Glc) − H]−, 285 [(M – 2 × Rha – 2 × Glc) − H]− |
ii [9] |
| 8 | Luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside) | 15.02 | 253, 265, 345 | 755 [M − H]−, 609 [(M − Rha) − H]−, 447 [(M − Rha − Glc) − H]−, 285 [(M − Rha – 2 × Glc) − H]− |
ii [9] |
| 9 | Eriodictyol-O-malonyl-hexoside (isomer) | 15.41 | 283 | 535 [M − H]−, 449 [(M − malonyl) − H]−, 287 [(M − malonyl − Hex) − H]− |
- |
| 10 | Eriodictyol-7-O-rutinoside (eriocitrin) | 16.21 | 284 | 595 [M − H]−, 449 [(M − Rha) − H]−, 287 [(M − Rha − Glc) − H]− |
i |
| 11 | Luteolin-7-O-rutinoside (scolymoside) | 17.02 | 252, 262, 345 | 593 [M − H]−, 447 [(M − Rha) − H]−, 285 [(M − Rha − Glc) − H]− |
i |
| 12 | Eriodictyol-7-O-glucoside | 17.58 | 283 | 899 [2M − H]−, 449 [M − H]−, 287 [(M − Glc) − H]− |
i |
| 13 | Luteolin-7-O-glucoside (cynaroside) | 19.48 | 254, 267, 348 | 895 [2M − H]−, 447 [M − H]−, 285 [(M − Glc) − H]− |
i |
| 14 | Luteolin-4′-O-glucoside | 20.64 | 260, 335 | 447 [M − H]−, 285 [(M − Glc) − H]− |
i |
| 15 | Apigenin-7-O-rutinoside (isorhoifolin) | 22.51 | 266, 334 | 577 [M − H]−, 431 [(M − Rha) − H]−, 269 [(M − Rha − Glc) − H]− |
i |
| 16 | Naringenin-7-O-glucoside (prunin) | 23.89 | 283 | 433 [M − H]−, 271 [(M − Glc) − H]− |
i |
| 17 | Apigenin-7-O-glucoside (cosmosiin) | 24.47 | 267, 336 | 863 [2M − H]−, 431 [M − H]−, 269 [(M − Glc) − H]− |
i |
| 18 | Apigenin-O-hexoside | 25.31 | 265, 334 | 431 [M − H]−, 269 [(M − Glc) − H]− |
iii [51] |
| 19 | Rosmarinic acid | 27.26 | 327 | 359 [M − H]−, 183 | i |
| 20 | Luteolin-O-acetyl-hexoside | 29.11 | 251, 263, 346 | 489 [M − H]−, 447 [(M − acetyl) − H]−, 285 [(M − acetyl − Hex) − H]− |
iii [52] |
| 21 | Eriodictyol | 35.03 | 283 | 287 [M − H]− | i |
| 22 | Acacetin-7-O-rutinoside (linarin) | 36.14 | 267, 330 | 591 [M − H]−, 445 [(M − Rha) − H]−, 283 [(M − Rha − Glc) − H]− |
i |
| 23 | Acacetin-7-O-glucoside (tilianin) | 36.72 | 266, 330 | 445 [M − H]−, 283 [(M − Glc) − H]− |
i |
| 24 | Luteolin | 37.72 | 253, 266, 347 | 285 [M − H]− | i |
| 25 | Acacetin-O-acetyl-hexoside | 38.10 | 266, 331 | 487 [M − H]−, 445 [(M − acetyl) − H]−, 283 [(M − acetyl − Hex) − H]− |
iii [52] |
| 26 | Naringenin | 38.32 | 283 | 271 [M − H]− | i |
| 27 | Apigenin | 39.75 | 267, 336 | 269 [M − H]− | i |
| 28 | Chrysoeriol | 40.21 | 266, 347 | 299 [M − H]− | i |
| 29 | Acacetin | 42.34 | 267, 330 | 283 [M − H]− | i |
| 30 | Isothymusin | 43.06 | 302, 330 | 329 [M − H]− | ii [46] |
| 31 | Salvigenin | 45.81 | 273, 330 | 327 [M − H]− | i |
| 32 | Genkwanin | 46.04 | 267, 335 | 283 [M − H]− | i |
a Reference compound used: i, commercial sample; ii, isolated compound; iii, literature data.
Two simple phenolic glycosides, compounds 1 and 2, were detected in D. palmatum for the first time. The quasi-molecular ions were [M + H]+ as well as sodium adducts [M + Na]+ in positive ion mode; both compounds had weak ionization in negative ion mode. Compound 2 was characterized as arbutin (hydroquinone-O-glucoside) due to intense [M + Na]+ at m/z 295 and specific absorbance in the UV spectrum compared with the reference compound [44]. Compound 1 gave the sodium adduct [M + Na]+ and quasi-molecular ion [M + H]+ at m/z 381 and 352, respectively (i.e., 86 amu more than arbutin indicating malonyl-derivative of 2 tentatively characterized as O-malonyl-arbutin) [45]. Data in the literature data demonstrated that the arbutin and its esters are rare components for the Lamiaceae family. Only Origanum majorana L. (formerly Majorana hortensis Moench.) is known as a good source of arbutin (2) as other Origanum species (O. onites L., O. microphyllum (Benth.) Vogel, O. saccatum P.H. Davis, O. solymicum P.H. Davis) only contain trace amounts of 2 [46]. Arbutin ester 1 was detected only in D. moldavica of Mexican origin [45].
Four chromatographic peaks (3, 4, 5 and 19) with the same UV spectra as the standards of 5-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, caffeic acid and rosmarinic acid were detected in all samples of D. palmatum. Two of them (3 and 5) showed the [M − H]− ions at m/z 353 and one (19) at m/z 359. Components 4, 5 and 19 were previously detected in D. palmatum [8], and 3 was discovered in this species for the first time in this study.
Flavone glycosides as usual components of the flavonoid complex in the Dracocephalum genus [11] were presented as derivatives of apigenin, acacetin and luteolin widely distributed in Lamiaceae family [47]. Luteolin-7-O-rutinoside (scolymoside, 11), luteolin-7-O-glucoside (cynaroside, 13), luteolin-4′-O-glucoside (14), apigenin-7-O-rutinoside (isorhoifolin, 15), apigenin-7-O-glucoside (cosmosiin, 17), acacetin-7-O-rutinoside (linarin, 22) and acacetin-7-O-glucoside (tilianin, 23) standards allowed the unequivocal identification of these seven flavone glycosides in D. palmatum extracts. Five of these flavonoids (11, 13–15, and 17) have already been characterized as components of D. palmatum [8]. Acacetin derivatives 22 and 23, described in D. palmatum for the first time, are not usual for Dracocephalum genus. Both components were also found in D. foetidum [48], D. moldavica [11] and D. peregrinum [49,50].
Two rare di-O-glycosides of luteolin, luteolin-7,4′-di-O-rutinoside (dracopalmaside, 7) and luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside, 8), previously isolated from D. palmatum of natural origin [9] were also detected in plants grown in greenhouse conditions.
Compound 18 was identified as apigenin-O-hexoside according to UV and ESI-MS spectra and literature information [51]. This compound demonstrated the same UV spectra pattern as flavone standards. The ESI-MS deprotonated ion [M − H]− at m/z 431 and the aglycone fragment at m/z 269 revealed the characteristic fragmentation of an apigenin-hexose derivative [53]. Due to the difference in the retention times of 18 (tR 25.31 min) and apigenin-7-O-glucoside (tR 24.47 min), component 18 may be tentatively determined as apigenin-4′-O-glucoside, previously identified in Elsholtzia rugulosa Hemsl [51].
Two components, 20 (tR 29.11 min) and 25 (tR 38.10 min), detected only in low-temperature samples of D. palmatum, were characterized as O-acetyl-hexosides of luteolin and acacetin, respectively, according to UV and ESI-MS fragmentation patterns [54]. The presence of acylation in structures 20 and 25 was explained by their late elution compared with deacylated analogs, such as luteolin-7-O-glucoside (13; tR 19.48 min) and acacetin-7-O-glucoside (23; tR 36.72 min), respectively. The ESI-MS spectra in negative ion mode showed the peaks of the [M − H]− ions and their deacylated fragments: 489→447 for 20 (Figure 1d) and 487→445 for 25, which were useful for the characterization of the type of acyl group as an acetyl-group [53]. The presence of acetylated hexosides of luteolin and acacetin was not shown in D. palmatum previously, but has been described in D. foetidum [48] and D. peregrinum [49,50].
Five compounds were detected within the group of flavanone glycosides, and three of them were identified as eriodictyol-7-O-rutinoside (eriocitrin, 10), eriodictyol-7-O-glucoside (12) and naringenin-7-O-glucoside (prunin, 16) by comparison with standards. Eriodictyol-7-O-glucoside (12) and naringenin-7-O-glucoside (16) were found previously in D. palmatum [8] and D. rupestre [11]. Eriodictyol-7-O-rutinoside (10) was a new component for D. palmatum and in the Dracocephalum genus.
ESI-MS and UV spectra of components 6 and 9 were identical and lead readily to the determination of both compounds as flavanone-O-acyl-hexosides [54]. Based on m/z values, these flavonoids should be isomeric O-malonyl-hexosides of eriodictyol. UV and MS product spectra confirmed the identity of 6 and 9 as aglycone using an eriodictyol standard. The type of acyl group (malonyl) was confirmed by the presence of both acylated and deacylated fragments in ESI-MS spectra in negative ion mode (Figure 1c). Known literature regarding glycosides of eriodictyol showed no information about the O-malonyl-hexoside of eriodictyol, demonstrating that 6 and 9 are new isomeric natural compounds. Even though malonyl-glycosyl derivatives of flavonoids are rare components of the Lamiaceae family, they have already been isolated from D. foetidum [48], indicating the ability of the Dracocephalum plant to synthesize acylated flavonoid glycosides.
Nine flavonoid aglycones were detected in D. palmatum samples, including two flavanones (21 and 26) and seven flavones (24, and 27–32). Eight of them were identified by comparing their UV and ESI-MS patterns with reference samples: eriodictyol (21), luteolin (24), naringenin (26), apigenin (27), chrysoeriol (28), acacetin (29), salvigenin (31) and genkwanin (32). Component 30 showed the [M − H]− ion at m/z 329 and a specific UV spectrum with maxima at 302 and 330 nm, which are characteristic for isothymusin [55]. Four flavonoid aglycones (21, 24, 26, and 27) were identified in wild samples of D. palmatum [8], while components 28–32 were discovered in this Dracocephalum species for the first time.
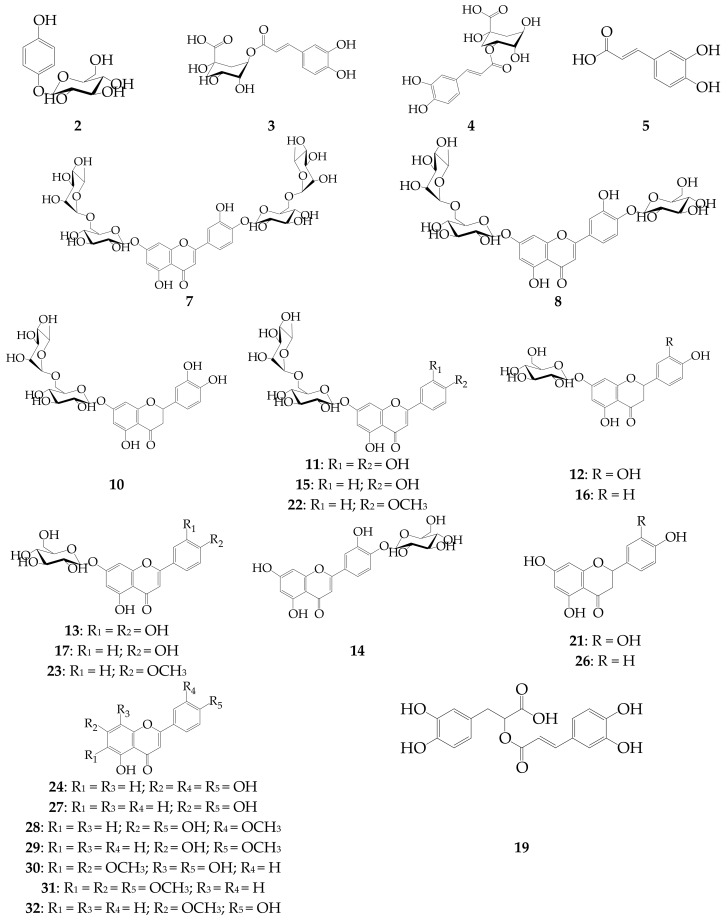
These results suggest that the phenolic profile of D. palmatum may be successfully described using the online technique of RP-HPLC-DAD-ESI-MS, which allowed the characterization of 32 components (Figure 2). Although some of the phenolics (4, 5, 7, 8, 11–17, 19, 21, 24, 26, and 27) have been previously described in D. palmatum, others (1–3, 10, 18, 20, 22, 23, 25, and 28–32) have been discovered in this Dracocephalum species for the first time, including two new acylated flavanone glycosides (6 and 9).
Figure 2.
Structures of phenolic compounds 2–5, 7, 8, 10–17, 19, 21–24, and 26–32 detected in D. palmatum herb.
Application of RP-HPLC-DAD for quantification of components 1–32 in D. palmatum showed that the composition of simple phenols, phenylpropanoids, flavone and flavanone aglycones and glycosides was significantly influenced by abiotic stress signals (e.g., cold-temperature conditions) (Table 5).
Table 5.
Content of phenolic compounds (mg/g DW ± SD) in D. palmatum herb under different temperatures of cultivation. 1
| Compound (No of Compounds) | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| Simple phenols | ||
| O-Malonyl-arbutin (1) | 0.39 ± 0.01 a | 1.24 ± 0.02 a |
| Arbutin (2) | 0.17 ± 0.00 | 0.22 ± 0.00 |
| Subtotal | 0.56 | 1.46 |
| Phenylpropanoids | ||
| 5-O-Caffeoylquinic acid (3) | 0.09 ± 0.00 | 0.05 ± 0.00 |
| 3-O-Caffeoylquinic acid (4) | 0.11 ± 0.00 | 0.09 ± 0.00 |
| Caffeic acid (5) | 0.61 ± 0.02 | 0.84 ± 0.02 |
| Rosmarinic acid (19) | 1.26 ± 0.03 | 1.68 ± 0.04 |
| Subtotal | 2.07 | 2.66 |
| Flavone glycosides. Apigenin derivatives | ||
| Apigenin-7-O-rutinoside (isorhoifolin, 15) | 1.11 ± 0.03 | 0.56 ± 0.02 |
| Apigenin-7-O-glucoside (cosmosiin, 17) | 0.53 ± 0.01 | 6.54 ± 0.17 |
| Apigenin-O-hexoside (18) | 0.47 ± 0.01 b | 8.34 ± 0.18 b |
| Subtotal | 2.11 | 15.44 |
| Flavone glycosides. Acacetin derivatives | ||
| Acacetin-7-O-rutinoside (linarin, 22) | 0.04 ± 0.00 | 0.06 ± 0.00 |
| Acacetin-7-O-glucoside (tilianin, 23) | 0.52 ± 0.01 | 1.27 ± 0.04 |
| Acacetin-O-acetyl-hexoside (25) | ND | 0.08 ± 0.00 c |
| Subtotal | 0.56 | 1.41 |
| Flavone glycosides. Luteolin derivatives | ||
| Luteolin-7,4′-di-O-rutinoside (dracopalmaside, 7) | 0.14 ± 0.00 | 0.52 ± 0.01 |
| Luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside, 8) | 0.82 ± 0.02 | 1.75 ± 0.04 |
| Luteolin-7-O-rutinoside (scolymoside, 11) | 2.27 ± 0.07 | 2.54 ± 0.07 |
| Luteolin-7-O-glucoside (cynaroside, 13) | 2.56 ± 0.07 | 29.56 ± 0.78 |
| Luteolin-4′-O-glucoside (14) | 0.67 ± 0.02 | 9.57 ± 0.19 |
| Luteolin-O-acetyl-hexoside (20) | ND | 0.92 ± 0.02 d |
| Subtotal | 6.46 | 44.86 |
| Flavanone glycosides. Eriodictyol derivatives | ||
| Eriodictyol-O-malonyl-hexoside (sum of 6 and 9) | ND | 1.84 ± 0.04 e |
| Eriodictyol-7-O-rutinoside (eriocitrin, 10) | 0.21 ± 0.00 | 1.35 ± 0.03 |
| Eriodictyol-7-O-glucoside (12) | 1.77 ± 0.03 | 15.82 ± 0.33 |
| Subtotal | 1.98 | 19.01 |
| Flavanone glycosides. Naringenin derivatives | ||
| Naringenin-7-O-glucoside (pruning, 16) | 1.02 ± 0.02 | 1.64 ± 0.03 |
| Subtotal | 1.02 | 1.64 |
| Flavone aglycones | ||
| Luteolin (24) | 1.19 ± 0.03 | 12.94 ± 0.30 |
| Apigenin (27) | 0.46 ± 0.01 | 1.03 ± 0.03 |
| Chrysoeriol (28) | 0.09 ± 0.00 | 0.14 ± 0.00 |
| Acacetin (29) | ND | 0.18 ± 0.00 |
| Salvigenin (31) | ND | 0.09 ± 0.00 |
| Isothymusin (30) | ND | 0.12 ± 0.00 |
| Genkwanin (32) | ND | 0.10 ± 0.00 |
| Subtotal | 1.74 | 14.60 |
| Flavanone aglycones | ||
| Eriodictyol (21) | 0.24 ± 0.00 | 0.69 ± 0.02 |
| Naringenin (26) | ND | 0.54 ± 0.01 |
| Subtotal | 0.24 | 1.23 |
| Total flavone glycosides | 9.13 | 61.71 |
| Total flavanone glycosides | 3.00 | 20.65 |
| Total flavonoids glycosides | 12.13 | 82.36 |
| Total flavonoids aglycones | 1.98 | 15.83 |
| Total flavonoids | 14.11 | 98.19 |
| Total phenolic compounds | 16.74 | 102.31 |
1 Averages ± standard deviation were obtained from three different experiments. a Expressed as arbutin equivalents; b expressed as apigenin-7-O-glucoside equivalents; c expressed as acacetin-7-O-glycoside equivalents; d expressed as luteolin-7-O-glucoside equivalents; e expressed as eryodictyol-7-O-glucoside equivalents; ND—not detected.
The amount of O-malonyl-arbutin (1) in a sample cultivated in NT conditions was 3.2 times lower than in cultivars grown in LT environments (1.24 mg/g). Arbutin content varied insignificantly in the both experimental groups. There were no qualitative differences in phenylpropanoid profiles of all experimental groups. The high amount of both caffeoylquinic acids (3 and 4) was noticed for the NT group. Their reduction was observed in LT conditions. However, the levels of caffeic (5) and rosmarinic acids (19) were the highest for LT samples and exceeded the amounts in samples from NT by 1.4 and 1.3 times, respectively. It should be noted that the high amount of 19 was noted earlier for D. kotschyi gathered from cold-temperature environments [56].
Significant changes in the flavonoid profiles of D. palmatum were observed. It has already been mentioned that flavones were represented by acacetin, apigenin and luteolin derivatives with the prevalence of the latter. Luteolin-7-O-glucoside (13) was the dominant flavonoid in NT group (2.56 mg/g) and LT group (29.56 mg/g). The highest amounts of other luteolin derivatives such as luteolin-7,4′-di-O-rutinoside (7), luteolin-7-O-rutinoside-4′-O-glucoside (8), luteolin-4′-O-glucoside (14) and luteolin-O-acetyl-hexoside (20) were noted for D. palmatum cultivated in LT environments. In addition, a significant increase in the apigenin derivatives apigenin-7-O-glucoside (17) and apigenin-O-hexoside (18) was observed under the same conditions. It is well known that flavonoid glycosides can be influenced by climate conditions such as temperature. Low temperature enhances total flavonoids, which act as shielding components and are responsible for protecting plants from damage as a result of enzymatic repair inhibition, combined with higher quantities of reactive oxygen species [57,58]. In the present experiment, induced biosynthesis of luteolin glycosides was assumed as a possible response. Furthermore, luteolin glycosides were shown to have higher antioxidant activity than their corresponding apigenin glycosides, underlining their importance in the cold-stress response [59].
The total amounts of minor components such as acacetin derivatives also increased to a maximum with decreasing temperature. It should be noted that acacetin-O-acetyl-hexoside (25) in NT conditions was not detected. The maximum amount of acacetin glycosides was observed in the LT group (1.41 mg/g) exceeded the amount of the same compounds in NT groups 2.5 times. Data in the literature indicate that the increased amount of methoxylated flavonoids can be paralleled with the increase in O-methyltransferase enzyme activity during cold acclimation [60].
The HPLC-detectable glycosides of flavanone types were represented by eriodictyol and naringenin derivatives. Eriodictyol-O-malonyl-hexosides (6 and 9) in NT conditions were not detected, but were revealed in cold-temperature environments. An increase in the amount of eriodictyol-7-O-glucoside (12) and naringenin-7-O-glucoside (16) in the LT group was found when compared to plants from NT.
Significant diversities in the flavone and flavanone aglycone profiles of D. palmatum grown in all temperature conditions were observed. Common aglycones such as apigenin (27) and luteolin (24) were detected in both experimental groups; however, the amount of luteolin in the LT plants exceeded the same amount in D. palmatum cultivated in NT conditions by more than 10 times.
In addition, the presence of rare methoxylated flavone aglycones such as acacetin (29), isothymusin (30), salvigenin (31) and genkwanin (32) was revealed only in plants cultivated in LT conditions. The sum of flavone aglycones in this group (14.6 mg/g) significantly exceeded the amount of aglycone compounds in NT environments (1.74 mg/g). Flavanone eriodictyol (21) was detected in the NT group in quantities less than 2.9 times compared to plants cultivated in LT conditions, while naringenin (26) was revealed only in the latter experimental group. Therefore, plants under LT are characterized by an increased concentration of free aglycones, including polymethoxylated flavonoids.
The high amount of lipophilic flavonoid aglycones can be explained by their ecophysiological role, which includes providing plants with protection from abiotic stress signals [61]. The lipophilic nature of flavonoid aglycones limited their distribution in plants, which is in contrast to water-soluble flavonoid glycosides. As they usually accumulate on the leaf surface and are extruded through the cuticle, they are known as surface or external flavonoids. Flavonoid aglycones, especially in the highly methylated form, accumulate in the Lamiaceae family [62]. The early note that species of the Dracocephalum genus are unable to accumulate external flavonoids as aglycons should be revised [63]. Low temperatures are known to positively affect the accumulation of the cuticular leaf wax. This mechanism protects plant tissues from cold stress [64]. Lipophilic flavonoid aglycons (polyhydroxylated and polymethoxylated) covalently bind to cutin or associate with waxes [65,66]. Thus, the presence of polymethoxylated flavonoid aglycones suggests its vital role as the first site of plant defense against abiotic stresses, such as LT. Thus, exposing D. palmatum plants to HT or LT resulted in significant changes in the phenolic composition of its metabolome.
2.5. Changes of Carbohydrates of D. palmatum during LT-Cultivation
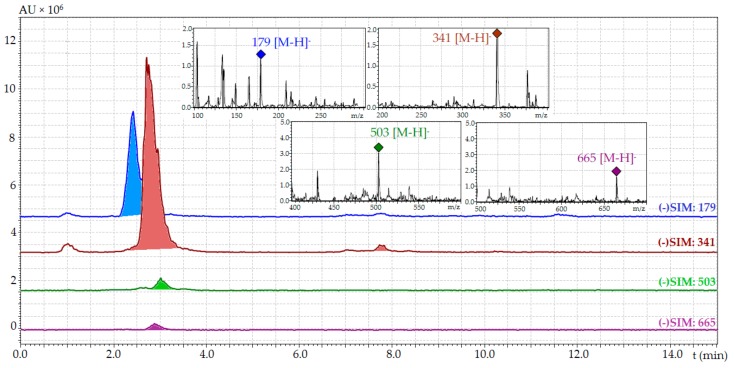
Carbohydrates of plants are key indicators of biochemical changes during temperature acclimation and the acquisition of cold tolerance [67]. Various forms of soluble sugars are involved in physiological reactions to temperature stress: simple sugars such as the mono- and oligosaccharides and polymeric components such as polysaccharides. Known data about soluble sugars of Lamiaceae display a wide distribution of glucose and sucrose as well as the raffinose family oligosaccharides (RFO) [68]. Application of RP-HPLC-MS in selected ion monitoring mode with negative ionization allowed detecting four carbohydrates in samples of D. palmatum (Figure 3).
Figure 3.
RP-HPLC-MS chromatograms in selected ion monitoring mode (SIM, negative ionization) of free sugars fraction of D. palmatum herb (low-temperature sample). SIM with following m/z value used: 179 for glucose, 341 for sucrose, 503 for raffinose, 665 for stachyose. On cuts, mass spectra of corresponding compounds; AU, absorbance units.
Components were identified as glucose, sucrose (α-d-glucopyranosyl-(1→2)-β-d-fructofuranoside), raffinose (α-d-galactopyranosyl-(1→6)-α-d-glucopyranosyl-(1→2)-β-d-fructofuranoside) and stachyose (α-d-galactopyranosyl-(1→6)-α-d-galactopyranosyl-(1→6)-α-d-glucopyranosyl-(1→2)-β-d-fructofuranoside) using HPLC and ESI-MS-data compared to standards (Table 6). Sucrose accumulated to a much higher extent at all temperatures of D. palmatum cultivation (35.54–169.21 mg/g) (Table 6). The concentrations of raffinose (0.87–9.36 mg/g) and stachyose (1.72–38.95 mg/g) were lower than glucose (16.86–26.39 mg/g).
Table 6.
HPLC parameters, electrospray ionization mass spectrometry (ESI-MS) data and the content of simple sugars (mg/g DW ± SD) in D. palmatum herb under different temperatures of cultivation.
| Compound | tR (min) | ESI-MS (m/z) | Content (mg/g) 1 | |
|---|---|---|---|---|
| Temperature (°C) | ||||
| 20 | 1 | |||
| Glucose | 2.38 | 179 [M − H]− | 16.86 ± 0.32 | 26.39 ± 0.52 |
| Sucrose | 2.73 | 341 [M − H]− | 35.54 ± 0.78 | 169.21 ± 3.72 |
| Stachyose | 2.89 | 665 [M − H]− | 1.72 ± 0.03 | 38.95 ± 0.82 |
| Raffinose | 3.04 | 503 [M − H]− | 0.87 ± 0.02 | 9.36 ± 0.18 |
| Total content | 54.99 | 243.91 | ||
1 Averages ± standard deviations were obtained from three different experiments.
The levels of glucose and oligosaccharides in D. palmatum gradually increased during the low-temperature experiment. Total concentration of mono- and oligosaccharides in D. palmatum increased from 54.99 mg/g in group with NT cultivation to 243.91 mg/g in the group with a LT of cultivation. The differences between separate sugar amounts in the 20 °C and 1 °C groups were 1.6-, 4.8-, 10.8- and 22.6-fold for glucose, sucrose, raffinose and stachyose, respectively.
Previous studies have demonstrated that soluble low-molecular weight sugars play multiple roles in LT tolerance of plants. Concentrations of glucose and sucrose may increase several fold during exposure to LT [69]. The accumulation of sucrose in cane sugar supports their function as an osmoprotectant that stabilizes cellular membranes and maintains turgor [70]. The RFOs (raffinose, stachyose) are especially associated with cold hardiness, LT and dormancy [71]. Reaction of the simple sugar profile of D. palmatum LT cultivation was typical for cold-tolerant crops: increases in mono- and oligosaccharide levels were observed. No dramatic changes occurred in D. palmatum sugars after HT cultivation (35 °C).
Cold acclimation of plants also induced changes both in non-structural or reserve polysaccharides as starch or inulin and cell wall polysaccharides like pectin and pectin-associated polymers [72]. For characterization of polymeric sugars of the herb D. palmatum, the high-molecular polysaccharides were isolated by ethanol precipitation, followed by dialysis to yield 2.29% and 9.86% (referring to the dried herb of the 20 °C and 1 °C plant groups, respectively) raw water-soluble polysaccharides (RWSP) (Table 7).
Table 7.
Yield of raw water soluble polysaccharide (RWSP) of D. palmatum herb under different temperatures of cultivation, their general characteristics and monosaccharide compositions.
| Parameter | Temperature (°C) | |
|---|---|---|
| 20 | 1 | |
| RWSP yield (%) a | 2.29 ± 0.04 c | 9.86 ± 0.20 c |
| RWSP general characteristics | ||
| Protein content, % b | 2.61 ± 0.07 c | 2.75 ± 0.09 c |
| Uronic acids, % b | 43.57 ± 1.01 c | 46.16 ± 1.14 c |
| Reaction with I2 (starch) | positive | positive |
| Reaction with resorcinol (inulin) | negative | negative |
| Reaction with Yariv’s reagent (AGP-complexes) | positive | positive |
| Reaction with Fehling’s reagent (mannans) | negative | negative |
| RWSP monosaccharide composition (mol %) | ||
| Ara | 10.1 | 10.2 |
| Gal | 26.1 | 27.7 |
| Glc | 14.4 | 10.2 |
| Fuc | 0.1 | 0.1 |
| Man | 4.3 | 4.0 |
| Rha | 1.9 | 1.7 |
| Rib | Tr. d | Tr. d |
| Xyl | Tr. d | Tr. d |
| GalA | 41.2 | 44.6 |
| GlcA | 1.8 | 1.4 |
a Percentage of dry plant weight ± SD; b Percentage of dry RWSP weight ± SD; c Averages ± standard deviations were obtained from three different experiments; d Tr., traces (<0.1 mol %).
The dialyzed RWSP contained a high amount of uronic acid, which increased from 43.57 in the 20 °C group to 46.16% in the 1 °C group and a low concentration of protein components (2.61–2.75%). All samples of RWSP gave a positive reaction with iodine and Yariv reagent, demonstrating the presence of starch and arabinogalactan-protein complexes, respectively. No reaction with either resorcinol and Fehling’s reagent indicated the absence of inulin and polymeric mannans. The polysaccharide fractions were composed of galacturonic acid, galactose, glucose and arabinose as main sugars with ratios of 4.1:2.6:1.4:1 and 4.4:2.7:1.0:1 for plants grown at NT and LT, respectively. Quantitatively minor components of RWSP were mannose, rhamnose, glucuronic acid and fucose and trace monomers such as xylose and ribose. Thus, the polysaccharide composition of D. palmatum seems to be composed of a mixture of starch, arabinogalactans and/or arabinogalactan–protein complexes and pectic components with high uronic amount.
Regarding monosaccharide composition of RWSP samples, two evident changes observed with the polysaccharide complex of D. palmatum growing at LT should be mentioned: (i) the amount of glucose in RWSP increased after cultivation temperature increased; and (ii) the amount of galacturonic acid, galactose and arabinose in RWSP increased after cultivation temperature decreased. The first event may be caused by the influence of the main plant glucose-containing polymer (starch), whose amount typically declines during exposure to LTs due to hydrolysis [73]. Cold-induced starch degradation was supported many times for various species as a process involved in the freezing tolerance enhancement of plants during an early phase of cold acclimation [74]. The cumulative effect of LT on galacturonic acid as a principal component of pectin and galactose and arabinose as monomers of satellite arabinogalactans was also previously discussed. Pectins appear to be a key element of the plant response to cold stress as shown by a number of studies on various species [75]. In Pisum sativum L., cold acclimation was accompanied by an increase in galacturonan and highly branched rhamnogalacturonan with branched and unbranched arabinans and galactans. The increased cold tolerance might be related to increased synthesis of arabinan and galactan side chains and galacturonan, which may act as a gelling component and a cold protectant [76].
2.6. Changes of Malondialdehyde, Antioxidant Enzymes and Antioxidant Potential of D. palmatum during LT-Cultivation
Low temperature not only caused various phenotypic changes but also initiates cellular damages reflected on increased electrolyte leakage and misbalance in reactive oxygen species (ROS) level and antioxidant content. Malondialdehyde (MDA) served as an indicator of cell membrane injury, elevated concentration of ROS and lipid peroxidative processes [77]. The level of MDA in NT plants was 92.74 nM/g fresh weight in opposite to LT plants with a value 197.02 nM/g fresh weight (Table 8). These results verified a significant raise in MDA content in leaf tissues after LT treatment, signifying that cold stress affected oxidative lipid injury. For scavenging of ROS, plants have a specific antioxidant mechanisms included enzymatic (superoxide dismutase, catalase and other) and non-enzymatic antioxidants [78]. Superoxide dismutase (SOD) plays a determinant role in protection against the toxic effects of oxidative stress by scavenging superoxide radicals and promoting their conversion into oxygen and hydrogen peroxide [79]. Catalase is an enzyme that functions in H2O2 degradation, which maintains hydrogen peroxide homeostasis in plants [80]. We measured the activities of two enzymatic antioxidants as superoxide dismutase and catalase in D. palmatum leaf tissue of NT an LT groups. The results indicated that the low temperature induced a significant increase of activity of both enzymes in 4.6 and 1.7 times comparing with NT plants (respectively, for SOD and catalase). The rising activity of antioxidant enzymes is a plant response to high level of the ROS, resulting in protection of cell membranes from increased MDA to accommodate LT stress [81].
Table 8.
Malondialdehyde (MDA) content, superoxide dismutase (SOD) and catalase activities of D. palmatum leaves and antioxidant activity of D. palmatum extracts obtained from the herb grown under different temperatures of cultivation and luteolin-7-O-glucoside as a reference compound a,b.
| Parameter | Luteolin-7-O-Glucoside | Temperature (°C) | |
|---|---|---|---|
| 20 | 1 | ||
| MDA content (nM/g) FW | - | 92.74 ± 7.41 | 197.02 ± 15.76 |
| SOD activity (U/g·min) FW | - | 57.90 ± 5.21 | 264.32 ± 12.35 |
| Catalase activity (U/g·min) FW | - | 0.93 ± 0.06 | 1.53 ± 0.12 |
| Total antioxidant capacity (mg-eq). luteolin-7-O-glucoside/g | 1000 iii | 280.98 ± 8.99 i | 682.26 ± 21.15 ii,iii |
| DPPH•-radical scavenging activity, IC50 (µg/mL) | 16.97 ± 0.34 iv,v | 33.28 ± 0.73 vi | 11.40 ± 0.24 iv |
| ABTS•+-radical scavenging activity, IC50 (µg/mL) | 9.86 ± 0.19 vii,viii | 14.62 ± 0.31 viii | 5.69 ± 0.11 vii |
| O2•−-radical scavenging activity, IC50 (µg/mL) | 14.92 ± 0.43 ix,x | 18.36 ± 0.56 x | 9.21 ± 0.21 ix |
| Br•-radical scavenging activity (mg-eq). luteolin-7-O-glucoside/g | 1000 xii | 150.19 ± 1.95 xi | 799.63 ± 11.19 xii |
| NO inactivating activity, IC50 (µg/mL) | >100 | 37.92 ± 1.59 | 21.37 ± 0.85 xiii |
| H2O2 inactivating activity (mM/g) | 0.53 ± 0.02 xiv | 1.56 ± 0.04 | 2.75 ± 0.06 xv |
| Fe2+-chelating activity (µM) Fe2+/g | 106.12 ± 3.18 xvi | 142.84 ± 4.42 xvi,xvii | 206.11 ± 4.78 xviii |
a Averages ± standard deviations (SD) were obtained from five different experiments; b Values with different roman letters (i–xviii) indicate statistically significant differences among groups at p < 0.05 by one-way analysis of variance.
The antioxidant properties of D. palmatum extracts were also analyzed by plenty methods both single-electron transfer (SET) and combination of SET with hydrogen-atom transfer techniques. To the latter methods total antioxidant capacity, DPPH• (2,2-diphenyl-1-picrylhydrazyl) and ABTS•+ (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) scavenging tests were related and successfully performed (Table 8). In turn among SET methods superoxide (O2•−) and bromine (Br•) radicals scavenging assays; nitric oxide (NO) and hydrogen peroxide (H2O2) inactivating tests and ferrous (II) ion (Fe2+) chelating assay were applied. In addition, all methods mentioned above were employed for evaluation of antioxidant potential of luteolin-7-O-glucoside, the dominant compound of D. palmatum herb with known property to prevent oxidation [82].
The total antioxidant capacity varied significantly in plant extracts and ranged from 280.98 mg/g (NT) to 682.26 mg/g (LT) (Table 8). In the DPPH• and ABTS•+ assays, the LT plants demonstrated high efficiency in the scavenging of free radicals (IC50 11.40 and 5.69 µg/mL, respectively).
The plants cultivated in NT conditions demonstrated less pronounced scavenging activity to inactivate DPPH• and ABTS•+ free radicals. The scavenging value against superoxide radicals was highest in the group cultivated in LT environments (IC50 9.21 µg/mL). The efficiency of luteolin-7-O-glucoside in this assay was lower (IC50 14.92 µg/mL). The extract obtained from the herb grown in LT manifested significant scavenging activity of NO molecules (21.37 µg/mL). The herb extracts from plants cultivated in NT conditions demonstrated less pronounced scavenging activity (IC50 37.92 µg/mL, respectively). In addition, the activity of the LT group in the H2O2 inactivating assay and Fe2+-chelating activity assay was characterized as very high in both methods, opposite of the low activity of luteolin-7-O-glucoside. It should be noted that herbal extract cultivated in the LT group was the most active antioxidant in all assays. Early information about the quantitative levels in the experimental groups allowed us to associate the significant antioxidant potential of this extract with the highest total phenolics levels.
Previously, the antioxidant properties of several Dracocephalum extractions were analysed. Thus, methanol extract from the aerial parts of D. moldavica revealed high efficiency in the scavenging of DPPH•, ABTS•+ and superoxide anion radicals (IC50 23.10, 8.0 and 445.5 µg/mL, respectively). The total phenolic value was 289.55 mg/g of dry extract, and rosmarinic acid was the major polyphenol (107.11 mg/g of dry extract) [83]. Luteolin-7-O-glucoside isolated from whole plant D. tanguticum exhibited the highest antioxidant effect in DPPH• (IC50 3.94 µM), ABTS•+ (IC50 56.46 µM) and ferrous ions radicals (IC50 1.10 mM) [84]. The methanol extract of D. heterophyllum demonstrated high efficiency in the scavenging of DPPH• radicals (IC50 37.0 µg/mL) [85]. The total phenolic amount of the D. kotschyi methanol extract from leaves was 175.6 mg/g and DPPH• scavenging capacity of the same extract was 88.99% [86]. The methanol extract from the aerial portion of D. polychaetum revealed effective scavenging of DPPH• radicals (IC50 5.6 mg/mL) [87].
Bioactivity of D. palmatum extracts was dependent from cultivation temperature level. In particular, the antiradical potential against various particles, including neutral 2,2-diphenyl-1-picrylhydrazyl radical and bromine radical, and charged 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) cation-radical and superoxide anion-radical was the highest for the samples cultivated in cold-temperature condition. Inactivating power of the same plant extract in relation nitric (II) oxide and hydrogen peroxide molecules as well as Fe2+ chelating ability significantly exceeded the results obtained in other experimental groups and was similar to or exceeded the activity of the reference antioxidant, luteolin-7-O-glucoside.
3. Materials and Methods
3.1. Chemicals
The following chemicals were purchased from Biosupplies Australia Ply Ltd. (Victoria, Australia): Yariv reagent kit (Cat. No. 100-4); Extrasynthese (Lyon, France): acacetin-7-O-rutinoside (linarin; Cat. No. 1169, ≥98.5%); apigenin-7-O-rutinoside (isorhoifolin; Cat. No. 1121, ≥98.5%); 5-O-caffeoyl quinic acid (neochlorogenic acid; Cat. No. 4961, ≥99%); 3-O-caffeoylquinic acid (chlorogenic acid; Cat. No. 4991, ≥99%); caffeic acid (Cat. No. 6034, ≥99%); luteolin-7-O-glucoside (cynaroside; Cat. No. 1126, ≥98%); luteolin-4′-O-glucoside (Cat. No. 1083, ≥95%); rosmarinic acid (Cat. No. 4957, ≥99%); ChemFaces (Wuhan, China): acacetin-7-O-glucoside (tilianin; Cat. No. CFN92764, ≥95%); chrysoeriol (Cat. No. CFN98785, ≥95%); isothymusin (Cat. No. CFN97562, ≥95%); salvigenin (Cat. No. CFN99883, ≥95%); raffinose (Cat. No. CFN90425, ≥98%); stachyose (Cat. No. CFN90424, ≥98%); Santa Cruz Biotechnology, Inc. (Dallas, TX, USA): β-phellandrene (Cat. No. sc-477582, ≥98%); Sigma-Aldrich (St. Louis, MO, USA): acacetin (Cat. No. 00017, ≥97%); apigenin (Cat. No. 10798, ≥95%); apigenin-7-O-glucoside (cosmosiin, Cat. No. 44692, ≥97%); arabinose (Cat. No. A3256, ≥99%); arbutin (Cat. No. A4256, ≥98%); 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (Cat. No. A1888, ≥98%); bornyl acetate (Cat. No. 45855, ≥99%); camphene (Cat. No. 456055, ≥95%); β-caryophyllene (Cat. No. 75541, ≥98.5%); caryophyllene oxide (Cat. No. 91034, ≥99%); 1,8-cineol (Cat. No. 29210, ≥99%); p-cymene (Cat. No. 30039, ≥99.5%); p-cumenol (Cat. No. 175404, ≥98%); cuminaldehyde (Cat. No. 16679, ≥97%); 2,2-diphenyl-1-picrylhydrazyl (Cat. No. D9132); eriodictyol (Cat. No. 74565, ≥95%); eriodictyol-7-O-rutinoside (eriocitrin; Cat. No. 45714, ≥98%); eriodictyol-7-O-glucoside (Cat. No. 19474, ≥99%); Fehling’s reagent I (Cat. No. 36018); fucose (Cat. No. F8150, ≥98%); galactose (Cat. No. G0750, ≥99%); galacturonic acid monohydrate (Cat. No. 48280, ≥97%); genkwanin (Cat. No. SMB00422, ≥98%); glucose (Cat. No. G8270, ≥99.5%); glucuronic acid (Cat. No. G5269, ≥98%); isoamyl acetate (Cat. No. 79857, ≥99.7%); limonene (Cat. No. 62118, ≥99%); linalool (Cat. No. 51782, ≥99%); lithium perchlorate (Cat. No. 431567, ≥99.99%); luteolin (Cat. No. L9283, ≥98%); luteolin-7-O-rutinoside (scolymoside; Cat. No. SMB00200, ≥95%); mannose (Cat. No. M8574, ≥99%); β-myrcene (Cat. No. W276200, ≥95%); myrtenyl acetate (Cat. No. 80699, ≥95%); naringenin (Cat. No. N58ra93, ≥95%); naringenin-7-O-glucoside (prunin; Cat. No. SMB00076, ≥95%); α-pinene (Cat. No. 80605, ≥98.5%); β-pinene (Cat. No. 80609, ≥98.5%); trans-pinocarveol (Cat. No. 80613, ≥96%); perchloric acid (Cat. No. 311421, ≥70%, 99.999% trace metals basis); resorcinol (Cat. No. 398047, ≥99%); ribose (Cat. No. R7500, ≥99%); rhamnose (Cat. No. W373011, ≥99%); sabinene (Cat. No. 275166, ≥99%); sucrose (Cat. No. S9378, ≥99.5%); γ-terpinene (Cat. No. 86476, ≥98.5%); terpinene-4-ol (Cat. No. 49598, ≥95%); and xylose (Cat. No. X1500, ≥99%). Luteolin-7,4′-di-O-rutinoside (dracopalmaside) and luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside) were isolated previously from D. palmatum [9].
3.2. Plant Material, Growth Conditions, and Stress Treatments
D. palmatum were grown from authenticated seeds obtained from Tsitsin’s Main Botanical Garden of the Russian Academy of Science (Moscow, Russia). Seeds were collected from the Republic Yakutia, Kobyaiskii region, village Kitchan (year of collection 1991). Seeds were sterilized by incubation for 1 min in 75% ethanol and then washed thoroughly with sterile water. The seeds were germinated in soil in peat pots at 20 °C (16 h light at photon flux 135 µmol m−2 s−1/8 h dark at 16 °C). Seedlings at the age of two-month old were subjected to environmental stress. Plants were divided into two groups, one group continued to grow in normal temperature conditions (20 °C). For low temperature treatments, seedlings were transferred to a temperature of 1 °C in an artificial climate box for 20 days. The following light and photoperiodic conditions have been used for seedlings growing: 16 h light at 20 °C (or 1 °C) and photon flux 490 µmol m−2 s−1/8 h dark at 20 °C (or 1 °C). The herbs were collected after 20 days of the environmental stress treatment. The experimental samples were immediately frozen in liquid nitrogen and stored at −80 °C until use for analysis of photosynthetic pigments, malondialdehyde and enzyme activity. A part of fresh material was shade dried at room temperature before analytical assays (phenolic compounds, free sugars), isolation procedures (fatty acids, essential oil, polysaccharides) and bioactivity determination (dry extracts).
3.3. Electrolyte Leakage
Electrolyte leakage was measured following method of Campos et al. [88]. Twenty freshly cut leaf discs (0.5 cm2 each) were rinsed 3 times (2–3 min) with demineralized water and subsequently floated at 4 °C on 10 mL of demineralized water in the dark in the growth chamber. The conductivity of the suspending solution was measured after 24 h using an electrical conductivity analyzer (Expert-002-2-6n; Econics-Expert Co., Ltd., Moscow, Russia) before and after autoclaving at 120 °C for 30 min to release the total electrolytes. Electrolyte leakage was calculated as a percentage of total electrolytes. The results were presented as the mean values ± SD (standard deviations) of three replicates.
3.4. Photosynthetic Pigments Analysis
Photosynthetic pigments (chlorophylls, carotenes, pheophytins) were determined using multi-wavelength spectrophotometric method. Frozen weighed plant leaves (0.5 g) were homogenized under liquid nitrogen and an aliquot of 2 g was weighed into a 50-mL centrifuge tube with 10 mL of acetone/water mixture (4:1). After vortexing (1 min), mixing, and shaking for 30 min, samples were centrifuged for 15 min at 3000× g at room temperature. The procedure of acetone/water extraction was repeated twice and organic fractions were combined in a volumetric flask (50 mL). The final volume of organic extract was reached to 50 mL by acetone/water mixture (4:1). The absorbance of the organic extract was measured using SF-2000 UV-Vis-spectrophotometer (OKB Specter; St. Petersburg, Russia) in a 1 cm quartz cuvette at 470/646.8/663.2 nm (chlorophylls, carotenes) and 536/666 nm (pheophytins) blanked with acetone/water mixture (4:1). Pigment concentrations were calculated using corresponding absorption coefficients [89,90]. The results were expressed in fresh weight basis and were presented as the mean values ± SD (standard deviations) of three replicates.
3.5. Chlorophyll Fluorescence and Carbon Assimilation Rate Measurement
Chlorophyll fluorescence was measured on the upper leaf surfaces by PAM fluorometer Junior PAM (Heinz Walz GmbH, Effeltrich, Germany). The following fluorescence parameters were measured: minimal fluorescence in the dark-adapted state (F0) and the maximal fluorescence in the dark adapted state (Fm). Maximal quantum yield of PSII photochemistry (Fv/Fm) was calculated according to equation: Fv/Fm = (Fm − F0)/Fm. The parameter of the carbon assimilation rate (as µM CO2/m2·s) was analyzed using infrared gas analyzer LCpro+ Portable Gas Exchange System (ADC BioScientific Ltd., Hertfordshire, UK) coupled with Small Leaf Chamber. The results were presented as the mean values ± SD (standard deviations) of ten replicates.
3.6. Fatty Acids Analysis
Fatty acids were extracted using a modified Folch method [91] following by the esterification into fatty acids methyl esters (FAMEs) [92]. The pulverized dried herb (0.1 kg) was extracted at 70 °C for 10 h by Soxhlet extractor with 500 mL of chloroform-methanol (2:1) mixture. The solvent was evaporated to dryness at 35 °C and to yield the fatty acid fraction (FAF). Samples of FAF (0.1 g) were dissolved in 4 mL of 0.5 N sodium hydroxide solution in methanol and boiled under reflux for 60 min. After saponification, 3 mL of boron trifluoride methanolic solution (14%, v/v) was added and the solution boiled for 15 min following by hexane (6 mL) addition and heating under reflux (10 min). Then saturated sodium chloride (1 mL) was added, vortexed (10 s) and centrifuged at 6000 rpm (15 min). The upper hexane layer was removed, concentrated (N2), and the resultant residue was dissolved in hexane (200 µL) before gas chromatography-mass spectrometry (GC-MS) analysis.
The GC-MS system was consisted of an Agilent 6890 N gas chromatograph and an Agilent Technologies 5973 N mass selective/quadrupole detector (Agilent Technologies Inc., Santa Clara, CA, USA). A capillary column HP-INNOWax with a length of 30 m, inner diameter of 0.25 mm, and film thickness of 0.5 µm was used for separation (Agilent Technologies Inc., Santa Clara, CA, USA) with helium at a 25 mL/min as a carrier gas. The temperature was 40 °C after injection then programmed at 2 °C/min to 300 °C, and maintained at that temperature for 45 min. A solvent delay of 9 min was set before MS acquisition began. The transfer line from GC column to MS was set to 180 °C, the source 230 °C, and the quadrupole 150 °C. MS detector was done by electron ionization at 70 eV, with a scan range of 30 a.m.u. to 700 a.m.u. (1 scan/s). Compounds were identified by using online NIST 05 and Wiley-7th library spectra, published MS data and analytical standards parameters of available FAMEs. Stock standard solutions of available FAMEs were prepares by dissolving FAME mixture (Supelco-37 mixture C4–C24, Sigma-Aldrich, St. Louis, MO, USA) in hexane at a concentration 0.2 mg/mL. Tridecanoic acid was used as the internal standard and was dissolved in hexane at concentration 0.5 mg/mL. The relative content of each FAME was calculated by normalization of the obtained total ion current peak areas as the percentages of total fatty acids.
3.7. Essential Oil Analysis
The pulverized dried herb (300 g) was placed in a 2000 mL round-bottom flask along with 600 mL distilled water and subjected to Clevenger hydrodistillation for 2.5 h. The oil was extracted from the distillate with hexane and then dried over anhydrous sodium sulfate. The solvent was evaporated using rotary evaporator gave the essential oil. The aliquot of essential oil (10 µL) was dissolved in hexane (500 µL) before gas chromatography-mass spectrometry (GC-MS) analysis.
The GC-MS system was consisted of an Agilent 6890 N gas chromatograph and an Agilent Technologies 5973 N mass selective/quadrupole detector (Agilent Technologies Inc., Santa Clara, CA, USA). A capillary column HP-5MS with a length of 30 m, inner diameter of 0.25 mm, film thickness of 0.5 µm and 5% diphenyl- and 95% dimethylpolysiloxane as stationary phase was used for separation (Agilent Technologies Inc., Santa Clara, CA, USA) with helium at a 1 mL/min as a carrier gas. The temperature was 150 °C after injection then programmed at 2 °C/min to 250 °C, and maintained at that temperature for 45 min. A solvent delay of 9 min was set before MS acquisition began. The transfer line from GC column to MS was set to 250 °C, the source 230 °C, and the quadrupole 150 °C. MS detector was done by electron ionization at 70 eV, with a scan range of 41 a.m.u. to 450 a.m.u. (1 scan/s). Compounds were identified by using online NIST 05 and Wiley-7th library spectra, published MS data and analytical standards parameters of available compounds. Stock standard solutions of available aliphatic compounds, phenolic and tepenes were prepared by dissolving each compound in hexane separately at a concentration 0.5 mg/mL. Decane was used as the internal standard and was dissolved in hexane at concentration 1.0 mg/mL. Component relative percentages were calculated based on normalization method without using correction factors.
3.8. Phenolic Compounds Analysis
Reversed-phase high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry (RP-HPLC-DAD-ESI-MS) procedure was used for phenolic compounds qualitative and quantitative analysis. Sample preparation for RP-HPLC-DAD-ESI-MS analysis: an accurately weighted, dried, and powdered D. palmatum herb samples (200 mg) were placed in a conical flasks. Then 5 mL of 60% methanol were added and the mixtures were weighted. The samples were then extracted in an ultrasonic bath for 60 min at 45 °C with an ultrasound power of 100 W and frequency of 35 kHz. After cooling, the flasks weights were reduced to initial sign, and the resultant extracts were filtered through a 0.22-µm polytetrafluoroethylene (PTFE) syringe filter before injection into the HPLC system for analysis.
Experiments were performed on an LCMS 8050 liquid chromatograph coupled with diode-array-detector and triple-quadrupole electrospray ionization detector (Shimadzu, Columbia, MD, USA), using a GLC Mastro C18 column (150 × 2.1 mm, Ø 3 µm; Shimadzu, Kyoto, Japan); the column temperature was 30 °C. Eluent A was water and eluent B was acetonitrile. The injection volume was 1 µL, and elution flow was 200 µL/min. Gradient program: 0–5 min, 5–18% B; 5–12 min, 18–20% B; 12–25 min, 20% B; 25–37 min, 20–45% B; 37–43 min, 45–70% B; 43–58 min, 70–100% B; and 58–60 min, 100–5% B. The DAD acquisitions were performed in the range of 200–600 nm and chromatograms were integrated at 280 nm. For ESI-MS, the parameters were set as follows: temperature levels of ESI interface, desolvation line and heat block were 300 °C, 250 °C and 400 °C, respectively; the flow levels of nebulizing gas (N2), heating gas (air) and collision-induced dissociation gas (Ar) were 3 L/min, 10 L/min and 0.3 mL/min, respectively. The capillary voltage was kept at +4 kV (simple phenols) in positive mode and at −4.5 kV (phenylpropanoids and flavonoids) in negative mode. ESI-MS spectra were recorded by scanning in the range of m/z 100–1900.
Quantification of phenolic compounds was realized in RP-HPLC-DAD experiments using chromatographic conditions mentioned above. To prepare the stock solutions of reference compounds, 1 mg each of arbutin, 5-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, caffeic acid, luteolin-7,4′-di-O-rutinoside (dracopalmaside), luteolin-7-O-rutinoside-4′-O-glucoside (cynarotriside), eriodictyol-7-O-rutinoside (eriocitrin), luteolin-7-O-rutinoside (scolymoside), eriodictyol-7-O-glucoside, luteolin-7-O-glucoside (cynaroside), luteolin-4′-O-glucoside, apigenin-7-O-rutinoside (isorhoifolin), naringenin-7-O-glucoside (prunin), apigenin-7-O-glucoside (cosmosiin), rosmarinic acid, eriodictyol, acacetin-7-O-rutinoside (linarin), acacetin-7-O-glucoside (tilianin), luteolin, naringenin, apigenin, chrysoeriol, acacetin, isothymusin, salvigenin, and genkwanin were accurately weighed and individually dissolved in DMSO/methanol mixture (1:4) in volumetric flasks (1 mL). The external standard calibration curve was generated using five data points, covering the concentration ranges 0.25–1.00 mg/mL. The calibration curves were created by plotting the peak area vs. the concentration levels. Taxifolin (tR 32.54 min) and gardenin B (tR 48.63 min) were used as the internal standards and was dissolved separately in DMSO/methanol mixture (1:4) at concentration 1 mg/mL. The concentrations of O-malonyl-arbutin, eriodictyol-O-malonyl-hexosides, apigenin-7-O-hexoside, luteolin-7-O-acetyl-hexoside and acacetin-7-O-acetyl-hexoside were calculated using arbutin, eriodictyol-7-O-glucoside, apigenin-7-O-glucoside, luteolin-7-O-glucoside and acacetin-7-O-glucoside, respectively, as reference compounds. The following correction coefficients were used to calculate contents of O-malonyl-arbutin, eriodictyol-O-malonyl-hexosides, luteolin-7-O-acetyl-hexoside and acacetin-7-O-acetyl-hexoside: 1.316, 1.191, 1.094 and 1.094, respectively. All analyses were carried out in triplicate and the data were expressed as mean value ± standard deviation (SD).
3.9. Carbohydrates Analysis
3.9.1. RP-HPLC-MS Analysis of Free Sugars
RP-HPLC-DAD-ESI-MS procedure was used for analysis of free sugars. Sample preparation for free sugar analysis: an accurately weighted, dried, and powdered D. palmatum herb samples (100 mg) were placed in a conical flasks. Then, 10 mL of 40% methanol were added and the mixtures were weighted. The samples were then extracted in an ultrasonic bath for 30 min at 50 °C with an ultrasound power of 100 W and frequency of 35 kHz. After cooling, the flasks weights were reduced to initial sign, and the resultant extracts were filtered through a 0.22-µm polytetrafluoroethylene (PTFE) syringe filter before injection into the HPLC system for analysis.
Experiments were performed on an LCMS 8050 liquid chromatograph (Shimadzu, Columbia, MD, USA) coupled with diode-array-detector (DAD) and triple-quadrupole electrospray ionization detector (ESI), using a GLC Mastro C18 column (150 × 2.1 mm, Ø 3 µm; Shimadzu, Kyoto, Japan); the column temperature was 25 °C. Eluent A was water and eluent B was acetonitrile–water mixture (20:80). The injection volume was 1 µL, and elution flow was 150 µL/min. Gradient program: 0–10 min, 2–8% B; and 10–16 min, 8–12% B. For selected ion monitoring mode (SIM) with negative ionization mass-spectrometry, the parameters were set as follows: SIM m/z values—179 a.m.u. for glucose, 341 a.m.u. for sucrose, 503 a.m.u. for raffinose, 665 a.m.u. for stachyose; temperature levels of ESI interface, desolvation line and heat block were 300 °C, 250 °C and 400 °C, respectively; the flow levels of nebulizing gas (N2), heating gas (air) and collision-induced dissociation gas (Ar) were 3 L/min, 10 L/min and 0.3 mL/min, respectively. The capillary voltage was kept at −4 kV in negative mode. RP-HPLC-MS quantification experiments were carried out at the same chromatographic conditions in SIM mode.
To prepare the stock solutions of reference sugars, 1 mg of glucose, sucrose, raffinose and stachyose were accurately weighed and individually dissolved in water in volumetric flasks (2 mL). The external standard calibration curve was generated using five data points, covering the concentration range 0.1–0.5 mg/mL. The calibration curves were created by plotting the peak area vs. the concentration levels. Quinic acid (tR 9.26 min) was used as the internal standard and was dissolved in water at concentration 0.4 mg/mL. All the analyses were carried out in triplicate and the data were expressed as mean value ± standard deviation (SD).
3.9.2. Raw Water Soluble Polysaccharide (RWSP) Analysis
Powdered dried D. palmatum herb (100 g) was extracted with 10 L of distilled water at 90 °C for 60 min. The water extract was filtered to remove plant material, concentrated in vacuo to 200 mL and finally centrifuged at 3000 rpm (20 min). The resultant supernatant was precipitated with 95% ethanol (1:5) and centrifuged at 3000 rpm (20 min). The crude polysaccharide pellets were redissolved in water (200 mL) and treated by Sevag method three times to remove protein contaminants [93]. Deproteinised polysaccharide solution was intensively dialyzed against distilled water in dialysis tubes (MW-cut off 2 kDa; Sigma-Aldrich, St. Louis, MO, USA). Finally non-dialysed solution was passed though cation-exchange resin KU-2-8 (H+-form, 200 g; Closed Joint-Stock Company Tokem, Kemerovo, Russia) to remove mineral contaminants. The volume of the polysaccharide solution was reduced in vacuo (200 mL) and liophylized using freeze-dry apparatus Scientz Ordinary 10N (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) to obtain off-white powder of RWSP.
Total carbohydrate content in RWSP was determined by a modified anthrone-H2SO4 spectrophotometric assay with glucose as a standard [94]. Estimation of uronic acid content was realized using 3,5-dimethylphenol-H2SO4 spectrophotometric assay with galacturonic acid as a standard [95]. To determine the protein concentration in RWSP the Bradford colorimetric method was used with BSA as a standard [96]. The tests of RWSP with iodine, resorcinol-HCl reagent, Yariv reagent and Fehling’s reagent to qualify the presence of starch, inulin, arabinogalactan-protein complexes and mannans were performed as described previously [97,98,99].
The monosaccharide composition of RWSP was determined after acidic hydrolysis with trifluoroacetic acid (TFA) following by 1-phenyl-3-methyl-5-pyrazolone (PMP) labeling and microcolumn HPLC with ultraviolet detection separation (HPLC-UV) of PMP-labeled hydrolyzates. To hydrolyze RWSP samples 10 mg was dissolved in 1 mL of 2 M TFA in a 5 mL ampoule, incubated at 120 °C for 2 h, the cooled reaction mixture was centrifuged at 2000 rpm for 5 min and evaporated to dryness under reduced pressure to remove TFA, and the hydrolyzed and dried samples were redissolved in 1 mL of distilled water for the following experiments. PMP-labeling was performed using Sun et al. protocol [100] with slight modification. The hydrolyzed RWSP samples (50 µL) were labeled by adding 15 µL of NaOH (1.5 M) and 75 µL of PMP solution (0.5 M in methanol). The mixtures were incubated at 70 °C for 2 h, cooled to room temperature, and neutralized with 60 µL of HCl (0.5 M), 1 mL of chloroform was added, and, after vigorous shaking (20 s) and centrifuging at 3000 rpm for 10 min, the organic phase was carefully removed and discarded. The aqueous layer was passed through a 0.45 µm syringe filter before HPLC analysis.
Microcolumn HPLC-UV chromatographic separation of PMP-labeled sugars were achieved on an MiLiChrom A-02 microcolumn chromatograph (Econova; Novosibirsk, Russia) equipped with UV-detector. A microcolumn ProntoSIL-120-5-C18 AQ with a length of 75 mm, inner diameter of 1 mm, and particles diameter of 1 µm was used for separation (Metrohm AG, Herisau, Switzerland). Separation was performed in a gradient mode with 100 mM CH3COONH4 solution (pH 6.9) as eluent A, and acetonitrile as eluent B using the following gradient program: 0–20 min, 20–26% B. The column temperature was 35 °C, injection volume was 1 µL, and elution was at 150 µL/min. Chromatograms were recorded at 250 nm. The retention times of PMP-labeled monosaccharides were followed: 6.94 min for mannose, 8.94 min for ribose, 9.15 min for rhamnose, 10.91 min for glucuronic acid, 11.38 min for galacturonic acid, 13.11 min for glucose, 13.73 min for galactose, 14.93 min for xylose, 15.11 min for arabinose, and 16.90 min for fucose.
To prepare the stock solutions of reference sugars, 1 mg of mannose, ribose, rhamnose, glucose, galactose, xylose, arabinose, fucose, galacturonic acid and glucuronic acid were accurately weighed and individually dissolved in water in volumetric flasks (2 mL). All sugar reference samples were PMP-labeled before analysis. The external standard calibration curve was generated using five data points, covering the concentration range 0.01–0.05 mg/mL. The calibration curves were created by plotting the peak area vs. the concentration levels. All the analyses were carried out in triplicate and the data were expressed as mean value ± standard deviation (SD).
3.10. Antioxidant Activity Assays
Frozen leaves (2 g) were homogenized with 10 mL of 0.05 M sodium phosphate buffer solution (pH 7.8) with 1% polyvivylpyrrolidone and was centrifuged at 12,000× g at 4 °C for 15 min. The supernatant was collected for malondialdehyde (MDA) and enzyme analysis. MDA content was measured using spectrophotometric assay based on the thiobarbituric acid reaction [101]. Superoxide dismutase activity was analyzed using spectrophotometric method of Giannopolitis-Ries with measurements of the inhibition levels of nitroblue tetrazolium reduction at 560 nm [102]. Catalase activity was measured using spectrophotometric method of Abassi et al. which include the measuring of H2O2 extinction decline at 240 nm [103].
The total antioxidant capacity of D. palmatum extracts was determined using the phosphomolybdic acid method [104]; the DPPH• radical scavenging activity and ABTS•+ radical scavenging activity was assessed as described early [105]; the determination of superoxide anion scavenging activity (O2•−-SA) was measured in phenazine methosulfate-nicotinamide adenine dinucleotide-nitroblue tetrazolium systems using the method of Ozen et al. [106]; the Br• radical scavenging activity (Br•-SA) was determined using culometric method [105] with electrogenerated bromine radicals; the potential to inactivate NO was assessed by the sodium nitropusside technique [107]; the ability to inactivate H2O2 was determined using the technique developed by Badami and Channabasavaraj [108]; to evaluate the chelating activity for Fe2+ ions the o-phenanthroline technique was applied [59].
For preparation of the extract, accurately-weighed dried sample of D. palmatum herb (10 g) was transferred in a conical flask for preparation of the extracts. After that, 150 mL of solvent (60% methanol solutions) was added with stirring and put in an ultrasonic bath. The extraction conditions were 60 min at 45 °C, ultrasound power of 100 W, the frequency 35 kHz. The extraction was repeated twice. The obtained extract were filtered through a cellulose filter and combined. The filtrates were evaporated in vacuo until dryness with the use of a rotary evaporator.
3.11. Statistical Analysis
The results were given as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with a post hoc least significant difference test was used to determine significance (p ≤ 0.05) with Statistica 5.5 software (Dell Technologies Inc., Round Rock, TX, USA) together with the correlation matrix with the use of elementary statistics.
4. Conclusions
Since D. palmatum is a typical plant of arctic territory of Yakutia, it is a suitable model plant to qualify and quantify the plant physiological and biochemical responses to temperature change by lipophilic and hydrophilic compound formation and bioactivity variation. In the past, such studies have not been carried out using Yakutian plant as an experimental object. The present study demonstrated that long-term cultivation (20 days) of D. palmatum seedlings at low temperature (1 °C) damaged plant organ integrity that affected on the rising level of electrolyte leakage and reactive oxygen species misbalance resulting in increasing concentration of malondialdehyde and antioxidant enzyme (superoxide dismutase and catalase). However, at the same time, accumulation of photosynthetic pigments, unsaturated fatty acids, EO and volatile monoterpenes; all groups of phenolic compounds including simple phenol derivatives, phenylpropanoids, flavone and flavanone derivatives; and soluble mono- and oligosaccharides and polysaccharides was observed in D. palmatum. All investigated antioxidant assays showed increased levels of bioactivity in the plant material collected at low temperature. For the first time, such multi-factor approach was used to research of D. palmatum and arctic plants as whole. While the relationship between temperature of plants cultivation and their chemical composition is complex, in the present study it was shown that the low temperature may cause specific chemical shifts achieving sustainable growth of the arctic plant. The scope and diverse of the information received allow to expand our knowledge about adaptive potential of arctic plants and their cold acclimation mechanisms. As far as the practical side is concerned, it is worth mentioning that the cold-induced accumulation of the various groups of compounds in D. palmatum confirmed the potential of low temperature cultivation for enrichment of arctic plants used in food and medicinal industries by bioactive components.
Acknowledgments
The authors acknowledge the financial support provided by the Ministry of Education and Science of Russia (Project No. 20.7216.2017/6.7), Project “Assessment, the main trends in change of the natural and socio-economic status, human development of the Arctic Economic Zone of the Republic of Sakha (Yakutia)” of the Program of Integrated Research in the Republic of Sakha (Yakutia) aimed at developing its productive forces and social sphere during 2016–2020, and FASO Russia (Project No. 0337-2016-0006).
Author Contributions
Daniil N. Olennikov and Nina I. Kashchenko designed the experiments; Tat’yana G. Gornostai and Inessa Yu. Selyutina performed the cultivation experiments; Nadezhda K. Chirikova and Nina I. Kashchenko performed the chemical and bioactivity experiments; Daniil N. Olennikov and Ifrat N. Zilfikarov analyzed the data; Daniil N. Olennikov and Nina I. Kashchenko wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ramakrishna A., Ravishankar G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011;6:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eremina M., Rozhon W., Poppenberger B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016;73:797–810. doi: 10.1007/s00018-015-2089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guy C., Kaplan F., Kopka J., Selbig J., Hincha D.K. Metabolomics of temperature stress. Physiol. Plant. 2008;132:220–235. doi: 10.1111/j.1399-3054.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 4.Safronov V.M. Climate change and mammals of Yakutia. Biol. Bull. 2016;43:1256–1270. doi: 10.1134/S1062359016110121. [DOI] [Google Scholar]
- 5.Desyatkin R., Fedorov A., Desyatkin A., Konstantinov P. Air temperature changes and their impact on permafrost ecosystems in Eastern Siberia. Therm. Sci. 2015;19:S351–S360. doi: 10.2298/TSCI150320102D. [DOI] [Google Scholar]
- 6.Doron’kin V.M., Kovtonyuk N.K., Zuev V.V. Flora of Siberia, 11. Nauka; Novosibirsk, Russia: 1997. pp. 170–185. [Google Scholar]
- 7.Efremova M.I., Chirikova N.K. Medicinal and Food Plants of Yakutia as Renewable Resources of Arctic. SVFU; Yakutsk, Russia: 2015. pp. 195–197. [Google Scholar]
- 8.Olennikov D.N., Chirikova N.K., Okhlopkova Z.M., Zulfugarov I.S. Chemical composition and antioxidant activity of Tánara Ótó (Dracocephalum palmatum Stephan), a medicinal plant used by the North-Yakutian Nomads. Molecules. 2013;18:14105–14121. doi: 10.3390/molecules181114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olennikov D.N., Chirikova N.K. Dracopalmaside, a new flavonoid from Dracocephalum palmatum. Chem. Nat. Compd. 2015;51:1067–1069. doi: 10.1007/s10600-015-1493-3. [DOI] [Google Scholar]
- 10.Hussein M.S., El-Sherbeny S.E., Khalil M.Y., Naguib N.Y., Aly S.M. Growth characters and chemical constituents of Dracocephalum moldavica L. plants in relation to compost fertilizer and planting distance. Sci. Hortic. 2006;108:322–331. doi: 10.1016/j.scienta.2006.01.035. [DOI] [Google Scholar]
- 11.Zeng Q., Jin H.-Z., Fu J.-J., Qin J.-J., Hu X.-J., Liu J.-H., Yan L., Chen M., Zhang W.-D. Chemical constituents of plants from the genus Dracocephalum. Chem. Biodivers. 2010;7:1911–1929. doi: 10.1002/cbdv.200900188. [DOI] [PubMed] [Google Scholar]
- 12.Alaei S., Khosh-Khui M., Kobraee S., Zaji B. Effect of different salinity levels on essential oil content and composition of Dracocephalum moldavica. Agric. Commun. 2014;2:42–46. [Google Scholar]
- 13.Said-Al Ahl H.A.H., Abdou M.A.A. Impact of water stress and phosphorus fertilizer on fresh herb and essential oil content of dragonhead. Int. Agrophys. 2009;23:403–407. [Google Scholar]
- 14.Zhang C.J., Li H.Y., Yun T., Fu Y.H., Liu C.M., Gong B., Neng B.J. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Tibetan herbal medicine Dracocephalum heterophyllum Benth. Nat. Prod. Res. 2008;22:1–11. doi: 10.1080/14786410701619076. [DOI] [PubMed] [Google Scholar]
- 15.Alaei S., Mahna N. Comparison of essential oil composition in Dracocephalum moldavica in greenhouse and field. J. Essent. Oil Bear. Plants. 2013;16:346–351. doi: 10.1080/0972060X.2013.813237. [DOI] [Google Scholar]
- 16.Monsef-Esfahani H.R., Karamkhani F., Nickavar B., Abdi K., Faramarzi M.A. The volatile constituents of Dracocephalum kotschyi oils. Chem. Nat. Compd. 2007;43:40–43. doi: 10.1007/s10600-007-0027-z. [DOI] [Google Scholar]
- 17.Murillo-Amador B., Morales-Prado L.E., Troyo-Diéguez E., Córdoba-Matson M.V., Hernández-Montiel L.G., Rueda-Puente E.O., Nieto-Garibay A. Changing environmental conditions and applying organic fertilizers in Origanum vulgare L. Front. Plant Sci. 2015;6:549. doi: 10.3389/fpls.2015.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murillo-Amador B., Nieto-Garibay A., López-Aguilar R., Troyo-Diéguez E., Rueda-Puente E.O., Flores-Hernández A., Ruiz-Espinosa F.H. Physiological, morphometric characteristics and yield of Origanum vulgare L. and Thymus vulgaris L. exposed to open-field and shade-enclosure. Ind. Crop. Prod. 2013;49:659–667. doi: 10.1016/j.indcrop.2013.06.017. [DOI] [Google Scholar]
- 19.Georgieva K., Lichtenthaler H.K. Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica. 2006;44:569–578. doi: 10.1007/s11099-006-0073-y. [DOI] [Google Scholar]
- 20.Glaszmann J.C., Kaw R.N., Khush G.S. Genetic divergence among cold tolerant rices (Oryza satva L.) Euphytica. 1990;45:95–104. doi: 10.1007/BF00033276. [DOI] [Google Scholar]
- 21.Krol M., Huner N.P.A., McIntosh A. Chloroplast biogenesis at cold hardening temperatures. Development of photosystem I and photosystem II activities in relation to pigment accumulation. Photosynth. Res. 1988;14:97–112. doi: 10.1007/BF00032315. [DOI] [PubMed] [Google Scholar]
- 22.Goli S.A.H., Sahafi S.M., Rashidi B., Rahimmalek M. Novel oilseed of Dracocephalum kotschyi with high n-3 to n-6 polyunsaturated fatty acid ratio. Ind. Crop. Prod. 2013;43:188–193. doi: 10.1016/j.indcrop.2012.07.036. [DOI] [Google Scholar]
- 23.Domokos J., Peredi J., Halasz-Zelnik K. Characterization of seed oils of dragonhead (Dracocephalum moldavica L.) and catnip (Nepeta cataria var. citriodora Balb.). Ind. Crop. Prod. 1994;3:91–94. doi: 10.1016/0926-6690(94)90081-7. [DOI] [Google Scholar]
- 24.Marin P.D., Sajdl V., Kapor S., Tatic B., Petkovic B. Fatty acids of the Saturejoideae, Ajugoideae and Scutellarioideae (Lamiaceae) Phytochemistry. 1991;30:2979–2982. doi: 10.1016/S0031-9422(00)98235-9. [DOI] [Google Scholar]
- 25.Tulucku E. Herbal tea fatty acid content of some medicinal plants grown in Konya, Turkey. Asian J. Chem. 2011;23:1369–1379. [Google Scholar]
- 26.Ahmad P. Oilseed Crops: Yield and Adaptations under Environmental Stress. John Wiley & Sons Ltd.; Chichester, UK: 2017. pp. 80–91. [Google Scholar]
- 27.Schulte L.R., Ballard T., Samarakoon T., Yao L., Vadlani P., Staggenborg S., Rezac M. Increased growing temperature reduces content of polyunsaturated fatty acids in four oilseed crops. Ind. Crop. Prod. 2013;51:212–219. doi: 10.1016/j.indcrop.2013.08.075. [DOI] [Google Scholar]
- 28.Tajabadi F., Khalighi-Sigaroodi F., Rezazadech S. Improving gas chromatography–mass spectrometry analysis of essential oils by multivariate curve resolution: Full identification of co-eluting compounds of Dracocephalum moldavica L. Chromatographia. 2017;80:1069–1077. doi: 10.1007/s10337-017-3322-2. [DOI] [Google Scholar]
- 29.Nezhadali A., Khazaeifar A., Akbarpour M., Masrournia M. Chemical composition of essential oil and antibacterial activity of Dracocephalum subcapitatum. J. Essent. Oil Bear. Plants. 2010;13:112–117. doi: 10.1080/0972060X.2010.10643798. [DOI] [Google Scholar]
- 30.Sonboli A., Esmaeili M.A., Gholipour A., Kanani M.R. Composition, cytotoxicity and antioxidant activity of the essential oil of Dracocephalum surmandinum from Iran. Nat. Prod. Commun. 2010;5:341–344. [PubMed] [Google Scholar]
- 31.Esmaeli M.A., Sonboli A., Mirjalili M.H. Oxidative stress protective effect of Dracocephalum multicaule essential oil against human cancer cell line. Nat. Prod. Res. 2014;28:848–852. doi: 10.1080/14786419.2014.881364. [DOI] [PubMed] [Google Scholar]
- 32.Sonboli A., Gholipour A., Yousefzadi M. Antibacterial activity of the essential oil and main components of two Dracocephalum species from Iran. Nat. Prod. Res. 2012;26:2121–2125. doi: 10.1055/s-0031-1282913. [DOI] [PubMed] [Google Scholar]
- 33.Lee S.B., Cha K.H., Kim S.N., Altantsetseg S., Shatar S., Sarangerel O., Nho C.W. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. J. Microbiol. 2007;45:53–57. [PubMed] [Google Scholar]
- 34.Mahmood U., Kaul V.K., Singh V., Brij L., Negi H.R., Ahuja P.S. Volatile constituents of the cold desert plant Dracocephalum heterophyllum Benth. Flavour Fragr. J. 2005;20:173–175. doi: 10.1002/ffj.1439. [DOI] [Google Scholar]
- 35.Suleimen E.M., Myrzagalieva A.B., Ibataev Z.A., Iskakova Z.B., Samarkhanov T.N., Medeubaeva B.Z. Constituent composition and biological activity of essential oil from Dracocephalum peregrinum. Chem. Nat. Compd. 2017;53:173–174. doi: 10.1007/s10600-017-1941-3. [DOI] [Google Scholar]
- 36.Sadraei H., Asghari G., Kasiri F. Comparison of antispasmodic effects of Dracocephalum kotschyi essential oil, limonene and α-terpineol. Res. Pharm. Sci. 2015;10:109–116. [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadi L., Mirza M. Volatile constituents of Dracocephalum aucherry Boiss. J. Essent. Oil Res. 2001;13:202–203. doi: 10.1080/10412905.2001.9699664. [DOI] [Google Scholar]
- 38.Agarwal S.G., Kapahi B.K., Thappa R.K. Essential oil constituents of Himalayan Dracocephalum speciosum Benth. J. Essent. Oil Res. 2005;17:94–95. doi: 10.1080/10412905.2005.9698841. [DOI] [Google Scholar]
- 39.Sangwan N.S.A., Farooqi H.A., Shabih F., Sangwan R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001;34:3–21. doi: 10.1023/A:1013386921596. [DOI] [Google Scholar]
- 40.Prakasa Rao E.V.S., Rao R.S.G., Ramesh S. Seasonal variation in oil content and its composition in two chemotypes of scented geranium (Pelaronium spp.) J. Essent. Oil Res. 1995;7:159–163. doi: 10.1080/10412905.1995.9698491. [DOI] [Google Scholar]
- 41.Putievsky E., Ravid U., Dudai N. The influence of season and harvest frequency on essential oil and herbal yields from a pure clone of sage grown under cultivated conditions. J. Nat. Prod. 1986;49:326–329. doi: 10.1021/np50044a023. [DOI] [Google Scholar]
- 42.Burbott A.J., Loomis W.D. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967;42:20–28. doi: 10.1104/pp.42.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark R.J., Menary R.C. Environmental effects on peppermint (M. piperita L.) effect of day length, photon flux density, night and day temperature on yield and composition of peppermint oil. Aust. J. Plant Physiol. 1980;7:685–692. doi: 10.1071/PP9800685. [DOI] [Google Scholar]
- 44.Qiao J.Q., Xu D., Lian H.Z., Ge X. Analysis of related substances in synthetical arbutin and its intermediates by HPLC–UV and LC–ESI–MS. Res. Chem. Intermed. 2015;41:691–703. doi: 10.1007/s11164-013-1221-1. [DOI] [Google Scholar]
- 45.Martínez-Vázquez M., Estrada-Reyes R., Martínez-Laurrabaquio A., López-Rubalcava C., Heinze G. Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: Sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 2012;141:908–917. doi: 10.1016/j.jep.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Lukas B., Schmiderer C., Mitteregger U., Novak J. Arbutin in marjoram and oregano. Food Chem. 2010;121:185–190. doi: 10.1016/j.foodchem.2009.12.028. [DOI] [Google Scholar]
- 47.Valant-Vetschera K.M., Roitman J.N., Wollenweber E. Chemodiversity of exudate flavonoids in some members of the Lamiaceae. Biochem. Syst. Ecol. 2003;31:1279–1289. doi: 10.1016/S0305-1978(03)00037-1. [DOI] [Google Scholar]
- 48.Selenge E., Murata T., Tanaka S., Sasaki K., Batkhuu J., Yoshizaki F. Monoterpene glycosides, phenylpropanoids, and acacetin glycosides from Dracocephalum foetidum. Phytochemistry. 2014;101:91–100. doi: 10.1016/j.phytochem.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Dai L.-M., Zhao C.-C., Jin H.-Z., Tang J., Shen Y.-H., Li H.-L., Peng C.-Y., Zhang W.-D. A new ferulic acid ester and other constituents from Dracocephalum peregrinum. Arch. Pharm. Res. 2008;31:1325–1329. doi: 10.1007/s12272-001-2113-2. [DOI] [PubMed] [Google Scholar]
- 50.Fu P., Zhao C.-C., Tang J., Shen Y.-H., Xu X.-K., Zhang W.-D. New flavonoid glycosides and cyanogenic glycosides from Dracocephalum peregrinum. Chem. Pharm. Bull. 2009;57:207–210. doi: 10.1248/cpb.57.207. [DOI] [PubMed] [Google Scholar]
- 51.She G., Guo Z., Lv H., She D. New flavonoid glycosides from Elsholtzia rugulosa Hemsl. Molecules. 2009;14:4190–4196. doi: 10.3390/molecules14104190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J.Y., Chang E.J., Kim H.J., Park J.H., Choi S.W. Antioxidative flavonoids from leaves of Carthamus tinctorius. Arch. Pharm. Res. 2002;25:313–319. doi: 10.1007/BF02976632. [DOI] [PubMed] [Google Scholar]
- 53.Abad-García B., Garmón-Lobato S., Berrueta L.A., Gallo B., Vicente F. On line characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta. 2012;99:213–224. doi: 10.1016/j.talanta.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 54.Abad-García B., Berrueta L.A., Garmón-Lobato S., Gallo B., Vicente F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A. 2009;1216:5398–5415. doi: 10.1016/j.chroma.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 55.Olennikov D.N., Akobirshoeva A.A. Flavonoids and phenylpropanoids of Nepeta glutinosa and Ziziphora pamiroalaica. Chem. Nat. Compd. 2016;52:909–912. doi: 10.1007/s10600-016-1812-3. [DOI] [Google Scholar]
- 56.Fattahi M., Nazeri V., Torras-Claveria L., Sefidkon F., Cusido R.M., Zamani Z., Palazon J. Identification and quantification of leaf surface flavonoids in wild-growing populations of Dracocephalum kotschyi by LC-DAD-ESI-MS. Food Chem. 2013;141:139–146. doi: 10.1016/j.foodchem.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Neugart S., Kläring H.-P., Zietz M., Schreiner M., Rohn S. The effect of temperature and radiation on flavonol aglycones and flavonol glycosides of kale (Brassica oleracea var. sabelica). Food Chem. 2012;133:1456–1465. doi: 10.1016/j.foodchem.2012.02.034. [DOI] [Google Scholar]
- 58.Klimov S.V., Burakhanova E.A., Dubinina I.M., Alieva G.P., Sal’nikova E.B., Olenichenko N.A., Zagoskina N.V., Trunova T.I. Suppression of the source activity affects carbon distribution and frost hardiness of vegetating winter wheat plants. Russ. J. Plant Physiol. 2008;55:308–314. doi: 10.1134/S1021443708030035. [DOI] [Google Scholar]
- 59.Olennikov D.N., Kashchenko N.I., Chirikova N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules. 2014;19:18296–18316. doi: 10.3390/molecules191118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moheb A., Ibrahim R.K., Roy R., Sarhan F. Changes in wheat leaf phenolome in response to cold acclimation. Phytochemistry. 2011;72:2294–2307. doi: 10.1016/j.phytochem.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 61.Mierziak J., Kostyn K., Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19:16240–16265. doi: 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomás-Barberán F.A., Husain S.Z., Gil M.I. The distribution of methylated flavones in the Lamiaceae. Biochem. Syst. Ecol. 1988;16:43–46. doi: 10.1016/0305-1978(88)90115-9. [DOI] [Google Scholar]
- 63.Tomás-Barberán F.A., Wollenweber E. Flavonoid aglycones of the leaf surfaces of some Labiatae species. Plant Syst. Evol. 1990;173:109–118. doi: 10.1007/BF00940856. [DOI] [Google Scholar]
- 64.Amid A., Lytovchenko A., Fernie A.R., Warren G., Thorlby G.J. The sensitive to freezing3 mutation of Arabidopsis thaliana is a cold-sensitive allele of homomeric acetyl-CoA carboxylase that results in cold-induced cuticle deficiencies. J. Exp. Bot. 2012;14:5289–5299. doi: 10.1093/jxb/ers191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeats T.H., Rose J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013;163:5–20. doi: 10.1104/pp.113.222737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfündel E.E., Agati G., Cerovic Z.G. Optical properties of plant surfaces. In: Riederer M., Müller C., editors. Biology of the Plant Cuticle. Blackwell; Oxford, UK: 2006. [Google Scholar]
- 67.Theocharis A., Clément C., Barka E.A. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235:1091–1105. doi: 10.1007/s00425-012-1641-y. [DOI] [PubMed] [Google Scholar]
- 68.Dos Santos R., Vergauwen R., Pacolet P., Lescrinier E., van den Ende W. Manninotriose is a major carbohydrate in red deadnettle (Lamium purpureum, Lamiaceae) Ann. Bot. 2013;111:385–393. doi: 10.1093/aob/mcs288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bohnert H.J., Sheveleva E. Plant stress adaptations—Making metabolism move. Curr. Opin. Plant Biol. 1998;1:267–274. doi: 10.1016/S1369-5266(98)80115-5. [DOI] [PubMed] [Google Scholar]
- 70.Jouve L., Hoffmann L., Hausman J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): Involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004;6:74–80. doi: 10.1055/s-2003-44687. [DOI] [PubMed] [Google Scholar]
- 71.Van den Ende W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013;4:247. doi: 10.3389/fpls.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Gall H., Philippe F., Domon J.M., Gillet F., Pelloux J., Rayon C. Cell wall metabolism in response to abiotic stress. Plants. 2015;16:112–166. doi: 10.3390/plants4010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollock C.J., Lloyd E.J. The effect of low temperature upon starch, sucrose and fructan synthesis in leaves. Ann. Bot. 1987;60:231–235. doi: 10.1093/oxfordjournals.aob.a087441. [DOI] [Google Scholar]
- 74.Yano R., Nakamura M., Yoneyama T., Nishid I. Starch-related α-glucan/water dikinase is involved in the cold-induced development of freezing tolerance in Arabidopsis. Plant Physiol. 2005;138:837–846. doi: 10.1104/pp.104.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solecka D., Zebrowski J., Kacperska A. Are involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 2008;101:521–530. doi: 10.1093/aob/mcm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baldwin L., Domon J.M., Klimek J.F., Fournet F., Sellier H., Gillet F., Pelloux J., Lejeune-Hénaut I., Carpita N.C., Rayon C. Structural alteration of cell wall pectins accompanies pea development in response to cold. Phytochemistry. 2014;104:37–47. doi: 10.1016/j.phytochem.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q., Chen Q., Wang S., Hong Y., Wang Z. Rice and cold stress: Methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice. 2014;7:24. doi: 10.1186/s12284-014-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan J., Ren J., Zhu W., Amombo E., Fu J., Chen L. Antioxidant responses and gene expression in bermudagrass under cold stress. J. Am. Soc. Hortic. Sci. 2014;139:699–705. [Google Scholar]
- 79.Soydam A.S., Büyük I., Aras S. Relationships among lipid peroxidation, SOD enzyme activity, and SOD gene expression profile in Lycopersicum esculentum L. exposed to cold stress. Genet. Mol. Res. 2013;12:3220–3229. doi: 10.4238/2013.August.29.6. [DOI] [PubMed] [Google Scholar]
- 80.Camejo D., Martí Mdel C., Nicolás E., Alarcón J.J., Jiménez A., Sevilla F. Response of superoxide dismutase isoenzymes in tomato plants (Lycopersicon esculentum) during thermo-acclimation of the photosynthetic apparatus. Physiol. Plant. 2007;131:367–377. doi: 10.1111/j.1399-3054.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 81.Esimi N., Atici Ö. Relationships between some endogenous signal compounds and the antioxidant system in response to chilling stress in maize (Zea mays L.) seedlings. Turk. J. Bot. 2016;40:37–44. doi: 10.3906/bot-1408-59. [DOI] [Google Scholar]
- 82.Brown J.E., Rice-Evans C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998;29:247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 83.Aprotosoaie A.C., Mihai C.T., Vochita G., Rotinberg P., Trifan A., Luca S.V., Petreus T., Gille E., Miron A. Antigenotoxic and antioxidant activities of a polyphenolic extract from European Dracocephalum moldavica L. Ind. Crop. Prod. 2016;79:248–257. doi: 10.1016/j.indcrop.2015.11.004. [DOI] [Google Scholar]
- 84.Wang S.-Q., Han X.-Z., Li X., Ren D.-M., Wang X.-N., Lou H.-X. Flavonoids from Dracocephalum tanguticum and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Bioorg. Med. Chem. Lett. 2010;20:6411–6415. doi: 10.1016/j.bmcl.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 85.Raj X.J., Chaurasia O.P., Vajpayee P.K., Murugan M.P., Bala S.S. Antioxidative activity and phytochemical investigation on a high altitude medicinal plant Dracocephalum heterophyllum Benth. Pharmacogn. J. 2010;2:112–117. [Google Scholar]
- 86.Sharifi B., Goli S.A.H., Maghsoudlou Y. Antioxidant activity and chemical composition of the methanolic extract and related fractions of Dracocephalum kotschyi leaves using liquid chromatography–tandem mass spectrometry. Ind. Crop. Prod. 2017;104:111–119. doi: 10.1016/j.indcrop.2017.04.030. [DOI] [Google Scholar]
- 87.Pouraboli I., Nazari S., Sabet N., Sharififar F., Jafari M. Antidiabetic, antioxidant, and antilipid peroxidative activities of Dracocephalum polychaetum shoot extract in streptozotocin-induced diabetic rats: In vivo and in vitro studies. Pharm. Biol. 2016;54:272–278. doi: 10.3109/13880209.2015.1033561. [DOI] [PubMed] [Google Scholar]
- 88.Campos P.S., Quartin V., Ramalh J.C., Nunes M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003;160:283–292. doi: 10.1078/0176-1617-00833. [DOI] [PubMed] [Google Scholar]
- 89.Lichtenthaler H.K., Buschmann C. Current Protocols in Food Analytical Chemistry. John Wiley & Sons; Weinheim, Germany: 2010. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Unit F4.3.1–Unit F4.3.8. [Google Scholar]
- 90.Vernon L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960;32:1144–1150. doi: 10.1021/ac60165a029. [DOI] [Google Scholar]
- 91.Olennikov D.N., Agafonova S.V., Penzina T.A., Borovskii G.B. Fatty acid composition of fourteen wood-decaying basidiomycete species growing in permafrost conditions. Rec. Nat. Prod. 2014;8:184–188. [Google Scholar]
- 92.Pedneault K., Angers P., Avis T.J., Gosselin A., Tweddell R.J. Fatty acids profiles of polar and non-polar lipids of Pleurotus ostreatus and P. cornucopiae var. ‘citrino-pileatus’ grown at different temperatures. Mycol. Res. 2007;111:1228–1234. doi: 10.1016/j.mycres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Sevag M.G., Lackman D.B., Smolens J. The isolation of the components of Streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938;124:425–436. [Google Scholar]
- 94.Olennikov D.N., Tankhaeva L.M., Samuelsen A.B. Quantitative analysis of polysaccharides from Plantago major using the Dreywood method. Chem. Nat. Compd. 2006;42:265–268. doi: 10.1007/s10600-006-0096-4. [DOI] [Google Scholar]
- 95.Usov A.T., Bilan M.I., Klochkova N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulitara P. et R. (Rhodophyta, Corallinaceae) Bot. Mar. 1995;35:43–51. doi: 10.1515/botm.1995.38.1-6.43. [DOI] [Google Scholar]
- 96.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;76:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 97.Olennikov D.N., Stolbikova A.V., Rokhin A.V., Khobrakova V.B., Tankhaeva L.M. Polysaccharides from Fabaceae. V. α-Glucan from Sophora flavescens roots. Chem. Nat. Compd. 2011;47:1–6. doi: 10.1007/s10600-011-9817-4. [DOI] [Google Scholar]
- 98.Olennikov D.N., Tankhaeva L.M. A quantitative assay for total fructans in burdock (Arctium spp.) roots. Russ. J. Bioorg. Chem. 2011;37:893–898. doi: 10.1134/S1068162011070181. [DOI] [Google Scholar]
- 99.Togola A., Inngjerdingen M., Diallo D., Barsett H., Rolstad B. Polysaccharides with complement fixing and macrophage stimulation activity from Opilia celtidifolia, isolation and partial characterization. J. Ethnopharmacol. 2007;115:423–431. doi: 10.1016/j.jep.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 100.Sun Z., Song C., Xia L., Wang X., Suo Y., You J. Comprehensive comparisons between 1-phenyl-3-methyl-5-pyrazolones, 1-(4-methoxyphenyl)-3-methyl-5-pyrazolones and 1-(2-naphthyl)-3-methyl-5-pyrazolones as labeling reagents used in LC-DAD-ESI-MS-MS analysis of neutral aldoses and uronic acids. Chromatographia. 2010;71:789–797. doi: 10.1365/s10337-010-1570-5. [DOI] [Google Scholar]
- 101.Chen J.W., Cao K.F. Changes in activities of antioxidative system and monoterpene and photochemical efficiency during seasonal leaf senescence in Hevea brasiliensis trees. Acta Physiol. Plant. 2008;30:1–9. doi: 10.1007/s11738-007-0070-1. [DOI] [Google Scholar]
- 102.Giannopolitis C.N., Ries S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abassi N.A., Kushad M.M., Endress A.G. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998;74:183–184. doi: 10.1016/S0304-4238(98)00077-6. [DOI] [Google Scholar]
- 104.Preito P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 105.Olennikov D.N., Kashchenko N.I., Chirikova N.K. Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species. Molecules. 2017;27:16. doi: 10.3390/molecules22010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozen T., Demirtas I., Aksit H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011;124:58–64. doi: 10.1016/j.foodchem.2010.05.103. [DOI] [Google Scholar]
- 107.Kumar S., Kumar D., Jusha M., Saroha K., Singif N., Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58:215–220. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 108.Badami S., Channabasavaraj K.P. In vitro antioxidant activity of thirteen medicinal plants of India’s Western Ghats. Pharm. Biol. 2007;45:392–396. doi: 10.1080/13880200701215141. [DOI] [Google Scholar]