Abstract
Alzheimer’s disease (AD) is characterized by extracellular plaques in the brain, mainly consisting of amyloid-β (Aβ), as derived from sequential cleavage of the amyloid precursor protein. Epidemiological studies suggest a tight link between hypovitaminosis of the secosteroid vitamin D and AD. Besides decreased vitamin D level in AD patients, an effect of vitamin D on Aβ-homeostasis is discussed. However, the exact underlying mechanisms remain to be elucidated and nothing is known about the potential effect of vitamin D analogues. Here we systematically investigate the effect of vitamin D and therapeutically used analogues (maxacalcitol, calcipotriol, alfacalcidol, paricalcitol, doxercalciferol) on AD-relevant mechanisms. D2 and D3 analogues decreased Aβ-production and increased Aβ-degradation in neuroblastoma cells or vitamin D deficient mouse brains. Effects were mediated by affecting the Aβ-producing enzymes BACE1 and γ-secretase. A reduced secretase activity was accompanied by a decreased BACE1 protein level and nicastrin expression, an essential component of the γ-secretase. Vitamin D and analogues decreased β-secretase activity, not only in mouse brains with mild vitamin D hypovitaminosis, but also in non-deficient mouse brains. Our results further strengthen the link between AD and vitamin D, suggesting that supplementation of vitamin D or vitamin D analogues might have beneficial effects in AD prevention.
Keywords: vitamin D, vitamin D analogues, amyloid precursor protein, amyloid-β, secretases, Aβ-degradation
1. Introduction
The most important pathological hallmarks of Alzheimer’s disease (AD), which is a progressive neurodegenerative disorder, are extracellular plaques that are composed of aggregated Aβ peptides and intracellular neurofibrillary tangles, consisting of hyperphosphorylated Tau proteins [1,2,3,4]. Aβ-generation depends on initial cleavage of the amyloid precursor protein (APP) by β-secretase 1 (BACE1), followed by intramembrane cleavage of APP by γ-secretase, a heterotetrameric protein complex consisting of presenilin 1 or 2 (PS1, PS2), nicastrin, anterior-pharynx-defective 1a or 1b (Aph1a, Aph1b), and presenilin-enhancer 2 (PEN2) [5,6,7,8]. Besides amyloidogenic, Aβ-releasing processing of APP, APP can be shed by α-secretases in a non-amyloidogenic pathway [9,10,11,12]. The α-secretases cleave APP within the Aβ domain and preclude the formation of Aβ peptides. Total Aβ level is not only dependent on the proteolytic activities of the APP cleaving secretases, but also on Aβ-degradation, involving e.g., the Aβ degrading enzymes neprilysin (NEP) and insulin-degrading enzyme (IDE) [13,14]. In addition to several lipids that influence the generation, degradation, and aggregation of Aβ peptides [15,16,17,18,19,20,21,22,23,24,25], it has recently been shown that fat soluble vitamins affect molecular mechanisms that are involved in AD pathogenesis, e.g., Aβ-induced neurotoxicity, oxidative stress, inflammatory processes, as well as Aβ-generation, Aβ-degradation, and Aβ-clearance [26,27,28,29,30,31]. Several vitamins, including vitamin A, provitamin Aβ-carotene, vitamin D3, vitamin K, and vitamin E, have also been reported to be reduced in plasma/serum of AD patients [30,32,33,34,35,36] and vitamin D hypovitaminosis affects up to 90% of the elderly population [37]. The biological activity of vitamin D can be attributed to binding interactions with the vitamin D receptor (VDR), which undergoes a conformational change, allowing for an interaction with the retinoid X receptor (RXR). The VDR-RXR heterodimer is considered to be the active complex that binds to vitamin D response elements in the DNA of target genes [38,39]. The VDR as well as the 1α-hydroxylase (CYP27B1), which converts 25(OH) vitamin D3 (calcifediol) into its active form 1,25(OH)2 vitamin D3 (calcitriol), have been shown to be expressed in human brain [40], and vitamin D and vitamin D metabolites have been reported to cross the blood-brain-barrier [41]. Recently, we and others could show that vitamin D deficiency causes an increase in amyloidogenic β-secretase cleavage of APP and a decrease in Aβ-degradation, resulting in elevated Aβ level [29,42]. In line, 25(OH) vitamin D supplementation elevated Aβ-degradation due to increased NEP expression and activity [29], supporting vitamin D supplementation as a novel approach to treat AD. In the present study, we compare these effects as mediated by vitamin D with vitamin D analogues on their amyloidogenic potential by investigating the APP processing pathways, as well as Aβ-degradation. Experiments were performed in neuroblastoma cells, revealing a concentration of approximately 2.5 ng/mL 25(OH) vitamin D3 [29]. However, it has to be pointed out that, although neuroblastoma cell lines have some neuronal properties, substantial differences when compared to neurons exist, which is a caveat of the study. Therefore, the main results were also validated ex vivo in homogenates of wildtype (wt) and hypovitaminosis D mouse brains. Further studies are needed to verify the potential positive effects of vitamin D and its analogues in vivo.
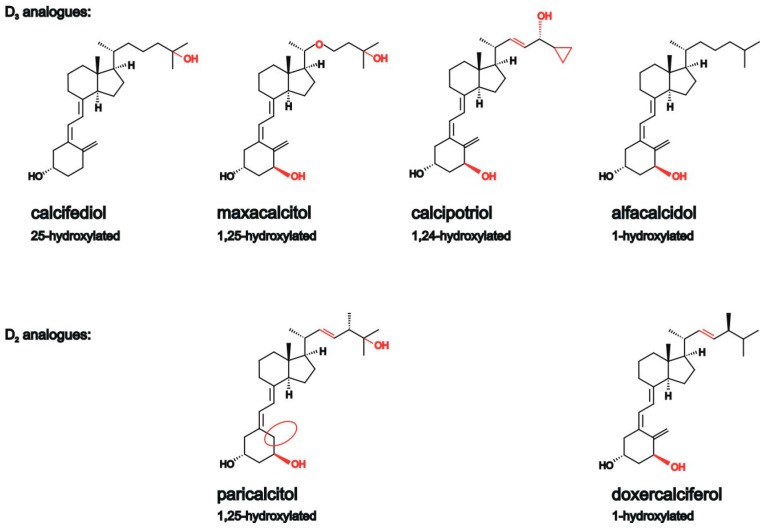
We selected 25(OH) vitamin D3 (calcifediol), which is converted by CYP27B1 to 1,25-dihydroxyvitamin D3, the natural vitamin D hormone, and therapeutically used analogues of vitamin D3 and D2 (Figure 1). Vitamin D analogues are modified in the side-chain portion of the molecule and exert a lower calcemic activity than natural vitamin D3, but retain many therapeutic properties of 1,25-dihydroxyvitamin D3. The 1,25-hydroxylated vitamin D3 analogue, maxacalcitol, is used to treat renal patients that are affected by secondary hyperparathyroidism, while the 1,24-hydroxylated vitamin D3 analogue calcipotriol for treatment of psoriasis and 1-hydroxylated alfacalcidol is administered to treat osteoporosis and secondary hyperparathyroidism. Paricalcitol and doxercalciferol were selected as vitamin D2 analogues. Identical to the vitamin D3 analogue maxacalcitol, paricalcitol contains a hydroxyl group at C1 and C25, but a vitamin D2 instead of a vitamin D3 side chain. 1-hydroxylated doxercalciferol is the vitamin D2 analogue that is comparable to the vitamin D3 analogue alfacalcidol in regard of the hydroxylation status. Both vitamin D2 analogues are used to treat elevated serum parathyroid hormone levels that are associated with secondary hyperparathyroidism [43,44].
Figure 1.
Chemical structure of 25(OH) vitamin D3 and different vitamin D analogues. Structural changes between the analogues are highlighted in red or with red cycles.
2. Results
2.1. Vitamin D Analogues Decrease Total Aβ Level
In order to analyze whether analogues of vitamin D3 and vitamin D2 have similar anti-amyloidogenic properties when compared to 25(OH) vitamin D3 [29], we examined total secreted Aβ level in the human neuroblastoma cell line SH-SY5Y stably transfected with human APP695, the major isoform that is found in neurons [45], in the presence of calcifediol (25(OH) vitamin D3), maxacalcitol, calcipotriol, and alfacalcidol (vitamin D3 analogues), as well as the vitamin D2 analogues paricalcitol and doxercalciferol (Figure 1). We decided to use 25(OH) vitamin D3 instead of active 1,25(OH)2 vitamin D3 as calcifediol has a long serum half-life of approximately three weeks when compared to the short serum half-life (4–6 h) of 1,25(OH)2 vitamin D3 [46]. Cells were treated for 24 h in presence of vitamin D or analogues and secreted Aβ level were examined by analyzing the conditioned media of treated cells or control cells, incubated with the solvent EtOH. Vitamin D and its analogues were incubated in a final concentration of 100 nM. The concentration that was used corresponds to physiological serum concentrations of approximately 75 nmol/L [47,48], and is frequently used for cell culture experiments [26,49,50]. Furthermore, no significant change in cell viability was found using 100 nM vitamin D as compared to the solvent control (supplement Figure S1). Moreover, it has to be mentioned that calcifediol is used as a therapeutical supplement to treat vitamin D hypovitaminosis [51].
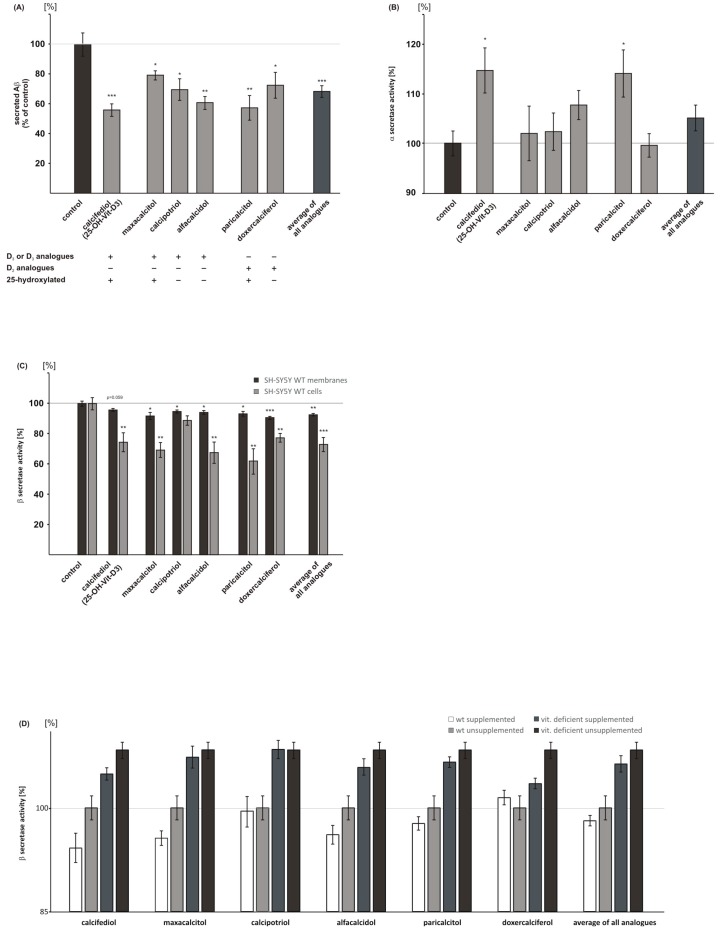
In presence of 25(OH) vitamin D3, calcifediol, which is converted by 1α-hydroxylase CYP27B1 to 1,25-dihydroxyvitamin D3, we found a significant reduction to 55.1% in total Aβ level when compared to cells that are treated with the solvent control (calcifediol: 55.1 ± 4.2%, p ≤ 0.001) (Figure 2A). The vitamin D3 analogues maxacalcitol (1,25-hydroxylated) and calcipotriol (1,24-hydroxylated) also showed significantly reduced Aβ level to 78.2% and 68.2%, respectively (maxacalcitol: 78.2 ± 3.1%, p = 0.031; calcipotriol: 68.2 ± 7.2%, p = 0.017). Alfacalcidol, a 1-hydroxylated vitamin D3 analogue, decreased total Aβ level to 60.3% (alfacalcidol: 60.3 ± 4.3%, p = 0.002). Vitamin D2 analogues also significantly reduced the Aβ level. The determination of total Aβ level of paricalcitol treated cells, a 1,25-hydroxylated vitamin D2 analogue, revealed a significant reduction to 56.3% (paricalcitol: 56.3 ± 8.2%, p = 0.005). The 1-hydroxylated vitamin D2 analogue doxercalciferol revealed a significant decrease to 70.9% (doxercalciferol: 70.9 ± 8.6%, p = 0.037). On average, mean analogues of vitamin D3 and D2 significantly reduced total Aβ level to 66.8% (mean analogues: 66.8 ± 3.9%, p ≤ 0.001) (Figure 2A). However, no significant differences in the Aβ level were obtained between single vitamin analogues as determined by ANOVA analysis.
Figure 2.
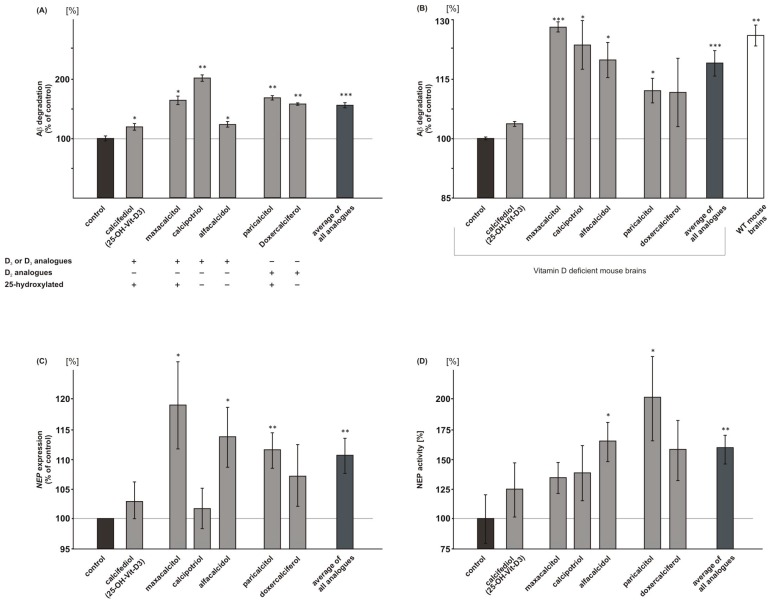
Effect of vitamin D and analogues on Aβ generation. Cells were treated with 25(OH) vitamin D3 (calcifediol), the vitamin D3 analogues maxacalcitol, calcipotriol, alfacalcidol, the vitamin D2 analogues paricalcitol, doxercalciferol in a final concentration of 100 nM or solvent control (EtOH). (A) Total secreted Aβ level in SH-SY5Y APP695 overexpressing cells (n = 3). Aβ of the conditioned media was analyzed by immunoprecipitation and Western Blot (WB) analysis. Using Post Hoc analysis, no significant differences between calcifediol and analogues were found in respect to their potential to reduce Aβ level. (B) Determination of α-secretase activity in living SH-SY5Y wt cells (n = 7). (C) Analysis of β-secretase activity in isolated membranes of SH-SY5Y wt cells (n = 7) and in living cells (n = 5). (D) β-secretase activity in three wt mouse brain and five vitamin D deficient mouse brain homogenates (n = 3). Vitamin D and analogues influence β-secretase activity in wt mouse brains and in vitamin D deficient mouse brains. (E) RT-PCR analysis of BACE1 in SH-SY5Y wt cells (n = 3). (F) Determination of BACE1 protein level in cell lysates of SH-SY5Y wt cells by WB analysis (n = 3). Control conditions were set to 100% and illustrated as a line in the graphic. Error bars represent the standard error of the mean. Asteriks show the statistical significance calculated by unpaired Student’s t test (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
2.2. Analysis of Non-Amyloidogenic APP Shedding in Presence of Vitamin D Analogues
Reduced total Aβ level can be caused by different mechanisms, including an increase in the non-amyloidogenic α-secretase dependent processing of APP. Therefore, we examined α-secretase activity in presence of 25(OH) vitamin D3 (calcifediol), vitamin D3 analogues, and vitamin D2 analogues in living SH-SY5Y wt cells. The vitamin D3 analogues maxacalcitol, calcipotriol, and alfacalcidol and the vitamin D2 analogue doxercalciferol showed no significant effect on α-secretase activity (Figure 2B). In contrast, 25(OH) vitamin D3 calcifediol and the vitamin D2 analogue paricalcitol, being also hydroxylated at C25 like calcifediol, revealed a significant increase in α-secretase activity, to 114.5% and 113.9%, respectively (calcifediol: 114.5 ± 4.5%, p = 0.015; paricalcitol: 113.9 ± 4.7%, p = 0.023).
2.3. Vitamin D Analogues Decrease Amyloidogenic β-Secretase Dependent APP Cleavage
Cleavage of APP by BACE1 is the initial step in Aβ-production, generating the N-terminus of Aβ peptides. To evaluate whether vitamin D analogues have a direct effect on β-secretase activity, we prepared purified membranes of SH-SY5Y wt cells, incubated them with the vitamin D analogues, and measured β-secretase activity. 25(OH) vitamin D3, calcifediol, as well as all vitamin D analogues only slightly affected β-secretase activity directly, however, except for calcifediol, the observed reduction in β-secretase activity was statistically significant (maxacalcitol: 91.7 ± 2.3%, p = 0.016; calcipotriol: 94.7 ± 1.0%, p = 0.023; alfacalcidol: 94.1 ± 1.3%, p = 0.018; paricalcitol: 93.2 ± 1.6%, p = 0.013; and doxercalciferol: 90.7 ± 1.0%, p ≤ 0.001) (Figure 2C). To validate these results, we analyzed β-secretase activity in living SH-SY5Y wt cells. Vitamin D or vitamin D analogue supplemented cells revealed a more pronounced effect on β-secretase activity. Calcifediol significantly reduced β-secretase activity to 74.4%, the vitamin D3 analogues maxacalcitol and alfacalcidol showed a reduction to 69.2% and 67.5%, respectively (calcifediol: 74.4 ± 6.3%, p = 0.009; maxacalcitol: 69.2 ± 5.0%, p = 0.0014; alfacalcidol: 67.5 ± 7.1%, p = 0.004) (Figure 2C). Calcipotriol showed a non-significant decrease in β-secretase activity to 88.8% (calcipotriol: 88.8 ± 3.1%, p = 0.06). Additionally, the vitamin D2 analogues, paricalcitol and doxercalciferol, provided with a markedly reduction to 61.9% and 77.3%, a more pronounced effect in metabolically active cells when compared to β-secretase measurement of supplemented isolated membranes (Figure 2C). This indicates that beside the direct effect, indirect mechanisms, like gene expression or protein stability, contribute to the observed decrease in β-secretase activity. This observation is substantiated by the finding that all of the analogues averaged significantly reduced β-secretase activity in living cells to 72.9% (mean analogues, living cells: 72.9 ± 4.7%, p ≤ 0.001), whereas this effect was alleviated in direct β-secretase activity measurements (mean analogues, cellular membranes: 92.5 ± 0.8%, p = 0.004) (Figure 2C). Notably, calcifediol treated cells, which showed a reduction in β-secretase activity to 74.4% in living cells, reveal a nearly identical decrease in soluble β-secreted APP (sAPPβ) level to 76.9% (sAPPβ: 76.9 ± 1.8%, p = 0.0018) (supplement Figure S2), further underlining the data observed by the fluorescence resonance energy transfer (FRET)-based β-secretase assay.
To further examine the potential of vitamin D analogues to decrease Aβ level by reducing β-secretase activity in an ex vivo situation, we analyzed vitamin D deficient mouse brains showing a 23% reduction in the 25(OH) vitamin D level as compared to wt mouse brains [29], either supplemented with vitamin D or unsupplemented. Therefore, we used homogenates of vitamin D deficient and wt mouse brains, and measured β-secretase activity in the presence or absence of vitamin D analogues. As already shown in our previous study [29] we found a significant increase in β-secretase activity in vitamin D deficient mouse brains when compared to wt mouse brains (Figure 2D). Supplementing of vitamin D deficient mouse brains with vitamin D3 or vitamin D3 and D2 analogues revealed the following results: five out of six analyzed vitamins showed a decrease in β-secretase activity, resulting in a partial rescue of β-secretase activity obtained for unsupplemented wt mouse brains. However, only calcifediol and doxercalciferol reached a level of significance (calcifediol, p = 0.026; doxercalciferol: p = 0.040; Table 1). Four out of six vitamins/analogues tended to decrease β-secretase activity when supplemented on wt mouse brains; three vitamin D species, calcifediol, alfacalcidol and maxacalcitol, revealed a significant reduction in β-secretase activity (calcifediol, p = 0.028; alfacalcidol, p = 0.050; maxacalcitol, p = 0.015). Analyzing the effect of all of the analogues averaged on either wt mouse brains or vitamin D deficient mouse brains, we found a similar decrease in β-secretase activity (Figure 2D), suggesting that both individuals with hypovitaminosis and individuals with normal vitamin D status, might profit from vitamin D supplementation in a similar range, which should be evaluated in further studies. A more detailed statistical analysis is given in Table 1 and Table 2.
Table 1.
Comparison between β-secretase activity in supplemented (WT+; 100 nM calcifediol or its analogues) and unsupplemented (WT-) wildtype mouse brains and in supplemented (deficient+; 100 nM calcifediol or its analogues) and unsupplemented (deficient-) vitamin D deficient mouse brains.
| Analogues | Statistical Test | WT+ | WT+ | WT+ | WT- | WT- | Deficient+ |
|---|---|---|---|---|---|---|---|
| WT− | Deficient+ | Deficient− | Deficient+ | Deficient− | Deficient− | ||
| calcifediol | t test 1 | 0.028 | 0.000 | 0.000 | 0.005 | 0.000 | 0.026 |
| Bonferroni 2 | 0.039 | 0.000 | 0.000 | 0.099 | 0.001 | 0.450 | |
| alfacalcidol | t test | 0.050 | 0.000 | 0.000 | 0.003 | 0.000 | 0.144 |
| Bonferroni | 0.239 | 0.000 | 0.000 | 0.010 | 0.000 | 0.878 | |
| calcipotriol | t test | 0.858 | 0.002 | 0.002 | 0.000 | 0.000 | 0.964 |
| Bonferroni | 1.000 | 0.001 | 0.001 | 0.002 | 0.002 | 1.000 | |
| doxercalciferol | t test | 0.573 | 0.499 | 0.014 | 0.145 | 0.000 | 0.040 |
| Bonferroni | 1.000 | 1.000 | 0.038 | 0.873 | 0.005 | 0.226 | |
| maxacalcitol | t test | 0.015 | 0.000 | 0.000 | 0.001 | 0.000 | 0.606 |
| Bonferroni | 0.167 | 0.000 | 0.000 | 0.001 | 0.000 | 1.000 | |
| paricalcitol | t test | 0.179 | 0.000 | 0.000 | 0.000 | 0.000 | 0.223 |
| Bonferroni | 0.945 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
1 Student’s t test was used to compare two groups. 2 Bonferroni was used to compare more than two groups.
Table 2.
Comparison of the effects of calcifediol and its analogues on β-secretase activity in either WT or vitamin D deficient mouse brains. No significant differences between calcifediol and the analogues tested with Post Hoc Bonferroni.
| Analogues | WT | Deficient | |
|---|---|---|---|
| calcifediol | alfacalcidol | 1.000 | 1.000 |
| calcipotriol | 0.466 | 0.967 | |
| doxercalciferol | 0.066 | 1.000 | |
| maxacalcitol | 1.000 | 1.000 | |
| paricalcitol | 1.000 | 1.000 | |
| alfacalcidol | calcipotriol | 1.000 | 1.000 |
| doxercalciferol | 0.515 | 1.000 | |
| maxacalcitol | 1.000 | 1.000 | |
| paricalcitol | 1.000 | 1.000 | |
| calcipotriol | doxercalciferol | 1.000 | 0.158 |
| maxacalcitol | 1.000 | 1.000 | |
| paricalcitol | 1.000 | 1.000 | |
| doxercalciferol | maxacalcitol | 0.316 | 0.686 |
| paricalcitol | 1.000 | 1.000 | |
| maxacalcitol | paricalcitol | 1.000 | 1.000 |
As described above, we observed a more pronounced reduction in β-secretase activity in living cells in presence of vitamin D analogues when compared to the direct effect of these substances on β-secretase activity. To examine whether vitamin D analogues decrease β-secretase activity by affecting the gene expression of BACE1, we performed qPCR analysis of BACE1 and determined BACE1 protein level by WB analysis in SH-SY5Y wt cells. Except for paricalcitol, all vitamin D3 and vitamin D2 analogues, as well as calcifediol, tended to decrease BACE1 gene expression (Figure 2E).
Significant results were only obtained for calcifediol, calcipotriol, and alfacalcidol (calcifediol: 80.8 ± 2.9%, p = 0.003; calcipotriol: 75.4 ± 8.1%, p = 0.040; alfacalcidol: 70.3 ± 5.4%, p = 0.006). The reason why paricalcitol showed no effect on BACE1 gene expression remains speculative. Paricalcitol, a third generation analogue of vitamin D2, is an activator of VDR and is used for secondary hyperparathyroidism. When compared to calcitriol, different effects were observed e.g., a reduced stimulation of the intestinal calcium transport proteins or a reduced effect on calbindin expression [52]. Similar mechanisms might be responsible for an absent effect on BACE1 expression in the presence of paricalcitol. However, the underlying mechanisms remain to be elucidated.
On average, all of the analogues revealed a significant reduction in BACE1 gene expression to 82.1% (mean analogues: 82.1 ± 4.6%, p = 0.005) (Figure 2E). In accordance with these results, analyzing BACE1 protein level by WB analysis, all vitamin D analogues revealed significant results. In the presence of calcifediol or the vitamin D3 and vitamin D2 analogues, BACE1 protein level was significantly reduced (Figure 2F and supplement Figure S3). 25(OH) vitamin D3, calcifediol, showed the most pronounced reduction in BACE1 protein level to 54.1% (calcifediol: 54.1 ± 5.7%, p = 0.004) (Figure 2F). The vitamin D3 analogues maxacalcitol, calcipotriol, and alfacalcidol revealed a 20 to 30% reduction in BACE1 protein level. For alfacalcidol, the decrease did not reach a significant level (maxacalcitol: 71.4 ± 5.8%, p = 0.020; calcipotriol: 68.9 ± 2.8%, p = 0.009; alfacalcidol: 77.6 ± 8.4%, p = 0.099). A significant reduction in BACE1 protein level to 63.2% and 74.3%, respectively, was also obtained for the vitamin D2 analogues paricalcitol and doxercalciferol (paricalcitol: 63.2 ± 4.2%, p = 0.005; doxercalciferol: 74.3 ± 5.4%, p = 0.025). All of the analogues averaged showed a significant decrease in BACE1 protein level to 71.1% (mean analogues: 71.1 ± 2.5%, p = 0.011) (Figure 2F), which is in line with the decreased BACE1 expression to 82.1%.
2.4. Vitamin D Analogues Decrease γ-Secretase Processing of APP
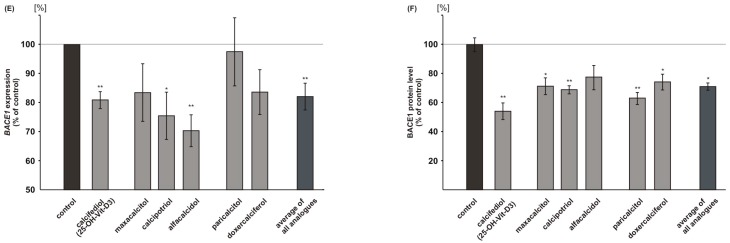
Beside the observed potency of vitamin D3 and vitamin D2 analogues to reduce amyloidogenic β-secretase dependent APP processing we analyzed whether γ-secretase activity, releasing Aβ peptides, is also affected in presence of these substances. As the effect for the vitamin D analogues was more prominent in cultured cells when compared to isolated membranes, and as effects on gene expression and protein level were found in case of β-secretase cleavage, we analyzed γ-secretase activity in metabolically active cells. When compared to cells treated with the solvent control calcifediol, maxacalcitol, alfacalcidol, paricalcitol, and doxercalciferol showed a significant reduction in γ-secretase activity, whereas calcipotriol did not affect γ-secretase activity (calcifediol: 85.3 ± 2.5%, p ≤ 0.001; maxacalcitol: 91.3 ± 3.0%, p = 0.039; alfacalcidol: 74.7 ± 3.3%, p ≤ 0.001; paricalcitol: 80.6 ± 4.0%, p = 0.0014; doxercalciferol: 80.2 ± 4.3%, p = 0.0017) (Figure 3A). All of the vitamin D analogues averaged significantly reduced γ-secretase activity to 86.1% (mean analogues: 86.1 ± 3.4%, p = 0.003). A significant reduction in gene expression of the γ-secretase component nicastrin was found for calcifediol, calcipotriol, alfacalcidol, paricalcitol, and doxercalciferol (calcifediol: 83.3 ± 4.1%, p = 0.015; calcipotriol: 83.9 ± 2.8%, p = 0.005; alfacalcidol: 83.1 ± 3.6%, p = 0.009; paricalcitol: 88.5 ± 3.2%, p = 0.023; doxercalciferol: 82.8 ± 2.7%, p = 0.003) (Figure 3B). Maxacalcitol showed a non-significant reduction in nicastrin mRNA levels to 91.3%. The average of all the analogues also revealed a significant reduction to 85.9% (mean analogues: 85.9 ± 1.7%, p ≤ 0.001), which is comparable to the observed reduction in secretase activity to 86.1%.
Figure 3.
Effect of vitamin D and analogues on γ-secretase. (A) Analysis of γ-secretase activity in living SH-SY5Y wt cells (n ≥ 5). (B) mRNA level of the γ-secretase component nicastrin determined by RT-PCR analysis (n = 3). Error bars represent the standard error of the mean. Asteriks show the statistical significance calculated by unpaired Student’s t test (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
2.5. Vitamin D3 and Vitamin D2 Analogues Increase Aβ-Degradation
In order to investigate whether vitamin D3 and vitamin D2 analogues affect Aβ-degradation, we used mouse neuroblastoma N2a cells and treated these cells with vitamin analogues in presence of human synthetic Aβ peptides. Remaining synthetic Aβ peptides were detected by WB analysis, using an antibody recognizing human but not endogenous mouse Aβ. In the presence of 25(OH) vitamin D3, calcifediol, Aβ-degradation was significantly increased to 119.6% (calcifediol: 119.6 ± 4.5%, p = 0.034) (Figure 4A). The 1,25-hydroxylated and 1,24-hydroxylated vitamin D3 analogues maxacalcitol and calcipotriol increased Aβ-degradation to 164.5% and 202.1%, whereas the 1-hydroxylated vitamin D3 analogue alfacalcidol revealed an increase in Aβ-degradation that was similar to calcifediol (maxacalcitol: 164.5 ± 6.3%, p = 0.009; calcipotriol 202.1 ± 4.2%, p = 0.002; alfacalcidol: 123.5 ± 3.5%, p = 0.046) (Figure 4A). An increased Aβ-degradation was also found for vitamin D2 analogues. 1,25-hydroxylated paricalcitol elevated Aβ-degradation to 168.7%, 1-hydroxylated doxercalciferol to 158.2% (paricalcitol: 168.7 ± 2.6%, p = 0.002; doxercalciferol: 158.2 ± 2.8%, p = 0.003). The average of all vitamin D analogues showed a significant increase in Aβ-degradation to 156.1% (mean analogues: 156.1 ± 3.2%, p ≤ 0.001) (Figure 4A). We analyzed whether vitamin D3 and vitamin D2 analogues also have the potential to increase Aβ-degradation in vitamin D deficient mouse brains. Therefore, we incubated homogenates of vitamin D deficient mouse brains with vitamin D analogues in an ex vivo experiment. Maxacalcitol, calcipotriol, alfacalcidol and paricalcitol also significantly increased Aβ-degradation in case of vitamin D deficiency (maxacalcitol: 128.3 ± 1.3%, p = 0.001; calcipotriol: 123.8 ± 6.2%, p = 0.041; alfacalcidol: 120.0 ± 4.5%, p = 0.027; paricalcitol: 112.2 ± 3.1%, p = 0.043), whereas calcifediol and doxercalciferol did not affect Aβ-degradation in mouse brain homogenates of vitamin D deficient mice (Figure 4B). Again, on average, all of the analogues revealed a significant increase in Aβ-degradation in vitamin D deficient mouse brains to 119.2% (mean analogues: 119.2 ± 3.2%, p ≤ 0.001).
Figure 4.
Effect of vitamin D and analogues on Aβ catabolism. Vitamin D and its analogues influence Aβ-degradation. Total Aβ-degradation in (A) mouse neuroblastoma N2a wt cells (n = 3) and (B) vitamin D deficient mouse brains (n = 5). Calcifediol and its analogues increased the Aβ-degradation compared to solvent control. (C) RT-PCR analysis of NEP expression in SH-SY5Y wt cells (n = 3). (D) NEP activity in N2a cells (n = 8). Error bars represent the standard error of the mean. Asteriks show the statistical significance calculated by unpaired Student’s t test (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
In line, RT-PCR analysis of treated human neuroblastoma cells SH-SY5Y revealed that vitamin D and all of the analogues tended to increase NEP expression, one of the main enzymes known to be involved in Aβ-degradation [13]. A significant increase in NEP gene expression was found for alfacalcidol, maxacalcitol, and paricalcitol (alfacalcidol: 113.8 ± 5.0%, p = 0.021; maxacalcitol: 119.1 ± 7.3%, p = 0.026; paricalcitol 111.6 ± 3.0%, p = 0.003) (Figure 4C). The average of all the analogues also showed a significant increase in NEP mRNA level to 110.7% (mean analogues: 110.7 ± 2.9%, p = 0.007). Similarly, vitamin D and the analogues tended to increase NEP activity (Figure 4D), which is again in line with elevated Aβ-degradation in presence of vitamin D or its analogues. Alfacalcidol and paricalcitol, which already showed a significant increase in NEP gene expression, also revealed a significant elevation in NEP activity (alfacalcidol: 165.2 ± 16.5%, p = 0.029; paricalcitol: 201.8 ± 35.3%, p = 0.029 (Figure 4D). All of the analogues averaged revealed a significant increase in NEP activity to 159.6% (mean analogues: 159.6 ± 12.0%, p = 0.0011).
2.6. Influence of Vitamin D3 and Vitamin D2 Analogues on Inflammatory Processes
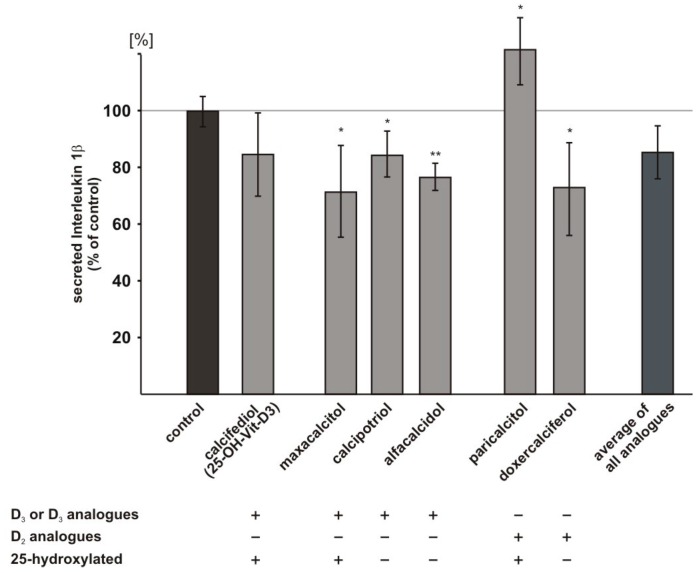
As inflammation plays a crucial role in AD, we also investigated whether vitamin D analogues interfere with inflammatory processes. We selected cytokine Interleukin-1β (IL-1β), which is known to initiate inflammatory responses [53] and reported to be elevated in brains of AD patients [54] to analyze whether vitamin D3 and vitamin D2 analogues might reduce inflammatory responses and used ELISA technique to determine IL-1β level. The vitamin D3 analogues maxacalcitol, calcipotriol, and alfacalcidol significantly reduced IL-1β level to 71.4%, 84.4%, and 76.6%, respectively (maxacalcitol: 71.4 ± 9.3%, p = 0.044; calcipotriol: 84.4 ± 4.6%, p = 0.050; alfacalcidol: 76.6 ± 2.7%, p = 0.005) (Figure 5). Calcifediol and doxercalciferol also showed the tendency to decrease IL-1β level, however, the observed decrease did not reach statistical significance (calcifediol: 84.7 ± 8.4%, p = 0.167; doxercalciferol: 73.1 ± 9.4%, p = 0.054). Paricalcitol significantly increased IL-1β level (paricalcitol: 121.7 ± 6.8%, p = 0.044) (Figure 5).
Figure 5.
Effect of vitamin D and analogues on Interleukin-1β level. Interleukin-1β (IL-1β) level was determined by enzyme-linked immunosorbent assay (ELISA) technique (n ≥ 3). IL-1β was analyzed in SH-SY5Y wt cells incubated with 100 nM calcifediol or analogues compared to cells treated with the solvent control. Error bars represent the standard error of the mean. Asteriks show the statistical significance calculated by unpaired Student’s t test (* p ≤ 0.05; ** p ≤ 0.01).
3. Discussion
Besides the central role of vitamin D in calcium and phosphate homeostasis, vitamin D is discussed to have neuroprotective and anti-inflammatory properties [55,56] and is important for brain development influencing proliferation, differentiation, neurite outgrowth, and neuronal density [57,58]. Several studies also observed an association between cognitive impairment and vitamin D hypovitaminosis [59,60,61,62,63,64]. Moreover, Sutherland et al. reported a link between vitamin D and AD, describing a reduction in VDR mRNA levels in hippocampal CA1 and CA2 pyramidal cells of AD patients when compared to patients that were suffering from Huntington disease [65]. Studies during the past years suggest that lower vitamin D concentrations are associated with a substantially increased risk of all-cause dementia and AD [66,67,68]. Recently, Mokry et al. identified four single nucleotide polymorphisms (SNPs) in the vitamin D pathway that are significantly linked to AD [69]. In our previous study, analyzing mild vitamin D deficiency, we found increased Aβ peptide level caused by increased β-secretase cleavage of APP and decreased Aβ-degradation in vitamin D deficient mouse brains [29]. The aim of the present study was to investigate whether therapeutically used analogues of vitamin D3 and vitamin D2 also shows anti-amyloidogenic properties, and whether differences exist between single vitamin D analogues. In agreement with increased Aβ peptide level in the case of mild 25(OH) vitamin D3 deficiency, calcifediol, which is converted by 1α-hydroxylase CYP27B1 to active 1,25(OH)2 vitamin D3, calcitriol, decreased total Aβ level to 55.1% in human neuroblastoma cells. Also, the vitamin D3 analogues maxacalcitol, calcipotriol and alfacalcidol and the vitamin D2 analogues paricalcitol and doxercalciferol significantly reduced Aβ level. No significant differences were obtained between single vitamin D analogues. In line with our results, a similar reduction of approximately 50% in Aβ40 and Aβ42 peptides and a decrease in the number of amyloid plaques has also been shown in APP transgenic mice that were fed for five months with a vitamin D enriched diet [70]. Beside the potential of vitamin D to reduce Aβ level it has been recently shown that vitamin D3 protects against Aβ peptide cytotoxicity by reverting the Aβ1–42 induced reduction in the sphingosine-1-phosphate/ceramide ratio [26]. We found that vitamin D3 and analogues of vitamin D3 and D2 reduce secreted Aβ peptide level by decreasing amyloidogenic APP processing and by an elevation of Aβ-degradation. Impaired amyloidogenic APP processing in presence of vitamin D and vitamin D analogues is caused by a slight direct effect of these vitamins on β-secretase activity, and by a decrease in BACE1 gene expression, accompanied by reduced BACE1 protein level. Besides transcriptional effects, it cannot be excluded that altered BACE1 protein stability in presence of vitamin D or vitamin D analogues might also contribute to the reduction in BACE1 protein level. BACE1 can be degraded by the lysosomal and ubiquitin-proteasome system (UPS) [71,72]. Interestingly, 1,25-dihydroxyvitamin D3 is involved in the regulation of numerous UPS genes, including ubiquitinating and deubiquitinating enzymes [73]. In this respect, is has to be mentioned that BACE1 degradation is dependent on ubiquitin carboxyl-terminal hydrolase L1, a deubiquitinating enzyme highly specific to neurons [74]. Furthermore, it could be recently shown that vitamin D deficiency down-regulates genes that are involved in protein catabolism [75]. However, so far it is unclear whether these mechanisms are also affected by vitamin D analogues, which has to be investigated in further studies.
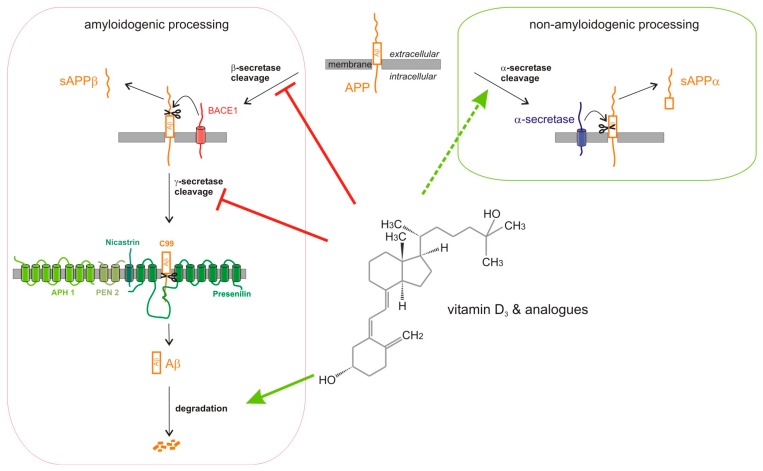
In analogy to our observed effect on BACE1 protein level, Briones et al. found a 24% reduction in BACE1 protein level in old rats supplemented with vitamin D3 as compared to control animals [76]. In accordance to decreased BACE1 protein level in presence of vitamin D, silencing VDR in E16 primary rat cortical neurons increased mRNA and protein level of BACE1 [42], resulting in an increased intracellular Aβ1–42 level. The vitamin D induced reduction in β-secretase APP cleavage could be further substantiated ex vivo by supplementing vitamin D deficient and wt mouse brain homogenates with vitamin D3 or analogues of vitamin D3 and vitamin D2, indicating that both, patients with vitamin D hypovitaminosis and patients with a normal vitamin D status, might profit from vitamin D supplementation. Beside the effect of vitamin D and its analogues on β-secretase, we found significantly reduced γ-secretase activity in metabolically active cells in the presence of vitamin D or vitamin D analogues. In accordance to reduced γ-secretase activity, we found significantly reduced mRNA levels of nicastrin, indicating that altered gene expression of nicastrin, which is necessary for the maturation of the γ-secretase complex [77,78], contributes to the observed reduction in γ-secretase activity. Notably, the mRNA level of nicastrin have been shown to be increased in VDR silenced primary rat cortical neurons [42], thus supporting our results. In addition to the important role of β- and γ-secretase processing of APP in Aβ anabolism, Aβ level can be also decreased by elevated non-amyloidogenic α-secretase processing of APP. However, the effect of vitamin D and its analogues on α-secretase shedding is not as congruent as found for amyloidogenic β-secretase cleavage. In our study, calcifediol and paricalcitol were the only vitamin D species elevating non-amyloidogenic APP processing, whereas the other vitamin D analogues showed no significant effect. Gezen-Ak et al. even found an increase in mRNA and protein level of α-secretase ADAM10 in VDR siRNA-treated cortical neurons [42]. However, as additional metalloproteinases of the ADAM family have been identified as α-secretases [9,12,79], further studies in vitamin D treated, vitamin D deficient or VDR deficient cells are necessary to clarify the role of vitamin D and vitamin D analogues in α-secretase APP processing. Beside Aβ anabolism impaired Aβ-degradation is discussed to contribute to sporadic AD. Vitamin D3, as well as all analyzed vitamin D3 and vitamin D2 analogues, significantly elevated Aβ-degradation in mouse neuroblastoma cells. Increased Aβ-degradation in presence of vitamin D3 or its analogues was also obtained in vitamin D deficient mouse brains. Notably, we observed an increased gene expression of NEP and elevated NEP activity in the presence of vitamin D and its analogues, indicating that the Aβ-degrading enzyme NEP is affected by vitamin D and vitamin D3 and D2 analogues. These results are in line with the finding of Briones et al. reporting an increase in the protein level of NEP in vitamin D supplemented old rats [76]. Based on these findings, vitamin D and its analogues reduce total Aβ level by pleiotropic mechanisms affecting Aβ anabolism and Aβ catabolism (Figure 6). Each of the individual effects of the vitamin D analogues seem to be rather small and in some cases a significant level is not reached. However, all of the observed mechanisms result in a reduction of total Aβ level, which is highly significant and much more pronounced.
Figure 6.
Model of the pleiotropic effects of vitamin D and analogues on Aβ-homeostasis. Vitamin D and analogues decrease amyloidogenic amyloid precursor protein (APP) processing by affecting β- and γ-secretase activity. The reduction of β-secretase activity is caused by a direct effect of vitamin D and its analogues on β-secretase activity combined with indirect effects on BACE1 gene expression and total BACE1 protein level. The γ-secretase activity is reduced by decreased gene expression of nicastrin responsible for the maturation of the heterotetrameric γ-secretase complex. A stimulation of the non-amyloidogenic α-secretase processing of APP was found for 25(OH) vitamin D3 and the vitamin D2 analogue paricalcitol. Total Aβ level in presence of vitamin D and analogues are further reduced by increased Aβ-degradation.
In summary, β-secretase cleavage is affected by a slight direct inhibitory effect on enzyme activity and a decrease in BACE1 expression and BACE1 protein level. γ-secretase activity is reduced by decreased gene expression of the γ-secretase component nicastrin. Furthermore, vitamin D and its analogues increase Aβ-degradation, substantiating the important role of vitamin D and its analogues in Aβ homeostasis, and that supplementation with vitamin D3 or analogues of vitamin D3 and D2 might be protective against biological processes that are associated with AD. Notably, we found a correlation between β-secretase activity, the rate-limiting step in Aβ-generation, Aβ-degradation, and total Aβ level in presence of vitamin D or its analogues, as determined by Pearson correlation (r = 0.699, p = 0.081). In line, BACE1 protein level and Aβ-degradation correlates with the Aβ level (r = 0.746, p = 0.054). However, the Pearson correlation did not reach significance. Regarding vitamin D supplementation to prevent or treat AD, it is important to note that vitamin D3 also interferes with inflammatory processes that are known to contribute to AD. A significant increase in cytokine IL-1β level has been found in post mortem samples from AD patients with a maximum response in those brain regions, frontal cortex, and hippocampus, where AD neuropathology is most prominent [54]. Analyzing IL-1β, initiating inflammatory responses, we could show that vitamin D3 and vitamin D2 analogues, except paricalcitol, significantly reduced the pro-inflammatory IL-1β level. This finding is consistent with the recent findings by Raha et al. reporting that vitamin D2 attenuates Aβ25–35 induced pro-inflammatory cytokines, such as IL-1β, IL-6, and TNFα [27]. Furthermore, a recent study, describing the increased level of pro-inflammatory IL-1β and decreased level of anti-inflammatory IL-10 in old rats as compared to young animals, shows that this age-related change in inflammatory states was mitigated by vitamin D supplementation [76]. This effect seems to be not limited to pro-inflammatory cytokines, as it has recently been shown that 1,25-dihydroxyvitamin D3 upregulates IL-34 known to provide strong neuroprotective and survival signs in brain injury and neurodegeneration [80].
In conclusion, our results substantiate vitamin D supplementation as an approach to prevent or treat AD by reducing Aβ anabolism, elevating Aβ catabolism and the reduction of pro-inflammatory cytokines. The analyzed vitamin D analogues reduce secreted Aβ level with a similar potency, but differ partially in the effect strength of the underlying mechanisms, illustrating that individual AD patients might profit in a different extend of vitamin D analogues. AD patients with reduced anti-amyloidogenic β-secretase activity might benefit the most from supplementation with species like calcifediol and paricalcitol, as these vitamin D species were the only vitamins increasing non-amyloidogenic APP processing. According to our results, individuals with impaired Aβ-degradation might have the highest benefits from vitamin D analogues, like calcipotriol and maxacalcitol, showing the strongest effect on Aβ-degradation. In respect to β- and γ-secretase processing all of the vitamin D analogues revealed similar results, indicating that individuals with increased amyloidogenic secretase activities might benefit from the vitamin D analogues in a similar way.
However, it has to be emphasized that the differences in the effect strength are rather small, and all of the analogues have been shown in respect to Aβ level to be similarly beneficial. Additionally, the use of vitamin D analogues in respect to AD seems to have no therapeutical advantage when compared to the use of calcifediol (or calcitriol), as no significant differences were found between vitamin D analogues and vitamin D. Therefore, further medical indications or pharmacological aspects of the vitamin D analogues should be taken into consideration as to which vitamin D analogue should be applied. Related to this, factors like plasma half-life of individual analogues, the ability to pass the blood-brain-barrier, the affinity for VDR as well as resorption, compatibleness, availability and potential side-effects have to be considered [43,44]. Especially pharmacological factors and the ability to pass the blood-brain-barrier might be even more relevant than the mechanistical differences of the individual analogues, which has to be further evaluated in in vivo experiments and clinical studies.
4. Materials and Methods
4.1. Chemicals and Reagents
Calcifediol, and its analogues, alfacalcidol, calcipotriol, doxercalciferol, maxacalcitol, and paricalcitol were purchased from MedChem Express (Monmouth Junction, NJ, USA), and all other chemicals used in this study were acquired from Sigma-Aldrich (Taufkirchen, Germany), if not stated otherwise.
4.2. Cell Culture and Mice
For the cell based experiments human neuroblastoma SH-SY5Y wt cells, SH-SY5Y APP695 cells and N2a cells were cultivated in Dulbecco’s Modified Eagle’s Medium (DMEM), containing 10% fetal calf serum (FCS, PAN-Biotech, Aidenbach, Germany) and 0.1% non-essential amino acid solution (MEM). For cultivating SH-SY5Y APP695, overexpressing the human APP695 isoform, 0.3 mg/mL hygromycin B (PAN-Biotech, Aidenbach, Germany) was added to the medium. The mouse neuroblastoma cell line N2a was maintained in DMEM/10% FCS/0.1% MEM supplemented with penicillin/streptomycin solution, 2 mM L-glutamine, and 1 mM sodium-pyruvat.
For the ex vivo experiments we used brains from female C57BL/6 wt mice (Charles River, Sulzfeld, Germany) and vitamin D deficient mice. All animal experiments were approved by the “Landesamt für Soziales, Gesundheit und Verbraucherschutz of the State of Saarland” (reference number 17/2011) following the national guidelines for animal treatment. To create the vitamin D deficit, C57BL/6 mice were fed as described, resulting in a 23% reduction in the 25(OH) vitamin D level [29]. After removing, the brains were washed in 0.9% sodium chloride and directly frozen in liquid nitrogen. To establish the homogenates, the mouse brains were slowly defrosted on ice, and afterwards were treated by Minilys (Peqlab, Erlangen, Germany) in HPLC-grade H2O.
4.3. Vitamin D Incubations
4.3.1. Cell Culture
To minimize the influence of 25(OH) vitamin D3 from serum, FCS in DMEM was reduced to 0.1%–2.5% 16 h before incubation, dependent on subsequent experiments. Incubation with 100 nM vitamin D or its analogues (dissolved in ethanol) was carried out for 24 h (8 + 16 h) in up to 2.5% FCS/DMEM. Controls were treated with ethanol in a final concentration of 1‰, corresponding to the concentration in the incubation media.
4.3.2. Mouse Brains or Purified Membranes
Equal amounts of mouse brain homogenates or postnuclear fractions, adjusted using bicinchoninic acid assay, were incubated with 100 nM of vitamin D, its analogues or ethanol for 15 min at 4 °C.
4.4. Determination of Protein Concentration
The determination of the protein concentration of the samples was performed by using the bicinchoninic acid assay (BCA), as described in [81].
4.5. Western Blot Experiments
Samples used for the WB experiments were adjusted to equal protein concentration in advance.
For the determination of BACE1, cell lysates were prepared by lysing cells in 150 mM NaCl, 50 mM Tris/HCl pH 7.4, 2 mM EDTA, 0.1% NP-40, 0.1% Triton-X 100. For the determination of total secreted Aβ level and sAPPβ conditioned media were used.
The following antibodies were used for WB analysis: W02 antibody (5 μg/mL; Millipore, Billerica, MA, USA), anti-sAPPβ: Mbs492139 (1:250; MyBioSource, San Diego, CA, USA), BACE1: ab2077 (1:1000; abcam, Cambridge, UK), anti-actin ab1801 (1:1000; abcam), anti-rabbit IgG HRP Conjugate W401B (1:5000; Promega, Mannheim, Germany) and anti-mouse P0260 (Dako, Hamburg, Germany).
Aβ levels were detected by performing immunoprecipitation of conditioned media before WB analysis. Therefore, 20 μL protein G-Sepharose and W02 antibody (5 μg/mL) were used.
Enhanced chemiluminescense (ECL)-method (Perkin Elmer, Rodgau-Jügesheim, Germany) was used to detect proteins. Densitometrically quantification was performed by using Image Gauge V3.45 software (Fujifilm, Düsseldorf, Germany).
4.6. Determination of Total Aβ-Degradation
For measuring the total degradation of Aβ, human synthetic Aβ40 (Bachem, Bubendorf, Switzerland) was added to murine samples. After a determined period of time, non-degraded human Aβ was detected by WB analysis using W02 antibody, which binds specifically to human Aβ, as described before [82]. Human Aβ differs in the W02 epitope compared to murine Aβ. Therefore, no endogenous produced Aβ is detected by the use of murine N2a cells.
4.6.1. Determination of Total Aβ-Degradation in N2a wt Cells.
After cultivation of mouse neuroblastoma N2a wt cells in reduced FCS (0.1%) /DMEM for 6 h, cells were incubated with 100 nM vitamin D, its analogues or ethanol for 18 h and additionally 6 h in the presence of 0.5 μg/mL human synthetic Aβ40.
4.6.2. Determination of Total Aβ-Degradation in Deficient Mouse Brains
Deficient mouse brain homogenates were incubated as described earlier and treated for 1 h with synthetic Aβ40 in a final concentration of 1 μg/mL, 1 μM β-inhibitor, and 20 μM γ-inhibitor (Merck Millipore, Billerica, MA, USA).
4.7. Secretase Activity Assays
4.7.1. Determination of α-, β- and γ-Secretase Activity in Living SH-SY5Y Cells
Secretase activity assays were performed, as described before [82,83]. Following the incubation, cells were washed twice with prewarmed imaging solution containing 140 mM NaCl, 5 mM KCl, 8 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, pH 7.4. Afterwards, 100 μL (α)/50 μL (β and γ) imaging solution mixed with 3 μM α-secretase substrate (Calbiochem, No. 565767), 20 μM β-secretase substrate (Calbiochem, No. 565758) or 6,25 μM γ-secretase substrate (Calbiochem, No. 565764) was added. The resulting fluorescence was determined continuously at excitation wavelengths of 340 ± 10 nm (α), 345 ± 5 nm (β), 355 ± 10 nm (γ) and emission wavelengths of 490 ± 10 nm (α), 500 ± 5 nm (β), 440 ± 10 nm (γ) under light preclusion and at 37 °C in a Safire2 Fluorometer (Tecan, Crailsheim, Germany).
4.7.2. Determination of β-Secretase Activity in Isolated SH-SY5Y Membranes
Measurements have been done as published in [23]. After homogenization of SH-SY5Y cells in sucrose buffer with Minilys (Peqlab, Erlangen, Germany) using ceramic beads, homogenates were adjusted to an equal protein amount by bicinchoninic acid assay and postnuclear fractions (PNFs) were isolated by sucrose density centrifugation. PNFs were incubated with calcifediol or its analogues for 15 min at 4 °C, and for pelleting membranes ultracentrifuged at 55,000 rpm for 75 min at 4 °C. Following, membranes were resuspended using glass beads in Minilys. For determination of β-secretase activity 20 μM specific β-secretase substrate (described above) was added to 125 μg of protein diluted 1:1 with 1 × PBS pH 4.5. Fluorescence was measured as described before.
4.8. RT-PCR Experiments
Quantitative real-time (RT) PCR has been done, as published in [19]. After isolation of total RNA from incubated SH-SY5Y cells using TRIzol Reagent (Thermo Fisher Scientific; Waltham, MA, USA), 2 μg RNA were reversed transcribed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). RT-PCR using the Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) was performed on a PikoReal Real-Time PCR System (Thermo Fisher Scientific) and the following primers were used. β-actin: forward 5′-CTT CCT GGG CAT GGA GTC-3′, reverse 5′-AGC ACT GTG TTG GCG TAC AG-3′; TATA-box binding protein (TBP): forward 5′-CGG AGA GTT CTG GGA TTG T-3′, reverse 5′-GGT TCG TGG CTC TCT TAT C-3′; β-site APP-cleaving enzyme 1 (BACE1): forward 5′-GCC TAT GCT GAG ATT GCC AGG-3′, reverse 5′-GGA GAA GAG GTT GGG AAC GTG-3′; nicastrin: forward 5′-CTG TAC GGA ACC AGG TGG AG-3′, reverse 5′-GAG AGG CTG GGA CTG ATT TG-3′; neprilysin (NEP): forward 5′-GAT CAG CCT CTC GGT CCT TG-3′, reverse 5′-TGT TTT GGA TCA GTC GAG CAG-3′ (Eurofins MWG Operon, Eberberg, Germany). Two house-keeping genes (β-actin and TBP) were used to exclude RT efficiency differences. The obtained results were normalized to the β-actin/TBP ratio and the 2−(ΔΔCt) method was used to calculate expression changes.
4.9. Neprilysin Activity Assay
Measurement of NEP activity was performed as published in Miners et al. [84], with minor modifications utilizing the anti-NEP antibody AF1182 (R&D Systems, Minneapolis, MN, USA) and 5 μM MCA-RPPGFSAFK(DNP)-OH fluorogenic peptide substrate (R&D Systems, Minneapolis, MN, USA). Cells were chemical lysed in lysis buffer containing 0.5% TritonX-100, 20 nM Tris pH 7.4, 10% sucrose and fluorescence at an excitation wavelength of 320 ± 10 nm, and an emission wavelength of 405 ± 10 nm was measured in a Safire2 Fluorometer (Tecan, Crailsheim, Germany).
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
The level of cytokine IL-1β was measured in the medium of incubated SH-SY5Y wt cells using the Human IL-1 beta ELISA Kit (Abcam, Cambridge, UK).
4.11. Lactate Dehydrogenase (LDH) Activity Assay
The Cytotoxicity Detection Kit (LDH) from Roche (Basel, Schweiz), a colorimetric assay for the measurement of lactate dehydrogenase (LDH) release from cells, was used according manufacturer’s instructions to quantify cell death and lysis after incubation with different vitamin D concentrations.
4.12. Data Analysis
All quantified data represent an average of at least three independent experiments. Error bars represent standard error of the mean. Statistical significance was calculated using two-tailed Student’s t test, ANOVA, and post hoc Tests for multiple comparison analysis. The normality of the data distribution was tested with Shapiro Wilk test. Significance was set at * p ≤ 0.05; ** p ≤ 0.01 and *** p ≤ 0.001.
Acknowledgments
We thank Matthias W. Laschke for providing us with wildtype mouse brains. According to the author guidelines, funding for the research leading to these results were received from: the EU FP7 project LipiDiDiet, Grant Agreement No. 211696. Moreover, funding for MG and TH was provided by Fundacio la Marato de TV3 20140931 and by JPND (EU Joint Programme-Neurodegenerative Disease Research) Mind AD 1ED1508.
Supplementary Materials
Supplementary materials can be found at www.mdpi.com/1422-0067/18/12/2764/s1.
Author Contributions
Andrea Thiel, Anna A. Lauer, Jakob Winkler, Johannes Lehmann, Liesa Regner, Christopher Nelke, Daniel Janitschke, Céline Benoist, Olga Streidenberger, Hannah Stötzel performed the experiments; Kristina Endres, Christian Herr, Christoph Beisswenger, Robert Bals, Frank Lammert, Tobias Hartmann provided material and/or helped to prepare the manuscript; Andrea Thiel, Anna A. Lauer, Heike S. Grimm, Marcus O. W. Grimm wrote the manuscript; Marcus O. W. Grimm, Tobias Hartmann designed the study.
Conflicts of Interest
The authors declare no conflict of interests
References
- 1.Glenner G.G., Wong C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophy. Res. Commun. 2012;425:534–539. doi: 10.1016/j.bbrc.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L., Beyreuther K. Amyloid plaque core protein in alzheimer disease and down syndrome. Proc. Natl. Acad. Sci. USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y.C., Zaidi M.S., Wisniewski H.M. Microtubule-associated protein tau. A component of alzheimer paired helical filaments. J. Biol. Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 5.Sinha S., Anderson J.P., Barbour R., Basi G.S., Caccavello R., Davis D., Doan M., Dovey H.F., Frigon N., Hong J., et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 6.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., et al. β-secretase cleavage of alzheimer’s amyloid precursor protein by the transmembrane aspartic protease bace. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 7.Kimberly W.T., LaVoie M.J., Ostaszewski B.L., Ye W., Wolfe M.S., Selkoe D.J. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc. Natl. Acad. Sci. USA. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haass C. Take five—BACE and the γ-secretase quartet conduct alzheimer’s amyloid β-peptide generation. EMBO J. 2004;23:483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxbaum J.D., Liu K.N., Luo Y., Slack J.L., Stocking K.L., Peschon J.J., Johnson R.S., Castner B.J., Cerretti D.P., Black R.A. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 10.Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M., Haass C., Fahrenholz F. Constitutive and regulated α-secretase cleavage of alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn P.H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J.W., Kremmer E., Rossner S., Lichtenthaler S.F. Adam10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koike H., Tomioka S., Sorimachi H., Saido T.C., Maruyama K., Okuyama A., Fujisawa-Sehara A., Ohno S., Suzuki K., Ishiura S. Membrane-anchored metalloprotease MDC9 has an α-secretase activity responsible for processing the amyloid precursor protein. Biochem. J. 1999;343:371–375. doi: 10.1042/bj3430371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., Gerard C., Hama E., Lee H.J., Saido T.C. Metabolic regulation of brain Aβ by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 14.Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E.A., Frosch M.P., Eckman C.B., Tanzi R.E., Selkoe D.J., Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm M.O., Grimm H.S., Patzold A.J., Zinser E.G., Halonen R., Duering M., Tschape J.A., De Strooper B., Muller U., Shen J., et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-β and presenilin. Nature Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 16.Grimm M.O., Kuchenbecker J., Grosgen S., Burg V.K., Hundsdorfer B., Rothhaar T.L., Friess P., de Wilde M.C., Broersen L.M., Penke B., et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011;286:14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm M.O., Zinser E.G., Grosgen S., Hundsdorfer B., Rothhaar T.L., Burg V.K., Kaestner L., Bayer T.A., Lipp P., Muller U., et al. Amyloid precursor protein (APP) mediated regulation of ganglioside homeostasis linking alzheimer’s disease pathology with ganglioside metabolism. PLoS ONE. 2012;7:e34095. doi: 10.1371/journal.pone.0034095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osenkowski P., Ye W., Wang R., Wolfe M.S., Selkoe D.J. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J. Biol. Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm M.O., Rothhaar T.L., Grosgen S., Burg V.K., Hundsdorfer B., Haupenthal V.J., Friess P., Kins S., Grimm H.S., Hartmann T. Trans fatty acids enhance amyloidogenic processing of the alzheimer amyloid precursor protein (APP) J. Nutr. Biochem. 2012;23:1214–1223. doi: 10.1016/j.jnutbio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Burg V.K., Grimm H.S., Rothhaar T.L., Grosgen S., Hundsdorfer B., Haupenthal V.J., Zimmer V.C., Mett J., Weingartner O., Laufs U., et al. Plant sterols the better cholesterol in alzheimer’s disease? A mechanistical study. J. Neurosci. 2013;33:16072–16087. doi: 10.1523/JNEUROSCI.1506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemkul J.A., Bevan D.R. Aggregation of alzheimer’s amyloid β-peptide in biological membranes: A molecular dynamics study. Biochemistry. 2013;52:4971–4980. doi: 10.1021/bi400562x. [DOI] [PubMed] [Google Scholar]
- 22.Grimm M.O., Haupenthal V.J., Mett J., Stahlmann C.P., Blumel T., Mylonas N.T., Endres K., Grimm H.S., Hartmann T. Oxidized docosahexaenoic acid species and lipid peroxidation products increase amyloidogenic amyloid precursor protein processing. Neurodegener. Dis. 2016;16:44–54. doi: 10.1159/000440839. [DOI] [PubMed] [Google Scholar]
- 23.Rothhaar T.L., Grosgen S., Haupenthal V.J., Burg V.K., Hundsdorfer B., Mett J., Riemenschneider M., Grimm H.S., Hartmann T., Grimm M.O. Plasmalogens inhibit app processing by directly affecting γ-secretase activity in alzheimer’s disease. Sci. World J. 2012;2012:141240. doi: 10.1100/2012/141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm M.O., Grimm H.S., Tomic I., Beyreuther K., Hartmann T., Bergmann C. Independent inhibition of alzheimer disease β- and γ-secretase cleavage by lowered cholesterol levels. J. Biol. Chem. 2008;283:11302–11311. doi: 10.1074/jbc.M801520200. [DOI] [PubMed] [Google Scholar]
- 25.Grimm M.O., Mett J., Stahlmann C.P., Haupenthal V.J., Blumel T., Stotzel H., Grimm H.S., Hartmann T. Eicosapentaenoic acid and docosahexaenoic acid increase the degradation of amyloid-β by affecting insulin-degrading enzyme. Biochem. Cell Biol. 2016;94:534–542. doi: 10.1139/bcb-2015-0149. [DOI] [PubMed] [Google Scholar]
- 26.Pierucci F., Garcia-Gil M., Frati A., Bini F., Martinesi M., Vannini E., Mainardi M., Luzzati F., Peretto P., Caleo M., et al. Vitamin D3 protects against Aβ peptide cytotoxicity in differentiated human neuroblastoma SH-SY5Y cells: A role for S1P1/p38MAPK/ATF4 axis. Neuropharmacology. 2017;116:328–342. doi: 10.1016/j.neuropharm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Raha S., Lee H.J., Yumnam S., Hong G.E., Saralamma V.V.G., Ha Y.L., Kim J.O., Kim Y.S., Heo J.D., Lee S.J., et al. Vitamin D2 suppresses amyloid-β 25–35 induced microglial activation in BV2 cells by blocking the NF-κB inflammatory signaling pathway. Life sci. 2016;161:37–44. doi: 10.1016/j.lfs.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y.X., He L.Y., Zhang M., Wang F., Liu F., Peng W.X. 1,25-dihydroxyvitamin D3 regulates expression of LRP1 and rage in vitro and in vivo, enhancing Aβ1–40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016;322:28–38. doi: 10.1016/j.neuroscience.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 29.Grimm M.O., Lehmann J., Mett J., Zimmer V.C., Grosgen S., Stahlmann C.P., Hundsdorfer B., Haupenthal V.J., Rothhaar T.L., Herr C., et al. Impact of vitamin D on amyloid precursor protein processing and amyloid-β peptide degradation in alzheimer’s disease. Neurodegener. Dis. 2014;13:75–81. doi: 10.1159/000355462. [DOI] [PubMed] [Google Scholar]
- 30.Grimm M.O., Mett J., Hartmann T. The impact of vitamin E and other fat-soluble vitamins on alzheimer’s disease. Int. J. Mol. Sci. 2016;17:1785. doi: 10.3390/ijms17111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P., Shah J. Role of vitamin D in amyloid clearance via LRP-1 upregulation in alzheimer’s disease: A potential therapeutic target? J. Chem. Neuroanat. 2017;85:36–42. doi: 10.1016/j.jchemneu.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez-Jimenez F.J., Molina J.A., de Bustos F., Orti-Pareja M., Benito-Leon J., Tallon-Barranco A., Gasalla T., Porta J., Arenas J. Serum levels of β-carotene, α-carotene and vitamin A in patients with alzheimer’s disease. Eur. J. Neurol. 1999;6:495–497. doi: 10.1046/j.1468-1331.1999.640495.x. [DOI] [PubMed] [Google Scholar]
- 33.Mangialasche F., Xu W., Kivipelto M., Costanzi E., Ercolani S., Pigliautile M., Cecchetti R., Baglioni M., Simmons A., Soininen H., et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. aging. 2012;33:2282–2290. doi: 10.1016/j.neurobiolaging.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Lopes da Silva S., Vellas B., Elemans S., Luchsinger J., Kamphuis P., Yaffe K., Sijben J., Groenendijk M., Stijnen T. Plasma nutrient status of patients with alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement. 2014;10:485–502. doi: 10.1016/j.jalz.2013.05.1771. [DOI] [PubMed] [Google Scholar]
- 35.Presse N., Shatenstein B., Kergoat M.J., Ferland G. Low vitamin k intakes in community-dwelling elders at an early stage of alzheimer’s disease. J. Am. Diet. Assoc. 2008;108:2095–2099. doi: 10.1016/j.jada.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y., Honda Y., Hayashida N., Iwamoto J., Kanoko T., Satoh K. Vitamin K deficiency and osteopenia in elderly women with alzheimer’s disease. Arch. Phys. Med. Rehabil. 2005;86:576–581. doi: 10.1016/j.apmr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Annweiler C., Souberbielle J.C., Schott A.M., de Decker L., Berrut G., Beauchet O. Vitamin D in the elderly: 5 points to remember. Geriatrie et Psychologie Neuropsychiatrie du Vieillissement. 2011;9:259–267. doi: 10.1684/pnv.2011.0288. [DOI] [PubMed] [Google Scholar]
- 38.Annweiler C., Beauchet O. Vitamin D-mentia: Randomized clinical trials should be the next step. Neuroepidemiology. 2011;37:249–258. doi: 10.1159/000334177. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee A., Khemka V.K., Ganguly A., Roy D., Ganguly U., Chakrabarti S. Vitamin D and alzheimer’s disease: Neurocognition to therapeutics. Int. J. Alzheimers Dis. 2015;2015:192747. doi: 10.1155/2015/192747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyles D.W., Smith S., Kinobe R., Hewison M., McGrath J.J. Distribution of the vitamin d receptor and 1 α-hydroxylase in human brain. J. Chem. Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Pardridge W.M., Sakiyama R., Coty W.A. Restricted transport of vitamin D and a derivatives through the rat blood-brain barrier. J. Neurochem. 1985;44:1138–1141. doi: 10.1111/j.1471-4159.1985.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 42.Gezen-Ak D., Atasoy I.L., Candas E., Alaylioglu M., Yilmazer S., Dursun E. Vitamin D receptor regulates amyloid beta 1–42 production with protein disulfide isomerase A3. ACS Chem. Neurosci. 2017;8:2335–2346. doi: 10.1021/acschemneuro.7b00245. [DOI] [PubMed] [Google Scholar]
- 43.Brown A.J. Therapeutic uses of vitamin D analogues. Am. J. Kidney Dis. 2001;38:S3–S19. doi: 10.1053/ajkd.2001.28111. [DOI] [PubMed] [Google Scholar]
- 44.Mazzaferro S., Goldsmith D., Larsson T.E., Massy Z.A., Cozzolino M. Vitamin D metabolites and/or analogs: Which D for which patient? Curren. Vasc. Pharmacol. 2014;12:339–349. doi: 10.2174/15701611113119990024. [DOI] [PubMed] [Google Scholar]
- 45.Rohan de Silva H.A., Jen A., Wickenden C., Jen L.S., Wilkinson S.L., Patel A.J. Cell-specific expression of β-amyloid precursor protein isoform mrnas and proteins in neurons and astrocytes. Mol. Brain Res. 1997;47:147–156. doi: 10.1016/S0169-328X(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 46.Wootton A.M. Improving the measurement of 25-hydroxy vitamin D. Clin. Biochemist. Rev. 2005;26:33–36. [PMC free article] [PubMed] [Google Scholar]
- 47.Vieth R. Why the minimum desirable serum 25-hydroxy vitamin D level should be 75 nmol/L (30 ng/mL) Best Pract. Res. Clin. Endocrinol. 2011;25:681–691. doi: 10.1016/j.beem.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Kennel K.A., Drake M.T., Hurley D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010;85:752–757. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu-Wong J.R., Nakane M., Gagne G.D., Brooks K.A., Noonan W.T. Comparison of the pharmacological effects of paricalcitol and doxercalciferol on the factors involved in mineral homeostasis. Int. J. Endocrinol. 2010;2010:621687. doi: 10.1155/2010/621687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piotrowska A., Wierzbicka J., Nadkarni S., Brown G., Kutner A., Zmijewski M.A. Antiproliferative activity of double point modified analogs of 1,25-dihydroxyvitamin D(2) against human malignant melanoma cell lines. Int. J. Mol. Sci. 2016;17:76. doi: 10.3390/ijms17010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandi M.L. Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes. Clin. Cases Miner. Bone Metab. 2010;7:243–250. [PMC free article] [PubMed] [Google Scholar]
- 52.Duplancic D., Cesarik M., Poljak N.K., Radman M., Kovacic V., Radic J., Rogosic V. The influence of selective vitamin D receptor activator paricalcitol on cardiovascular system and cardiorenal protection. Clin. Interv. Aging. 2013;8:149–156. doi: 10.2147/CIA.S38349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cacabelos R., Alvarez X.A., Fernandez-Novoa L., Franco A., Mangues R., Pellicer A., Nishimura T. Brain interleukin-1 β in alzheimer’s disease and vascular dementia. Methods Find. Exp. Clin. Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- 55.Perez-Lopez F.R., Chedraui P., Fernandez-Alonso A.M. Vitamin D and aging: Beyond calcium and bone metabolism. Maturitas. 2011;69:27–36. doi: 10.1016/j.maturitas.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Amer M., Qayyum R. Relation between serum 25-hydroxyvitamin D and c-reactive protein in asymptomatic adults (from the continuous national health and nutrition examination survey 2001 to 2006) Am. J. Cardiol. 2012;109:226–230. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 57.Cannell J.J., Hollis B.W., Zasloff M., Heaney R.P. Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 2008;9:107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 58.McGrath J. Hypothesis: Is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr. Res. 1999;40:173–177. doi: 10.1016/S0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 59.Annweiler C. Vitamin D-mentia: Is vitamin D optional or essential for preventing late-life cognitive decline? J. Am. Geriatr. Soc. 2017;65:2155–2157. doi: 10.1111/jgs.15056. [DOI] [PubMed] [Google Scholar]
- 60.Lemire P., Brangier A., Beaudenon M., Duval G.T., Annweiler C. Cognitive changes under memantine according to vitamin D status in alzheimer patients: An exposed/unexposed cohort pilot study. J. Steroid Biochem. Mol. Biol. 2016;175:151–156. doi: 10.1016/j.jsbmb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Etgen T., Sander D., Bickel H., Sander K., Forstl H. Vitamin D deficiency, cognitive impairment and dementia: A systematic review and meta-analysis. Dement. Geriatr. Cogn. 2012;33:297–305. doi: 10.1159/000339702. [DOI] [PubMed] [Google Scholar]
- 62.Van der Schaft J., Koek H.L., Dijkstra E., Verhaar H.J., van der Schouw Y.T., Emmelot-Vonk M.H. The association between vitamin D and cognition: A systematic review. Ageing Res. Rev. 2013;12:1013–1023. doi: 10.1016/j.arr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Dickens A.P., Lang I.A., Langa K.M., Kos K., Llewellyn D.J. Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs. 2011;25:629–639. doi: 10.2165/11593080-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Annweiler C., Allali G., Allain P., Bridenbaugh S., Schott A.M., Kressig R.W., Beauchet O. Vitamin D and cognitive performance in adults: A systematic review. Eur. J. Neurol. 2009;16:1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 65.Sutherland M.K., Wong L., Somerville M.J., Yoong L.K., Bergeron C., Parmentier M., McLachlan D.R. Reduction of calbindin-28k mrna levels in alzheimer as compared to huntington hippocampus. Mol. Brain Res. 1993;18:32–42. doi: 10.1016/0169-328X(93)90171-K. [DOI] [PubMed] [Google Scholar]
- 66.Balion C., Griffith L.E., Strifler L., Henderson M., Patterson C., Heckman G., Llewellyn D.J., Raina P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afzal S., Bojesen S.E., Nordestgaard B.G. Reduced 25-hydroxyvitamin d and risk of alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014;10:296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 68.Littlejohns T.J., Henley W.E., Lang I.A., Annweiler C., Beauchet O., Chaves P.H., Fried L., Kestenbaum B.R., Kuller L.H., Langa K.M., et al. Vitamin D and the risk of dementia and alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mokry L.E., Ross S., Morris J.A., Manousaki D., Forgetta V., Richards J.B. Genetically decreased vitamin D and risk of alzheimer disease. Neurology. 2016;87:2567–2574. doi: 10.1212/WNL.0000000000003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J., Gattoni-Celli M., Zhu H., Bhat N.R., Sambamurti K., Gattoni-Celli S., Kindy M.S. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J. Alzheimer’s Dis. 2011;25:295–307. doi: 10.3233/JAD-2011-101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koh Y.H., von Arnim C.A., Hyman B.T., Tanzi R.E., Tesco G. Bace is degraded via the lysosomal pathway. J. Biol. Chem. 2005;280:32499–32504. doi: 10.1074/jbc.M506199200. [DOI] [PubMed] [Google Scholar]
- 72.Qing H., Zhou W., Christensen M.A., Sun X., Tong Y., Song W. Degradation of bace by the ubiquitin-proteasome pathway. FASEB J. 2004;18:1571–1573. doi: 10.1096/fj.04-1994fje. [DOI] [PubMed] [Google Scholar]
- 73.Alvarez-Diaz S., Larriba M.J., Lopez-Otin C., Munoz A. Vitamin D: Proteases, protease inhibitors and cancer. Cell Cycle. 2010;9:32–37. doi: 10.4161/cc.9.1.10266. [DOI] [PubMed] [Google Scholar]
- 74.Zhang M., Deng Y., Luo Y., Zhang S., Zou H., Cai F., Wada K., Song W. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J. Neurochem. 2012;120:1129–1138. doi: 10.1111/j.1471-4159.2011.07644.x. [DOI] [PubMed] [Google Scholar]
- 75.Max D., Brandsch C., Schumann S., Kuhne H., Frommhagen M., Schutkowski A., Hirche F., Staege M.S., Stangl G.I. Maternal vitamin D deficiency causes smaller muscle fibers and altered transcript levels of genes involved in protein degradation, myogenesis, and cytoskeleton organization in the newborn rat. Mol. Nutr. Food Res. 2014;58:343–352. doi: 10.1002/mnfr.201300360. [DOI] [PubMed] [Google Scholar]
- 76.Briones T.L., Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflamm. 2012;9:244. doi: 10.1186/1742-2094-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takasugi N., Tomita T., Hayashi I., Tsuruoka M., Niimura M., Takahashi Y., Thinakaran G., Iwatsubo T. The role of presenilin cofactors in the γ-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 78.Dries D.R., Yu G. Assembly, maturation, and trafficking of the γ-secretase complex in alzheimer’s disease. Curr. Alzheimer Res. 2008;5:132–146. doi: 10.2174/156720508783954695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allinson T.M., Parkin E.T., Condon T.P., Schwager S.L., Sturrock E.D., Turner A.J., Hooper N.M. The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur. J. Biochem. 2004;271:2539–2547. doi: 10.1111/j.1432-1033.2004.04184.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhang D., Li M., Dong Y., Zhang X., Liu X., Chen Z., Zhu Y., Wang H., Liu X., Zhu J., et al. 1α, 25-dihydroxyvitamin D3 up-regulates Il-34 expression in SH-SY5Y neural cells. Innate Immun. 2017;23:584–591. doi: 10.1177/1753425917725391. [DOI] [PubMed] [Google Scholar]
- 81.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 82.Grimm M.O., Stahlmann C.P., Mett J., Haupenthal V.J., Zimmer V.C., Lehmann J., Hundsdorfer B., Endres K., Grimm H.S., Hartmann T. Vitamin E: Curse or benefit in alzheimer’s disease? A systematic investigation of the impact of α-, γ- and δ-tocopherol on ass generation and degradation in neuroblastoma cells. J. Nutr. Health Aging. 2015;19:646–656. doi: 10.1007/s12603-015-0506-z. [DOI] [PubMed] [Google Scholar]
- 83.Grimm M.O., Haupenthal V.J., Rothhaar T.L., Zimmer V.C., Grosgen S., Hundsdorfer B., Lehmann J., Grimm H.S., Hartmann T. Effect of different phospholipids on α-secretase activity in the non-amyloidogenic pathway of alzheimer’s disease. Int. J. Mol. Sci. 2013;14:5879–5898. doi: 10.3390/ijms14035879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miners J.S., Verbeek M.M., Rikkert M.O., Kehoe P.G., Love S. Immunocapture-based fluorometric assay for the measurement of neprilysin-specific enzyme activity in brain tissue homogenates and cerebrospinal fluid. J. Neurosci. Methods. 2008;167:229–236. doi: 10.1016/j.jneumeth.2007.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.