Abstract
Background
Elevated peripheral blood neutrophil-to-lymphocyte ratio (NLR) is associated with poor oncologic outcomes in patients with stage IV melanoma and other solid tumors, but its impact has not been characterized for patients with high-risk, non-metastatic melanoma.
Methods
Retrospective review of a melanoma database identified patients with high-risk melanoma who underwent operation with curative intent at a single institution. NLR was calculated from blood samples obtained within two-weeks prior to operation. Multiple primary melanomas and concurrent hematologic or other metastatic malignancies were excluded. Cumulative incidence of death due to disease was estimated, and Gray’s test was used to examine the effect of NLR on melanoma disease specific death (DOD). Multivariable competing risks regression models assessed associated factors.
Results
Data on 1,431 patients with high-risk non-metastatic melanoma were analyzed. Median follow-up for survivors was 4 years. High NLR (≥3 or as continuous variable) was associated with older age, male sex, thicker primaries, higher mitotic index and more advanced nodal status. On multivariate analysis, high NLR (≥ 3 or as a continuous variable), older age, male sex, ulcerated primary, lymphovascular invasion and positive nodal status were all independently associated with worse DOD.
Conclusions
NLR is a readily available blood test that was independently associated with DOD in patients with high risk non-metastatic melanoma. It is unclear whether high NLR is a passive indicator of poor prognosis, or a potential therapeutic target. Further studies to evaluate the prognostic role of NLR to potentially identify those more likely to benefit from adjuvant immunotherapy may prove informative.
Graphical abstract
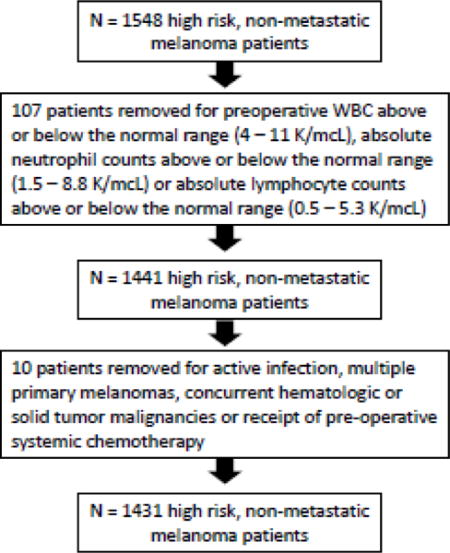
Introduction
Immunologic influence on carcinogenesis, tumor growth, invasion and metastasis is a growing area of clinical and scientific investigation. Melanoma has long been known as an immunogenic tumor, and was the first example of metastatic cancer to be cured by immunotherapy [1]. Concurrent with our increased understanding of tumor immunology, the incidence of melanoma has been increasing such that now it represents the fifth and seventh most common cancer diagnosis in men and women, respectively. An estimated 73,870 cases were diagnosed in 2015 and nearly 1 in 8 people diagnosed with melanoma died from the disease [2].
The interaction between the tumor and the host immune system has become a focus of increasing basic-science cancer research. In melanoma, the lymphoid cellular component of the immune system has been studied and exploited for treatment more extensively than the myeloid component. The association of tumor infiltrating lymphocytes (TILs) within primary melanomas with improved prognosis led to the incorporation of TILs into the histopathologic reporting of melanoma [3, 4]. In addition, interleukin-2, checkpoint inhibition and adoptive T-cell therapy (ACT) are examples of T-cell manipulation with apparent clinical benefit [5]. However, despite the growth in data of the lymphocyte’s role in tumor regression there currently remains a paucity of data on the influence of neutrophils in melanoma and other cancers.
Recently, the direct relationship between cancer related inflammation and oncologic outcome has become evident [6, 7]. Systemic inflammation is tied to alterations in circulating peripheral blood leukocytes. An easily obtainable, pre-treatment biomarker of systemic inflammation is the peripheral blood neutrophil-to-lymphocyte ratio (NLR). An elevated NLR has been associated with poor prognosis in a number of solid organ malignancies including colorectal, esophageal, gastric, pancreatic, and primary liver cancers, gastrointestinal stromal tumor and other soft tissue sarcomas, and metastatic melanoma [6, 8–15]. The aims of this study are to characterize the outcomes of patients with high-risk, non-metastatic melanoma, to determine if NLR correlates with known prognostic factors, and to examine the association of preoperative NLR with long-term outcome. We postulated that an elevated NLR, as in other malignancies, would be associated with inferior disease-specific mortality.
Methods
Following approval by our Institutional Review Board, a prospectively maintained melanoma database was queried to identify patients with cutaneous melanoma operated consecutively at our institution from 1998 through 2012. Patients with pathologic features deemed high risk for recurrence were included for the study; these included patients with T2b and greater primaries, and patients with regional nodal disease based on final pathologic staging. Preoperative blood samples were routinely obtained within two weeks of operation. Patients were excluded when preoperative blood cell counts were not available, preoperative WBC was above or below the normal range (4 – 11 K/mcL), absolute neutrophil counts were above or below the normal range (1.5 – 8.8 K/mcL) or absolute lymphocyte counts were above or below the normal range (0.5 – 5.3 K/mcL). Other exclusions included patients with active infection, multiple primary melanomas, concurrent hematologic or solid tumor malignancies or receipt of pre-operative systemic chemotherapy. Blood NLR was calculated on every eligible patient as the absolute neutrophil count (number of neutrophils per microliter) divided by absolute lymphocyte count (number of lymphocytes per microliter). NLR was evaluated as a continuous variable. For clinical utility, the method of maximally selected rank statistics was utilized to optimally dichotomize NLR [18]. An optimal cut point of 2.9 was identified and rounded to 3 for relevance in clinical practice. Neutrophil and lymphocyte values were also evaluated separately for their possible role as prognostic markers. Because NLR tends to inflate with increased age and many patients died of non-melanoma causes, we evaluated disease specific death (DOD) as the outcome in order to determine the influence of NLR on melanoma related death. Clinicopathologic variables included patient age and sex, tumor site, mitotic index, thickness, ulceration, lymphovascular invasion, nodal stage and nodal characteristic (clinical node status and number of pathologically positive nodes). Nodal characteristics were classified as the following 1) no clinically evident lymphadenopathy, no pathologic lymph node metastases, 2) no clinically evident lymphadenopathy, positive pathologic lymph node metastases and 3) clinically evident lymphadenopathy, positive pathologic lymph node metastases.
Statistical analysis
The primary endpoint of the study was disease specific death (DOD), defined as the time interval from surgical resection to the time of melanoma specific death. Death of unknown causes and death of other causes were treated as competing risks. Patients alive were censored at their last followup. The cumulative incidence of DOD was estimated along with 95% confidence intervals. The associations between DOD and preoperative/perioperative variables were assessed using Grey’s test and competing risks regression based on Fine and Grey’s method [16], in univariate and multivariable models. Backward model selection was used in multivariable analyses with significance level at 0.05. Maximally selected rank statistics, taking into account multiple testing issues, were used to find a cutoff value (optimal cut point = 2.9) of NLR to best differentiate survival groups [17]. Fisher’s exact test and Wilcoxon rank sum test were used to examine associations of NLR (high or low) with categorical and continuous clinical characteristics, respectively. Median values are reported with corresponding ranges or interquartile ranges (IQR). P-values less than 0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (The SAS Institute, Cary, NC) and R version 3.1 (The R Foundation for Statistical Computing).
Results
Demographic and Tumor Characteristics
Total Group
Our prospective database identified 1548 high risk, non-metastatic melanoma patients treated consecutively over a fourteen-year period. Following exclusions, 1,431 consecutive patients were available for analysis. Patient and tumor characteristics are summarized in Table 1. Most patients were male (61.6%) and the median age at time of treatment was 63.4 years (range, 4 - 99.6). The most common tumor location was the extremity (37.3%) followed by the trunk (27.5%), head/neck (23.2%), and hand/foot (12%). The median mitotic index was 4 (range, 0 – 58). Tumor thickness, as measured in millimeters, included 0 – 1 (7.4%), 1.1 – 2 (22%), 2.1 – 4 (39.6%) and > 4 (31%). Further pathologic characteristics included primary melanoma ulceration (44.1%), tumor infiltrating lymphocytes (45.4%) and lymphovascular invasion (LVI) (15.4%). Nodal sub-stage, as defined by AJCC classification, included N0 (50.7%), N1a (18.4%), N1b (7.2%), N2a (4.5%), N2b (4.9%), N2c (4.5%) and N3 (9.8%). Lymph node involvement in the study cohort revealed 56.5% with clinically negative / pathologically negative nodes, 31.9% with clinically negative / pathologically positive nodes and 11.7% with clinically positive / pathologically positive nodes. In-transit disease was present in 120/1431 patients (8.4%).
Table 1.
Patient and tumor characteristics for 1,431high risk, non-metastatic melanoma patients.
(key: nodal characteristic: Clin(−)/Path(−)/#(0) = no clinically evident lymphadenopathy, no pathologic lymph node metastases. Clin(−)/Path(+)/#(≥1) = no clinically evident lymphadenopathy, positive pathologic lymph node metastases. Clin(−)/Path(+)/#(≥1) = clinically evident lymphadenopathy, positive pathologic lymph node metastases.)
| Total Cohort | Neutrophil-to-Lymphocyte Ratio | p-value | ||
|---|---|---|---|---|
| < 3 | ≥ 3 | |||
| (n = 1,431) | (n = 847) | (n = 584) | ||
| Age (years), median (range) | 63.4 (4 – 99.6) | 60.2 (4 – 99.6) | 67.35 (16.3 – 95.5) | <0.001 |
| Sex | ||||
| Female | 550 (38.4%) | 375 (44.3%) | 175 (30%) | <0.001 |
| Male | 881 (61.6%) | 472 (55.7%) | 409 (70%) | |
| Tumor Site | ||||
| Extremity | 534 (37.3%) | 322 (38%) | 212 (36.3%) | 0.275 |
| Hand/Foot | 172 (12%) | 111 (13.1%) | 61 (10.4%) | |
| Head/Neck | 332 (23.2%) | 193 (22.8%) | 139 (23.8%) | |
| Trunk | 393 (27.5%) | 221 (26.1%) | 172 (29.5%) | |
| Tumor Thickness (mm) | ||||
| N/A* | 56 | 29 | 27 | |
| 0 – 1 | 102 (7.4%) | 63 (7.7%) | 39 (7%) | 0.004 |
| 1.1 – 2 | 302 (22%) | 196 (24%) | 106 (19%) | |
| 2.1 – 4 | 545 (39.6%) | 335 (41%) | 210 (37.7%) | |
| >4 | 426 (31%) | 224 (27.4%) | 202 (36.3%) | |
| Mitotic index, median(range) | 4 (0 – 58) | 3 (0 – 58) | 4 (0 – 36) | <0.001 |
| Ulceration | ||||
| Identified | 631 (44.1%) | 361 (42.6%) | 270 (46.2%) | 0.194Ɨ |
| Not identified | 798 (55.8%) | 485 (57.3%) | 313 (53.6%) | |
| Unknown | 2 (0.1%) | 1 (0.1%) | 1 (0.2%) | |
| TILs | ||||
| Identified | 649 (45.4%) | 393 (46.4%) | 256 (43.8%) | 0.366Ɨ |
| Not identified | 780 (54.5%) | 453 (53.5%) | 327 (56%) | |
| Unknown | 2 (0.1%) | 1 (0.1%) | 1 (0.2%) | |
| LVI | ||||
| Identified | 220 (15.4%) | 122 (14.4%) | 98 (16.8%) | 0.250Ɨ |
| Not identified | 1209 (84.5%) | 724 (85.5%) | 485 (83%) | |
| Unknown | 2 (0.1%) | 1 (0.1%) | 1 (0.2%) | |
| In-transit status | ||||
| Negative | 1311 (91.6%) | 783 (92.4%) | 528 (90.4%) | 0.205 |
| Positive | 120 (8.4%) | 64 (7.6%) | 56 (9.6%) | |
| N substage | ||||
| N0 | 726 (50.7%) | 439 (51.8%) | 287 (49.1%) | 0.034 |
| N1a | 264 (18.4%) | 165 (19.5%) | 99 (17%) | |
| N1b | 103 (7.2%) | 65 (7.7%) | 38 (6.5%) | |
| N2a | 64 (4.5%) | 40 (4.7%) | 24 (4.1%) | |
| N2b | 70 (4.9%) | 30 (3.5%) | 40 (6.8%) | |
| N2c | 64 (4.5%) | 34 (4%) | 30 (5.1%) | |
| N3 | 140 (9.8%) | 74 (8.7%) | 66 (11.3%) | |
| Nodal Characteristic | ||||
| Clin(−)/Path(−)/#(0) | 808 (56.5%) | 481 (56.8%) | 327 (56%) | 0.169 |
| Clin(−)/Path(+)/#(≥1) | 456 (31.9%) | 278 (32.8%) | 178 (30.5%) | |
| Clin(+)/Path(+)/#(≥1) | 167 (11.7%) | 88 (10.4%) | 79 (13.5%) | |
p value was from the test combining unknown and not identified.
N/A was not included in the test.
NLR Low vs. NLR High
NLR was evaluated as a continuous variable. For ease of implementation into clinical practice a statistical optimal cutoff point of NLR equal to 2.9 was derived. Therefore, we classified high NLR as ≥ 3 and low NLR as < 3. The NLR low population included 847 patients as compared to NLR high, 584. High NLR was associated with older age (p <0.001), male sex (p <0.001), thicker primary tumors (p = 0.004), higher mitotic index (p <0.001) and increased nodal substage (p = 0.034). There were no associations between high NLR and tumor site, ulceration, tumor infiltrating lymphocytes, lymphovascular invasion, nodal characteristics, or the presence of in-transit disease.
Disease Status
At last follow-up, patients were found as alive with unknown disease status (n = 3/1431, 0.2%), alive with disease (69/1431, 4.8%), alive with no evidence of disease (736/1431, 51.4%), dead of disease (427/1431, 29.8%), dead of unknown causes (109/1431. 7.6%), and dead of other causes (87/1431, 6.1%). Median follow up for survivors was 4.0 years (range, 0.01 – 16.7).
Disease-Specific Mortality
Univariate analysis revealed the following factors to be significantly associated with DOD: older age (HR 1.01, 95% CI: 1.00 - 1.01; p = 0.015), male sex (HR 1.5, 95% CI 1.22 - 1.85); p < 0.001), increasing tumor thickness (HR 1.02, 95% CI 1 - 1.04); p = 0.013), ulceration (HR 1.42, 95% CI 1.18 - 1.72); p < 0.001), LVI (HR 2.02, 95% CI 1.61 - 2.54); p < 0.001), nodal characteristics of clinically negative/pathologic positive and clinically positive/pathologic positive (HR 1.75 95% CI 1.42 - 2.17); p < 0.001), increasing absolute lymphocyte count (HR 0.83, 95% CI 0.69 - 0.99); p = 0.038), and high NLR (HR 1.39, 95% CI 1.15 - 1.68; p = 0.001) (Table 2). Moreover, when assessed as a continuous variable, NLR remained significantly associated with DOD (p<0.001).
Table 2.
Univariate Survival Analysis for 1,431 high risk, non-metastatic melanoma patients.
(Key: NLR = neutrophil to lymphocyte ratio, LVI = lymphovascular invasion. nodal characteristic: Clin(−)/Path(−)/#(0) = no clinically evident lymphadenopathy, no pathologic lymph node metastases. Clin(−)/Path(+)/#(≥1) = no clinically evident lymphadenopathy, positive pathologic lymph node metastases. Clin(−)/Path(+)/#(≥1) = clinically evident lymphadenopathy, positive pathologic lymph node metastases.)
| Characteristic | N (%) | Univariate | p-value |
|---|---|---|---|
| (HR, 95% CI) | |||
| Age (years), median (IQR) | 63.4 (49.9 – 73.8) | 1.01 (1.00 – 1.01) | 0.015 |
| Gender | |||
| Female | 550 (38.4%) | 1 | <0.001 |
| Male | 881 (61.6%) | 1.5 (1.22 – 1.85) | |
| White blood cell count, median (IQR) | |||
| Absolute neutrophil count | 4.5 (3.6 – 5.4) | 1.04 (0.97 – 1.11) | 0.310 |
| Absolute lymphocyte count | 1.7 (1.3 – 2.1) | 0.83 (0.69 – 0.99) | 0.038 |
| Absolute monocyte count (n=1108) | 0.3 (0.3 – 0.4) | 1.97 (0.91 – 4.24) | 0.084 |
| Tumor thickness, median (IQR) | 2.9 (1.8 – 4.6) | 1.02 (1 – 1.04) | 0.013 |
| Tumor Site | |||
| Extremity | 534 (37.3%) | 1 | 0.065 |
| Hand/Foot | 172 (12%) | 1.42 (1.07 – 1.89) | |
| Head and Neck | 332 (23.2%) | 0.96 (0.73 – 1.24) | |
| Trunk | 393 (27.5%) | 1.14 (0.9 – 1.44) | |
| Ulceration | |||
| Not identified/Unknown | 631 (44.1%) | 1 | <0.001 |
| Identified | 800 (55.9%) | 1.42 (1.18 – 1.72) | |
| LVI | |||
| Not identified/Unknown | 220 (15.4%) | 1 | <0.001 |
| Identified | 1211 (84.6%) | 2.02 (1.61 – 2.54) | |
| Nodal Characteristic | |||
| Clin(−)/Path(−)/#(0) | 808 (56.5%) | 1 | <0.001 |
| Clin(−)/Path(+)/#(≥1) | 456 (31.9%) | 1.75 (1.42 – 2.17) | |
| Clin(+)/Path(+)/#(≥1) | 167 (11.7%) | 3.49 (2.67 – 4.55) | |
| NLR | |||
| < 3 | 847(59.2%) | 1 | 0.001 |
| ≥ 3 | 584 (40.8%) | 1.39 (1.15 – 1.68) |
Multivariate regression analysis found the following factors to remain as independent predictors of DOD: older age (HR 1.01, 95% CI 1.00 – 1.01; p = 0.011), male sex (HR 1.31, 95% CI 1.06 - 1.63; p = 0.012), ulceration (HR 1.48, 95% CI 1.21 - 1.83; p < 0.001), LVI (HR 1.52, 95% CI 1.18 - 1.96; p = 0.001), nodal characteristics of clinically negative/pathologic positive and clinically positive/pathologic positive (HR 1.86 95% CI 1.48 - 2.33); p < 0.001) and high NLR (HR 1.25, 95% CI 1.02 - 1.53; p = 0.03) (Table 3). Additionally, when assessed as a continuous variable, NLR remained significantly associated with DOD controlling for the same set of factors in our multivariate model. Cumulative incidences of DOD by these factors are depicted in Figure 1.
Table 3.
Multivariate survival analysis for 1,431 high risk, non-metastatic melanoma patients.
(Key: NLR = neutrophil to lymphocyte ratio, LVI = lymphovascular invasion. nodal characteristic: Clin(−)/Path(−)/#(0) = no clinically evident lymphadenopathy, no pathologic lymph node metastases. Clin(−)/Path(+)/#(≥1) = no clinically evident lymphadenopathy, positive pathologic lymph node metastases. Clin(−)/Path(+)/#(≥1) = clinically evident lymphadenopathy, positive pathologic lymph node metastases.)
| Characteristic | Multivariate | p-value |
|---|---|---|
| HR (95% CI) | ||
| Age (continuous) | 1.01 (1 – 1.01) | 0.011 |
| Gender | ||
| Female | 1 | 0.012 |
| Male | 1.31 (1.06 – 1.63) | |
| Ulceration | ||
| Not identified/Unknown | 1 | <0.001 |
| Identified | 1.48 (1.21 – 1.83) | |
| LVI | ||
| Not identified/Unknown | 1 | 0.001 |
| Identified | 1.52 (1.18 – 1.96) | |
| Nodal Characteristic | ||
| Clin(−)/Path(−)/#(0) | 1 | <0.001 |
| Clin(−)/Path(+)/#(≥1) | 1.86 (1.48 – 2.33) | |
| Clin(+)/Path(+)/#(≥1) | 3.71 (2.78 – 4.95) | |
| NLR | ||
| < 3 | 1 | 0.030 |
| ≥ 3 | 1.25 (1.02 – 1.53) |
Figure 1.
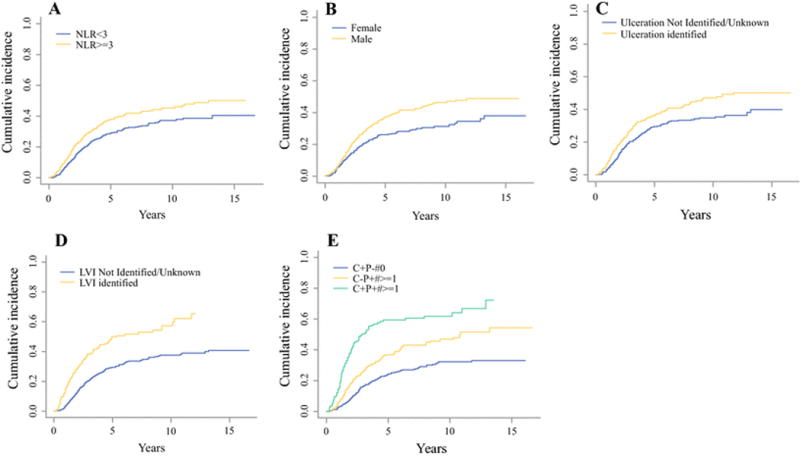
Cumulative incidence of death due to disease for 1,431 high risk, non-metastatic melanoma patients. (A): NLR, (B): Gender, (C): Ulceration, (D): LVI, (E): nodal characteristic.
Discussion
This analysis of 1,431 consecutive patients undergoing surgical treatment for high-risk Stage II and III primary cutaneous melanoma demonstrated that high NLR was independently associated with significantly higher risk of DOD when compared to low NLR. A secondary objective of this study was the assessment for any correlation of known prognostic pathologic features of melanoma and NLR. High NLR did correlate with many known predictors of melanoma specific survival such as older age, male sex, thicker primary tumors, higher mitotic index, and increased nodal substage; however high NLR was not associated with ulceration, TILs or LVI. In a multivariate analysis of survival, high NLR (≥ 3 or as a continuous variable), older age, male gender, ulcerated primaries, presence of LVI, and nodal characteristics were associated with increased risk of DOD. The lack of association of DOD with tumor thickness on multivariate analysis is believed to stem from the high proportion of patients with positive nodal status. Overall, these associations support our hypothesis that an elevated NLR is prognostic of inferior disease specific survival, independent of other known poor prognostic clinicopathologic variables.
The link between inflammation and neoplasia has been recognized since the time of Virchow and is felt to be related to the lack of homeostasis within immune cell compartments leading to chronic inflammation and carcinogenesis [18, 19]. More recently, in a systematic review of 15 studies and over 8500 patients, Guthrie et al found the systemic inflammation-based NLR to have prognostic value in predicting oncologic outcomes in patients with cancer [7]. Specifically, the NLR was elevated in patients with more advanced / aggressive disease, evidenced by increased tumor stage, nodal status and extent of disease [7]. As such, NLR may reflect a more advanced stage of disease with potentially more aggressive tumor behavior [7]. However, the method that links tumor and host biology remains unclear.
Although peripheral blood neutrophilia has been associated with poor cancer outcomes in many tumor types, the role of neutrophils in tumor development and progression has not been fully elucidated. Within the myeloid cell lineage, neutrophils, macrophages and myeloid-derived suppressor cells (MDSC) have been described as suppressors of T-lymphocyte function. In addition, these cells have been ascribed effector function in tumor angiogenesis, tumor invasion and metastasis. A proposed mechanism of neutrophil-mediated tumorigenesis and metastasis includes the production of cytokines by melanoma cells that interact with receptors expressed on neutrophils and melanoma cells (autocrine effect) [20]. Recently, Motomura et al. found that an elevated NLR was associated with an increase in serum IL-17 and an increase in the peri-tumoral infiltration of macrophages [7, 21]. Moreover, Kantola et al reported that an elevated NLR was associated with elevations in IL-1ra, IL-6, IL-7, IL-8, IL-12, MCP-1 and PDGFBB [7, 22]. Therefore, theoretically, an elevated NLR may represent some level of up-regulation of the innate immune system [7]. Additionally, in other solid tumors the expression of chemokine receptors such as the IL-8 receptor (CXCR1), have been correlated with numbers of tumor-infiltrating neutrophils [23]. Neutrophil-mediated tumor progression may occur via so-called neutrophil extracellular traps (NETs) that are thought to promote tumor metastasis by trapping circulating tumor cells within webs of extracellular DNA [24].
What is not known from clinical studies of peripheral blood neutrophilia is whether an elevated circulating ANC in cancer patients includes a population of granulocytic myeloid derived suppressor cells (MDSC) that exhibit an immature neutrophil phenotype. The pretreatment NLR may be a window through which to observe the balance between the host’s pro-tumor neutrophil and the anti-tumor lymphocyte cellular compartments. The cut-offs used to define high and low NLR (whether by receiver operating characteristic, binary partitioning, rank statistics, median, quartile or as continuous variable) may simply represent the threshold at which the tumor suppressive effects of lymphocytes are overcome by the immunosuppressive influences of neutrophils and their myeloid relatives. The ratio, or more likely the changes in specific cell population numbers, may have a potential to be used to monitor or predict response to specific immune-based therapies. Recently, Ferrucci et al found the baseline NLR to be strongly and independently associated with progression free and overall survival following the treatment with anti-CTLA4 antibody. Specifically, they found that an NLR ≥ 3 had a significantly and independently increased risk of death [hazard ratio (HR) = 5.76; 95% CI 4.29-7.75] and of progression (HR = 4.10; 95% CI 3.08-5.46) compared with patients with a lower NLR [6, 25]. However, whether NLR remains a general prognostic factor or predictive for ipilimumab benefit in the metastatic setting remains unclear. Moreover, Zaragoza confirmed that an elevated NLR (≥4) before initiating ipilimumab treatment in patients with metastatic melanoma to be an independent prognostic indicator of poor survival [26]. Additionally, treatments aimed at abrogating the suppressive function of neutrophils and other myeloid-derived cells could shift the balance in favor of immune system dominance over tumor progression. Given these observations it is therefore reasonable to propose that this readily available hematological test could be investigated as a predictive as well as prognostic biomarker in those who require adjuvant treatment or who do not appear clinically suitable for surgical intervention and would therefore be useful in the improved stratification of patients with cancer [7, 27].
There are several limitations to the current study. As with any retrospective analysis this study is subject to selection bias since we analyzed only patients at high-risk for melanoma recurrence and DOD. Although overall survival would allow for a more frequent event rate, this study includes more than 400 DOD events providing sufficient power in the analyses. Additionally, we elected to exclude the low risk primary melanoma population due to the low event rate (DOD) among this group and possibly different group of prognostic factors. However, the inclusion of all melanoma patients (non-high risk), could potentially provide additional insight into a threshold for immune relevance. Lastly, elevated NLR has been linked to postoperative complications, however we did not assess or correct for complications. Despite these limitations our sample size and prospective data collection strengthen the results and add to the current available literature.
Conclusions
We reported the characteristics and outcomes of 1,431 patients with high-risk, non-metastatic melanoma resected at a single institution over fourteen years. An elevated preoperative NLR (≥ 3 or as a continuous variable) correlated with some known poor prognostic indicators of melanoma survival and was also an independent predictor of DOD. Our results are consistent with previous studies reporting the prognostic significance of NLR in metastatic melanoma and other solid organ malignancies. As an independent indicator of DOD, and a readily available blood test, NLR can be used to assist in risk stratification and potentially predicting immunotherapy treatment response of patients with high-risk non-metastatic melanoma. Further studies to evaluate the prognostic and predictive value of NLR, and its component peripheral blood neutrophils and lymphocytes, and their correlation with response to immunotherapy may prove informative. Given the remarkably consistent association of elevated NLR with poor disease-specific outcome across broad variety of solid tumors, the critical question to be addressed in the future is whether NLR is merely an indicator/prognosticator or a targetable governor of outcome.
Supplementary Material
Synopsis.
Elevated pre-operative neutrophil-to-lymphocyte ratio portends poor survival in high-risk, non-metastatic melanoma.
Acknowledgments
This study was supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant)
Footnotes
Disclosures: None
Presented: SSO 2015
References
- 1.Rosenberg SA. IL-2: The First Effective Immunotherapy for Human Cancer. J Immunol. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Azimi F, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30(21):2678–83. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 4.Tuthill RJ, et al. Risk assessment in localized primary cutaneous melanoma: a Southwest Oncology Group study evaluating nine factors and a test of the Clark logistic regression prediction model. Am J Clin Pathol. 2002;118(4):504–11. doi: 10.1309/WBF7-N8KH-71KT-RVQ9. [DOI] [PubMed] [Google Scholar]
- 5.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrucci PF, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112(12):1904–10. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guthrie GJ, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Xue TC, et al. Prognostic Significance of the Neutrophil-to-Lymphocyte Ratio in Primary Liver Cancer: A Meta-Analysis. PLoS One. 2014;9(5):e96072. doi: 10.1371/journal.pone.0096072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol. 2014;21(3):778–85. doi: 10.1245/s10434-013-3418-3. [DOI] [PubMed] [Google Scholar]
- 10.Cananzi FC, Dalgleish A, Mudan S. Surgical management of intraabdominal metastases from melanoma: role of the neutrophil to lymphocyte ratio as a potential prognostic factor. World J Surg. 2014;38(6):1542–50. doi: 10.1007/s00268-013-2418-6. [DOI] [PubMed] [Google Scholar]
- 11.Malietzis G, et al. A Preoperative Neutrophil to Lymphocyte Ratio of 3 Predicts Disease-Free Survival After Curative Elective Colorectal Cancer Surgery. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 12.Perez DR, et al. Blood neutrophil-to-lymphocyte ratio is prognostic in gastrointestinal stromal tumor. Ann Surg Oncol. 2013;20(2):593–9. doi: 10.1245/s10434-012-2682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharaiha RZ, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18(12):3362–9. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szkandera J, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One. 2013;8(11):e78225. doi: 10.1371/journal.pone.0078225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SC, et al. Pretreatment Neutrophil to Lymphocyte Ratio Independently Predicts Disease-specific Survival in Resectable Gastroesophageal Junction and Gastric Adenocarcinoma. Ann Surg. 2016;263(2):292–7. doi: 10.1097/SLA.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng SC, Fine JP, Wei LJ. Prediction of cumulative incidence function under the proportional hazards model. Biometrics. 1998;54(1):219–28. [PubMed] [Google Scholar]
- 17.Lausen BH, Bretz T, Schumacher M. Assessment of Optimal Selected Prognostic Factors. Biometrical Journal. 2004;46(3):364–374. [Google Scholar]
- 18.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72(1):9–18. [PMC free article] [PubMed] [Google Scholar]
- 21.Motomura T, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58(1):58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Kantola T, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–36. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellocq A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152(1):83–92. [PMC free article] [PubMed] [Google Scholar]
- 24.Cools-Lartigue J, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrucci PF, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–8. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 26.Zaragoza J, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174(1):146–51. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 27.Eggermont AM, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016 doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


