Abstract
OBJECTIVE
Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications. Depending on a child’s age and previous influenza vaccination history, they are recommended to receive either 1 dose or 2 doses of influenza vaccine to be considered fully vaccinated against influenza for the season. We compared estimates of full (complete) influenza vaccination coverage of children 6 to 23 months across 10 consecutive influenza seasons (2002–2012), by race/ethnicity, age group, and by number of doses required to be fully vaccinated given child’s vaccination history.
METHODS
National Immunization Survey data were used to estimate full influenza vaccination status among children 6 to 23 months on the basis of provider report. Estimates were computed by using Kaplan-Meier survival analysis methods.
RESULTS
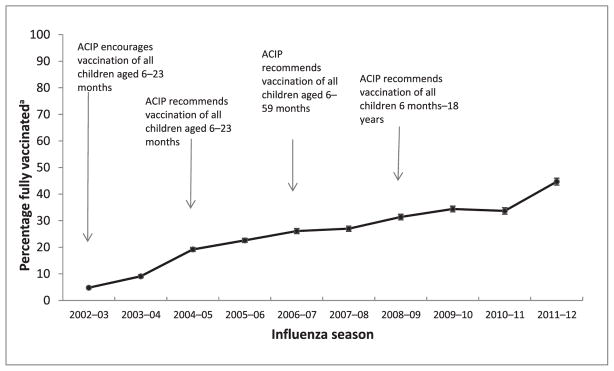
Full influenza vaccination coverage among children 6 to 23 months increased from 4.8% in the 2002–2003 influenza season to 44.7% in the 2011–2012 season. In all 10 influenza seasons studied, non-Hispanic black children and Hispanic children had lower full influenza vaccination coverage than non-Hispanic white children. For all 10 influenza seasons, full influenza vaccination coverage was higher among children requiring only 1 dose compared with those requiring 2 doses.
CONCLUSIONS
Less than half of children 6 to 23 months in the United States, and an even a smaller percentage of Hispanic and non-Hispanic black children, are fully vaccinated against influenza. More implementation of evidence-based strategies that increase the percentage of children who are fully vaccinated is needed.
Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications. Before 2002, recommendations for influenza vaccination of children in the United States focused on those with health conditions that confer increased risk of severe illness from influenza.1 Recommendations for the routine annual influenza vaccination of all children were first made in 2002, when the Advisory Committee on Immunization Practices (ACIP) voted to encourage providers to vaccinate all children aged 6 to 23 months, regardless of medical conditions.2 In 2004, the ACIP explicitly recommended vaccination for all children 6 to 23 months.3 Later, in 2006, these recommendations were expanded to include all children 6 to 59 months and in 2008 to include all children 6 months to 18 years.4,5
Children 6 months through 8 years who are influenza vaccine–naive require 2 doses of vaccine for optimal immune response.6,7 The ACIP recommends that children 6 months through 8 years receive either 1 dose or two doses spaced at least 4 weeks apart, depending on previous influenza vaccination history. The recommendations for determining the needed number of doses required to be considered fully vaccinated against influenza have changed over time (Table 1). For the 2002–2003 through the 2006–2007 seasons, 2 doses were recommended for children who had never received an influenza vaccination previously, otherwise 1 dose was recommended. In the 2007–2008 season the recommendation for 2 doses expanded to include those who should have received 2 doses in the previous season but received only 1 dose. The emergence of the pandemic 2009 H1N1 virus (H1N1pdm09) ahead of the 2009–2010 influenza season led to the development of the monovalent pandemic vaccine, and 2 influenza vaccines were recommended during 2009–2010: the trivalent seasonal influenza vaccination and the pandemic influenza A(H1N1)pdm09 (pH1N1) monovalent vaccine.8 For the 2010–2011 season, given the relative antigenic novelty of the p(H1N1) virus, the recommended approach for determining the number of doses needed for young children was modified to consider both previous exposure to seasonal influenza vaccines and the pH1N1 vaccine.9 During subsequent seasons up through the 2014–2015 season, the procedure for determining the number of doses needed continued to consider receipt of both seasonal influenza vaccine and the receipt of H1N1pdm09-containing vaccine (Table 1), with some further variations depending on whether vaccine viruses had changed compared with the previous season.10–13
TABLE 1.
Full Influenza Vaccination Recommendations and Number of Doses Needed to be Fully Vaccinated, National Immunization Survey (NIS), Provider-Report, United States, 2002–2003 Through 2011–2012 Influenza Seasons
| Influenza Season | Recommendationsa | Children Who Needed 2 Doses, % ± 95% CIb |
|---|---|---|
| 2002–2003 | “Among previously unvaccinated children aged <9 years, two doses administered >1 months apart are recommended for satisfactory antibody responses.“2 | 99.2 ± 0.2 |
| 2003–2004 | “Among previously unvaccinated children aged <9 years, two doses administered >1 month apart are recommended for satisfactory antibody responses.”14 | 96.5 ± 0.4 |
| 2004–2005 | “Among previously unvaccinated children aged <9 years, 2 doses administered >1 month apart are recommended for satisfactory antibody responses. If possible, the second dose should be administered before December. If a child aged <9 years receiving vaccine for the first time does not receive a second dose of vaccine within the same season, only 1 dose of vaccine should be administered the following season. Two doses are not required at that time.”3 | 92.0 ± 0.6 |
| 2005–2006 | “Among previously unvaccinated children aged <9 years, 2 doses administered >1 month apart are recommended for satisfactory antibody responses. If possible, the second dose should be administered before December. If a child aged <9 years receiving vaccine for the first time does not receive a second dose of vaccine within the same season, only 1 dose of vaccine should be administered the following season. Two doses are not required at that time.”15 | 81.5 ± 0.8 |
| 2006–2007 | “Among previously unvaccinated children aged 6 months–<9 years, 2 doses of inactivated vaccine administered >1 month apart are recommended for eliciting satisfactory antibody responses. If possible, the second dose should be administered before the onset of influenza season. If a child aged 6 months–<9 years receiving influenza vaccine for the first time does not receive a second dose of vaccine within the same season, only 1 dose of vaccine should be administered the following season. Two doses are not required at that time.”16 | 81.0 ± 1.0 |
| 2007–2008 | “ACIP recommends 2 vaccine doses for children aged 6 months–8 years who received an influenza vaccine (either TIV or LAIV) for the first time in the previous season but who did not receive the recommended second dose of vaccine within the same season. ACIP recommendations are now harmonized with regard to this issue with those of AAP. This recommendation represents a change from the 2006 recommendations, in which children aged 6 months–8 years who received only 1 dose of vaccine in their first year of vaccination were recommended to receive only a single dose in the following season. ACIP does not recommend that a child receive influenza vaccine for the first time in the spring with the intent of providing a priming dose for the following season. Children recommended for vaccination who are in their third or more year of being vaccinated and who received only 1 dose in each of their first 2 years of being vaccinated should continue receiving a single annual dose.”17 | 88.7 ± 0.6 |
| 2008–2009 | “All children aged 6 months–8 years who have not received vaccination against influenza previously should receive 2 doses of vaccine the first influenza season that they are vaccinated. The second dose should be administered 4 or more weeks after the initial dose. For example, children aged 6 months–8 years who were vaccinated for the first time during the 2007–08 influenza season but only received 1 dose during that season should receive 2 doses of the 2008–09 influenza vaccine. All other children aged 6 months–8 years who have previously received 1 or more doses of influenza vaccine at any time should receive 1 dose of the 2008–09 influenza vaccine. Children aged 6 months–8 years who only received a single vaccination during a season before 2007–08 should receive 1 dose of the 2008–09 influenza vaccine.”18 | 87.6 ± 0.8 |
| 2009–2010 | “All children aged 6 months–8 years who have not received vaccination against influenza previously should receive 2 doses of vaccine the first influenza season that they are vaccinated. The second dose should be administered 4 or more weeks after the initial dose. When only 1 dose is administered to children aged 6 months–8 years during their first year of vaccination, 2 doses should be administered in the following season. However, 2 doses should only be administered in the first season of vaccination, or in the season that immediately follows if only 1 dose is administered in the first season. For example, children aged 6 months–8 years who were vaccinated for the first time with the 2008–09 influenza vaccine but received only 1 dose should receive 2 doses of the 2009–10 influenza vaccine. All other children aged 6 months–8 years who have previously received 1 or more doses of influenza vaccine at any time should receive 1 dose of the 2009–10 influenza vaccine. Children aged 6 months–8 years who received only a single vaccination during a season before 2007–08 should receive 1 dose of the 2009–10 influenza vaccine.”5 | 84.4 ± 0.8 |
| 2010–2011 | “All children aged 6 months–8 years who receive a seasonal influenza vaccine for the first time should be administered 2 doses. Children aged 6 months–8 years who received a seasonal vaccine for the first time during 2009–2010 but who received only 1 dose should receive 2 doses, rather than 1, during 2010–2011. In addition, for the 2010–11 influenza season, children aged 6 months–8 years who did not receive at least 1 dose of an influenza A (H1N1) 2009 monovalent vaccine should receive 2 doses of a 2010–11 seasonal influenza vaccine, regardless of previous influenza vaccination history. Children aged 6 months–8 years for whom the previous 2009–10 seasonal or influenza A (H1N1) 2009 monovalent vaccine history cannot be determined should receive 2 doses of a 2010–11 seasonal influenza vaccine.”9 | 90.3 ± 0.6 |
| 2011–2012 | “Vaccination providers should note that, in previous seasons, children aged 6 months through 8 years who received only 1 dose of influenza vaccine in their first year of vaccination required 2 doses the following season. However, because the 2011–12 vaccine strains are unchanged from the 2010–11 season, children in this age group who received at least 1 dose of the 2010–11 seasonal vaccine will require only 1 dose of the 2011–12 vaccine. Children in this age group who did not receive at least 1 dose of the 2010–11 seasonal influenza vaccine, or for whom it is not certain whether the 2010–11 seasonal vaccine was received, should receive 2 doses of the 2011–12 seasonal influenza vaccine.”10 | 70.1 ± 1.2 |
CI, confidence interval.
Recommendations regarding which children required 2 doses versus 1 dose to be fully vaccinated against influenza, as published in the annual “Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP),” published in Morbidity and Mortality Weekly Report.
Aged 6 to 23 months.
Influenza vaccination coverage with at least 1 dose based solely on parental report (ie, no provider verification) has been routinely assessed and reported since 2009, but full vaccination coverage has not been assessed since then.19 Obtaining full vaccination coverage measures based on parental report is more complicated because it requires the parent to remember and report the child’s lifetime history of influenza vaccination. Before the 2009–2010 season, coverage with at least 1 dose and full coverage based solely on provider-reported vaccination histories had been assessed among children 6 to 23 months, but these reports were discontinued due to lack of timeliness, that is, the estimates for 1 season were not available before the start of the next season due to the extended time needed to obtain provider-reported vaccination data. Furthermore, nationally representative provider-reported data were not available for all of the age groups recommended to receive annual influenza vaccination as the recommendations expanded from children 6 to 23 months to all children 6 months and older.
This study focuses on children 6 to 23 months because, among children, this age group has the highest risk of influenza-related complications, they were the first group of children for which a routine influenza recommendation was made, and this is an age group for which nationally representative provider-reported influenza vaccination histories are available from survey data. The aims of this study were to examine the percentage of children 6 to 23 months who were fully vaccinated against influenza during each of 10 consecutive influenza seasons, by race/ethnicity, age group, and number of doses required to be fully vaccinated given the child’s vaccination history. The estimates in this report can serve as a benchmark against which full vaccination coverage based on the more timely parental report can be compared once these measures are developed for all children 6 months through 8 years.
METHODS
Survey Description
Data from the 2003 through 2012 National Immunization Survey (NIS) were analyzed. The NIS is an ongoing, national, list-assisted random-digit-dialed, landline and cellular telephone survey of households with children 19 to 35 months.20 Cellular telephones were added to the NIS in 2011.21 The household telephone survey is followed by a mailed questionnaire, the Immunization History Questionnaire, to all of the immunization providers identified during the telephone survey and for which permission to contact them was granted by the parent. The influenza vaccination coverage estimates in this report are based only on the provider-reported vaccinations. Providers reported the entire lifetime history of influenza vaccination on the Immunization History Questionnaire for every influenza season for which the child had received vaccination. Age was defined based on age on November 1 of the influenza season under study, with this study being limited to children who were 6 to 23 months on November 1, realizing that some children would age out of this age range during the influenza season, but they and all of their vaccinations were included. For each influenza season, 2 full calendar years of NIS data files were combined for analysis of the influenza season beginning the previous calendar year. For example, to obtain estimates for the 2002–2003 influenza season, the 2003 and 2004 NIS data files were combined. This was necessary to obtain an equal distribution of children throughout the age range of 6 to 23 months. The race/ethnicity variable used in this study (Hispanic, non-Hispanic white only, non-Hispanic black only, non-Hispanic other or multiple race) is based on parent report of the child’s race and ethnicity; children of Hispanic ethnicity may be of any race. The household response rates for the NIS data included in this study, as defined by the Council of American Survey Research Organizations, ranged from 61.6% to 73.1% for the landline sample (2003–2012) and 25.2% to 30.6% for the cellular telephone sample (2011–2012).22 The percentage for which adequate provider vaccination records were obtained among children with completed household interviews ranged from 63.6% to 72.3%.22 Methodological details of the NIS have been previously published.20
Statistical Methods
The Kaplan-Meier survival analysis procedure was used to calculate the percentage of children fully vaccinated. The event variable was defined as the provider-reported month of the influenza vaccination dose that made the child fully vaccinated against influenza, either the first (or only) dose or the second dose. The number of doses needed was determined on the basis of the season-specific recommendations for number of doses required, as summarized in Table 1. The Kaplan-Meier method was also used to calculate the percentage of children who received at least 1 dose of influenza vaccination (≥1 dose coverage), with the event variable defined as the provider-reported month of the child’s first influenza dose (regardless of their need for 1 or 2 doses). Influenza vaccinations received during the entire influenza season from July through May were included in the coverage estimates. This method was used to estimate the percentage of children fully vaccinated against influenza overall and by age group, racial/ethnic group, and number of doses required to be fully vaccinated, and for the 2011–2012 season only, by state.
Differences between influenza seasons within each group were tested by using t tests. Likewise, differences between groups within each influenza season were tested by using pairwise comparison t tests. Comparisons reported as being increases or decreases were statistically significant, whereas comparisons that were not statistically significant are reported as not being different. Reported percentages and corresponding 95% confidence intervals were weighted, and reported sample sizes were unweighted. All analyses were weighted to population totals, unit nonresponse, and noncoverage of nontelephone households and to adjust for households having multiple telephone lines. Tests for linear trend were performed using a weighted linear regression on the season-specific estimates using season number as the independent variable and weights as the inverse of the estimated variance of the estimated vaccination coverage. Pairwise comparisons of estimates were conducted by using t tests assuming large degrees of freedom and used the value of 1.96 for the critical value. Analyses were conducted by using SAS release 9.3 (SAS Inc, Cary, NC) and SUDAAN release 11.0.0 (Research Triangle Institute, Research Triangle Park, NC) statistical software to take into account the complex survey design. All statistical tests were 2-tailed with the significance level set at α < 0.05. Institutional review board approval for conducting the NIS was obtained through the National Center for Health Statistics Research Ethics Review Board and through the IRB of NORC at the University of Chicago.
RESULTS
Percentage of Children Requiring 2 Doses
Among children 6 to 23 months, the percentage of children requiring 2 doses to be considered fully vaccinated varied by influenza season as the dosage recommendations changed (Table 1). The percentage of children 6 to 23 months requiring 2 doses to be fully vaccinated against influenza ranged from 70.1% for 2011–2012 to 99.2% for the 2002–2003 season.
Trends in Full Influenza Vaccination Coverage Overall
Full influenza vaccination coverage among children 6 to 23 months overall increased from the 2002–2003 influenza season to the 2011–2012 season (trend test P < .001; Table 2; Fig 1). The average annual increase was 4.2 percentage points based on the slope of the trend test. The estimates and annual changes from the previous season are denoted in Table 2. In only 2 of the 10 influenza seasons (the 2007–2008 and the 2010–2011 seasons), there was no increase observed in the percentage of children fully vaccinated compared with the previous season. Full influenza vaccination coverage by state for the 2011–2012 influenza season is presented in Table 3; coverage ranged from 23.6% in Mississippi to 72.2% in Massachusetts.
TABLE 2.
Full Influenza Vaccination Coverage Among Children Aged 6 to 23 Months by Influenza Season, Age Group, Racial/Ethnic Group, and Number of Doses Needed to Be Fully Vaccinated, NIS, Provider Report, United States, 2002–2003 Through 2011–2012 Influenza Seasons
| Influenza Season | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 2002–2003 | 2003–2004 | 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | 2009–2010 | 2010–2011 | 2011–2012 | Trend Test* | |
|
|
|||||||||||
| n = 28 967 | n = 25 316 | n = 26 181 | n = 26 981 | n = 21 991 | n = 23 825 | n = 22 961 | n = 23 747 | n = 23 098 | n = 19 490 | ||
|
|
|||||||||||
| % ± 95% CI** | % ± 95% CI, Change† | % ± 95% CI, Change | % ± 95% CI, Change | % ± 95% CI, Change | % ± 95% CI, Change | % ± 95% CI, Change | % ± 95% CI, Change | % ± 95% CI, Change | % ± 95% CI, Change | Slope ± 95% CI | |
| Full vaccination coverage | |||||||||||
| Overall | 4.8 ± 0.4 | 9.1 ± 0.6 +4.3‡ |
19.2 ± 0.8 +10.1‡ |
22.6 ± 0.8 +3.4§ |
26.1 ± 1.0 +3.5§ |
27.0 ± 1.0 +0.9 |
31.4 ± 1.1 +4.4§ |
34.4 ± 1.1 +3.0§ |
33.7 ± 1.2 −0.7 |
44.7 ± 1.3 +11.0§ |
4.2 ± 0.6 |
| Age group | |||||||||||
| a. 6–11 mo | 5.3 ± 0.7c | 9.4 ± 1.1 +4.1§ |
19.8 ± 1.3 +10.4§ |
22.7 ± 1.6 +2.9§ |
25.8 ± 1.7 +3.1§ |
30.5 ± 1.8bc +4.7§ |
35.6 ± 1.9bc +5.1§ |
36.0 ± 1.9b +0.4 |
41.9 ± 2.2bc +5.9§ |
45.4 ± 2.4 +3.5§ |
4.7 ± 0.5 |
| b. 12–16 mo | 4.7 ± 0.6 | 8.9 ± 1.1 +4.2§ |
19.5 ± 1.4 +10.6§ |
22.6 ± 1.5 +3.1§ |
24.7 ± 1.8 +2.1 |
26.0 ± 1.7a +1.3 |
30.4 ± 1.8a +4.4§ |
33.0 ± 1.9a +2.6 |
32.8 ± 2.0ac −0.2 |
44.6 ± 2.4 +11.8§ |
4.1 ± 0.6 |
| c. 17–23 mo | 4.3 ± 0.6a | 9.1 ± 1.0 +4.8§ |
18.1 ± 1.4 +9.0§ |
22.3 ± 1.3 +4.2§ |
27.0 ± 1.8 +4.7§ |
24.1 ± 1.6a −2.9§ |
27.9 ± 1.8a +3.8§ |
34.0 ± 1.9 +6.1§ |
26.4 ± 1.7ab −7.6§ |
43.9 ± 2.2 +17.5§ |
3.8 ± 0.9 |
| Race/ethnicity | |||||||||||
| a. Hispanic | 2.7 ± 0.6bd | 6.6 ± 1.1bcd +3.9§ |
15.1 ± 1.6b +8.5§ |
16.9 ± 1.7bd +1.8 |
21.7 ± 2.2bcd +4.8§ |
22.4 ± 2.2bcd +0.7§ |
26.6 ± 2.4bcd +4.2§ |
30.6 ± 2.5bcd +4.0§ |
31.5 ± 2.7bc +0.9 |
40.2 ± 3.1bcd +8.7§ |
4.0 ± 0.4 |
| b. White only, non-Hispanic | 6.1 ± 0.5ac | 11.4 ± 0.8ac +5.3§ |
23.3 ± 1.1acd +11.9§ |
27.3 ± 1.1acd +4.0§ |
30.3 ± 1.3ac +3.0§ |
31.3 ± 1.3ac +1.0 |
36.2 ± 1.3ac +4.9§ |
38.3 ± 1.4ac +2.1§ |
36.8 ± 1.4acd −1.5 |
49.0 ± 1.6ac +12.2§ |
4.6 ± 0.8 |
| c. Black only, non-Hispanic | 2.7 ± 0.9bd | 4.1 ± 1.3abd +1.4 |
13.0 ± 2.1bd +8.9§ |
15.4 ± 2.1bd +2.4 |
17.8 ± 2.6abd +2.4 |
17.6 ± 2.4abd −0.2 |
21.3 ± 2.6abd +3.7§ |
24.1 ± 2.6abd +2.8 |
25.6 ± 3.1abd +1.5 |
35.3 ± 3.5abd +9.7§ |
3.3 ± 0.5 |
| d. Other/multiple race, non-Hispanic | 6.5 ± 1.9ac | 10.7 ± 2.5ac +4.2§ |
17.1 ± 2.3bc +6.4§ |
23.4 ± 2.6abc +6.3§ |
27.5 ± 3.6ac +4.1 |
30.0 ± 3.3ac +2.5 |
33.7 ± 3.9ac +3.7 |
39.2 ± 3.9ac +5.5 |
35.5 ± 3.7bc −3.7 |
48.7 ± 4.2ac +13.2§ |
4.3 ± 0.6 |
| Doses needed | |||||||||||
| a. One | 45.3 ± 10.6b | 54.3 ± 5.5b +9.0 |
58.0 ± 3.5b +3.7 |
52.8 ± 2.3b −5.2§ |
58.9 ± 2.7b +6.1§ |
70.0 ± 3.2b +11.1§ |
72.7 ± 3.0b +2.7 |
76.6 ± 2.5b +3.9 |
81.7 ± 2.7b +5.1§ |
74.1 ± 2.4b −7.6§ |
3.8 ± 1.3 |
| b. Two | 4.4 ± 0.4a | 7.5 ± 0.6a +3.1§ |
15.9 ± 0.8a +8.4§ |
15.7 ± 0.8a −0.2 |
18.4 ± 1.0a +2.7§ |
21.5 ± 1.0a +3.1§ |
25.5 ± 1.1a +4.0§ |
26.5 ± 1.1a +1.0 |
28.4 ± 1.2a +1.9§ |
32.2 ± 1.5a +3.8§ |
3.2 ± 0.4 |
| ≥1 dose coverage | |||||||||||
| Overall | 8.1 ± 0.5 | 19.7 ± 0.9 +11.6§ |
37.1 ± 1.0 +17.4§ |
36.6 ± 1.0 −0.5 |
40.4 ± 1.2 +3.8§ |
45.3 ± 1.2 +4.9§ |
49.7 ± 1.2 +4.4§ |
53.6 ± 1.2 +3.9§ |
55.9 ± 1.2 +2.3§ |
57.5 ± 1.4 +1.6§ |
6.0 ± 1.3 |
| Race/ethnicity | |||||||||||
| a. Hispanic | 6.4 ± 1.0bd | 16.0 ± 1.7bd +9.6§ |
33.3 ± 2.2b +17.3§ |
31.3 ± 2.2bd −2.0 |
37.7 ± 2.6bcd +6.4§ |
42.7 ± 2.7bcd +5.0§ |
47.6 ± 2.7bc +4.9§ |
54.3 ± 2.7cd +6.7§ |
56.6 ± 2.9cd +2.3 |
55.6 ± 3.3bcd −1.0 |
6.3 ± 1.1 |
| b. White only, non-Hispanic | 9.3 ± 0.6ac | 22.4 ± 1.1ac +13.1§ |
41.0 ± 1.3acd +18.6§ |
40.6 ± 1.3ac −0.4 |
43.1 ± 1.4ac +2.5§ |
48.0 ± 1.4ac +4.9§ |
52.9 ± 1.4ac +4.9§ |
54.2 ± 1.4cd +1.3 |
56.7 ± 1.5cd +2.5§ |
59.4 ± 1.6ac +2.7§ |
6.0 ± 1.5 |
| c. Black only, non-Hispanic | 5.1 ± 1.2bd | 13.6 ± 2.5bd +8.5§ |
30.1 ± 2.9bd +16.5§ |
30.3 ± 2.8bd +0.2 |
31.8 ± 3.2abd +1.5 |
36.6 ± 3.1abd +4.8§ |
39.8 ± 3.1abd +3.2 |
45.4 ± 3.2abd +5.6§ |
46.9 ± 3.4abd +1.5 |
50.5 ± 3.7abd +3.6 |
5.4 ± 1.0 |
| d. Other/multiple race, non-Hispanic | 10.4 ± 2.2ac | 24.0 ± 3.2ac +13.6§ |
36.3 ± 3.3bc +12.3§ |
39.2 ± 3.5ac +2.9 |
46.0 ± 4.0ac +6.8§ |
50.6 ± 3.7ac +4.6 |
51.6 ± 4.0c +1.0 |
59.4 ± 3.9abc +7.8§ |
61.5 ± 3.6abc +2.1 |
61.8 ± 4.0ac +0.3 |
5.9 ± 1.2 |
CI, confidence interval half-width.
The presence or absence of superscripted letters denotes whether that estimate was statistically significantly different at P < .05 from another row, and denotes which row it differed from (a, b, c, or d) based on pairwise comparison t tests. For example, in 2002–2003, the percentage of fully vaccinated 6- to 11-month-olds (a) was statistically significantly different from the percentage of fully vaccinated 17- to 23-month-olds (c).
Tests for linear trend were performed using a weighted linear regression on the season-specific estimates using season number as the independent variable and weights as the inverse of the estimated variance of the estimated vaccination coverage. The estimated slope coefficients were interpreted as the average change across seasons assuming a linear increase. Slopes and their 95% confidence intervals are presented; all of the tests for linear trend were significant at P < .001.
Estimates obtained using Kaplan-Meier survival analysis methods.
Change in percentage points compared with the previous influenza season.
Pair-wise comparisons between adjacent influenza seasons were conducted using t tests; statistically significant (P < .05) differences are footnoted.
Age on November first of the influenza season under study.
FIGURE 1.
Full influenza vaccination coverage among children aged 6 to 23 months by influenza season, NIS, provider report, United States, 2002–2003 through 2011–2012 influenza seasons. aEstimates obtained using Kaplan-Meier survival analysis methods and reported with 95% confidence interval half-widths.
TABLE 3.
Full Influenza Vaccination Coverage Among Children 6 to 23 Months by State, NIS, provider report, United States, 2011–2012 Influenza Season
| Statea | Full Influenza Vaccination % ± 95% CIb | State | Full Influenza Vaccination % ± 95% CIa | State | Full Influenza Vaccination % ± 95% CIa |
|---|---|---|---|---|---|
| Massachusetts | 72.2 ± 6.1 | Colorado | 50.6 ± 7.2 | Florida | 39.1 ± 7.1 |
| Connecticut | 71.1 ± 6.4 | New York | 50.4 ± 4.5 | Alaska | 38.9 ± 6.1 |
| Rhode Island | 65.3 ± 6.7 | Arizona | 49.9 ± 7.0 | Kansas | 38.7 ± 6.2 |
| Vermont | 64.9 ± 6.1 | New Mexico | 48.2 ± 6.5 | Tennessee | 38.6 ± 6.5 |
| Delaware | 64.5 ± 6.7 | Illinois | 48.0 ± 4.7 | Utah | 38.3 ± 6.4 |
| Minnesota | 63.6 ± 6.9 | Washington | 47.8 ± 7.2 | Texas | 36.7 ± 4.1 |
| New Hampshire | 62.7 ± 6.3 | Michigan | 47.7 ± 7.0 | Iowa | 34.9 ± 6.9 |
| South Dakota | 60.1 ± 6.8 | Virginia | 47.6 ± 8.6 | Kentucky | 34.5 ± 6.7 |
| Hawaii | 57.7 ± 6.6 | Ohio | 45.7 ± 6.6 | Louisiana | 33.8 ± 6.3 |
| Wisconsin | 57.5 ± 6.6 | North Carolina | 44.1 ± 6.7 | West Virginia | 33.5 ± 6.9 |
| Pennsylvania | 56.7 ± 5.3 | Maryland | 44.0 ± 7.0 | Georgia | 33.2 ± 7.0 |
| Nebraska | 56.6 ± 6.4 | Oregon | 43.7 ± 6.2 | Alabama | 32.9 ± 7.1 |
| North Dakota | 56.2 ± 6.5 | California | 43.0 ± 6.8 | Wyoming | 32.2 ± 6.7 |
| Maine | 55.4 ± 6.8 | Montana | 42.1 ± 6.8 | Oklahoma | 31.8 ± 6.3 |
| New Jersey | 55.2 ± 6.3 | Indiana | 42.0 ± 6.7 | South Carolina | 31.6 ± 6.3 |
| District of Columbia | 54.3 ± 7.1 | Idaho | 41.6 ± 7.2 | Arkansas | 26.6 ± 6.3 |
| Missouri | 51.6 ± 7.4 | Nevada | 40.8 ± 6.2 | Mississippi | 23.6 ± 6.3 |
CI, confidence interval half-width.
Ordered from highest to lowest full influenza vaccination coverage.
Estimates obtained using Kaplan-Meier survival analysis methods.
Trends in Full Influenza Vaccination Coverage by Age Group
Within each age group studied, full influenza vaccination coverage increased from the 2002–2003 influenza season to the 2011–2012 season (trend test P < .001 each; Table 2). In 5 of the 10 influenza seasons studied (2003–2004, 2004–2005, 2005–2006, 2006–2007, and 2011–2012), there were no differences between the age groups in full influenza vaccination coverage. In the remaining 5 seasons, children aged 6 to 11 months had higher full influenza vaccination coverage than children in the older age groups.
Trends in Full Influenza Vaccination Coverage by Race/Ethnicity
Within all 4 racial/ethnic groups studied, full influenza vaccination coverage increased from the 2002–2003 influenza season to the 2011–2012 season (trend test P < .001 each; Table 2). The change in vaccination coverage from one season to the next within each group is indicated in Table 2 including whether these changes were statistically significant.
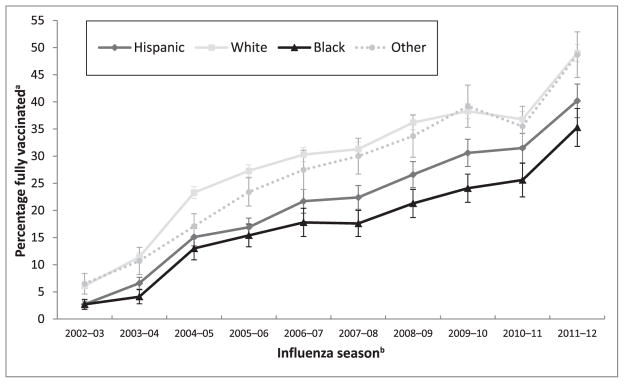
In all 10 influenza seasons studied, non-Hispanic black children (hereafter referred to as black children) and Hispanic children had lower full influenza vaccination coverage than non-Hispanic white children (hereafter referred to as white children; Table 2; Fig 2). The gap in these racial/ethnic differences does not appear to decrease in magnitude over time. For example the percentage of black children fully vaccinated in the 2002–2003 season was 2.7%, whereas the percentage of white children fully vaccinated was 6.1%, a difference of −3.4 percentage points; for the remaining seasons these black-white differences widen as follows: −7.3, −10.3, −11.9, −12.5, −13.7, −14.9, −14.2, −11.2, and −13.7 percentage points. The Hispanic-white differences by season were as follow: −3.4, −4.8, −8.2, −10.4, −8.6, −8.9, −9.6, −7.7, −5.3, and −8.8). In 7 of the 10 seasons, black children also had lower full influenza vaccination coverage than Hispanic children.
FIGURE 2.
Full influenza vaccination coverage among children aged 6 to 23 months by influenza season and racial/ethnic group, NIS, provider report, United States, 2002–2003 through 2012–2013 influenza seasons. aEstimates obtained using Kaplan-Meier survival analysis methods and reported with confidence interval half-widths. Estimates for the 2009–2010 season include only the seasonal influenza vaccine and exclude the 2009 H1N1 vaccine. bStatistically significant (P < .05) differences between groups as well as the direction of the differences are as follows: 2002–2003: H < W; H < O; B < W; B < O; 2003–2004: H < W; H < O; B < H; B < W; B < O. 2004–2005: H < W; B < W; O < W; B < O; 2005–2006: H < W; H < O; B < W; B < O; O < W; 2006–2007: H<W; B < H; H < O; B < W; B < O; 2007–2008: H < W; B < H; H < O; B < W; B < O; 2008–2009: H < W; B < H; H < O; B < W; B < O; 2009–2010: H < W; B < H; H < O; B<W; B < O; 2011–2012: H < W; B < H; B < W; W < O; B < O; 2012–2013: H < W; B < H; H < O; B < W; B < O, where H, Hispanic; W, white only, non-Hispanic; B, black only, non-Hispanic; O, other or multiple race, non-Hispanic. All other pairwise comparisons not noted were not statistically significant.
Similar racial/ethnic differences were also seen among the estimates of coverage with at least 1 dose of influenza vaccination (Table 2). In all 10 influenza seasons, black children had lower ≥1 dose influenza vaccination coverage than white children. In 8 of the 10 seasons Hispanic children had lower coverage than white children, whereas in the 2009–2010 and 2010–2011 seasons they had similar coverage.
Full Influenza Vaccination Coverage by Number of Doses Required
Among children who required 1 dose to be fully vaccinated and among those who required 2 doses, full influenza vaccination coverage increased from the 2002–2003 influenza season to the 2011–2012 season (trend test P < .001 each; Table 2). The change in vaccination coverage from one season to the next within each group and whether these changes were statistically significant is indicated in Table 2. For all 10 influenza seasons, full influenza vaccination coverage was higher among children requiring only 1 dose compared with those requiring 2 doses (Table 2). For the 2011–2012 season, among the subset of children 6 to 23 months who required 2 doses and received 1 dose, 63.7% received their required second dose, which can be viewed as the completion rate among those who began the series of 2 doses (not shown in tables).
DISCUSSION
This study found that full influenza vaccination coverage of children 6 to 23 months increased from 4.8% in the 2002–2003 influenza season to 44.7% in the 2011–2012 season. This is to be expected because previously published reports of coverage with at least 1 dose of influenza vaccination have shown increases during this time.19,23 Despite the increase, the majority of children 6 to 23 months in the United States were not fully vaccinated against influenza.
Previous reports of full influenza vaccination coverage of children 6 to 23 months have been published using NIS provider-reported data, but these reports used a different estimation methodology (proportion estimate, subset of children 6 to 23 months during September–December and only included vaccinations through January).24–29 The estimates from previous reports compared with the estimates in this report are as follows for the 2002–2003 season through the 2009–2010 season: 4.4% versus 4.8%, 8.4% versus 9.1%, 17.8% versus 19.2%, 20.6% versus 22.6%, 21.3% versus 26.1%, 23.4% versus 27.0%, 24.7% versus 31.4%, 30.1% versus 34.4%, respectively.24–29 Note that the previous reports slightly underestimated full influenza vaccination coverage as expected because they did not count any vaccinations past January; reanalyzing these data using the improved Kaplan-Meir method rather than the proportion estimate, the censored nature of the data could be taken into account and all vaccinations counted. These reports were discontinued due to lack of timeliness of NIS provider-reported influenza data (ie, the estimates were not available before the start of the next influenza season due to the survey time needed to obtain provider-reported vaccination histories) and due to a need for influenza vaccination estimates for all children 6 months to 17 years. To address this, the NIS-Flu was developed, which can provide estimates of parental report of influenza vaccination coverage for children 6 months to 17 years for an influenza season before the start of the following influenza season.19 Parental report of at least 1 dose of influenza vaccination has been reported based on the NIS-Flu data; however, parental report of full vaccination coverage has not yet been assessed. Full influenza vaccination coverage of children 6 to 23 months based on Immunization Information Systems sentinel site data compared with the national estimates from this report likewise showed slight differences: 35.9% versus 33.7%, respectively, for the 2010–2011 season and 44.3% versus 44.7%, respectively, for the 2011–2012 season.30
NIS estimates based on provider report are not as timely as estimates based on parental report due to the time needed to obtain vaccination information from all immunization providers. Additionally, for this study, 2 full calendar years of interviews were used for each influenza season to have a more equal age distribution of children 6 months, 7 months, and up to 23 months; if only 1 calendar year was used, the sample would be weighted more heavily toward older children. Use of parental reported influenza vaccination allows for the timely production of influenza vaccination coverage estimates.19 Obtaining parental report of full influenza vaccination, however, is more complicated because it requires the parental report of several past seasons of influenza vaccination, which may be more subject to recall bias. A thorough analysis of the validity of parent- versus provider-reported child influenza vaccination is needed, including examining whether there are differences by race/ethnicity. Initial indications, when examining the estimated number of people vaccinated, are that parent report overestimates influenza vaccination coverage.19 At least 1 study did find differences in parental versus provider report by various sociodemographic characteristics, including race/ethnicity.31 The ≥1 dose coverage estimates in this report, based on provider report, are lower than those based on the parental-reported NIS-Flu estimates; a detailed validity study to quantify the extent of parental overreporting is warranted.19
Racial/ethnic differences in influenza vaccination coverage among adults have persisted over many influenza seasons.32 The finding that such differences also exist for full influenza vaccination coverage of young children is concerning. Broad use of evidence-based strategies that enhance access to vaccination services, increase demand for vaccinations, and use provider or system interventions are important components of efforts to increase vaccination coverage and reduce these differences.33 Furthermore, providers are encouraged to begin offering influenza vaccination soon after vaccine becomes available and to continue vaccination efforts throughout the influenza season. This is especially important for children who require 2 doses within the season to be fully protected.
This study is subject to at least 3 limitations. First, the NIS is a telephone survey followed by a mail survey to immunization providers. Although statistical adjustments compensate for nonresponse and households without telephones, bias might remain. Second, the NIS relies on provider reported vaccination histories; incomplete records and reporting might affect estimates. However, a previous study has shown that nearly all (97%) vaccinated children aged 6 to 23 months received their influenza vaccination at a medical place (74% at a doctor’s office, 18% at a clinic or health center, and 5% at a hospital or emergency department).30 Third, there was a change in the NIS sampling design from landline to dual frame (landline and cellular telephone), which should be taken into account when interpreting trends over time in NIS estimates.
CONCLUSIONS
Less than half of children aged 6 to 23 months in the United States are fully vaccinated against influenza, and even a smaller percentage of Hispanic and non-Hispanic black children. Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated. Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications.
WHAT’S KNOWN ON THIS SUBJECT
Influenza vaccination coverage with ≥1 dose based on parental report has been routinely assessed and reported since 2009 in the United States at http://www.cdc.gov/flu/fluvaxview, but full influenza vaccination coverage (by parent or provider report) has not been assessed since then.
WHAT THIS STUDY ADDS
This study reports the percentage of children 6 to 23 months in the United States who were fully vaccinated against influenza during each of 10 consecutive influenza seasons (2002–2003 through 2011–2012) by race/ethnicity based on provider reported data.
Acknowledgments
FUNDING: No external funding.
ABBREVIATIONS
- ACIP
Advisory Committee on Immunization Practices
- NIS
National Immunization Survey
Footnotes
Dr Santibanez conceptualized and designed the study, carried out the analyses, and drafted and revised the manuscript; Dr Grohskopf, Mr Zhai, and Ms Kahn critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Burney LE. Influenza immunization: statement. Public Health Rep. 1960;75(10):944. [PMC free article] [PubMed] [Google Scholar]
- 2.Bridges CB, Fukuda K, Uyeki TM, Cox NJ, Singleton JA Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2002;51(RR-3):1–31. [PubMed] [Google Scholar]
- 3.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004;53(RR-6):1–40. [PubMed] [Google Scholar]
- 4.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA Advisory Committee on Immunization Practices. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-10):1–42. [PubMed] [Google Scholar]
- 5.Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 6.Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics. 2005;116(1):153–159. doi: 10.1542/peds.2005-0049. [DOI] [PubMed] [Google Scholar]
- 7.Allison MA, Daley MF, Crane LA, et al. Influenza vaccine effectiveness in healthy 6- to 21-month-old children during the 2003–2004 season. J Pediatr. 2006;149(6):755–762. doi: 10.1016/j.jpeds.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC) Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-10):1–8. [PubMed] [Google Scholar]
- 9.Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(33):1128–1132. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61(32):613–618. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2013–2014. MMWR Recomm Rep. 2013;62:1–43. [PubMed] [Google Scholar]
- 13.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2003;52:1–36. [Google Scholar]
- 15.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-8):1–40. [PubMed] [Google Scholar]
- 16.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA Advisory Committee on Immunization Practices. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-10):1–42. [PubMed] [Google Scholar]
- 17.Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR-6):1–54. [PubMed] [Google Scholar]
- 18.Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) [Accessed March 9, 2015];FluVaxView: flu vaccination coverage, United States, 2013–14 influenza season. 2014 Sep 18; Available at: http://www.cdc.gov/flu/fluvaxview/coverage-1314estimates.htm.
- 20.Centers for Disease Control and Prevention (CDC) [Accessed August 6, 2015];Datasets and related documentation for the National Immunization Survey. 2015 Mar 30; Available at: http://www.cdc.gov/nchs/nis/datasets.htm.
- 21.Centers for Disease Control and Prevention (CDC) [Accessed August 6, 2015];National Immunization Survey, a user’s guide for the 2011 public-use data file. 2012 Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISPUF11_DUG PDF.
- 22.Centers for Disease Control and Prevention (CDC) [Accessed August 6, 2015];National Immunization Survey, a user’s guide for the 2012 public-use data file. 2015 Feb; Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NIS/NISPUF12_DUG.PDF.
- 23.Santibanez TA, Lu PJ, O’Halloran A, Meghani A, Grabowsky M, Singleton JA. Trends in childhood influenza vaccination coverage—U.S., 2004–2012. Public Health Rep. 2014;129(5):417–427. doi: 10.1177/003335491412900505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Influenza vaccination coverage among children aged 6–23 months—United States, 2005–06 influenza season. MMWR Morb Mortal Wkly Rep. 2007;56(37):959–963. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Influenza vaccination coverage among children aged 6–23 months—United States, 2006–07 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57(38):1039–1043. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Childhood influenza-vaccination coverage—United States, 2002–03 influenza season. MMWR Morb Mortal Wkly Rep. 2004;53(37):863–866. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Childhood influenza vaccination coverage—United States, 2003–04 influenza season. MMWR Morb Mortal Wkly Rep. 2006;55(4):100–103. [PubMed] [Google Scholar]
- 28.CDC. Childhood influenza vaccination coverage—United States, 2004–2005 influenza season. MMWR Morb Mortal Wkly Rep. 2006;55:1061–1065. [PubMed] [Google Scholar]
- 29.Santibanez TA, Santoli JM, Bridges CB, Euler GL. Influenza vaccination coverage of children aged 6 to 23 months: the 2002–2003 and 2003–2004 influenza seasons. Pediatrics. 2006;118(3):1167–1175. doi: 10.1542/peds.2006-0831. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Surveillance of influenza vaccination coverage—United States, 2007–2008 through 2011–2012 influenza seasons. MMWR CDC Surveill Summ. 2013;62:1–28. [PubMed] [Google Scholar]
- 31.Brown C, Clayton-Boswell H, Chaves SS, et al. New Vaccine Surveillance Network (NVSN) Validity of parental report of influenza vaccination in young children seeking medical care. Vaccine. 2011;29(51):9488–9492. doi: 10.1016/j.vaccine.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Lu PJ, O’Halloran A, Bryan L, et al. Trends in racial/ethnic disparities in influenza vaccination coverage among adults during the 2007–2008 through 2011–2012 seasons. Am J Infect Control. 2014;42(7):763–769. doi: 10.1016/j.ajic.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guide to Community Preventive Services. [Accessed August 6, 2015];Increasing appropriate vaccination. 2015 Jun 22; http://wwwthecommunityguide.org/vaccines/index.html.