Abstract
Background
Traditional predictors of neurological prognosis after cardiac arrest are unreliable after targeted temperature management. Absence of pupillary reflexes remains a reliable predictor of poor outcome. Diffusion-weighted imaging has emerged as a potential predictor of recovery, and here we compare imaging characteristics to pupillary exam.
Methods
We identified 69 patients who had MRIs within seven days of arrest and used a semi-automated algorithm to perform quantitative volumetric analysis of apparent diffusion coefficient (ADC) sequences at various thresholds. Area under receiver operating characteristic curves (ROC-AUC) were estimated to compare predictive values of quantitative MRI with pupillary exam at days 3, 5 and 7 post-arrest, for persistence of coma and functional outcomes at discharge. Cerebral Performance Category scores of 3–4 were considered poor outcome.
Results
Excluding patients where life support was withdrawn, ≥2.8% diffusion restriction of the entire brain at an ADC of ≤650 × 10−6 m2/s was 100% specific and 68% sensitive for failure to wake up from coma before discharge. The ROC-AUC of ADC changes at ≤450 × 10−6 mm2/s and ≤650 × 10−6 mm2/s were significantly superior in predicting failure to wake up from coma compared to bilateral absence of pupillary reflexes. Among survivors, >0.01% of diffusion restriction of the entire brain at an ADC ≤450 × 10−6 m2/s was 100% specific and 46% sensitive for poor functional outcome at discharge. The ROC curve predicting poor functional outcome at ADC ≤450 × 10−6 mm2/s had an AUC of 0.737 (0.574–0.899, p = 0.04).
Conclusion
Post-anoxic diffusion changes using quantitative brain MRI may aid in predicting persistent coma and poor functional outcomes at hospital discharge.
Keywords: Quantitative brain MRI, Cardiac arrest, Pupillary exam, Clinical outcomes, Coma
Introduction
Cardiac arrest can be associated with significant neurologic burden due to hypoxic-ischemic encephalopathy [1]. Prior to the ubiquitous use of targeted temperature management (TTM), clinical guidelines to predict functional outcome were widely accepted [2]. However, many predictors have been shown to have significant false positive rates in the post-TTM era. Bilaterally absent pupillary light reflexes remain predictive of poor outcome even after TTM [3–6]. More recently, diffusion-weighted imaging (DWI) sequence on magnetic resonance imaging (MRI) has emerged as a potential novel tool to predict recovery [7–11]. In this study, we investigate the utility of quantifying apparent diffusion coefficient (ADC) injury burden within the first seven days post-resuscitation using a semi-automated algorithm in predicting awakening from coma and functional outcome at discharge, and compare it to pupillary exam for the same time period.
Methods
Subjects
We retrospectively identified 460 adult cardiac arrest patients admitted to an intensive care unit at Columbia University Medical Center between January 2007 and November 2015 who remained comatose and were candidates for TTM. Post-arrest MRIs were obtained in 104 patients (22.6%), and 72 of these (69.2% with MRI) had an MRI within seven days of the arrest. One patient was excluded because of unavailability of ADC sequences, and two because of significant acute structural abnormalities, leaving 69 total scans analysed. None of the 69 patients suffered a second arrest within the seven days after index arrest. The following variables from the Utstein reporting guidelines [12] were recorded: age, sex, race, initial cardiac rhythm, whether arrest was witnessed or bystander cardiopulmonary resuscitation (CPR) administered, time to return of spontaneous circulation (ROSC), and whether TTM was used. Additional data collected included time to first MRI, withdrawal of life-sustaining therapies (WLST), and cerebral performance categories (CPC) [13] prior to arrest and at discharge. Poor functional outcome was defined as CPC of 3–5. WLST was defined as terminal extubation and/or withdrawal of vasopressors, inotropes and any mechanical circulatory assistance. Coma was defined as Glasgow Coma Scale ≤8. Pupillary exams at days 3, 5 and 7 post-arrest were collected. Our TTM protocol appears in supplementary material. The Columbia University institutional review board approved the collection of these data.
MR techniques
In this retrospective study, MRI was obtained for clinical purposes at the discretion of treating attending. All MRIs were de-identified and diffusion restriction was identified on DWI 1000 sequences and volumetric analysis calculated on corresponding ADC sequences at thresholds of ≤450 × 10−6 mm2/s and ≤650 × 10−6 mm2/s, thresholds shown to be significant in prior studies on MRI after cardiac arrest [7,11]. Total brain volume was calculated for each subject to standardize injury burden in each patient. Information on the semi-automated algorithm, which is highly correlated with a neuroradiologist's manual technique (Pearson correlation of 0.995) [14], is available in supplementary material.
Statistical analysis
All segmented ADC volumes were expressed as percent of total brain volume [7,11]. As distributions were non-normal, medians were compared using the Mann–Whitney U test. Categorical variables were compared using Fisher's exact test or Pearson chi-square, as appropriate. Predictive value of pupillary exam and radiographic parameters were analysed using receiver operator characteristics (ROC) curve analysis and compared using the Hanley and McNeil method [15]. A p value of <0.05 was considered significant. SPSS version 23 (Chicago, IL) was used for all analyses.
Results
Patient characteristics
Of the 69 patients included, 85% had a good functional status prior to arrest and 87% successfully completed TTM. WLST occurred in 30%. Compared to 391 patients who remained comatose post-resuscitation but did not undergo MRI within the first week, these patients were younger, more likely to have good functional status prior to arrest and survive to discharge with severe disability or in comatose/persistent vegetative state (Table 1). Among patients that underwent MRI within one week of the arrest, 80% (n = 55) were comatose at the time the MRI was obtained and the remaining 20% (n= 14) were awake but not yet back to their baseline mental status. MRI was performed a median of four days after arrest (IQR 3, 6 days).
Table 1.
Patient Demographics.
| Characteristic | Patients with MRI within first 7 days (n = 69) | Patients without MRI in first 7 days (n = 391) | P-value |
|---|---|---|---|
| Median age, years (IQR) | 60 (50, 73) | 65 (55, 78) | 0.03 |
| Female sex | 46% (n = 32) | 46% (n = 178) | NS |
| Race | |||
| White, non-Hispanic | 39% (n = 27) | 36% (n = 142) | NS |
| Black, non-Hispanic | 23% (n= 16) | 26% (n = 100) | |
| Hispanic | 32% (n = 22) | 32% (n = 126) | |
| Asian | 1% (n= 1) | 3% (n = 10) | |
| Other | 0% | 0.6% (n = 2) | |
| Unknown | 4% (n = 3) | 3% (n = 10) | |
| Pre-arrest good functional status (CPC 1–2) | 85% (n = 59) | 74% (n = 290) | 0.04 |
| Out-of-hospital arrest | 77% (n = 53) | 67% (n = 262) | NS |
| Witnessed arrest | 84% (n = 58) | 76% (n = 297) | NS |
| Bystander CPR | 58% (n = 40) | 63% (n = 248) | NS |
| Initial rhythm | NS | ||
| VT/VF | 23% (n= 16) | 22% (n = 84) | |
| PEA | 42% (n = 29) | 47% (n = 182) | |
| Asystole | 28% (n= 19) | 25% (n = 97) | |
| Unknown | 7% (n = 5) | 7% (n = 28) | |
| Median ROSC, min (IQR) | 21 (12, 28) | 35 (24, 30) | NS |
| TTM completed | 87% (n = 60) | 72% (n = 280) | <0.01 |
| WLST | 30% (n = 21) | 43% (n = 168) | NS |
| Survival to discharge | 52% (n = 36) | 29% (n = 112) | <0.01 |
| Good functional status at discharge | 13% (n = 9) | 15% (n = 59) | NS |
| CPC at discharge | |||
| 1 | 4% (n = 3) | 5% (n = 19) | <0.01 |
| 2 | 9% (n = 6) | 10% (n = 40) | |
| 3 | 22% (n= 15) | 9% (n = 35) | |
| 4 | 17% (n= 12) | 5% (n = 18) | |
| 5 | 48% (n = 33) | 71% (n = 279) | |
IQR, interquartile range; CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; VT/VF, ventricular tachycardia/ventricular fibrillation; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; TTM, targeted temperature management; WLST, withdrawal of life-sustaining therapy; NS, not significant.
MRI diffusion abnormalities
The supplementary table lists location-specific diffusion changes. Diffusion changes were never found in the brainstem. Notably, diffusion changes in the thalamus were seen in 20% (n = 14) patients and were always associated with concomitant cortical and/or subcortical abnormalities. Presence of ADC changes in the thalamus at an ADC threshold of ≤650 × 10−6 mm2/s had a 100% specificity in predicting poor outcome (CPC 3–5) regardless of WLST. Sensitivity ranged from 18% (n = 69) to 21% (excluding patients with WLST; n = 22).
Comparing MRI to pupillary exam
Among patients who were comatose at the time of MRI (n = 55), the median percent of total brain volume affected at both ADC ≤450 and ≤650 × 10−6 mm2/s was significantly different between those who were awake versus comatose or dead at discharge (Table 2). This difference was present even when excluding 22 patients with WLST. The AUC of quantitative ADC changes at a threshold of ≤450 × 10−6 mm2/s and ≤650 × 10−6 mm2/s in predicting failure to wake up before discharge were 0.878 (95% CI 0.786–0.970, p < 0.001) and 0.884 (95% CI 0.798–0.971, p < 0.001), respectively. Both were significantly superior in predicting persistence of coma or death than bilaterally absent pupillary light reflexes on day 3 (0.617,95%CI 0.420–0.813, p = 0.316), day 5 (0.580, 95%CI 0.374–0.786, p = 0.482), or day 7 (0.568, 95%CI 0.354–0.782, p = 0.557), (p<0.03) (Fig. 1). When excluding patients with WLST, a burden of ≥2.8% of diffusion restriction of the entire brain at an ADC of ≤650 × 10−6 m2/s was 100% specific and 68% sensitive for failure to wake up from coma before discharge.
Table 2.
Consciousness after coma.*
| Characteristic | Conscious at discharge (n = 11) | Including WLST (n = 44) | Excluding WLST (n = 22) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Unconscious/dead at discharge | p-value, if significant** | Unconscious/dead at discharge | p-value, if significant** | ||
| Median age, years (IQR) | 57 (47, 73) | 63 (46, 73) | NS | 56 (29, 63) | NS |
| Female sex | 36% (n = 4) | 45% (n = 20) | NS | 41% (n = 9) | NS |
| Pre-arrest good functional status | 82% (n = 9) | 84% (n = 37) | NS | 91% (n = 20) | NS |
| Witnessed arrest | 91% (n = 10) | 77% (n = 34) | NS | 77% (n= 17) | NS |
| Out-of-hospital arrest | 64% (n = 7) | 86% (n = 38) | NS | 95% (n = 21) | 0.03 |
| Bystander CPR | 45% (n = 5) | 59% (n = 26) | NS | 59%(n=13) | NS |
| Shockable rhythm | 55% (n = 6) | 16% (n = 7) | 0.01 | 23% (n = 5) | NS |
| Median ROSC, min (IQR) | 15 (10, 25) | 25 (17, 30) | 0.05 | 30 (18, 40) | 0.02 |
| TTM completed | 82% (n = 9) | 93% (n = 41) | NS | 91% (n = 20) | NS |
| Total percent of brain involved at ADC ≤ 450, median (IQR) | 0.001% (<0.001%, 0.007%) | 0.53% (0.02%, 5.15%) | <0.01 | 1.3% (0.01%, 17.77%) | <0.01 |
| Total percent of brain involved at ADC ≤ 650, median (IQR) | 0.11% (<0.001%, 0.35%) | 7.03% (1.68%, 21.44%) | <0.01 | 7.50% (1.22%, 43.42%) | <0.01 |
WLST, withdrawal of life-sustaining therapy; IQR, interquartile range; CPR, cardiopulmonary resuscitation; ROSC, return of spontaneous circulation; TTM, targeted temperature management; NS, not significant.
Among 55 patients who were comatose at time of MRI.
Compared to those conscious at discharge.
Fig. 1.
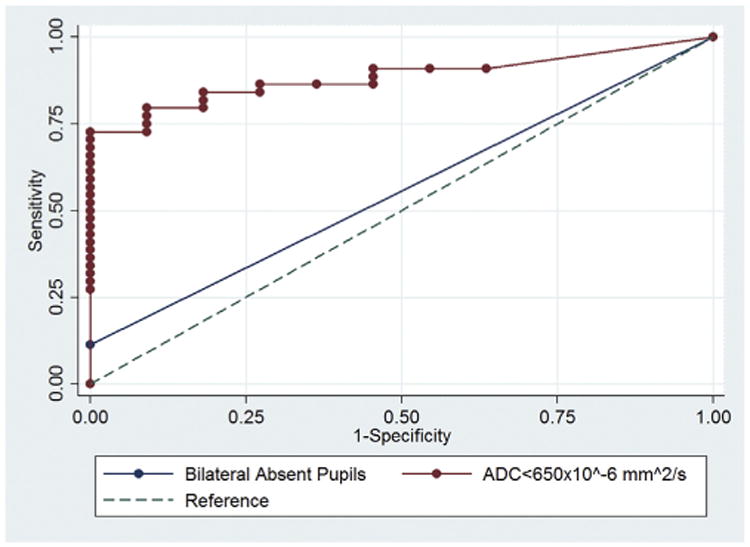
Receiver operating curves comparing MRI findings at ADC ≤650 × 10−6 to presence of bilaterally absent pupils in predicting waking up from coma by time of hospital discharge. AUC of MRI changes at ADC ≤650 × 10−6 (0.884 (95% CI 0.798–0.971, p<0.001)), compared to AUC of bilaterally absent pupillary reflexes at day 3 (0.617, 95%CI 0.420–0.813, p = 0.316).
Among 36 survivors (52% of cohort), only MRI changes at an ADC threshold ≤450 × 10−6 mm2/s were significantly different between those who were independent on discharge (CPC 1–2) and those who were not (CPC 3–4) (0.00% (IQR 0.000, 0.001) vs 0.01% (IQR<0.001, 0.093), p = 0.04). Among survivors, a burden of >0.01% of diffusion restriction of the entire brain at an ADC of ≤450 × 10−6 m2/s was 100% specific and 46% sensitive for poor functional outcome at discharge. The ROC curve predicting poor functional outcome (CPC 3–4) at the same threshold had an AUC of 0.737 (95% CI 0.574–0.899, p = 0.04), while ROC curves for bilaterally absent pupillary light reflexes at days 3, 5 and 7 were not statistically different from the null hypothesis.
Discussion
In this study, we found that diffusion restriction burden within the entire brain at ADC ≤650 × 10−6 mm2/s was predictive of failure to wake up from coma. Median diffusion restriction burden at ADC ≤450 × 10−6 mm2/s was different among survivors with good versus poor functional status. These findings are consistent with previous studies [7,11]. Notably, we used a semi-automated algorithm because of the potential for interrater disagreements [16]. Our findings were confirmed when excluding patients with WLST. Compared with pupillary exam, MRI was superior in predicting persistence of coma. Previous studies show that absence of pupillary reflex is extremely specific but only about 20% sensitive [6], which is consistent with our finding of 16% sensitivity. At 100% specificity, MRI findings had a sensitivity of 68%. It is possible that MRI may identify patients with preserved pupillary reflexes who will not wake up.
Further, we found that thalamic changes were never found in isolation. This is supported by animal studies which show regional differences in cerebral blood flow in the cortex versus thalamus both during hypoperfusion and reperfusion, suggesting the thalamus is more protected from hypoxia than the cortex [17,18]. Consistent with one animal study showing decreased neuronal apoptosis in the thalamus, cerebellum and medulla after cardiac arrest when compared with the cortex and hippocampus [19], we believe that thalamic involvement represents more severe hypoxic-ischemic injury.
There are several limitations of this retrospective study. First, the cardiac arrest literature is plagued by high rates of WLST, resulting in low numbers when analyzing functional outcome in an unbiased group and making it difficult to perform predictive analyses. An additional self-fulfilling prophecy can occur when an MRI shows significant diffusion restriction and physicians use that information to prognosticate for families. We attempted to account for this by repeating analyses while excluding patients with WLST. Second, MRIs were obtained at the discretion of treating physicians. Our data show a bias in ordering early MRIs in younger and more functional patients, perhaps reflecting more aggressive care. Patients who remained comatose or had focal abnormalities may have been more likely to get an MRI. Additionally, patients with good exams after rewarming may not have MRIs, so the true rates of diffusion abnormalities in those with good outcome may not be accurate. Our cohort likely reflects the current practice of most of the academic centers where MRIs are not ordered routinely. Third, in this study MRIs could have been done any time within the first seven days after arrest. One case series has shown diffusion abnormalities in the post-arrest can temporally and spatially evolve over a 12 day time period [20], and perhaps MRIs done within the first seven days are not comparable to each other. Last, we do not have long-term functional or cognitive outcomes in this cohort.
Conclusion
Quantitative MRI diffusion changes within the first seven days post-arrest may be predictive of remaining in coma or functional outcomes at discharge. Thalamic changes may signify more severe hypoxic-ischemic injury.
Supplementary Material
Acknowledgments
We want to thank the nurses, physician assistants, nurse practitioners and physicians who take care of these patients. Dr. Reynolds acknowledges support of the NIH/NINDS (R25 NS070697) and Dr. Park acknowledges support of the NIH/NINDS (K01ES026833). Dr. Claassen acknowledges support by the DANA Foundation.
Footnotes
Disclosures: The authors report no conflicts of interest relevant to the data presented in this paper.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.resuscitation.2017.06.010.
References
- 1.Greer DM. Mechanisms of injury in hypoxic-ischemic encephalopathy: implications to therapy. Semin Neurol. 2006;26:373–9. doi: 10.1055/s-2006-948317. [DOI] [PubMed] [Google Scholar]
- 2.Wijdicks EFM, Hijdra A, Young GB, Bassetti CL, Wiebe S. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–10. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 3.Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–14. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 4.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010;67:301–7. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- 5.Kamps MJ, Horn J, Oddo M, Fugate JE, Storm C, Cronberg T, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med. 2013;39:1671–82. doi: 10.1007/s00134-013-3004-y. [DOI] [PubMed] [Google Scholar]
- 6.Greer DM, Yang J, Scripko PD, Sims JR, Cash S, Wu O, et al. Clinical examination for prognostication in comatose cardiac arrest patients. Resuscitation. 2013;84:1546–51. doi: 10.1016/j.resuscitation.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijman CAC, Mlynash M, Caulfield AF, Hsia AW, Eyngorn I, Bammer R, et al. rognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65:394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mlynash M, Campbell DM, Leproust EM, Fischbein NJ, Bammer R, Eyngorn I, et al. Temporal and spatial profile of brain diffusion-weighted MRI after cardiac arrest. Stroke. 2010;41:1665–72. doi: 10.1161/STROKEAHA.110.582452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SP, Park KN, Park HK, Kim JY, Youn CS, Ahn KJ, et al. Diffusion-weighted magnetic resonance imaging for predicting the clinical outcome of comatose survivors after cardiac arrest: a cohort study. Crit Care. 2010;14:R17. doi: 10.1186/cc8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch KG, Mlynash M, Jansen S, Persoon S, Eyngorn I, Krasnokutsky MV, et al. Prognostic value of a qualitative brain MRI scoring system after cardiac arrest. J Neuroimaging. 2015;25:430–7. doi: 10.1111/jon.12143. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch KG, Mlynash M, Eyngorn I, Pirsaheli R, Okada A, Komshian S, et al. Multi-center study of diffusion-weighted imaging in coma after cardiac arrest. Neurocrit Care. 2016;24:82–9. doi: 10.1007/s12028-015-0179-9. [DOI] [PubMed] [Google Scholar]
- 12.Cummins RO, Chamberlain DA, Abramson NS, Allen M, Baskett PJ, Becker L, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style. A statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation. 1991;84:960–75. doi: 10.1161/01.cir.84.2.960. [DOI] [PubMed] [Google Scholar]
- 13.Jastremski M, Sutton-Tyrrell K, Vaagenes P, Abramson N, Heiselman D, Safar P. Glucocorticoid treatment does not improve neurological recovery following cardiac arrest. Brain Resuscitation Clinical Trial I Study Group. JAMA. 1989;262:3427–30. [PubMed] [Google Scholar]
- 14.Chang K, Zhang B, Guo X, Zong M, Rahman R, Sanchez D, et al. Multimodal imaging patterns predict survival in recurrent glioblastoma patients treated with bevacizumab. Neuro Oncol. 2016;18:1680–7. doi: 10.1093/neuonc/now086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 16.Greer D, Scripko P, Bartscher J, Sims J, Camargo E, Singhal A, et al. Clinical MRI interpretation for outcome prediction in cardiac arrest. Neurocrit Care. 2012;17:240–4. doi: 10.1007/s12028-012-9716-y. [DOI] [PubMed] [Google Scholar]
- 17.Drabek T, Foley LM, Janata A, Stezoski J, Hitchens TK, Manole MD, et al. Global and regional differences in cerebral blood flow after asphyxial versus ventricular fibrillation cardiac arrest in rats using ASL-MRI. Resuscitation. 2014;85:964–71. doi: 10.1016/j.resuscitation.2014.03.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manole MD, Kochanek PM, Bayır H, Alexander H, Dezfulian C, Fink EL, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr Res. 2014;75:295–301. doi: 10.1038/pr.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananiadou OG, Drossos GE, Bibou KN, Palatianos GM, Johnson EO. Acute regional neuronal injury following hypothermic circulatory arrest in a porcine model. Interact Cardiovasc Thorac Surg. 2005;4:597–601. doi: 10.1510/icvts.2005.112813. [DOI] [PubMed] [Google Scholar]
- 20.Greer D, Scripko P, Bartscher J, Sims J, Camargo E, Singhal A, et al. Serial MRI changes in comatose cardiac arrest patients. Neurocrit Care. 2011;14:61–7. doi: 10.1007/s12028-010-9457-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


