Abstract
Dogs have been studied for many years as a medical diagnostic tool to detect a pre-clinical disease state by sniffing emissions directly from a human or an in vitro biological sample. Some of the studies report high sensitivity and specificity in blinded case-control studies. However, in these studies it is completely unknown as to which suites of chemicals the dogs detect and how they ultimately interpret this information amidst confounding background odors. Herein, we consider the advantages and challenges of canine olfaction for early (meaningful) detection of cancer, and propose an experimental concept to narrow the molecular signals used by the dog for sample classification to laboratory-based instrumental analysis. This serves two purposes; first, in contrast to dogs, analytical methods could be quickly up-scaled for high throughput sampling. Second, the knowledge gained from identifying probative chemicals could be helpful in learning more about biochemical pathways and disease progression. We focus on exhaled breath aerosol, arguing that the semi-volatile fraction should be given more attention. Ultimately, we conclude that the interaction between dog-based and instrument-based research will be mutually beneficial and accelerate progress towards early detection of cancer by breath analysis.
Keywords: cancer diagnostics, canine olfaction, sniffing dog, breath aerosol, breath analysis
Overview
Dogs have a highly developed sense of smell as is evident in their historical use in security and police investigative applications for detecting drugs, weapons, bombs, and for tracking criminals (Sloane 1955, Settle et al 1994, Furton and Myers 2001, Mesloh et al 2002). More recently, dogs have been trained to detect a variety of diagnosed medical conditions in humans (Sonoda et al 2011, Ehman et al 2012, Jezierski et al 2015, Hackner et al 2016, Neerincx et al 2016). The focus here is to explore a strategy of reaching pre-clinical (very early) instrumental detection of cancer based on breath analysis by taking advantage of the dog’s superior odor detection capabilities (Buszewski et al 2012, Ehman et al 2012). It is very important that this strategy is studied, because of the supreme importance of early cancer detection for improving treatment outcomes (Etzioni et al 2003, McCulloch et al 2006). The big questions are, what does the cancer-trained dog actually detect when alerting on a human sample such as breath, are the compounds consistent in the continuum from preclinical to medically diagnosed disease, and how likely is the dog to be consistent over time?
Certainly there are challenges in using dogs regardless of their effectiveness; unlike analytical instruments, dogs have personality and physical needs (Walczach et al 2012, Hackner and Pleil 2016). They require a great deal of care and generally can only work for short periods of time as they may get bored, distracted, hungry, thirsty and sleepy. Furthermore, each dog needs to be individually trained for his job (group training of dogs is out of the question); this can take months; and refresher training may be important. Finally, dogs must be continually evaluated for performance using known (prior) and new challenge samples to assure stability of results (Moser and McCulloch 2010, Johnen et al 2013, Elliker et al 2014, Hall et al 2015, Polgar et al 2016).
Concept
The basic concept for breath cancer diagnostics, expressed as a roadmap, is to: (1) assume that the dog (actually several dogs in agreement) can detect cancer (in general, or a certain type of cancer) with a reasonable degree of sensitivity and specificity by sniffing breath samples; (2) collect these multi-dog diagnostic results with careful control and recording of conditions and observations; (3) obtain samples in duplicate from subjects, and test one with a dog and one with an instrument; (4) use agnostic ‘discovery’ approach to identify the chemicals that correlate (individually or as a pattern) with dog alerts; (5) conduct a trial instrumental breath test for early cancer detection; and, hopefully, (6) establish and implement this test.
Why not skip the dogs and just proceed with metabolomic analysis of human breath samples, along with evaluation against onset of cancer? The short answer to this question is that the dog might provide a shortcut to developing a useful instrumental test for early detection of cancer. Assuming that the dog can indicate disease state with reasonable reliability before clinical classification takes place, the dog/instrumental results might provide a head start both on selecting the right instrumental signals for chemical identification, and good hypotheses for evaluation
How does the instrument benefit the dog testing? The short answer to this question is that the instrument provides an opportunity to improve the initial classification of the samples by the dogs, and to improve the canine training. The classification results from the canine testing will certainly include false positive and false negatives, and one needs to learn what is causing each. For example, the canine breath test for cancer that we describe below tends to give positive results for pregnant and breast-feeding women. What is the dog detecting?
We proceed using the following assumptions and limitations:
The dog senses chemicals (or patterns of chemicals) that are reproducibly related to cancer to a reasonable degree.
In this sensing, the sniffing by the dog provides turbulent, moist conditions that make semi-volatiles airborne.
The format for sample collection must be amenable to both dog and instrument.
The first point addresses one of the biggest issues in the field. The dog can be trained using known cancer samples, but how does this translate to real-world unknowns? We need to make the assumption that the dog can classify cancer with a reasonable degree of reliability.
Secondly, we need to recognize that any probative compounds must become airborne in one way or another under the conditions of dog sniffing. This does not mean that they need to be gas-phase only, as many organic compounds can be carried by aerosols or small particles, but at some point, they must have been airborne for the dog to detect them. The turbulent, moist sniffing conditions of the dog could certainly enhance the production of airborne compounds from the substrate it is analyzing. If dog breathing is like a summer breeze, dog sniffing is like a hurricane.
Finally, some form of interim sampling format is certainly a requirement: direct breath comparisons (neither breathing by a subject directly at a dog or an instrument is practical or has any future).
Approach
There are many different ways that breath-based disease diagnostics could be studied. To address the concept outlined above, we propose to implement a very specific approach. This would invoke using the information gained from dog diagnostics to accelerate development of targeted analytical methods.
Breath
Breath contains volatiles, semi-volatiles, particles and aerosol, and each of these can be free or combined with the others. The ‘breath aerosol’ is comprised of small liquid particles enriched in the semi-volatiles, dissolved ionic material, bacteria, and larger organic compounds. Although much attention so far for cancer detection by breath analysis has been on the collection and analysis or on-line analysis of the volatiles (Filipiak et al 2014, Schroeder 2015, Gasparri et al 2016, Schallschmidt et al 2016), we believe that the semi-volatile constituents of the breath aerosol should be given more attention for two reasons. First, collection of the aerosols in breath can (via filtration, adsorption, or absorption) can be simpler than collecting the gas-phase with a focus on the volatiles. We concede that analysis of breath volatiles for diagnostics with an electronic nose can be convenient and is an important area of research (Nagle et al 1998, Fens et al 2013, Bikov et al 2015, Nakhleh et al 2016.) Second, the ability of dogs to alert on ‘old samples’ implies that they are detecting semi-volatiles. Consider that dogs can track an escaped prisoner or a fox through the forest; apparently they are cueing in on less volatile chemicals slowly releasing from surfaces rather than on purely gas-phase compounds.
Dogs
To start, there are some academic and private organizations that train and/or use dogs to perform cancer diagnostics, for example CancerDogs Inc. (http://cancerdogs.ca/index.html), American Scent Dog Association (http://scentdogassociation.com/research.html), University of Arkansas Medical Sciences (http://uams.edu/), University of Pennsylvania (http://pennvetwdc.org/), and Auburn University (http://vetmed.auburn.edu/research/cps/).
In one strategy, the dogs develop an internal ‘library’ of odor patterns based on samples from diagnosed cancer patients and from control subjects. Subsequently, samples from patients with unknown cancer status are presented to the dog for assignment into a case or control category. Overall most of the attention has been given to detecting odors in urine, breath, cell lines, or tissue samples (Phillips et al 2003, Willis et al 2004, Cornu et al 2011, Shirasu and Touhara 2011, Capelli et al 2016, Pleil 2016). One innovative approach to breath collection in general is the use of conventional isolation face masks as a sampling medium, and has been applied to virus collection from human breath (Mitchell et al 2016). The company CancerDogs Inc. has now implemented similar masks for breath odor collection for canine analysis; we will consider this approach below in more detail.
Instrumentation
Dogs can detect chemicals at trace levels, and the putative cancer signals no doubt arise from trace compounds. Such chemicals need to be characterized structurally, which means that primarily mass spectrometry is required for the instrumental part of the analysis. Considering the need, as we have argued, to include or even emphasize semi-volatiles in the detection, then liquid chromatography-mass spectrometry instruments will be important to employ. Nevertheless, many semi-volatiles, especially after derivatization, can be detected by gas-chromatography-mass spectrometry, so it will play a role as well. The most sensitive mass spectrometry techniques will probably be required, especially if limited breath samples are acquired from the subject for convenience.
Samples assessment
The first step would be to collect breath aerosol samples in duplicate from subjects, one for canine assessment and the other, companion sample, for instrument discovery analysis. Ideally this would take place with both blinded ordinary samples (cancer status unknown), samples with early cancer, and samples from other diseases. No doubt the dogs will sort the samples into subgroups of positives and negatives within each group to at least some degree, providing a tentative disease classification. Upon instrument analysis, the analytical data would then be composited into the categorical bins provided by the dogs and assessed for differences in chemical composition. The compounds that similarly differentiate the groups would then be the first indication as to what the dogs consider to be probative for sorting samples.
Preliminary observations
There are two components to this exploratory work: dog results and instrumental results. So far, these results have not been linked directly other than both being amenable to analysis of the underlying sample collection format of a mask.
Dog results
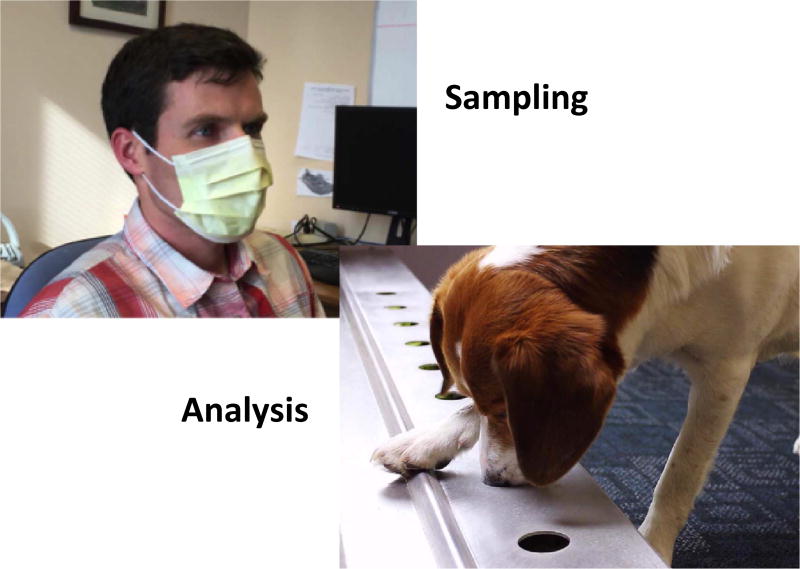
The canine classification project at CancerDogs has been ongoing for about 5 years. Subjects wear an isolation face mask for ten minutes; place it in a metal foil bag; and mail it to CancerDogs for analysis by dog sniffing (figure 1). In 2016, about 7000 samples were tested. Samples are scored as positive if at least 3 of 4 dogs alert on them both in an initial test, and in a repeat test of a second sample collected 1–2 months later. The program is a prospective study to begin to learn more about the ability of dogs to sniff early cancer. The noninvasive, overall convenience of the test for the subjects is an advantage. It will take time to learn the clinical value of the test, and the best classification criteria. The mask format clearly provides sufficient signal for the group of dogs to classify unknown samples (but with a tentative meaning) based on training sets from known cases and controls.
Figure 1.
Collecting breath aerosol sample using hospital mask and dog performing odor diagnostic tasks. (Dog photo courtesy Glenn Ferguson from CancerDogs Inc.)
Preliminary analytical results
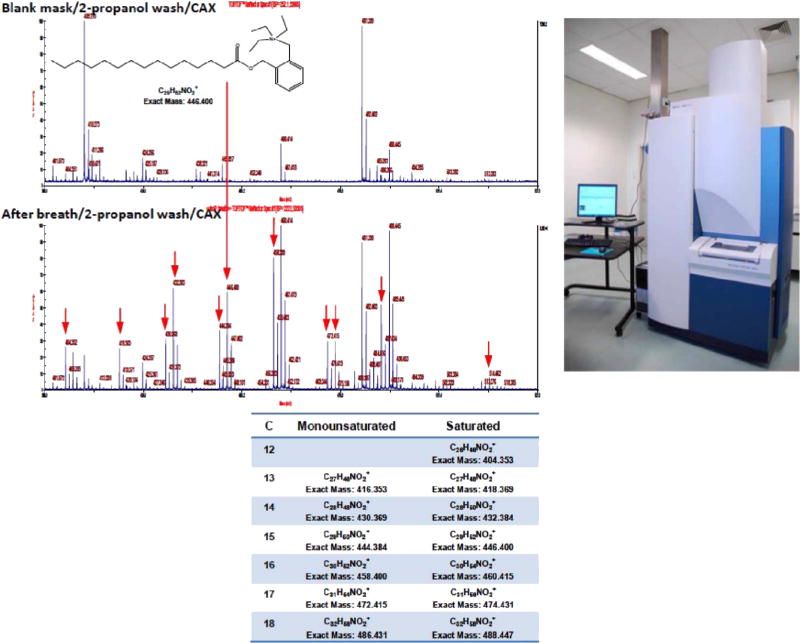
Personnel at Northeastern University wondered whether biochemicals could be detected by mass spectrometry on the same brand of mask employed by CancerDogs after similar exposure to human breath. Under an existing methods development protocol at US EPA for collection of anonymous biological specimens, a mask (prewashed briefly with isopropanol, unlike practice by CancerDogs) was worn by a subject for 20 min. This and an unexposed mask were eluted with isopropanol, and each eluent was subjected to chemical analysis by CAX-B labeling/MALDI-TOF/TOF-MS (Wang et al 2015).
Indeed, many more peaks are seen in the sample from the exposed mask, with fatty acids as the prominent peaks (figure 2), even without sample cleanup by HPLC. Note the prominent peak for pentadecanoic acid (or some isomer of this compound), which apparently comes from a bacterium. It will be of interest to elute an exposed mask in the future under conditions that mimic the turbulent, moist sniffing of a dog as one way to potentially favor detection of the chemicals that alert the dog. Also, thorough pre-washing of the mask, and testing other mask materials, remain to be studied.
Figure 2.
Upper left: MALDI-TOF-MS spectra from unused and used masks, where the eluent (2-propanol) after evaporation was reacted with CAX-B prior to detection. Lower left: assignment of most of the major peaks to fatty acids, assuming linear chain isomers. Upper right: MALDI-TOF/TOF-MS (Model 5800 from AB Sciex).
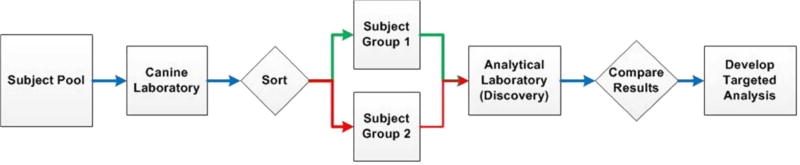
Figure 3 illustrates the case-control workflow path for using a dog laboratory as the triage component for developing targeted analytical methods. Here, many samples are drawn from a broad population and presented to the dogs for sorting into groups according to their particular training (for a specific cancer or disease). Once a sufficient number of case samples are identified, subjects are resampled to achieve equal case-control groups that are then analyzed with laboratory instrumentation according to discovery protocols. The results from the case and control groups are then compared and only the analytical features corresponding to differences are kept for targeted methods development.
Figure 3.
Proposed workflow for developing targeted analytical methods using a dog laboratory sorting procedure. Many samples from the total subject pool are needed to achieve equal case and control groups; subsets are resampled to achieve balanced groups for the analytical laboratory.
Caveats (what could go wrong)
The proposed workflow is sound under the assumption that the instrument can detect some representatives of the putative critical compounds, and that at least some of these compounds are actually cancer or disease signals.
In regard to a mask for collection of the breath condensate, a potential problem is the underlying variability of routine isolation face masks as the collection substrate. Certainly, there is no expectation that mass-produced masks are completely clean; there could easily be background variability that may override certain parts of the analytical results, and if the background is heterogeneous, there may be spurious results. However, it could be a simple matter to clean the masks in bulk before use, as already pointed out.
A third caveat revolves around the sampling or analysis locations; for example, the different ambient environments surrounding the subject, and later the dog and laboratory analyses, may influence the analytical results. This effect should be minimal as both cancer and not-cancer samples are analyzed at both locations in a blinded form.
A fourth issue could be the techniques for extraction of chemicals from the mask for presentation to the dog or the analytical system. Especially if the dog is detecting a pattern of chemicals, this should be as consistent as possible. Nevertheless, while it is apparently turbulent headspace for the dog, it might need to be liquid extraction for the instrument. Bias needs to be considered for potential losses of semi-volatiles having significant volatility.
Fifth is the matter of qualitative versus quantitative results. One should rely on the usual techniques employed in metabolomics for quantifying the data as much as possible at the discovery stage (Wu and Li 2016). If the dog primarily recognizes qualitative patterns, this might reduce the need for quantitation, but this is as yet unknown. It is conceivable that the breath aerosol might reach a steady state on the mask, which could be helpful.
Conclusions
Breath aerosol
Using the aerosols in exhaled breath as the vehicle for probative chemicals of cancer has two advantages. First, it is easy to collect aerosol in contrast to volatile organic compounds (VOCs) because the collection medium is a simple, inexpensive medical mask rather than some form of gas container, and second, the masks maintain sample integrity and are easy to ship as the compounds, by definition, will not readily evaporate. Furthermore, VOCs have not been entirely reproducible in previous studies, and so there is a potential advantage strictly from changing the molecules detected. However, some of the reasons for the prior irreproducibility with VOCs may also impact the reliability of classifying based on detection of semi-volatiles.
Sample triage
Using the dogs as the sampling triage for case-control discovery analysis in the laboratory is an elegant solution to the conundrum of developing an early warning system for disease occurrence that requires a known (diagnosed) set of training samples. Consider that once the disease state is diagnosed by standard techniques, we no longer require pre-clinical laboratory assessment. Currently, we take it on faith that any specific chemical biomarkers indicating an adverse outcome pathway are the same in the very early stages; this may not be true. Because the dogs alert similarly on known outcomes and unknown samples that have later on been validated to some extent, it is more likely that whatever suite of specific laboratory biomarkers that distinguish the sample classes as provided by the dogs are consistent in disease progression.
Biochemical validation
The ultimate discovery of probative chemicals using laboratory instrumentation will certainly help understand the adverse outcome pathways involved in disease progression. This has implications well-beyond early disease diagnosis; in fact, knowledge of molecular changes from early adverse effects could be used to develop in vitro (cell-based) toxicity tests for manufactured chemicals in commerce, and also for pharmaceuticals development. Dogs can be trained to alert on a variety of different sample sets, and so aerosol effluents from cellular bioreactors could be used as well.
Feedback validation
This proposed approach in no way diminishes the value of canine olfaction. In fact, confirming that the diagnostics provided by the dogs are based in plausible biochemical pathways will ultimately help establish the validity of the early warning provided by samples identified by dog-based analysis.
Summary
Using canine olfaction for case-control sample classification in conjunction with instrumental analysis is an exciting approach for accelerating the development of a presumed breath test for early detection of cancer. Both approaches are valuable to study, and both are improved with their interaction. We suggest that this complementary approach be studied in future to better understand the biochemistry of adverse health progressions from in vivo and in vitro samples, especially those based on larger molecules as transported by aerosols.
Acknowledgments
The authors are grateful for valuable insights regarding canine olfaction from Klaus Hackner, University Hospital, Krems Austria, Boguslaw Buszewski, Nicolaus Copernicus University, Torun Poland, and Glenn Ferguson of CancerDogs Inc., Ottawa, Canada. Poguang Wang from Northeastern University conducted the experiment for figure 2. We thank Matthew Stiegel from Duke University for advice regarding the workflow in figure 3 and Adam Biales from US EPA for expert comments. This article was reviewed in accordance with the policy of the National Exposure Research Laboratory, US Environmental Protection Agency, and approved for publication. Mention of trade names or commercial products do not constitute endorsement or recommendation for use. This work was supported in part by NIEHS Grant P42ES017198 at Northeastern University. The authors declare they have no competing financial interests.
References
- Bikov A, Lazar Z, Horvath I. Established methodological issues in electronic nose research: how far are we from using these instruments in clinical settings of breath analysis? J. Breath Res. 2015;9:034001. doi: 10.1088/1752-7155/9/3/034001. [DOI] [PubMed] [Google Scholar]
- Buszewski B, Rudnicka J, Ligor T, Walczak M, Jezierski T, Amann A. Analytical and unconventional methods of cancer detection using odor. TRAC Trends Anal. Chem. 2012;38:1–2. [Google Scholar]
- Capelli L, et al. Application and uses of electronic noses for clinical diagnosis on urine samples: a review. Sensors. 2016;16:1708. doi: 10.3390/s16101708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu JN, Cancel-Tassin G, Ondet V, Girardet C, Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: a step forward in early diagnosis. Eur. Urol. 2011;59:197–201. doi: 10.1016/j.eururo.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ehmann R, Boedeker E, Friedrich U, Sagert J, Dippon J, Friedel G, Walles T. Canine scent detection in the diagnosis of lung cancer: revisiting a puzzling phenomenon. Eur. Respiratory J. 2012;39:669–76. doi: 10.1183/09031936.00051711. [DOI] [PubMed] [Google Scholar]
- Elliker KR, Sommerville BA, Broom DM, Neal DE, Armstrong S, Williams HC. Key considerations for the experimental training and evaluation of cancer odour detection dogs: lessons learnt from a double-blind, controlled trial of prostate cancer detection. BMC Urol. 2014;14:22. doi: 10.1186/1471-2490-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat. Rev. Cancer. 2003;3:243–52. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- Fens N, Schee MP, Brinkman P, Sterk PJ. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy. 2013;43:705–15. doi: 10.1111/cea.12052. [DOI] [PubMed] [Google Scholar]
- Filipiak W, et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014;8:027111. doi: 10.1088/1752-7155/8/2/027111. [DOI] [PubMed] [Google Scholar]
- Furton KG, Myers LJ. The scientific foundation and efficacy of the use of canines as chemical detectors for explosives. Talanta. 2001;54:487–500. doi: 10.1016/s0039-9140(00)00546-4. [DOI] [PubMed] [Google Scholar]
- Gasparri R, et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016;10:016007. doi: 10.1088/1752-7155/10/1/016007. [DOI] [PubMed] [Google Scholar]
- Hackner K, Errhalt P, Mueller MR, Speiser M, Marzluf BA, Schulheim A, Schenk P, Bilek J, Doll T. Canine scent detection for the diagnosis of lung cancer in a screening-like situation. J. Breath Res. 2016;10:046003. doi: 10.1088/1752-7155/10/4/046003. [DOI] [PubMed] [Google Scholar]
- Hackner K, Pleil JD. Canine olfaction as an alternative to analytical instruments for disease diagnosis: understanding ‘dog personality’ to achieve reproducible results. J. Breath Res. 2016;11:012001. doi: 10.1088/1752-7163/aa5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NJ, Glenn K, Smith DW, Wynne CD. Performance of Pugs, German Shepherds, and Greyhounds (Canis lupus familiaris) on an odor-discrimination task. J. Comp. Psychol. 2015;129:237–46. doi: 10.1037/a0039271. [DOI] [PubMed] [Google Scholar]
- Jezierski T, Walczak M, Ligor T, Rudnicka J, Buszewski B. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. Perspectives and limitations. J. Breath Res. 2015;9:027001. doi: 10.1088/1752-7155/9/2/027001. [DOI] [PubMed] [Google Scholar]
- Johnen D, Heuwieser W, Fischer-Tenhagen C. Canine scent detection—fact or fiction? Appl. Animal Behav. Sci. 2013;148:201–8. [Google Scholar]
- McCulloch M, Jezierski T, Broffman M, Hubbard A, Turner K, Janecki T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr. Cancer Ther. 2006;5:30–9. doi: 10.1177/1534735405285096. [DOI] [PubMed] [Google Scholar]
- Mesloh C, Wolf R, Henych M. Scent as forensic evidence and its relationship to the law enforcement canine. J. Forensic Identif. 2002;52:169–82. [Google Scholar]
- Mitchell AB, Mourad B, Tovey E, Buddle L, Peters M, Morgan L, Oliver BG. Spirometry filters can be used to detect exhaled respiratory viruses. J. Breath Res. 2016;10:046002. doi: 10.1088/1752-7155/10/4/046002. [DOI] [PubMed] [Google Scholar]
- Moser E, McCulloch M. Canine scent detection of human cancers: a review of methods and accuracy. J. Veterinary Behav.: Clin. Appl. Res. 2010;5:145–52. [Google Scholar]
- Nagle HT, Gutierrez-Osuna R, Schiffman SS. The how and why of electronic noses. IEEE Spectr. 1998;5:22–31. [Google Scholar]
- Nakhleh MK, et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano. 2016 doi: 10.1021/acsnano.6b04930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerincx AH, et al. Detection of Staphylococcus aureus in cystic fibrosis patients using breath VOC profiles. J. Breath Res. 2016;10:046014. doi: 10.1088/1752-7155/10/4/046014. [DOI] [PubMed] [Google Scholar]
- Phillips M, Cataneo RN, Cummin AR, Gagliardi AJ, Gleeson K, Greenberg J, Maxfield RA, Rom WN. Detection of lung cancer with volatile markers in the breath. Chest. 2003;123:2115–23. doi: 10.1378/chest.123.6.2115. [DOI] [PubMed] [Google Scholar]
- Pleil JD. Cellular respiration: replicating in vivo systems biology for in vitro exploration of human exposome, microbiome, and disease pathogenesis biomarkers. J. Breath Res. 2016;10:010201. doi: 10.1088/1752-7155/10/1/010201. [DOI] [PubMed] [Google Scholar]
- Polgar Z, Kinnunen M, Ujvary D, Miklosi A, Gacsi M. A test of canine olfactory capacity: comparing various dog breeds and wolves in a natural detection task. PLoS One. 2016;11:e0154087. doi: 10.1371/journal.pone.0154087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallschmidt K, Becker R, Jung C, Bremser W, Walles T, Neudecker J, Leschber G, Frese S, Nehls I. Comparison of volatile organic compounds from lung cancer patients and healthy controls—challenges and limitations of an observational study. J. Breath Res. 2016;10:046007. doi: 10.1088/1752-7155/10/4/046007. [DOI] [PubMed] [Google Scholar]
- Schroeder W. Volatile S-nitrosothiols and the typical smell of cancer. J. Breath Res. 2015;9:016010. doi: 10.1088/1752-7155/9/1/016010. [DOI] [PubMed] [Google Scholar]
- Settle RH, Sommerville BA, McCormick J, Broom DM. Human scent matching using specially trained dogs. Animal Behav. 1994;48:1443–8. [Google Scholar]
- Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011;150:257–66. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- Sloane CF. Dogs in war, police work and on patrol. J. Crim. L. Criminology Police Sci. 1955;46:385–95. [Google Scholar]
- Sonoda H, et al. Colorectal cancer screening with odour material by canine scent detection. Gut. 2011;60:814–9. doi: 10.1136/gut.2010.218305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak M, Jezierski T, Górecka-Bruzda A, Sobczyńska M, Ensminger J. Impact of individual training parameters and manner of taking breath odor samples on the reliability of canines as cancer screeners. J. Veterinary Behav.: Clin. Appl. Res. 2012;7:283–94. [Google Scholar]
- Wang P, Zhang Q, Yao Y, Giese RW. Cationic xylene tag for increasing sensitivity in mass spectrometry. J. Am. Soc. Mass Spectrom. 2015;26:1713–21. doi: 10.1007/s13361-015-1200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CM, Church SM, Guest CM, Cook WA, McCarthy N, Bransbury AJ, Church MR, Church JC. Olfactory detection of human bladder cancer by dogs: proof of principle study. Brit. Med. J. 2004;329:712. doi: 10.1136/bmj.329.7468.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li L. Sample normalization methods in quantitative metabolomics. J. Chromatogr. A. 2016;1430:80–95. doi: 10.1016/j.chroma.2015.12.007. [DOI] [PubMed] [Google Scholar]