Abstract
Background
The genetic characterization of wild-type measles viruses plays an important role in the description of viral transmission pathways and the verification of measles elimination. The 450 nucleotides that encode the carboxyl-terminus of the nucleoprotein (N-450) are routinely sequenced for genotype analysis.
Objectives
The objectives of this study were to develop improved primers and controls for RT-PCR reactions used for genotyping of measles samples and to develop a method to provide a convenient, safe, and inexpensive means to distribute measles RNA for RT-PCR assays and practice panels.
Study design
A newly designed, genetically defined synthetic RNA and RNA isolated from cells infected with currently circulating genotypes were used to compare the sensitivity of primer pairs in RT-PCR and nested PCR. FTA® cards loaded with lysates of measles infected cells were tested for their ability to preserve viral RNA and destroy virus infectivity.
Results
A new primer pair, MeV216/MeV214, was able to amplify N-450 from viruses representing 10 currently circulating genotypes and a genotype A vaccine strain and demonstrated 100-fold increased sensitivity compared to the previously used primer set. A nested PCR assay further increased the sensitivity of detection from patient samples. A synthetic positive control RNA was developed that produced PCR products that are distinguishable by size from PCR products amplified from clinical samples. FTA® cards completely inactivated measles virus and stabilized RNA for at least six months.
Conclusions
These improved molecular tools will advance molecular characterization of circulating measles viruses globally and provide enhanced quality control measures.
Keywords: Measles, Molecular diagnostics, FTA® cards, Genotyping
1. Background
Molecular characterization of MeV has provided a valuable tool for measuring the effectiveness of measles control programs [1–3]. The WHO Measles and Rubella Laboratory Network (LabNet) supports genetic characterization of circulating wild-type viruses throughout the world [2,4]. Genetic characterization of MeVs is based on sequence analysis of the 450 nucleotides coding for the 150 amino acids at the carboxyl terminus of the nucleoprotein (N-450) [5,6]. Based on these sequences, 24 genotypes have been identified [6,7]. Since 1998, the standard genotyping RT-PCR assay from the CDC recommended a primer pair designated MV60/MV63.3 [8] though other primers were also used throughout LabNet [9–14]. Several new MeV genotypes have been described since the introduction of the standard genotyping method [15–17]; however, only limited sequence data were available for the primer binding sites of the more recent strains and the analytic sensitivity of the primers had not been evaluated. Several laboratories have introduced nested PCR assays, some of which do not amplify the complete N-450 [11,18–21] or do not provide data for sensitivity and specificity [9,10].
2. Objectives
The first objective was to determine the sensitivity of primers MV60/MV63.3 for viruses representing 10 currently circulating genotypes and genotype A and to develop primer pairs for RT-PCR and nested PCR that amplify N-450 with increased sensitivity. A second objective was to develop reagents for a quality control program for molecular techniques in LabNet. This includes a genetically defined positive control RNA, which can be differentiated from positive samples. It also includes the development of an RNA test panel that could be provided in a convenient, safe and inexpensive format to allow laboratories to build competence with the methods for measles genotyping.
3. Study design
3.1. Viruses and cells
Viruses (Table 1) were derived from the WHO Strain Bank at CDC. Vero/hSLAM cells (generous gift of Dr. Yanagi) [22] were maintained and infected as described previously [23]. To produce viral lysates, infected cells were scraped into cell culture medium and stored at −70 °C.
Table 1.
MeV Isolates used in this study.
| Genotype | Virus |
|---|---|
| A | MVi/Edmonston-wt.USA/54a,b |
| B3 | MVi/New Jersey.USA/45.05b,c |
| D2 | MVi/NewYork.USA/11.00b |
| D3 | MVi/Illinois.USA/89/1a,b |
| D4 | MVi/New York.USA/14.08/1b |
| D4 | MVi/New York.USA/26.09/3c |
| D5 | MVi/Pennsylvania.USA/34.07b |
| D6 | MVi/St. Petersburg.RUS/16.04/4b |
| D8 | MVi/Vellore.IND/08.05b |
| D8 | MVi/Virginia.USA/15.09c |
| G2 | MVi/Amsterdam.NET/49.97a,b |
| G3 | MVi/Gresik.INO/18.02a,b |
| H1 | MVi/Wisconsin.USA15.08/1b |
| H1 | MVi/Pennsylvania.USA/20.09c |
Reference strain [6].
Strain used for primer sensitivity tests.
Strain used for FTA® practice panel.
3.2. RNA extraction, RT-qPCR and sequencing
Total cellular RNA from infected or uninfected cells was isolated using Trizol (Invitrogen) and stored at −70 °C. A one-step RT-qPCR was used to determine the copy number of N gene-containing RNA (positive and negative strand) as described previously [24]. Synthetic MeV N gene RNA was used to establish a standard curve [24]. RNA extracts were diluted to defined copy numbers/μL in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.0). In all dilutions, the total RNA concentration was normalized through addition of RNA extracted from uninfected cells to a concentration of 4.4 ng/μL. After dilution, RT-qPCR was repeated to confirm expected concentrations of N gene-containing RNA. RNA from patient samples was extracted using the QIAamp Viral RNA Mini Kit (Qiagen) or the MagNA Pure LC total nucleic acid isolation kit (Roche).
For sequence determination, a fragment containing nucleotides 1–3470 of the MeV genome was amplified from each RNA preparation. Reverse transcriptase reactions and sequencing were performed as described previously [25].
3.3. Genotyping RT-PCR and nested PCR
The Superscript One-step RT-PCR kit with Platinum Taq (Invitrogen) was used for genotyping RT-PCR with 2 mM MgSO4, 10 units RNase inhibitor (Applied Biosystems) and 5 μL sample. The concentration of primers (Table 2) was 0.2 μM. Cycling conditions were 55 °C for 30 min, 94 °C for 2 min, 40 cycles of 94 °C for 15 s, 55 °C for 30 s, 72 °C for 30 s followed by final extension at 72 °C for 7 min. In all template dilutions, except for patient samples, the total RNA content was normalized through addition of RNA extracted from uninfected cells to a concentration of 4.4 ng/μL. For the nested PCR, Platinum Taq DNA Polymerase High Fidelity (Invitrogen) was used according to the manufacturer’s recommendations with 0.4 μM primers and 2 μL unpurified RT-PCR product as template. Cycling conditions were 94 °C for 30 s, 40 cycles of 94 °C for 15 s, 50 °C for 30 s, 68 °C for 1 min followed by final extension at 72 °C for 5 min. The sensitivity of RT-PCR combined with nested PCR was compared with the method by Kühne et al. [11] using a different assay formulation (see Supplementary methods). PCR products were separated on 1% agarose gels stained with GelRed (Biotium) and visualized using an imaging system (UVP).
Table 2.
Primer sequences.
| Name | Sequence (5′–3′) | Locationa |
|---|---|---|
| MV60 | GCT ATG CCA TGG GAG TAG GAG TGG | 1108–1131 |
| MV63.3 | CTG GCC CTC GGC CTC TCG CAC | 1686–1706 |
| MeV214 | TAA CAA TGA TGG AGG GTA GG | 1718–1737 |
| MeV216 | TGG AGC TAT GCC ATG GGA GT | 1104–1123 |
| MeV210 | GCT ATG CCA TGG GAG TRG GAG TGG | 1108–1131 |
| MeV217 | CAA TGA TGG AGG GTA GG | 1718–1734 |
Nucleotide position in reference to Edmonston-wt (accession number AF266288) genomic sequence.
3.4. Synthetic RNA with insert
A plasmid containing the nucleocapsid (N) and phosphoprotein (P) genes of a genotype D3 virus [26] (accession number EU29350) and a cloned version of the chloramphenicol-acetyl-transferase gene (pMVplusCAT [27]) were used as templates. An overlapping PCR fragment strategy [28,29] was used to amplify nucleotides 533–1521 (genomic numbering) of the N gene followed by nucleotides 1109–1328 of pMVplusCAT, followed by nucleotide 1522–1765 of the N–P gene region. The resulting PCR fragment, MeV-N3in, was inserted into the vector pTM1 [30] using restriction sites SacI and SpeI. Sequences of amplification primers are available upon request. Identity of the cloned construct was confirmed by sequencing. The Megascript T7 and Megaclear kits (Ambion) were used to generate RNA transcripts from the plasmid. The concentration of the synthetic RNA transcript was measured spectrophotometrically. Dilutions of the transcript were made in TE buffer and stored in aliquots at −70 °C.
3.5. Preparation of FTA® test panels
Indicating FTA® micro cards (Whatman, part number WB120311) with 25 mm diameter circles were loaded with 200 μL lysate and dried at RT for 1 h. Disks of 6 mm diameter were cut out with a stainless steel puncher (Staples). To avoid cross-contamination, the punch and forceps used to handle the filters were cleaned by incubation in 10% bleach for 1 min, rinsed with water, then with 70% ethanol for 1 min and air dried between samples. Disks were stored at 4 °C sealed in airtight bags with desiccant packs. RNA was extracted from the disks using the QIAamp Viral RNA Mini Kit (Qiagen) with the following modifications: 150 μL PBS and 600 μL AVL buffer were added to each disk, mixed for 15 s, then incubated at RT for 10 min. After centrifugation at 15,700 × g for 2 min at RT, 700 μL of the solution was transferred to a fresh tube and 560 μL of ethanol were added. The mixture was loaded onto a spin column and the purification was completed according to the manufacturer’s recommendations.
To measure the viability of measles viruses on FTA® cards, a 6 mm punch was mixed with inoculation medium [23], incubated at RT for 10 min and mixed again. The medium (without the filter) was used to inoculate Vero/hSLAM cells which were monitored for cpe for five days. Cultures without cpe were passaged by trypsinization twice before they were determined to be negative.
The genotyping practice panel consisted of four 1.5 mL tubes containing one FTA® disk each loaded with a different measles genotype and one tube with a disk loaded with lysate of uninfected cells. Kits were transported at RT to participating LabNet members.
4. Results and discussion
4.1. Comparison of primers MV60/MV63.3 with primers MeV216/MeV214
Ten MeVs representing currently circulating genotypes were chosen for analysis (Table 1). The Edmonston-wt strain, a representative of genotype A, was included because all vaccine strains have been derived from genotype A viruses and thus, are all contained within this single genotype [31]. The sequence of 150 nucleotides 5′ of N-450 and of the 3′ noncoding region of the N gene (in message sense RNA) were determined. The sequence of the Edmonston-wt strain was obtained from GenBank (accession number AF266288). Fig. 1 shows the sequence variability in the binding sites of MV60, MV63.3 and the reduced nucleotide variability in the binding sites of MeV216 and MeV214. The position of MeV214 increases the distance between the primer binding site and the 3′ terminus of N-450, which improves sequence quality. Unlike several previously published primer pairs [11,18–21], the new primers allow sequencing of the entire N-450 which is required to assign a genotype [32] and to submit the sequence to MeaNS [2], the global measles sequence database. In this study, only one representative for each genotype was sequenced. As the variability within genotypes can be as high as 5.3% [33], it is possible that other viruses belonging to the same genotype may have additional substitutions in the primer binding sites. However the new primer set performed very well for routine molecular surveillance for measles in the US (see below).
Fig. 1.
Genetic variability of primer binding sites among 11 genotypes. (A) Schematic representation of measles genome and location of N-450. N, P/C/V, M, F, H, L are measles genes. (B) Nucleotide sequence of primer binding sites of 11 genotypes. Left side of figure depicts forward primer binding sites, right side of figure depicts reverse primer binding sites. Dots below the consensus sequence indicate variable positions. Numbers above the consensus sequence refer to nucleotide positions in the measles genome.
4.2. Sensitivity and specificity of one-step RT-PCR with MeV216/MeV214
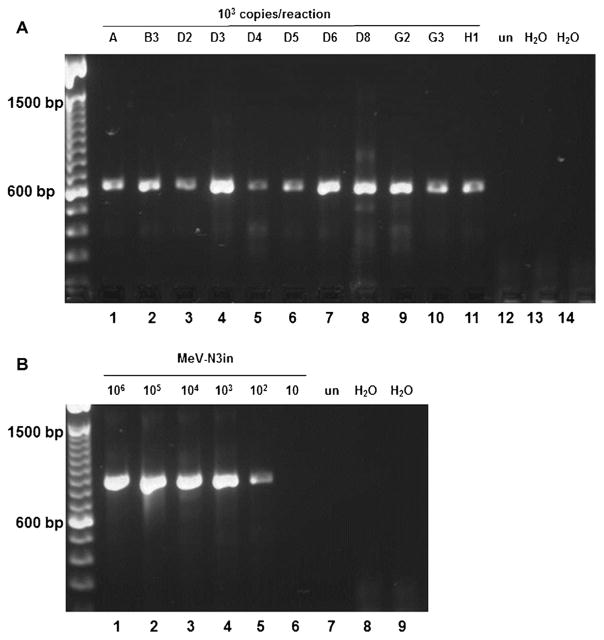
The synthetic RNA (MeV-N3in) comprises nt 533–1764 of the genome of a D3 virus (MVi/Davis.USA/87), including the entire 3′ non-coding-region of the N gene where the reverse primer (MeV214) binding site is located. The synthetic construct contains a 220 nt insertion in N-450 (Fig. 2). Serial dilutions of RNA preparations from cells infected with 11 genotypes and of MeV-N3in were used to determine the sensitivity of the primer pairs. The limit of sensitivity for primers MV60/MV63.3 was 106 copies for the 11 genotypes (Fig. 3A) and MeV-N3in (Fig. 3B). RT-PCR with 105 copies did not reproducibly amplify all genotypes (data not shown). The increased molecular weight of the 854 nt amplicon generated from MeV-N3in was clearly distinguishable in a 1% agarose gel from the 634 nt amplicon generated from RNA extracted from infected cells (Fig. 3B). This facilitates differentiation between amplicons generated from patient samples and from control RNA and rules out cross-contamination with control RNA. The limit of sensitivity for primers MeV216/MeV214 was 104 copies for the 11 genotypes (Fig. 4A). Amplification from 103 copies was not reproducibly positive for all genotypes tested (data not shown) but was positive for MeV-N3in (Fig. 4B). The difference in sensitivity between the serially diluted MeV-N3in and amplifications with all 11 genotypes may be due to slight differences in the concentration of RNA in the preparations from different genotypes. MV60/MV63.3 amplified all 11 genotypes with comparable sensitivity, despite the high number of substitutions in primer binding sites. However, primers MeV216/MeV214 demonstrated 100-fold increased sensitivity. Determining the genotype of each chain of transmission is recommended by WHO [34]. Achieving this goal will be facilitated by using an updated standard genotyping assay containing primers MeV216/MeV214. Primers MeV216/MeV214 were used in routine testing of 324 samples submitted to the CDC for domestic measles surveillance. Viruses from genotypes A, D4, B3, D8, D9, H1 and G3 were detected at concentrations as low as 104 copies per reaction (data not shown).
Fig. 2.
Schematic representation of synthetic RNA MeV-N3in. RNA consists of nucleotides 533–1765 of the measles genome including a 220 nucleotide insert derived from the bacterial chloramphenicol-acetyl-transferase gene. The location of the qRT-PCR product and the product of genotyping RT-PCR are also indicated.
Fig. 3.
RT-PCR with primers MV60/MV63.3. (A) Viral RNA. Lanes 1–11: genotypes as listed in Table 1, 106 copies/reaction. (B) MeV-N3in. Lanes 1–6: 10-fold serial dilution of MeV-N3in, 106–10 copies/reaction. un, RNA from uninfected cells; H2 O, water control.
Fig. 4.
RT-PCR with primers MeV216/MeV214. (A) Viral RNA. Lanes 1–11: genotypes as listed in Table 1, 104 copies/reaction. (B) MeV-N3in. Lanes 1–6: 10-fold serial dilution of MeV-N3in, 106–10 copies/reaction. un, RNA from uninfected cells; H2 O, water control.
Primers MeV216/MeV214 were tested with RNA extracted from samples obtained from five rubella cases, four mumps cases, one case each of human parainfluenza virus 1 and 4, and one case of parvovirus B19 infection. DNA from five different herpes viruses (varicella zoster virus, human herpes virus 6B, herpes simplex virus 1, Epstein Barr virus and cytomegalovirus) was also tested. All samples had tested positive in assays for the respective pathogens. None of the reactions produced amplicons with MeV216/MeV214, demonstrating the specificity of the primer pair (data not shown).
Based on the results described above, an MeV genotyping kit was designed to contain primers MeV216/MeV214, vacuum-dried MeV-N3in positive control RNA and TE buffer for rehydration of the synthetic RNA. The kit is provided to LabNet members upon request.
4.3. Nested PCR
To further increase sensitivity of detection, primers MeV210/MeV217 were designed to amplify the DNA fragment generated with MeV216/MeV214 (Table 2). A thermostable DNA polymerase with reduced error rate was chosen to decrease the likelihood of introduction of polymerase errors. RT-PCR with MeV216/MeV214 followed by nested PCR with MeV210/MeV217 amplified 102 copies of serially diluted MeV-N3in RNA and reproducibly amplified 103 copies of all 11 genotypes (Fig. 5A and B). Not all genotypes reproducibly amplified when using 102 copies as starting material (data not shown). Sequencing of nested PCR products from genotype A produced sequences that were identical to the published sequence (AF266288), indicating that no PCR errors were detectable in the consensus sequence (data not shown).
Fig. 5.
Nested PCR with primers MeV210/MeV217. (A) Lanes 1–11: genotypes as listed in Table 1, 103 copies/reaction. (B) MeV-N3in. Lanes 1–6: 10-fold serial dilution of synthetic RNA, 106–10 copies/reaction. un, RNA from uninfected cells; H2 O, water control.
Nested PCR was used to amplify N-450 from seven patient samples (four urine samples, two nasopharyngeal aspirates and one throat swab). Two of these had previously been genotyped using RT-PCR with primers MV60/MV63.3. Sequencing of the nested PCR products confirmed the previously obtained sequence, again indicating that no PCR errors were detectable in the consensus sequence (data not shown). Of the 5 samples which were negative with primers MV60/MV63.3, three were genotyped as D4, D8 and H1, respectively, using the nested PCR protocol. In addition, 36 patient samples that had been genotyped using the nested PCR assay described by Kühne [11] were amplified with the new nested PCR assay to compare the sensitivity of the two assays. The 36 samples containing genotypes A, B3, D8, D9, G3 and H1 included three nasopharyngeal aspirates, two throat swabs, two cell culture fluids, one throat/nasal swab, one urine sample and 27 sera. Thirty-five of the samples were positive with the new nested PCR assay, one sample (serum, genotype G3) was negative. The assay described by Kühne provides sequence information for only 444 of the 450 nt of N-450. The new nested assay demonstrated comparable sensitivity while allowing the sequencing of the entire N-450. In addition, the primers MeV210/MeV217 demonstrated fewer mismatches with the 11 genotypes than primers described in other nested assays [9,10].
4.4. Utility of FTA® cards as practice panels
FTA® cards inactivate infectious material and stabilize nucleic acids [35,36]. FTA® cards have been used to transport or store a wide variety of virologic samples which can be shipped as non-infectious material at ambient temperatures [37–43]. To test the stability of measles RNA stored on FTA® cards, lysates of four genotypes (Table 1) were loaded on FTA® cards. RNA extracted from one FTA® disk for each genotype was analyzed by RT-qPCR. The average Ct of all disks was 17.8 on day 1 and 18.1 after storage at 4 °C for 6 months (Fig. 6). Storage of FTA® disks at 37 °C for one week did not affect RNA stability (data not shown). Lysate eluted from disks was also used to inoculate Vero/hSLAM cells to test for virus viability. Repeated virus isolation attempts with two genotypes did not produce MeV cpe after three blind passages in cell culture, demonstrating that the FTA® cards had inactivated the virus [36,44].
Fig. 6.
Stability of RNA on FTA® disks. RNA extracted from one FTA® disk loaded with the indicated genotype (Table 1) was analyzed by RT-qPCR after incubation at 4 °C for one day or six months. Ct values are averages of three replicate wells. Error bars indicate one standard deviation.
FTA® practice panels consisted of 1.5 mL tubes containing one 6 mm disk loaded with a lysate of one of four MeV genotypes and one disk loaded with a lysate of uninfected cells. These panels were used in training workshops for 37 laboratories from all 6 WHO regions. In most cases, the FTA panels were transported at room temperature. None of the laboratories experienced difficulties with recovering RNA from the FTA cards and almost all of the laboratories were able to detect all samples by RT-PCR and to correctly identify the genotypes. The success of the practice panels demonstrated the utility of FTA® cards as a means to ship RNA at ambient temperature. FTA® card panels may be used to develop proficiency panels for LabNet members with PCR and genotyping capacities to ensure reliable results from virologic surveillance.
Supplementary Material
Acknowledgments
Funding
LabNet regional reference laboratories are supported in part by WHO.
The authors are grateful to Luis Lowe for providing sequence information. We also gratefully acknowledge the LabNet members who tested the practice panels.
Abbreviations
- nt
nucleotides
- RT
room temperature
- MeV
measles virus
- WHO
World Health Organization
- Ct
threshold cycle
- CDC
Centers for Disease Control and Prevention
- cpe
cytopathic effect
- LabNet
WHO Measles and Rubella Laboratory Network
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2013.05.018.
Footnotes
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Rota JS, Heath JL, Rota PA, King GE, Celma ML, Carabana J, et al. Molecular epidemiology of measles virus: identification of pathways of transmission and implications for measles elimination. J Infect Dis. 1996;173:32–7. doi: 10.1093/infdis/173.1.32. [DOI] [PubMed] [Google Scholar]
- 2.Rota PA, Brown KE, Hubschen JM, Muller CP, Icenogle J, Chen MH, et al. Improving global virologic surveillance for measles and rubella. J Infect Dis. 2011;204(Suppl 1):S506–13. doi: 10.1093/infdis/jir117. [DOI] [PubMed] [Google Scholar]
- 3.Rota PA, Featherstone DA, Bellini WJ. Molecular epidemiology of measles virus. Curr Top Microbiol Immunol. 2009;330:129–50. doi: 10.1007/978-3-540-70617-5_7. [DOI] [PubMed] [Google Scholar]
- 4.Featherstone DA, Rota PA, Icenogle J, Mulders MN, Jee Y, Ahmed H, et al. Expansion of the global measles and rubella laboratory network 2005–09. J Infect Dis. 2011;204(Suppl 1):S491–8. doi: 10.1093/infdis/jir107. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Nomenclature for describing the genetic characteristics of wild-type measles viruses (update). Part I. Wkly Epidemiol Rec. 2001;76:242–7. [PubMed] [Google Scholar]
- 6.World Health Organization. Measles virus nomenclature update: 2012. Wkly Epidemiol Rec. 2012;87:73–81. [PubMed] [Google Scholar]
- 7.Rota PA, Brown K, Mankertz A, Santibanez S, Shulga S, Muller CP, et al. Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis. 2011;204(Suppl 1):S514–23. doi: 10.1093/infdis/jir118. [DOI] [PubMed] [Google Scholar]
- 8.Katz RS, Premenko-Lanier M, McChesney MB, Rota PA, Bellini WJ. Detection of measles virus RNA in whole blood stored on filter paper. J Med Virol. 2002;67:596–602. doi: 10.1002/jmv.10144. [DOI] [PubMed] [Google Scholar]
- 9.Chibo D, Birch CJ, Rota PA, Catton MG. Molecular characterization of measles viruses isolated in Victoria, Australia, between 1973 and 1998. J Gen Virol. 2000;81:2511–8. doi: 10.1099/0022-1317-81-10-2511. [DOI] [PubMed] [Google Scholar]
- 10.Kremer JR, Nguyen GH, Shulga SV, Nguyen PH, Nguyen UT, Tikhonova NT, et al. Genotyping of recent measles virus strains from Russia and Vietnam by nucleotide-specific multiplex PCR. J Med Virol. 2007;79:987–94. doi: 10.1002/jmv.20827. [DOI] [PubMed] [Google Scholar]
- 11.Kuhne M, Brown DW, Jin L. Genetic variability of measles virus in acute and persistent infections. Infect Genet Evol. 2006;6:269–76. doi: 10.1016/j.meegid.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Mosquera MM, Ory F, Echevarria JE. Measles virus genotype circulation in Spain after implementation of the national measles elimination plan 2001–2003. J Med Virol. 2005;75:137–46. doi: 10.1002/jmv.20248. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama T, Fujino M, Yoshida N. Molecular epidemiology of measles virus in Japan. Pediatr Int. 2004;46:214–23. doi: 10.1046/j.1442-200x.2004.01864.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Fujino M, Inou Y, Kumada A, Aoki Y, Iwata S, et al. H1 genotype of measles virus was detected in outbreaks in Japan after 2000. J Med Virol. 2003;70:642–8. doi: 10.1002/jmv.10443. [DOI] [PubMed] [Google Scholar]
- 15.Kouomou DW, Nerrienet E, Mfoupouendoun J, Tene G, Whittle H, Wild TF. Measles virus strains circulating in Central and West Africa: geographical distribution of two B3 genotypes. J Med Virol. 2002;68:433–40. doi: 10.1002/jmv.10222. [DOI] [PubMed] [Google Scholar]
- 16.Muwonge A, Nanyunja M, Rota PA, Bwogi J, Lowe L, Liffick SL, et al. New measles genotype, Uganda. Emerg Infect Dis. 2005;11:1522–6. doi: 10.3201/eid1110.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Ding Z, Wang H, Li L, Pang Y, Brown KE, et al. New measles virus genotype associated with outbreak, China. Emerg Infect Dis. 2010;16:943–7. doi: 10.3201/eid1606.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alla A, Waku-Kouomou D, Benjouad A, Elaouad R, Wild TF. Rapid diversification of measles virus genotypes circulating in Morocco during 2004–2005 epidemics. J Med Virol. 2006;78:1465–72. doi: 10.1002/jmv.20720. [DOI] [PubMed] [Google Scholar]
- 19.Atrasheuskaya AV, Kulak MV, Neverov AA, Rubin S, Ignatyev GM. Measles cases in highly vaccinated population of Novosibirsk, Russia, 2000–2005. Vaccine. 2008;26:2111–8. doi: 10.1016/j.vaccine.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Djebbi A, Bahri O, Mokhtariazad T, Alkhatib M, Ben Yahia A, Rezig D, et al. Identification of measles virus genotypes from recent outbreaks in countries from the Eastern Mediterranean Region. J Clin Virol. 2005;34:1–6. doi: 10.1016/j.jcv.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Woo GK, Wong AH, Lee WY, Lau CS, Cheng PK, Leung PC, et al. Comparison of laboratory diagnostic methods for measles infection and identification of measles virus genotypes in Hong Kong. J Med Virol. 2010;82:1773–81. doi: 10.1002/jmv.21888. [DOI] [PubMed] [Google Scholar]
- 22.Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001;75:4399–401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bankamp B, Lopareva EN, Kremer JR, Tian Y, Clemens MS, Patel R, et al. Genetic variability and mRNA editing frequencies of the phosphoprotein genes of wild-type measles viruses. Virus Res. 2008;135:298–306. doi: 10.1016/j.virusres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Hummel KB, Lowe L, Bellini WJ, Rota PA. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J Virol Methods. 2006;132:166–73. doi: 10.1016/j.jviromet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Bankamp B, Fontana JM, Bellini WJ, Rota PA. Adaptation to cell culture induces functional differences in measles virus proteins. Virol J. 2008;5:129. doi: 10.1186/1743-422X-5-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bankamp B, Hodge G, McChesney MB, Bellini WJ, Rota PA. Genetic changes that affect the virulence of measles virus in a rhesus macaque model. Virology. 2008;373:39–50. doi: 10.1016/j.virol.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu MS, Chan J, Kaelin K, Spielhofer P, Radecke F, Schneider H, et al. Rescue of synthetic measles virus minireplicons: measles genomic termini direct efficient expression and propagation of a reporter gene. Virology. 1995;208:800–7. doi: 10.1006/viro.1995.1215. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucl Acids Res. 1988;16:7351–67. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–8. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 30.Moss B, Elroy-Stein O, Mizukami T, Alexander WA, Fuerst TR. Product review. New mammalian expression vectors. Nature. 1990;348:91–2. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 31.Rima BK, Earle JA, Yeo RP, Herlihy L, Baczko K, ter Meulen V, et al. Temporal and geographical distribution of measles virus genotypes. J Gen Virol. 1995;76(Pt 5):1173–80. doi: 10.1099/0022-1317-76-5-1173. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Expanded Programme on Immunization (EPI). Standardization of the nomenclature for describing the genetic characteristics of wild-type measles viruses. Wkly Epidemiol Rec. 1998;73:265–9. [PubMed] [Google Scholar]
- 33.Zhang Y, Zhu Z, Rota PA, Jiang X, Hu J, Wang J, et al. Molecular epidemiology of measles viruses in China, 1995–2003. Virol J. 2007;4:14. doi: 10.1186/1743-422X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Monitoring progress towards measles elimination. Wkly Epidemiol Rec. 2010;85:490–4. [PubMed] [Google Scholar]
- 35.Burgoyne LA. Solid medium and method for DNA storage. 5,496,562 US patent. 1996 Mar;
- 36.GE Lifesciences. HIV-1 inactivation on FTA elute media. 2008 available at: http://www.gelifesciences.co.jp/whatman/product/bioscience/pdf/HIV1_Inactivation_study_on_FTA_Elute.pdf.
- 37.Abdelwhab EM, Luschow D, Harder TC, Hafez HM. The use of FTA(R) filter papers for diagnosis of avian influenza virus. J Virol Methods. 2011;174:120–2. doi: 10.1016/j.jviromet.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Keeler SP, Ferro PJ, Brown JD, Fang X, El-Attrache J, Poulson R, et al. Use of FTA sampling cards for molecular detection of avian influenza virus in wild birds. Avian Dis. 2012;56:200–7. doi: 10.1637/9862-072611-Reg.1. [DOI] [PubMed] [Google Scholar]
- 39.Linhares DC, Rovira A, Torremorell M. Evaluation of Flinders Technology Associates cards for collection and transport of samples for detection of Porcine reproductive and respiratory syndrome virus by reverse transcription polymerase chain reaction. J Vet Diagn Invest. 2012;24:328–32. doi: 10.1177/1040638711429492. [DOI] [PubMed] [Google Scholar]
- 40.Moscoso H, Raybon EO, Thayer SG, Hofacre CL. Molecular detection and serotyping of infectious bronchitis virus from FTA filter paper. Avian Dis. 2005;49:24–9. doi: 10.1637/7220. [DOI] [PubMed] [Google Scholar]
- 41.Muthukrishnan M, Singanallur NB, Ralla K, Villuppanoor SA. Evaluation of FTA cards as a laboratory and field sampling device for the detection of foot-and-mouth disease virus and serotyping by RT-PCR and real-time RT-PCR. J Virol Methods. 2008;151:311–6. doi: 10.1016/j.jviromet.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Perozo F, Villegas P, Estevez C, Alvarado I, Purvis LB. Use of FTA filter paper for the molecular detection of Newcastle disease virus. Avian Pathol: J WVPA. 2006;35:93–8. doi: 10.1080/03079450600597410. [DOI] [PubMed] [Google Scholar]
- 43.Picard-Meyer E, Barrat J, Cliquet F. Use of filter paper (FTA) technology for sampling, recovery and molecular characterisation of rabies viruses. J Virol Methods. 2007;140:174–82. doi: 10.1016/j.jviromet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Whatman. Discover the power of FTA elute. 2006 available at: http://www.whatman.com/References/FTA%20Elute%20Brochure.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.