Abstract
Substance (alcohol, marijuana, opioids, cocaine, etc.) use often initiates during adolescence, a critical period of physiological and social development marked by an increase in risk-taking due, in part, to heightened motivation to obtain arousal from rewards. Substance use during adolescence has been associated with a greater risk of substance use disorders (SUD) in adulthood. Although use rates for most substances have remained relatively stable, the frequency of marijuana use and perception that regular marijuana use is not harmful has increased in adolescents. Furthermore, the non-medical use of opioids has increased, particularly in the South, Midwest, and rural low-income communities. Substance use in adolescence has been associated with adverse structural and functional brain changes, and may exacerbate the natural “imbalance” between frontal/regulatory and cortical-subcortical circuits, leading to further heightened impulsive and reward-driven behaviors. Exercise increases growth and brain-derived neurotrophic factors that stimulate endogenous dopaminergic systems that, in turn, enhance general plasticity, learning, and memory. Exercise may help to reinforce the “naïve” or underdeveloped connections between neurological reward and regulatory processes in adolescence from the “bottom up” and “offset” reward seeking from substances, while concomitantly improving cardiovascular health, as well as academic and social achievement. In this review, we provide an overview of the current state of substance use in adolescents and, rationale for the utilization of exercise, particularly ‘assisted’ exercise, which we have shown increases neural activity in cortical-subcortical regions and may modulate brain dopamine levels during adolescence, a unique window of heightened reward sensitivity and neural plasticity, for the prevention and adjunctive treatment of SUD.
Keywords: substance use, adolescence, exercise, ‘assisted’ exercise
Introduction
Substance use disorders (SUD) and related psychopathology are persistent public health problems (Compton et al., 2005), with over 20.3 million adults and 1.3 million children in the U.S. estimated to have a SUD (Substance Abuse and Mental Health Services Administration (SAMHSA), 2014). Worldwide, there are over 3.4 billion illicit drug users, accounting for over 12% of all deaths each year (World Health Organization, 2016). Drug overdose, which has been mostly linked to opioids, is the leading cause of unintentional injury deaths in the U.S. An estimated 2 million Americans, ages 12 and older, have had an opioid use disorder involving prescription opioids, and approximately 600,000 have had an opioid use disorder involving heroin (National Academy of Sciences, 2017). In addition, the number of overdose deaths from illicit opioids (including heroin and synthetic opioids) tripled from 2011 to 2015 in the U.S. (National Academy of Sciences, 2017). Overall, the consequences of substance use costs the U.S. more than $600 billion every year (National Institute on Drug Abuse, National Institutes of Health, U.S. Department of Health and Human Services, 2017; Office of National Drug Control Policy, 2004).
Substance use often initiates during adolescence, and over 80% of drug users began using during adolescence (Lenoue and Riggs, 2016). Unfortunately, only about 11% of individuals ages 12 and older and only about 6% of adolescents 12–17 years old who need treatment for substance misuse, actually receive it (Lipari et al., 2016; SAMHSA, 2014). Furthermore, over half of the adolescents receiving treatment will relapse within one year of treatment (Cornelius et al., 2003; McLellan et al., 2000; Ramo and Brown, 2008), suggesting more accessible and effective programs are needed to prevent and treat substance use in adolescents.
Substance Use Rates in Adolescents
Substance use is often initiated during adolescence, which is a sensitive period of biological and social development marked by an increase in risk taking behavior due, in part, to heightened motivation to obtain arousal from rewards (Eaton et al., 2012; Galvan, 2010; Willoughby et al., 2013). Although use rates for many substances have remained relatively stable or have decreased somewhat in adolescents, the frequency of some substances and the perception that regular use of these substances is not harmful has increased in adolescents. According to the Monitoring the Future (MTF) Survey of drug use and attitudes, self-reported daily use of marijuana in 12th graders increased from 2006 to 2011, and has remained at about 6% as of 2016 in the U.S. (Johnston, 2017; National Institute on Drug Abuse, National Institutes of Health and U.S. Department of Health and Human Services, 2016). Importantly, only 31.1% of 12th graders believe regular marijuana use is harmful, which is 27.2% less than that reported in 2000 (Johnston, 2017; National Institute on Drug Abuse et al., 2016). Data from the National Survey on Drug Use and Health (NSDUH) indicate 12 to 17 year olds represent 7–13% of all frequent (21 or more days in the past month) marijuana users (Burns et al., 2013; Peiper et al., 2016). Although frequent marijuana use appears to be decreasing in Caucasians, rates in African-Americans and Hispanics are increasing (Burns et al., 2013; Peiper et al., 2016). In 2016, 81% of 12th graders reported marijuana could be obtained “fairly easily” or “very easily” but less accessibility to marijuana was reported among 8th (35%) and 10th (64%) graders (Johnston, 2017). Interestingly, past year use of marijuana in 12th graders was about 5% higher in medical marijuana use states compared to non-medical marijuana use states (38.3% vs. 33.3%) (Johnston, 2017; National Institute on Drug Abuse et al., 2016), and this trend is expected to continue as more states pass medical and non-medical marijuana use laws. Furthermore, the potency of marijuana has been steadily increasing, which may be partially responsible for the increase in marijuana-related emergency room visits (Mehmedic et al., 2010; Zhu and Wu, 2016).
The overall abuse of opioids and deaths attributed to opioid misuse has continued to increase markedly in the U.S. and, among adolescents, the non-medical use of opioids appears to be increasing in the South and Midwest and in rural communities with lower socioeconomic status. According to the 2016 MTF, the prevalence of OxyContin use was 0.9%, 2.1%, and 3.4% in 8th, 10th, and 12th grades, respectively; and, the use of Vicodin was 0.8%, 1.7%, and 2.9% for 8th, 10th, and 12th graders, respectively (Johnston, 2017). Heroin use remains low with only about 1% or less of adolescents reporting use in the past year in the 2016 NTF (Johnston, 2017). Importantly, opioid use before/during high school is associated with a 33% increase in the risk of future opioid misuse after high school (Miech et al., 2015), and is associated with initiation of other illicit drugs as early as age 13 (Becker et al., 2008), suggesting opioids may be providing the “gateway” to the abuse of other substances. Furthermore, nearly 7 out of every 10 non-medical prescription opioid adolescent users reported co-ingestion of marijuana (58.5%), alcohol (52.1%), cocaine (10.6%), tranquilizers (10.3%), and amphetamines (9.5%) in the past year (McCabe et al., 2012). Interestingly, the prevalence of non-medical prescription pain drugs is higher among adolescents than it has ever been, and data suggest the younger adolescents (middle school) may be particularly affected (Miech et al., 2013). Moreover, data from the NSDUH indicate that adolescents ages 12–17 living in rural areas (6.8%) have 35% greater odds of past-year prescription opioid misuse than those living in large urban areas (5.3%) (Monnat et al., 2017). A recent systematic review of 15 studies with nationally representative populations found that low family income, poor mental health, and lack of treatment for mental health issues were positively associated with adolescent prescription opioid misuse (Young et al., 2012). Unfortunately, adolescents in rural regions with lower income who are misusing opioids may be less likely to seek and receive treatment (Oser and Harp, 2015).
Although alcohol remains the most commonly used substance among adolescents, the percentage of students in grades 8, 10, and 12 reporting alcohol use in the past year (17.6%, 38.3%, and 55.6%, respectively, in 2016 MTF), and those reporting binge drinking (5 drinks in a row in the past 2 weeks) continues to decline (Johnston, 2017; National Institute on Drug Abuse et al., 2016). The daily use of cigarettes has also been steadily declining (0.9%, 1.9%, and 4.8% among 8th, 10th, and 12th graders in 2016) (Johnston, 2017). The use of electronic cigarettes (e-cigarettes) and little cigars has been increasing, but recent data from the 2016 MTF indicate use in adolescents is declining (Johnston, 2017; Peiper et al., 2016).
Overall, the prevalence of past-year substance abuse or dependence of any substance among 12–17 year olds was 5.3% and 5.2% in boys and girls, respectively, in the 2013 NSDUH, which represents a decline since the 2006 NSDUH (8.0% in boys, 8.1% in girls) (Peiper et al., 2016). Furthermore, substance use and concurrent substance use (using two or more substances during the same time period) is associated with a greater risk of SUD and adolescents using alcohol, cigarettes, and marijuana and alcohol and marijuana, compared to alcohol alone, are more strongly associated with development of SUD in adulthood (Green et al., 2016; Moss et al., 2014). This appears to be reflected in the NSDUH data, which show an increase in SUD in young adults 18 to 25 and among those ages 26 and older (Peiper et al., 2016). Therefore, intervening during adolescence is necessary to prevent SUD in emerging and young adulthood.
Adolescence is a Critical Period of Development
Adolescence is an important developmental period between childhood and adulthood, which is marked by many physiological and social changes including, but not limited to, puberty and increased risk-taking and sensation (reward)-seeking behaviors (Crone and Dahl, 2012; Willoughby et al., 2013). The ages used to define adolescence or, more specifically, achieving independence and mature social and personal responsibilities (Cohen et al., 2016), varies with broader definitions, including ages spanning from 10 to 22 years and narrower ranges encompassing ages 12–17 years (as seen above with data on the substance use rates in the U.S.). Thus, age by itself may be a poor proxy for development and maturation to adulthood (Cohn, Westenberg and Cohn, 2004).
Normal brain development during adolescence involves an overall decrease in gray matter volume and a concomitant increase in white matter volume, as well as cortical thinning (Bava et al., 2013; Gogtay et al., 2004; Paus, 2005; Raznahan et al., 2014). The decreases in gray matter are believed to occur to enable greater synaptic pruning (or elimination of unnecessary connections), and the increases in white matter are believed to provide for increased myelination of axons to produce more efficient connections between brain regions (Bava et al., 2013; Paus, 2005; Raznahan et al., 2014). Furthermore, the cortical thinning may occur to help eliminate weak synaptic connections (Gogtay et al., 2004; Paus, 2005). Mesocorticolimbic dopamine systems actively mature during adolescents, with more immature interactions between dopamine D1 and D2 receptors and an overall increase in dopaminergic availability (Dwyer and Leslie, 2016; Wahlstrom et al., 2010). These structural changes during adolescence are believed to help drive normal adaptive changes in cognitive function, working memory, and social cognition (Fuhrmann et al., 2015).
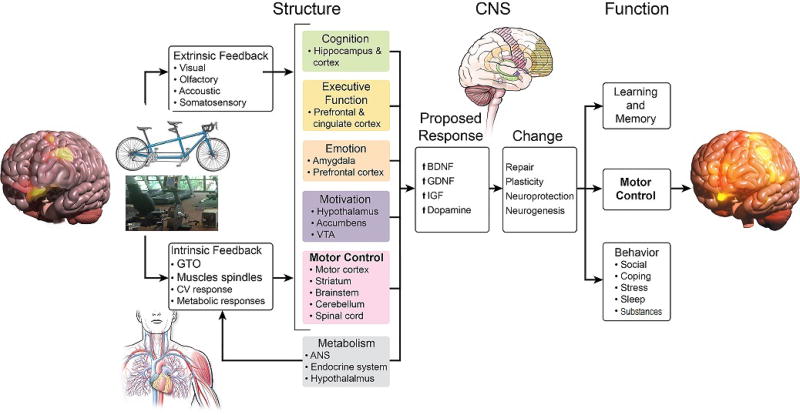
In adolescence, there is a general “imbalance” in functional development across brain regions, with earlier development occurring in posterior regions and anterior regions progressing later, which leads to underdeveloped connections between mid-brain cortico-limbic (reward) and frontal (inhibitory) region circuits (Casey et al., 2008; Ernst, 2014; Gogtay et al., 2004). More specifically, the “dual systems model” suggests an imbalance in neural maturation between cognitive control systems that regulate thoughts and emotions (prefrontal cortex) and the limbic system that drives affective and reward-related motivation (ventral striatum), such that cortical-subcortical connections precede top-down regulatory control connections (Casey et al., 2008; Casey, 2015; Somerville et al., 2010). This “imbalance” enables heightened risk-taking behavior, particularly when the behavior results in immediate rewards (Defoe et al., 2015; van Duijvenvoorde et al., 2010). However, others argue that this imbalance may not be present in all adolescents and, contextual factors, such as peer presence, may be more influential on the actual undertaking of risky behaviors (Shulman et al., 2016). The “triadic model” Figure 1) builds upon the “dual systems model” by adding a third system responsible for emotional intensity and avoidance (possibly involving the amygdala), which may serve to boost or dampen impulsive decision making (Ernst, 2014; Eldreth et al., 2013). Furthermore, complex interactions between affective-motivational processes, cognitive control, and social cognition likely drive decisions to engage in risky behavior (Kilford et al., 2016).
Figure 1.
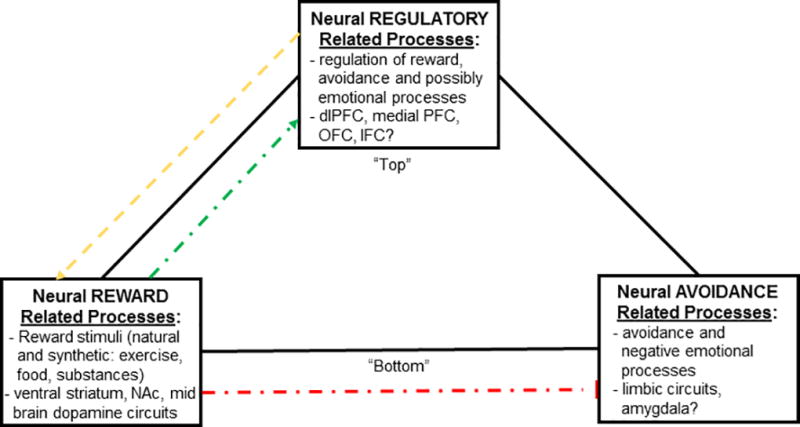
Proposed effects of exercise on the “triad model”, which relates reward (positive, affective stimuli), avoidance (negative, adversive stimuli) and regulatory related neural processes. Connections less developed (weaker) during adolescence (yellow) and connections that could be reinforced with exercise (green). ‘Assisted’ exercise may induce greater exercise-related reward and motivation, while concomitantly dampening reward-seeking from substances and, may inhibit avoidance/negative emotions (red) while enhancing overall “bottom up” inhibitory control. Adapted from Ernst (Ernst, 2014) and Eldreth et al. (Eldreth et al., 2013).
Neuroimaging studies show a fairly consistent pattern of functional activation connecting subcortical and cortical (ventral striatum, nucleus accumbens, amygdala, insula) and frontal (medial, lateral, ventral medial, and dorsolateral prefrontal cortex (dlPFC) and orbitofrontal cortex (OFC)) brain regions, in response to anticipation and receipt of rewards in adolescents and adults; however, adolescents have exhibited higher levels of activation in subcortical and cortical regions (Liu et al., 2011; Sescousse et al, 2013; Bartra et al, 2013; Silverman et al., 2015; Liu et al., 2011; Sescousse et al., 2013; Bartra et al., 2013). This “reward sensitivity” is regulated, in part, by dopamine levels and the number of dopamine receptors (Buckholtz et al., 2010; Pessiglione et al., 2006; Schultz, 2010; Sevy et al., 2006), and the dopamine response is critical for cognitive function in the prefrontal cortex (Braver and Cohen, 1999). Thus, adolescence provides a vulnerable state for reward-seeking behaviors and the potential misuse of substances (Koob & Zorrilla, 2010; Volkow & Baler, 2015); however, this period of neural plasticity also affords a window of opportunity to invoke and reinforce reward from healthy behaviors such as sport and exercise (Figure 1; discussed further below).
Abnormal Structural and Functional Brain Development May Predict Substance Use during Adolescence
Abnormal structural and functional brain development during childhood may increase the risk of substance misuse in adolescence. Lower frontal gray matter volume, lower cerebellar white matter volume, and lower white matter integrity in frontal and cortico-limbic regions have all been shown to predict alcohol and/or marijuana use in adolescence (Cheetham et al., 2012; Jacobus et al., 2013; Squeglia et al., 2014; Weiland et al., 2014). Interestingly, adolescents with smaller nucleus accumbens (NAc) volumes have been shown to be more likely to initiate substance use (Urosevic et al., 2015). In addition, a dose-dependent decrease in OFC volume has been associated with higher levels of substance use by age 18 (Cheetham et al., 2017). Moreover, thinner cortical thickness in childhood has been associated with marijuana use in adolescence and, a thinner dlPFC in earlier adolescence predicted binge drinking in late adolescence (Brumback et al., 2016; Jacobus et al., 2016).
Longitudinal functional MRI studies in childhood and earlier in adolescence have shown that differential neural activation, in response to inhibitory control and working memory tasks, may predict substance use later in adolescence and emerging adulthood. For example, less frontal (middle frontal gyrus) and parietal (inferior parietal lobe) brain activation in response to a working memory Go/No-Go task predicted heavier alcohol use (Squeglia et al., 2009; Wetherill, Squeglia et al., 2013). In addition, lower baseline ventromedial prefrontal cortex activation in response to a Go/No-Go response inhibition task predicted higher alcohol use later in adolescence (Mahmood et al., 2013). Furthermore, poorer working memory on the N-back task has predicted greater marijuana use (Cousijn et al., 2014).
Studies are emerging suggesting that resting-state cerebral blood flow may also lend insight towards predicting substance use in adolescence. For example, lower neural activation in reward and default mode networks (DMN) in 12–15 year olds has been associated with greater alcohol consumption three years later (Ramage et al.,. In addition, lower connectivity between the amygdala and OFC predicted alcohol use during adolescence (Peters et al., 2017). Interestingly, the presence of parents versus peers may also affect functional connections since, during “safe decision making”, in the presence of mothers, there was greater functional connectivity between the lateral prefrontal cortex and the ventral striatum (Telzer et al., 2015).
Other factors may increase the likelihood of early substance use, including prenatal substance exposure as well as other psychosocial and environmental factors, such as distress, depression, family conflict, violence, and exposure from environmental toxins (e.g., air pollution) (Anthony and Petronis, 1995; Behrendtet al., 2009; Buu et al., 2009; Delaney-Black et al., 2011; Frank et al., 2011; Kelly et al., 2011; Kilpatrick et al., 2000; Minnes et al., 2015; Reinherz et al., 2000; Richardson et al, 2002; Tucker et al, 2012). In particular, prenatal cocaine exposure is known to disrupt the monoaminergic neurotransmitter system (dopamine, serotonin, norepinephrine) in the prefrontal cortex (Mayes et al., 2007; Thompsonet al., 2009). Therefore, prenatal cocaine exposure may disrupt the developmental programming of an infant’s behavioral regulatory system, potentially resulting in maladaptive behavioral regulation, early substance use (Minnes et al., 2011) and general inhibitory control problems (Lambert and Bauer, 2012; Minnes et al., 2010; Thompson et al., 2009; Richardson et al., 2011). Adolescents with prenatal cocaine exposure, compared to adolescents without prenatal cocaine exposure (matched on several potential confounding factors including head circumference), had thinner cortical thickness, particularly in the dorsolateral prefrontal cortex, which correlated with impulsive behavior (Liu et al., 2013). Fetal alcohol exposure leads to a wide range of birth defects and difficulties in learning and memory, attention, visuospatial skills, language and speech disabilities, and mood disorders that may increase substance use during adolescence (Nguyen et al., 2017). The fetal endogenous cannabinoid signaling system, which is important for modulating neurodevelopment and the regulation of cardiovascular processes, is altered by prenatal marijuana exposure, particularly forms with higher delta 9 (D9)-tetrahydro-cannabinol (THC) to non-psychoactive cannabinol (CBD) ratios, providing a “first hit” that may manifest into cognitive impairment and emotional control issues (impulsivity, hyperactivity, externalization) in the presence of other stressors during adolescence (Richardson et al., 2016). In addition, human fetuses exposed to marijuana have lower dopamine receptor 2 (D2) mRNA expression in the ventral striatum, and this leads to increased substance-seeking behavior in animals (DiNieri et al., 2011; DiNieri & Hurd, 2012).
Structural Brain Effects from Substance Use during Adolescence
Because alcohol is and has been the most commonly used substance followed by marijuana during adolescence, most structural and functional neuroimaging studies have focused on the effects of these substances. Larger striatum and cerebellum and smaller prefrontal cortex and amygdala volumes have been observed in chronic marijuana users (Lorenzetti et al., 2016). In heavy drinkers, the decreases in gray matter volume occurring normally during adolescence are accelerated in frontal regions, potentially leading to non-beneficial pruning, and the normal increases in white matter volume are attenuated, particularly in the hippocampal region (Squeglia et al., 2015). Lower gray matter volume in the prefrontal cortex, amygdala, and striatum have been observed in heavy chronic marijuana users (Battistella et al., 2014). Preclinical studies suggest adolescent cocaine exposure impairs hippocampal cell genesis (proliferation and survival) (Garcia-Fuster et al., 2017), and adult hippocampal neurogenesis plays a role in cocaine addiction (Castilla-Ortega et al., 2016). Further, substantia nigra hyperechogenicity was observed in young adults with a history of illicit stimulant use (cocaine, methamphetamine) who were currently abstinent and, such morphological changes in the substantia nigra, which may alter dopamine levels, are associated with developing Parkinson's disease later in life (Todd et al., 2013). In addition, greater cortical thickness, particularly in frontal and parietal lobes, has been observed in adolescent marijuana users and some, but not all, studies suggest the adverse effects may not be reversible (Jacobus et al., 2014; Jacobus et al., 2015; Ganzer et al.,, 2016). Furthermore, poorer white matter integrity has been observed in adolescents with exposure to alcohol and/or marijuana (Bava et al., 2013; Jacobus et al., 2013; Jacobus et al., 2013). In addition, the THC in marijuana exposure during adolescence affects cannabinoid receptor (CB1) density and has other adverse effects on the cannabinoid system, which is vital for neurodevelopmental processes including neuronal genesis, neuronal migration, glial formation, and axonal elongation (Fuhrmann et al., 2015).
Functional Brain Effects from Substance Use during Adolescence
Substance exposure during adolescence may adversely affect cognitive functioning, as measured by neuropsychological tests and functional neuroimaging tasks. Adolescent alcohol and marijuana users have poorer performance on working memory, verbal learning and memory, visuospatial functioning and psychometric motor speed tasks, and poorer performance has been observed with higher doses and an earlier age-of-onset (Ganzer et al., 2016; Hanson et al.,, Medina et al., 2011; Nguyen-Louie et al., 2015). Moreover, marijuana users appear to suffer additional attentional deficits (Randolph et al., 2013), and the cognitive deficits may be sustained in chronic users (Ganzer et al., 2016; Renard et al., 2014).
Heavy alcohol users have shown decreased brain activation in frontal and parietal regions, in response to inhibition and working memory tasks (Wetherill et al., 2013; Squeglia et al., 2012) and decreased activity in the cerebellum in response to a reward processing task (Wheel of Fortune) (Cservenka et al., 2015). In addition, when winning money, higher activity in the ventral striatum was associated with higher amounts of alcohol consumption in adolescents (Braams et al., 2016). Higher activation in the anterior cingulate cortex (ACC) and cerebellum has been observed in response to alcohol cues in alcohol abusers (Brumback et al., 2015). These responses are supported by preclinical studies that show alcohol use disrupts inputs to the dopamine system and, the use of alcohol during adolescence alters risk-based decision making by modulating incentive learning (Kruse et al., 2017).
Greater activation in the dorsolateral prefrontal cortex has been observed in response to cannabis images, and these effects predicted greater cannabis use and cravings in cannabis users (Cousijn et al., 2013). Furthermore, heavy cannabis users compared with controls showed higher activation during “wins” from Iowa gambling task in core areas associated with decision making, and win-related activity and activity anticipating loss outcomes in executive function areas predicted a change in cannabis use after 6 months (Cousijn et al., 2013).
Gaps in Understanding Effects of Substance Use on the Brain
The majority of prior studies have focused on alcohol and marijuana, the two substances most commonly used during adolescence; however, additional studies are needed to evaluate potential adverse effects of other substances, including the non-medical use of prescription opioids, which is becoming particularly problematic in the South, Midwest, and low-income rural areas (Young et al., 2012). Furthermore, many studies have not evaluated the potential synergistic effects of concurrent use of two or more substances within the same time-period (Lorenzetti et al., 2016), marijuana potency (THC content), which has been increasing over time, and co-occurring psychological disorders. In addition, many prior studies have had small sample sizes and prospective studies have had relatively short amounts of follow-up time. Moreover, very few studies have integrated physiological (e.g., puberty) and social maturity as well as contextual influences into their evaluations. Importantly, however, there are several large multi-site trials underway, which will undoubtedly help to better understand the neurobiology of substance exposure and concurrent substance use in childhood and adolescence, including the IMAGEN (Schumann et al., 2010), National Consortium on Alcohol and Neuro-Development in Adolescence (NCANDA) (Brown et al., 2015) and Adolescent Brain Cognitive Development (ABCD) (Bjork et al., 2017; Volkow et al., 2017) studies. The IMAGEN study has been following 2,000 youth from Europe for over eight years, and the NCANDA study will be following over 800 youth at five sites in the U.S. for at least 10 years (Brown et al., 2015; Schumann et al., 2010; Dwyer and Leslie, 2016; Wahlstrom et al., 2010). The ABCD study, initiated recently, will be following 11,500 youth at 21 sites across the U.S. for at least 10 years. By utilizing common instruments, neuropsychological tests, and neuroimaging tasks (Kwako, Momenan et al., 2016), these ongoing large cohort studies will be able to establish more comprehensive normative data and understand the effects of substance use in more diverse ethnic samples (as most prior studies have predominantly evaluated Caucasians). These large studies will also be poised to better estimate substance dose and causal dose-response effects using biomarkers, smart-phone, and ecological momentary assessment (EMA) techniques, and to evaluate potential mediating and moderating effects by gender, family history of substance use and genetic variation.
Treatment Programs for Substance Use Disorders
Earlier initiation of substance use in adolescence is associated not only with an increased risk of SUDs but, also with antisocial behavior and impairments of adaptive functioning, including legal and relationship difficulties, incarceration, academic failure, unemployment, and mental health issues (Anthony & Petronis, 1995; Behrendt et al., 2009; DuRant et al., 1999; Fergusson and Horwood, 1997; Gordon et al., 2004; Guttmannova et al., 2012; Slade et al., 2008). Emerging studies also suggest that adolescents with obesity are at higher risk of engaging in substance misuse (Lanza et al., 2015). Furthermore, prevention of substance use is a “Healthy People 2020” initiative with the goal “to reduce substance abuse to protect the health, safety, and quality of life for all, especially children” (U.S. Department of Health and Human Services).
A variety of treatment modalities are typically utilized in current programs, including behavioral therapy (cognitive behavioral therapy, CBT), family-based therapy, 12-step programs (e.g., Alcoholics Anonymous), motivational interviewing, contingency management, and combinations of these components (Schuckit, 2009; Brewer et al., 2017). However, most of these programs were initially developed for adults and have been modified for adolescents. Nevertheless, most trials have shown the importance of multiple component programs and including parents to effectively reduce and/or prevent substance use during adolescence (Brewer et al., 2017; Newton et al., 2017). Furthermore, web- and smartphone-based programs as well as gamified Cognitive Bias Modification (CBM) treatments are emerging, and have shown some promise in preventing and treating substance misuse (Boendermaker et al., 2015).
Pharmacological agents may also be used in the treatment of SUD in adolescents. The Food and Drug Administration (FDA) has approved nicotine replacement therapy, sustained-release bupropion, and varenicline for tobacco use disorders, benzodiazepines, disulfiram, naltrexone, and acamprosate for alcohol use disorders and, methadone, naltrexone, and buprenorphine/naloxone for opioid use disorders (Hammond, 2016). No medications are currently FDA indicated for cannabis use disorder (Hammond, 2016), but the efficacy of N-acetylcysteine (NAC) compared to placebo has been shown in one trial of cannabis dependent adolescents (Gray et al., 2012). However, interestingly, NAC did not significantly reduce cannabis use in a subsequent trial of adults with cannabis dependence (Gray et al., 2017). Recently, the American Academy of Pediatrics recommended medication-assisted treatment for adolescents with severe opioid use disorders, however, only 0.4% (95% CI: 0.2%–0.7%) of adolescents in treatment for prescription opioids actually received medication, compared to 12.0% (95% CI: 11.7%–12.2%) of adults (Feder et al., 2017). Furthermore, the pharmacokinetic and pharmacodynamics responses to many of these agents and their potential side effects when taken during adolescence, a sensitive period of development, are not fully understood (Hammond, 2016).
Although exercise is not typically a component of current treatment programs, individuals with SUDs are interested in engaging in exercise as part of their treatment to help reduce substance cravings and boredom, and improve their overall physical and mental health (Abrantes et al., 2011; Gimenez-Meseguer et al., 2015; Neale, Nettleton and Pickering, 2012). Many patients with SUDs have expressed the preference to begin an exercise program early in their recovery (within the first 3 months) (Abrantes et al., 2011; Neale et al., 2012). Interestingly, some patients have reported that exercising was actively discouraged during treatment (Neale et al., 2012). Unfortunately, very little is currently known about the specific exercise preferences, motivators and barriers of adolescents with SUDs.
Overall, regardless of the type of treatment program offered, most individuals with SUDs do not take advantage of available treatment programs, as only about 6–7% of U.S. adolescents who need treatment actually receive it (Lipari et al., 2016; SAMHSA, 2014). Furthermore, unfortunately, over 50–60% of adolescents with SUDs are likely to relapse within one year of treatment (McLellan et al., 2000; Ramo and Brown, 2008; Cornelius et al., 2003). Thus, there is clearly a need for more accessible and improved treatment approaches for substance misuse and co-occurring psychiatric disorders in adolescents.
Rationale for Therapeutic Effects of Exercise in Substance Use Disorders
As mentioned above, substance misuse causes cognitive impairments in the same brain regions that are necessary to be engaged to initiate and maintain behavior change. Therefore, treatments to overcome these cognitive deficits are needed in conjunction with, or perhaps before, conventional behavioral therapies are initiated to help “prime” and/or repair structural and functional brain deficits induced by substance abuse.
Exercise has been shown to improve memory, inhibitory control, and attention in adults with mild cognitive impairments (Cammisuli et al., 2017), but statistically significant improvements in motor function, cognitive speed, and auditory and visual attention have been somewhat inconsistent in healthy adults (Angevaren et al., 2008; Young et al., 2015). There have been substantially fewer randomized trials in healthy children and adolescents, but several studies have shown improvements in attention, cognitive flexibility, and executive function with structured physical activity and exercise (Ardoy et al., 2014; Berse et al., 2015; Budde et al., 2008; Lambrick et al., 2016; Landry and Driscoll, 2012; Mahar, 2011; Song et al. 2016; Donnelly et al., 2016). Furthermore, cognitive improvements may be dose dependent, as children engaging in a 40-minute compared to a 20-minute daily exercise program were shown to have better performance on mathematic and anti-saccade eye tracking tasks (Davis et al., 2011).
Exercise induces structural and functional changes in the brain, including neurogenesis, angiogenesis, and improved neuronal health (Mueller, 2007), which may, in turn, affect overall brain health and function. Exercise is known to improve overall cardiovascular function, which increases cerebral blood flow and restores homeostasis to the neuronal microenvironment (Baek, 2016; Tarumi and Zhang, 2017). Animal studies have shown that exercise stimulates an interactive cascade of peripheral signaling molecules (insulin growth factor (IGF)-1) that cross the blood-brain barrier to increase neurotrophic factors (brain-derived neurotrophic factor, BDNF) in the hippocampus (a brain region central to learning and memory) potentiating synaptic transmission and plasticity (Cotman and Berchtold, 2002). A meta-analysis of 29 human studies has found BDNF levels increase with a single bout of exercise and even higher BDNF levels have been observed with chronic exercise (Szuhany et al., 2015). Recent data are also emerging that show levels of the neuropeptide, orexin-A, increase with exercise in humans and may enhance hippocampal neurogenesis and function (Chieffi et al., 2017). Exercise has also been shown to increase circulating and hippocampal levels of IGF-1, but data in human studies are somewhat inconsistent (Reinhardt and Bondy, 1994; Rojas, 2010; Griffin et al., 2011; Patterson, 2013). Vascular endothelial-derived growth factor (VEGF) is also upregulated with exercise, leading to blood vessel growth in the hippocampus as well as in the cortex and cerebellum (Fabel et al., 2003; van et al., 2005; Ding et al., 2006; Black et al., 1990). Furthermore, larger hippocampal volume and greater caudate and putamen volumes (two structures in the basal ganglia) have been observed in higher fit compared to less fit adolescents (Chaddock et al., 2010; Chaddock et al., 2010).
Exercise may exert neuroprotective effects and/or may help restore damage from substance use exposure. For example, exercise has been shown to restore hippocampal function following neurotoxicity from alcohol binging in rats (Leasure and Grider, 2010; Maynard and Leasure, 2013). Exercise has also been shown to increase gliogenesis in the prefrontal cortex and may help counteract the executive function impairments associated with an increased chance of relapse from methamphetamines and other substances (Mandyam et al., 2007). Chronic inflammation, which can arise from obesity and potentially SUDs, may impair IGF-1 and BDNF signaling, leading to “neurotrophin resistance” via upregulation of pro-inflammatory cytokines, such as IL-β (Tong et al., 2008; Cotman et al., 2007).
Exercise is known to evoke neurotransmitters and endogenous opioids in the brain (Greenwood and Fleshner, 2011) that are similar to those induced by substances of abuse. For example, exercise increases the levels of dopamine, epinephrine, norepinephrine, and β-endorphins, which are the same neurotransmitters activated by drugs and alcohol, and may be attributed to the “runners high” or neural reward received from higher intensity exercise in some individuals (Volkow et al., 2010; Volkow et al., 2010; Volkow et al., 2012; Volkow et al., 2012; Herrera et al., 2016; Boecker et al., 2008; Heitkamp, 1993; Bortz et al., 1981). The increase in these neurotransmitters may also contribute to the improvement in positive affect and mood reported with exercise (Matta Mello et al., 2013). In addition, exercise may help to normalize the glutamatergic system, which has been shown to be dysregulated, particularly during substance withdrawal periods, in preclinical studies (Lynch et al., 2013).
Furthermore, exercise provides many other physical and mental health benefits. Exercise improves cardiovascular health (Penedo et al., 2004; Wilson et al., 2016), which may be compromised in SUDs even after abstinence (Degenhardt et al., 2014; Huang et al., 2016; Karila et al., 2014; Maisch, 2016; Thylstrup et al., 2015). Exercise has also been shown to reduce depression, distress, and anxiety in adolescents (Schuch et al., 2016; Schuch et al., 2016; Nabkasorn et al., 2006; Blumenthal et al., 1999; Salmon, 2001), all of which may be more prevalent in adolescents with SUDs (Kelly et al., 2015; Rhew et al., 2017). Exercise improves self-efficacy (Parschau et al., 2013; McAuley and Blissmer, 2000), which may enhance adherence and overall effectiveness of behavioral treatment programs (Selzler et al., 2016). Physical activity also reduces the risk of several cancers (e.g., breast, liver, colon) (Courneya and Friedenreich, 2011; Moore et al., 2016) where risk may be elevated from the exposure to substances such as cigarettes, alcohol, and/or opioids (Afsharimani et al., 2011; Joshi et al., 2016; Liang et al., 2009; Moussas and Papadopoulou, 2017; Wang et al.,, 2016).
Evidence for Exercise to Prevent and Reduce Substance Misuse
Epidemiological studies suggest that higher levels of physical activity may decrease the use of substance misuse, although the results for alcohol are somewhat inconsistent. For example, cross-sectional studies have shown that higher levels of physical activity and participation in sports is inversely associated with smoking among adolescents (Rodriguez and Audrain-McGovern, 2004; Verkooijen et al., 2008). Further, data from the MTF study found that participation in physical activity in adolescence was associated with a lower use of cigarettes and marijuana into early adulthood, but there was an increased use of alcohol in those participating in school athletics (Terry-McElrath and O'Malley, 2011). The International Health Behavior in School-aged Children study of Polish adolescents found that boys who engaged in moderate physical activity for at least one hour, four days per week had a lower use of marijuana (Tabak et al., 2015). In addition, a study of Norwegian adolescents found that sports training, but not other hobbies, conducted one or more times per week, significantly decreased the risk of lifetime cannabis use among those exposed to cannabis use opportunities (OR=0.68; 95% CI: 0.50–0.92) (Burdzovic and Bretteville-Jensen, 2017). Thus, engaging in physical activity can decrease the use of substances in those with substance use opportunities, and this effect appears to be attributable to more than just the time occupied by the activities.
Acute exercise has been shown to reduce cigarette cravings and attentional bias to smoking images in adults (Haasova et al., 2013; Van Rensburg et al., 2009; Roberts et al., 2012). In particular, acute exercise (10 minutes of moderate intensity cycling) decreased activation in reward (caudate nucleus), motivation (orbitofrontal cortex), and visuo-spatial attention (parietal lobe, parahippocampal, fusiform gyrus) brain regions, in response to smoking versus neutral images (Van Rensburg et al., 2009). In addition, acute exercise significantly reduced anxiety in smokers following quit attempts (Abrantes et al., 2017). Furthermore, a 2-week aerobic exercise program (10, 30-minute moderate intensity treadmill bouts) reduced marijuana cravings in non-treatment seeking cannabis-dependent adults (Buchowski et al., 2011). Trials evaluating the acute effects of exercise on cravings and attentional bias in adolescents are needed.
Several randomized trials of chronic exercise, predominantly aerobic (cardiovascular) types, as an adjunctive treatment have been conducted in adults with SUDs, but trials in adolescents with SUDs are scant. Most trials in adults have shown better abstinence rates in the exercise versus control (education) arms in patients with alcohol and illicit drug use disorders (Linke and Ussher, 2015; Zschucke et al., 2012; Giesen et al., 2015). A meta-analysis of three trials found that exercise decreased the number of drinks per week in adults with alcohol use disorders (Hallgren et al., 2017). A review including 20 randomized trials conducted by the Cochrane Tobacco Addiction Group concluded that only two trials provided evidence that exercise improved long-term smoking cessation, and the other trials were too small or were not of sufficient quality to reliably include (Ussher et al., 2014). A subsequent randomized trial revealed that the beneficial effects of vigorous intensity exercise on point prevalence and prolonged abstinence may be limited to those with high anxiety at baseline (Smits et al., 2016). The Stimulant Reduction Intervention using Dosed Exercise (STRIDE) trial, which was conducted in 9 residential addiction treatment programs across the U.S., found that adult participants in the exercise arm had a 4.8% higher abstinence rate (78.7%) compared to those in the health education arm (73.9%), when controlling for treatment adherence and baseline stimulant use (Trivedi et al., 2017). Exercise has also been shown to improve secondary physiological (e.g., fitness, strength) and psychosocial (depression, anxiety) outcomes in these adult exercise trials (Linke and Ussher, 2015; Zschucke et al., 2012; Giesen et al., 2015).
Potential Therapeutic Effects of ‘Assisted’ Exercise for Substance Use Prevention and Treatment
Although studies presented above suggest that the “imbalance” between the development of subcortical-cortical and frontal/regulatory circuitry during adolescence may increase risky-behavior and substance abuse, following Telzer (Telzer, 2016), this period of heightened reward (ventral striatum) sensitivity may also provide a unique window of opportunity to promote positive health behaviors. Telzer suggested using “prosocial rewards to offset the rewarding nature of engaging in risky behaviors” (Telzer, 2016); however, we propose that exercise be utilized to promote adaptive (positive, healthy) behaviors and offset (suppress) maladaptive substance use and other adverse (negative, unhealthy) reward-seeking behaviors (Figure 1).
‘Assisted’ exercise compared to voluntary rate exercise may be able to provide even greater effects in suppressing reward from substance use, due to potentially larger increases in neurotransmitters (e.g., dopamine) and neurotrophic factors (e.g., BDNF), which may be particularly beneficial in adolescents with SUD having a “dopamine deficit” due to genetic variation and/or lower levels of striatal dopamine receptors (D2/D3) during substance abstinence (Volkow et al., 2001; Volkow et al., 2013; Volkow and Baler, 2014). We have previously shown that ‘assisted’ exercise, which provides mechanical assistance to pedal up to 35% faster than voluntary rates, provides global improvements in motor function and increased activity in cortical and subcortical regions consistent with neural activation patterns, after applying a dopamine agonist in Parkinson’s disease (PD) patients, suggesting ‘assisted’ exercise may be modulating dopamine levels in the brain (Ridgel et al., 2009; Alberts et al., 2011; Alberts et al., 2016). More specifically, ‘assisted’ cycling resulted in altered patterns of activation in the primary motor cortex, supplementary motor area, thalamus, globus pallidus, and putamen in individuals with PD, similar to activation patterns seen after levodopa administration (Alberts et al., 2011; Alberts et al., 2016). These human data are consistent with animal studies that show wheel running in rodents has strong rewarding and reinforcing properties, which are modulated through the activity of dopamine in the striatum (de et al., 2005). Further, the higher exercise rates that are afforded by ‘assisted’ or forced exercise may elicit greater BDNF levels, which may be due to increased dopamine in the dorsal striatum (Alomari et al., 2013; de et al., 2005; Herrera et al., 2016; Petzinger et al., 2010) (Figure 2). Interestingly, in animals, chronic forced exercise during adolescence has been shown to attenuate cocaine use (Thanos et al., 2010). We are currently evaluating the effects of ‘assisted’ compared to voluntary cycling exercise on food-related neural reward in obese endometrial cancer survivors, and hypothesize that ‘assisted’ exercise will further enhance the reward received from exercise providing increased (intrinsic) motivation to exercise and lead to greater sustained weight loss (Nock et al., 2014).
Figure 2.
Hypothesized effects of ‘assisted’ exercise on the brain. Higher rates of pedaling, afforded by ‘assisted’ exercise (on tandem bicycles or ‘smart’ motor-driven cycles) may provide enhanced intrinsic neuromuscular feedback leading to higher levels of neurotrophic factors (BDNF, brain-derived neurotrophic factor; GDNF, glial-derived neurotrophic factor; IGF-1/3, insulin-like growth factor) and neurotransmitters (dopamine), as well as increased striatal dopamine receptor availability providing increased neural activation in cortical-subcortical brain regions (see text). These effects may help to “dampen” reward-seeking from substances, particularly in individuals with a dopamine or neurotrophic deficit, during adolescence, a window of heightened reward sensitivity and neural plasticity and, improve overall cardiovascular, cognitive and psychosocial health. Adapted from Alberts et al. (Alberts et al., 2011).
Although no trials have yet to evaluate the effects of ‘assisted’ exercise in humans with substance use disorders, a recent trial has shown an increase in striatal D2/D3 availability (non-displaceable binding potential) after an 8-week exercise program, compared to an education control condition in adult methamphetamine users (Robertson et al., 2016). Therefore, if ‘assisted’ exercise can further enhance dopamine, BDNF, and striatal dopamine receptor availability, it may provide additional benefits beyond voluntary rate exercise, particularly in patients with dopamine and/or dopamine receptor deficits. Furthermore, given the data from preclinical studies, we propose that the exercise be initiated during early abstinence (Beiter et al., 2016) and started before integrating other cognitive behavioral treatment components, to help “prime” the brain and/or repair structural and functional brain deficits induced by substance abuse (Figure 3). Exercise may help to enhance adaptive (vs. maladaptive) decision making by reinforcing the circuitry connecting frontal and cortical-subcortical regions. Furthermore, treatment programs incorporating exercise should be offered as a preventative measure to adolescents at high risk for substance abuse, based on mental health, family history, genetic profiles, neurocognitive profiles, and other factors.
Figure 3.
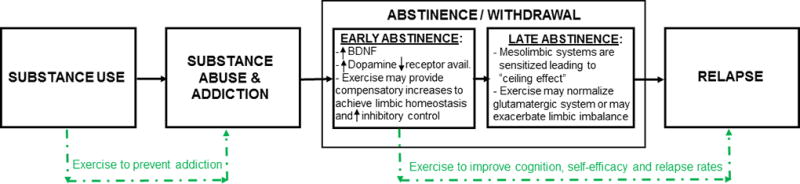
Proposed utilization of exercise as an adjunctive treatment in adolescents using and addicted to substances. Preclinical models indicate intervening with exercise during early but not late abstinence may provide improvements in relapse rates (Beiter et al., 2016). Taken together with data showing exercise can improve executive function, cognitive flexibility and attention (Davis et al., 2011; Donnelly et al., 2016; Landry and Driscoll, 2012), which are processes needed for behavior change, we propose that exercise be integrated into treatment programs during early abstinence and before cognitive behavioral therapies are initiated to help “prime” the brain and/or repair structural and functional brain deficits induced by substance abuse.
Summary
Substance abuse is an important public health issue, particularly during adolescence, a critical period of neurobiological and social development. Incorporating exercise into SUD treatment programs during early abstinence may provide additional therapeutic benefit in improving relapse rates and could help to prevent substance abuse in adolescents. If integrated during adolescence, a window of heightened reward sensitivity and neural plasticity, exercise may help to reinforce “naïve” or underdeveloped connections between neurological reward and regulatory processes from the “bottom up” and, in turn, help offset or dampen reward seeking from substances while concomitantly improving cardiovascular health, as well as academic and social achievement. ‘Assisted’ exercise can potentially further enhance dopamine, BDNF, and striatal dopamine receptor availability, and may provide additional benefits beyond voluntary rate exercise, particularly in patients with dopamine and/or dopamine receptor deficits. Improving healthy behaviors during adolescents may not only dampen desire for other reward-seeking behaviors, but may enhance the likelihood healthy behaviors are sustained into adulthood. Given the dearth of randomized trials in adolescents, additional studies are clearly needed to determine what dose (frequency, intensity, duration, length), type (aerobic, resistance), and format (‘assisted’, voluntary) of exercise can improve relapse rates and potentially prevent substance misuse during adolescence and emerging adulthood.
Acknowledgments
Funding: This work was supported, in part, by National Institutes of Health (NIH) grant no. R01-CA175100 [awarded to NLN]
Footnotes
Conflicts of Interest:
The authors declare that they have no conflicts of interest to disclose.
Reference List
- Abrantes AM, Battle CL, Strong DR, Ing E, Dubreuil ME, Gordon A, et al. EXERCISE PREFERENCES OF PATIENTS IN SUBSTANCE ABUSE TREATMENT. Ment. Health Phys. Act. 2011;4:79–87. doi: 10.1016/j.mhpa.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes AM, Farris SG, Minami H, Strong DR, Riebe D, Brown RA. Acute Effects of Aerobic Exercise on Affect and Smoking Craving in the Weeks Before and After a Cessation Attempt. Nicotine Tob. Res. 2017 doi: 10.1093/ntr/ntx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–238. doi: 10.1007/s10555-011-9285-0. [DOI] [PubMed] [Google Scholar]
- Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike, it is about the pedaling: forced exercise and Parkinson's disease. Exerc Sport Sci. Rev. 2011;39:177–186. doi: 10.1097/JES.0b013e31822cc71a. [DOI] [PubMed] [Google Scholar]
- Alberts JL, Phillips M, Lowe MJ, Frankemolle A, Thota A, Beall EB, et al. Cortical and motor responses to acute forced exercise in Parkinson's disease. Parkinsonism. Relat Disord. 2016;24:56–62. doi: 10.1016/j.parkreldis.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomari MA, Khabour OF, Alzoubi KH, Alzubi MA. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav. Brain Res. 2013;247:34–39. doi: 10.1016/j.bbr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane. Database. Syst. Rev. 2008 doi: 10.1002/14651858.CD005381.pub2. CD005381. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug and alcohol dependence. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Ardoy DN, Fernandez-Rodriguez JM, Jimenez-Pavon D, Castillo R, Ruiz JR, Ortega FB. A physical education trial improves adolescents' cognitive performance and academic achievement: the EDUFIT study. Scand. J Med. Sci. Sports. 2014;24:e52–e61. doi: 10.1111/sms.12093. [DOI] [PubMed] [Google Scholar]
- Baek SS. Role of exercise on the brain. J Exerc Rehabil. 2016;12:380–385. doi: 10.12965/jer.1632808.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcoholism, Clinical and Experimental Research. 2013;37(Suppl 1):E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA, Merrill JO, Schulman B, Finkelstein R, Olsen Y, et al. Opioid use disorder in the United States: insurance status and treatment access. Drug and alcohol dependence. 2008;94:207–213. doi: 10.1016/j.drugalcdep.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Behrendt S, Wittchen HU, Hofler M, Lieb R, Beesdo K. Transitions from first substance use to substance use disorders in adolescence: is early onset associated with a rapid escalation? Drug and alcohol dependence. 2009;99:68–78. doi: 10.1016/j.drugalcdep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Beiter RM, Peterson AB, Abel J, Lynch WJ. Exercise during early, but not late abstinence, attenuates subsequent relapse vulnerability in a rat model. Transl. Psychiatry. 2016;6:e792. doi: 10.1038/tp.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berse T, Rolfes K, Barenberg J, Dutke S, Kuhlenbaumer G, Volker K, et al. Acute physical exercise improves shifting in adolescents at school: evidence for a dopaminergic contribution. Front Behav. Neurosci. 2015;9:196. doi: 10.3389/fnbeh.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Straub LK, Provost RG, Neale MC. The ABCD study of neurodevelopment: Identifying neurocircuit targets for prevention and treatment of adolescent substance abuse. Curr. Treat. Options. Psychiatry. 2017;4:196–209. doi: 10.1007/s40501-017-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, et al. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, et al. The runner's high: opioidergic mechanisms in the human brain. Cerebral cortex (New York, N.Y.: 1991) 2008;18:2523–2531. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- Boendermaker WJ, Prins PJ, Wiers RW. Cognitive Bias Modification for adolescents with substance use problems--Can serious games help? J Behav. Ther. Exp Psychiatry. 2015;49:13–20. doi: 10.1016/j.jbtep.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Bortz WM, Angwin P, Mefford IN, Boarder MR, Noyce N, Barchas JD. Catecholamines, dopamine, and endorphin levels during extreme exercise. N. Engl. J Med. 1981;305:466–467. [PubMed] [Google Scholar]
- Braams BR, Peper JS, van der Heide D, Peters S, Crone EA. Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Dev Cogn Neurosci. 2016;17:83–93. doi: 10.1016/j.dcn.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Prog. Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Brewer S, Godley MD, Hulvershorn LA. Treating Mental Health and Substance Use Disorders in Adolescents: What Is on the Menu? Current psychiatry reports. 2017;19:5. doi: 10.1007/s11920-017-0755-0. [DOI] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T, Squeglia LM, Jacobus J, Pulido C, Tapert SF, Brown SA. Adolescent heavy drinkers' amplified brain responses to alcohol cues decrease over one month of abstinence. Addictive Behaviors. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback TY, Worley M, Nguyen-Louie TT, Squeglia LM, Jacobus J, Tapert SF. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev Psychopathol. 2016;28:1209–1216. doi: 10.1017/S0954579416000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, et al. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PloS one. 2011;6:e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde H, Voelcker-Rehage C, Pietrabyk-Kendziorra S, Ribeiro P, Tidow G. Acute coordinative exercise improves attentional performance in adolescents. Neurosci Lett. 2008;441:219–223. doi: 10.1016/j.neulet.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Burdzovic AJ, Bretteville-Jensen AL. Ready, willing, and able: the role of cannabis use opportunities in understanding adolescent cannabis use. Addiction (Abingdon, England) 2017;112:1973–1982. doi: 10.1111/add.13901. [DOI] [PubMed] [Google Scholar]
- Burns RM, Caulkins JP, Everingham SS, Kilmer B. Statistics on cannabis users skew perceptions of cannabis use. Front Psychiatry. 2013;4:138. doi: 10.3389/fpsyt.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu A, Dipiazza C, Wang J, Puttler LI, Fitzgerald HE, Zucker RA. Parent, family, and neighborhood effects on the development of child substance use and other psychopathology from preschool to the start of adulthood. Journal of studies on alcohol and drugs. 2009;70:489–498. doi: 10.15288/jsad.2009.70.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammisuli DM, Innocenti A, Franzoni F, Pruneti C. Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch. Ital. Biol. 2017;155:54–62. doi: 10.12871/000398292017126. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla-Ortega E, Serrano A, Blanco E, Araos P, Suarez J, Pavon FJ, et al. A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neurosci Biobehav. Rev. 2016;66:15–32. doi: 10.1016/j.neubiorev.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Vanpatter M, Voss MW, Pontifex MB, et al. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev Neurosci. 2010;32:249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons J, Yucel M, Lubman DI. Orbitofrontal Cortex Volume and Effortful Control as Prospective Risk Factors for Substance Use Disorder in Adolescence. Eur. Addict. Res. 2017;23:37–44. doi: 10.1159/000452159. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Chieffi S, Messina G, Villano I, Messina A, Esposito M, Monda V, et al. Exercise Influence on Hippocampal Function: Possible Involvement of Orexin-A. Front Physiol. 2017;8:85. doi: 10.3389/fphys.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, et al. When Is an Adolescent an Adult? Assessing Cognitive Control in Emotional and Nonemotional Contexts. Psychol Sci. 2016;27:549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Cohn LD, Westenberg PM, Cohn LD. Intelligence and maturity: meta-analytic evidence for the incremental and discriminant validity of Loevinger's measure of ego development. J Pers. Soc Psychol. 2004;86:760–772. doi: 10.1037/0022-3514.86.5.760. [DOI] [PubMed] [Google Scholar]
- Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. The Journal of clinical psychiatry. 2005;66:677–685. doi: 10.4088/jcp.v66n0602. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Maisto SA, Pollock NK, Martin CS, Salloum IM, Lynch KG, et al. Rapid relapse generally follows treatment for substance use disorders among adolescents. Addictive Behaviors. 2003;28:381–386. doi: 10.1016/s0306-4603(01)00247-7. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. Physical activity and cancer: an introduction. Recent Results Cancer Res. 2011;186:1–10. doi: 10.1007/978-3-642-04231-7_1. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2013;18:570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum. Brain Mapp. 2014;35:2470–2482. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Porrino LJ, et al. Individual differences in decision making and reward processing predict changes in cannabis use: a prospective functional magnetic resonance imaging study. Addict. Biol. 2013;18:1013–1023. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci. 2015;16:110–120. doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, et al. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30:91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de VL, van den Bos R, Spruijt BM. Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behav. Brain Res. 2005;160:382–388. doi: 10.1016/j.bbr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Defoe IN, Dubas JS, Figner B, van Aken MA. A meta-analysis on age differences in risky decision making: adolescents versus children and adults. Psychol Bull. 2015;141:48–84. doi: 10.1037/a0038088. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Larney S, Randall D, Burns L, Hall W. Causes of death in a cohort treated for opioid dependence between 1985 and 2005. Addiction (Abingdon, England) 2014;109:90–99. doi: 10.1111/add.12337. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicology and teratology. 2011;33:110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc. Res. 2006;3:15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- DiNieri JA, Hurd YL. Rat models of prenatal and adolescent cannabis exposure. Methods Mol Biol. 2012;829:231–242. doi: 10.1007/978-1-61779-458-2_14. [DOI] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JE, Hillman CH, Castelli D, Etnier JL, Lee S, Tomporowski P, et al. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016;48:1223–1224. doi: 10.1249/MSS.0000000000000966. [DOI] [PubMed] [Google Scholar]
- DuRant RH, Smith JA, Kreiter SR, Krowchuk DP. The relationship between early age of onset of initial substance use and engaging in multiple health risk behaviors among young adolescents. Archives of Pediatrics & Adolescent Medicine. 1999;153:286–291. doi: 10.1001/archpedi.153.3.286. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Leslie FM. Adolescent Maturation of Dopamine D1 and D2 Receptor Function and Interactions in Rodents. PloS one. 2016;11:e0146966. doi: 10.1371/journal.pone.0146966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, et al. Youth risk behavior surveillance - United States, 2011. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C.: 2002) 2012;61:1–162. [PubMed] [Google Scholar]
- Eldreth D, Hardin MG, Pavletic N, Ernst M. Adolescent transformations of behavioral and neural processes as potential targets for prevention. Prevention science : the official journal of the Society for Prevention Research. 2013;14:257–266. doi: 10.1007/s11121-012-0322-1. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Feder KA, Krawczyk N, Saloner B. Medication-Assisted Treatment for Adolescents in Specialty Treatment for Opioid Use Disorder. J Adolesc Health. 2017;60:747–750. doi: 10.1016/j.jadohealth.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction (Abingdon, England) 1997;92:279–296. [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Crooks D, Cabral HJ, Gerteis J, Hacker KA, et al. Adolescent initiation of licit and illicit substance use: Impact of intrauterine exposures and post-natal exposure to violence. Neurotoxicology and teratology. 2011;33:100–109. doi: 10.1016/j.ntt.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a Sensitive Period of Brain Development. Trends Cogn Sci. 2015;19:558–566. doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front Hum. Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzer F, Broning S, Kraft S, Sack PM, Thomasius R. Weighing the Evidence: A Systematic Review on Long-Term Neurocognitive Effects of Cannabis Use in Abstinent Adolescents and Adults. Neuropsychol. Rev. 2016;26:186–222. doi: 10.1007/s11065-016-9316-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuster MJ, Parsegian A, Watson SJ, Akil H, Flagel SB. Adolescent cocaine exposure enhances goal-tracking behavior and impairs hippocampal cell genesis selectively in adult bred low-responder rats. Psychopharmacology. 2017;234:1293–1305. doi: 10.1007/s00213-017-4566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesen ES, Deimel H, Bloch W. Clinical exercise interventions in alcohol use disorders: a systematic review. J Subst. Abuse Treat. 2015;52:1–9. doi: 10.1016/j.jsat.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Gimenez-Meseguer J, Tortosa-Martinez J, Fernandez-Valenciano de los Remedios. Benefits of Exercise for the Quality of Life of Drug-Dependent Patients. J Psychoactive Drugs. 2015;47:409–416. doi: 10.1080/02791072.2015.1102991. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MS, Kinlock TW, Battjes RJ. Correlates of early substance use and crime among adolescents entering outpatient substance abuse treatment. The American Journal of Drug and Alcohol Abuse. 2004;30:39–59. doi: 10.1081/ada-120029865. [DOI] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug and alcohol dependence. 2017;177:249–257. doi: 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Musci RJ, Johnson RM, Matson PA, Reboussin BA, Ialongo NS. Outcomes associated with adolescent marijuana and alcohol use among urban young adults: A prospective study. Addictive Behaviors. 2016;53:155–160. doi: 10.1016/j.addbeh.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci. Rev. 2011;39:140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Guttmannova K, Hill KG, Bailey JA, Lee JO, Hartigan LA, Hawkins JD, et al. Examining explanatory mechanisms of the effects of early alcohol use on young adult alcohol dependence. Journal of studies on alcohol and drugs. 2012;73:379–390. doi: 10.15288/jsad.2012.73.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van RK, Faulkner G, Cropley M, et al. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction (Abingdon, England) 2013;108:26–37. doi: 10.1111/j.1360-0443.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- Hallgren M, Vancampfort D, Giesen ES, Lundin A, Stubbs B. Exercise as treatment for alcohol use disorders: systematic review and meta-analysis. Br. J Sports Med. 2017;51:1058–1064. doi: 10.1136/bjsports-2016-096814. [DOI] [PubMed] [Google Scholar]
- Hammond CJ. The Role of Pharmacotherapy in the Treatment of Adolescent Substance Use Disorders. Child Adolesc Psychiatr Clin N. Am. 2016;25:685–711. doi: 10.1016/j.chc.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst. Abuse. 2011;20:135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkamp HC, Schmid K, Scheib K. Beta-endorphin and adrenocorticotropic hormone production during marathon and incremental exercise. Eur. J Appl. Physiol Occup. Physiol. 1993;66:269–274. doi: 10.1007/BF00235105. [DOI] [PubMed] [Google Scholar]
- Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, et al. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur. J Neurosci. 2016;43:1190–1202. doi: 10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Yang SY, Lin SK, Chen KY, Chen YY, Kuo CJ, et al. Risk of Cardiovascular Diseases and Stroke Events in Methamphetamine Users: A 10-Year Follow-Up Study. J Clin Psychiatry. 2016;77:1396–1403. doi: 10.4088/JCP.15m09872. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Castro N, Squeglia LM, Meloy MJ, Brumback T, Huestis MA, et al. Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol Teratol. 2016;57:20–29. doi: 10.1016/j.ntt.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214:374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3:396–414. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, et al. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, D O, P M, M R, A B, J G, S JE. Monitoring the Future national survey results on drug use, 1975–2016: Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2017. [Google Scholar]
- Joshi K, Kohli A, Manch R, Gish R. Alcoholic Liver Disease: High Risk or Low Risk for Developing Hepatocellular Carcinoma? Clin Liver Dis. 2016;20:563–580. doi: 10.1016/j.cld.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, et al. Acute and long-term effects of cannabis use: a review. Curr. Pharm. Des. 2014;20:4112–4118. doi: 10.2174/13816128113199990620. [DOI] [PubMed] [Google Scholar]
- Kelly AB, Chan GC, Mason WA, Williams JW. The relationship between psychological distress and adolescent polydrug use. Psychol Addict. Behav. 2015;29:787–793. doi: 10.1037/adb0000068. [DOI] [PubMed] [Google Scholar]
- Kelly AB, O'Flaherty M, Connor JP, Homel R, Toumbourou JW, Patton GC, et al. The influence of parents, siblings and peers on pre- and early-teen smoking: a multilevel model. Drug and Alcohol Review. 2011;30:381–387. doi: 10.1111/j.1465-3362.2010.00231.x. [DOI] [PubMed] [Google Scholar]
- Kilford EJ, Garrett E, Blakemore SJ. The development of social cognition in adolescence: An integrated perspective. Neurosci Biobehav. Rev. 2016;70:106–120. doi: 10.1016/j.neubiorev.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of consulting and clinical psychology. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr. Opin. Investig. Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Kruse LC, Schindler AG, Williams RG, Weber SJ, Clark JJ. Maladaptive Decision Making in Adults with a History of Adolescent Alcohol use, in a Preclinical Model, Is Attributable to the Compromised Assignment of Incentive Value during Stimulus-Reward Learning. Front Behav. Neurosci. 2017;11:134. doi: 10.3389/fnbeh.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol Psychiatry. 2016;80:179–189. doi: 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert BL, Bauer CR. Developmental and behavioral consequences of prenatal cocaine exposure: a review. Journal of perinatology : official journal of the California Perinatal Association. 2012;32:819–828. doi: 10.1038/jp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrick D, Stoner L, Grigg R, Faulkner J. Effects of continuous and intermittent exercise on executive function in children aged 8–10 years. Psychophysiology. 2016;53:1335–1342. doi: 10.1111/psyp.12688. [DOI] [PubMed] [Google Scholar]
- Landry BW, Driscoll SW. Physical activity in children and adolescents. PM.R. 2012;4:826–832. doi: 10.1016/j.pmrj.2012.09.585. [DOI] [PubMed] [Google Scholar]
- Lanza HI, Grella CE, Chung PJ. Adolescent obesity and future substance use: Incorporating the psychosocial context. J Adolesc. 2015;45:20–30. doi: 10.1016/j.adolescence.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Grider M. The effect of mild post-stroke exercise on reactive neurogenesis and recovery of somatosensation in aged rats. Exp Neurol. 2010;226:58–67. doi: 10.1016/j.expneurol.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Lenoue SR, Riggs PD. Substance Abuse Prevention. Child Adolesc Psychiatr Clin N. Am. 2016;25:297–305. doi: 10.1016/j.chc.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- Linke SE, Ussher M. Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am J Drug Alcohol Abuse. 2015;41:7–15. doi: 10.3109/00952990.2014.976708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari RN, Park-Lee E, van Horn S. America's Need for and Receipt of Substance Use Treatment in 2015. National Survey on Drug Use and Health. Research Triangle Park, NC: Substance Abuse and Mental Health Services Administration (SAMHSA), U.S. Department of Health and Human Services; 2016. [PubMed] [Google Scholar]
- Liu J, Lester BM, Neyzi N, Sheinkopf SJ, Gracia L, Kekatpure M, et al. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. 2013;167:348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav. Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Alonso-Lana S, Youssef GJ, Verdejo-Garcia A, Suo C, Cousijn J, et al. Adolescent Cannabis Use: What is the Evidence for Functional Brain Alteration? Curr. Pharm. Des. 2016;22:6353–6365. doi: 10.2174/1381612822666160805155922. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Yucel M. The Role of Cannabinoids in Neuroanatomic Alterations in Cannabis Users. Biol Psychiatry. 2016;79:e17–e31. doi: 10.1016/j.biopsych.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav. Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar MT. Impact of short bouts of physical activity on attention-to-task in elementary school children. Prev. Med. 2011;52(Suppl 1):S60–S64. doi: 10.1016/j.ypmed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents' fMRI activation to a response inhibition task predicts future substance use. Addictive Behaviors. 2013;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch B. Alcoholic cardiomyopathy : The result of dosage and individual predisposition. Herz. 2016;41:484–493. doi: 10.1007/s00059-016-4469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta Mello PE, Cevada T, Sobral Monteiro-Junior R, Teixeira GT, da Cruz RE, Lattari E, et al. Neuroscience of exercise: from neurobiology mechanisms to mental health. Neuropsychobiology. 2013;68:1–14. doi: 10.1159/000350946. [DOI] [PubMed] [Google Scholar]
- Mayes L, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school-aged children exposed in utero to cocaine. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2007;13:205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- Maynard ME, Leasure JL. Exercise enhances hippocampal recovery following binge ethanol exposure. PloS one. 2013;8:e76644. doi: 10.1371/journal.pone.0076644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci. Rev. 2000;28:85–88. [PubMed] [Google Scholar]
- McCabe SE, West BT, Teter CJ, Boyd CJ. Co-ingestion of prescription opioids and other drugs among high school seniors: results from a national study. Drug and alcohol dependence. 2012;126:65–70. doi: 10.1016/j.drugalcdep.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA : the journal of the American Medical Association. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Miech R, Bohnert A, Heard K, Boardman J. Increasing use of nonmedical analgesics among younger cohorts in the United States: a birth cohort effect. J Adolesc Health. 2013;52:35–41. doi: 10.1016/j.jadohealth.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Johnston L, O'Malley PM, Keyes KM, Heard K. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics. 2015;136:e1169–e1177. doi: 10.1542/peds.2015-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Lang A, Singer L. Prenatal tobacco, marijuana, stimulant, and opiate exposure: outcomes and practice implications. Addict. Sci. Clin Pract. 2011;6:57–70. [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Kirchner HL, Short E, Lewis B, Satayathum S, et al. The effects of prenatal cocaine exposure on problem behavior in children 4–10 years. Neurotoxicology and teratology. 2010;32:443–451. doi: 10.1016/j.ntt.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S, Singer LT, Lang A, Wu M. Effects of prenatal cocaine exposure and externalizing behavior on adolescent substance use (15–17 years). Neurobehavioral Teratology Society Annual Meeting; Montreal, Quebec, Canada. June 27–July1, 2015; Ref Type: Abstract. [Google Scholar]
- Monnat SM, Lounsbery MA, McKenzie TL, Chandler RF. Associations between demographic characteristics and physical activity practices in Nevada schools. Prev. Med. 2017;95S:S4–S9. doi: 10.1016/j.ypmed.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and alcohol dependence. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Moussas GI, Papadopoulou AG. Substance abuse and cancer. Psychiatriki. 2017;28:234–241. doi: 10.22365/jpsych.2017.283.234. [DOI] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol. Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M, et al. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J Public Health. 2006;16:179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences, E. a. M. Pain Management and Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- National Institute on Drug Abuse, National Institutes of Health, and U.S. Department of Health and Human Services. Monitoring the Future Survey: High School and Youth Trends. 2016 from http://www.drugabuse.gov/publications/drugfacts/monitoring-future-survey-high-school-youth-trends.
- National Institute on Drug Abuse National Institutes of Health U.S. Departmenr of Health and Human Services. Principles of Drug Addiction: A Research-Based Guide. 2017 (Rep. No. Third Edition) [Google Scholar]
- Neale J, Nettleton S, Pickering L. Heroin users' views and experiences of physical activity, sport and exercise. Int J Drug Policy. 2012;23:120–127. doi: 10.1016/j.drugpo.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Newton NC, Champion KE, Slade T, Chapman C, Stapinski L, Koning I, et al. A systematic review of combined student- and parent-based programs to prevent alcohol and other drug use among adolescents. Drug Alcohol Rev. 2017;36:337–351. doi: 10.1111/dar.12497. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Chong S, Tieng QM, Mardon K, Galloway GJ, Kurniawan ND. Radiological studies of fetal alcohol spectrum disorders in humans and animal models: An updated comprehensive review. Magn Reson. Imaging. 2017;43:10–26. doi: 10.1016/j.mri.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, Tapert SF. Effects of Emerging Alcohol and Marijuana Use Behaviors on Adolescents' Neuropsychological Functioning Over Four Years. J Stud Alcohol Drugs. 2015;76:738–748. doi: 10.15288/jsad.2015.76.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock NL, Dimitropoulos A, Rao SM, Flask CA, Schluchter M, Zanotti KM, et al. Rationale and design of REWARD (revving-up exercise for sustained weight loss by altering neurological reward and drive): a randomized trial in obese endometrial cancer survivors. Contemp. Clin Trials. 2014;39:236–245. doi: 10.1016/j.cct.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of National Drug Control Policy. The Economic Costs of Drug Abuse in the United States, 1992–2002. Washington, DC: Executive Office of the President. Office of National Drug Control Policy; 2004. Â Publication No. 207303. [Google Scholar]
- Oser CB, Harp KL. Treatment outcomes for prescription drug misusers: the negative effect of geographic discordance. J Subst. Abuse Treat. 2015;48:77–84. doi: 10.1016/j.jsat.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parschau L, Fleig L, Koring M, Lange D, Knoll N, Schwarzer R, et al. Positive experience, self-efficacy, and action control predict physical activity changes: a moderated mediation analysis. Br. J Health Psychol. 2013;18:395–406. doi: 10.1111/j.2044-8287.2012.02099.x. [DOI] [PubMed] [Google Scholar]
- Patterson SD, Leggate M, Nimmo MA, Ferguson RA. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur. J Appl. Physiol. 2013;113:713–719. doi: 10.1007/s00421-012-2479-5. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peiper NC, Ridenour TA, Hochwalt B, Coyne-Beasley T. Overview on Prevalence and Recent Trends in Adolescent Substance Use and Abuse. Child Adolesc Psychiatr Clin N. Am. 2016;25:349–365. doi: 10.1016/j.chc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Schneiderman N, Dahn JR, Gonzalez JS. Physical activity interventions in the elderly: cancer and comorbidity. Cancer Invest. 2004;22:51–67. doi: 10.1081/cnv-120027580. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Peper JS, Van Duijvenvoorde ACK, Braams BR, Crone EA. Amygdala-orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev Sci. 2017;20 doi: 10.1111/desc.12448. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AE, Lin AL, Olvera RL, Fox PT, Williamson DE. Resting-state regional cerebral blood flow during adolescence: associations with initiation of substance use and prediction of future use disorders. Drug and alcohol dependence. 2015;149:40–48. doi: 10.1016/j.drugalcdep.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Brown SA. Classes of substance abuse relapse situations: a comparison of adolescents and adults. Psychol Addict. Behav. 2008;22:372–379. doi: 10.1037/0893-164X.22.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph K, Turull P, Margolis A, Tau G. Cannabis and cognitive systems in adolescents. Adolesc Psychiatry. 2013;3(2):135–147. Ref Type: Journal (Full) [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1592–1597. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Hauf AM, Wasserman MS, Paradis AD. General and specific childhood risk factors for depression and drug disorders by early adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:223–231. doi: 10.1097/00004583-200002000-00023. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Le PG, Jay TM. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci. 2014;8:361. doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhew IC, Fleming CB, Vander SA, Nicodimos S, Zheng C, McCauley E. Examination of cumulative effects of early adolescent depression on cannabis and alcohol use disorder in late adolescence in a community-based cohort. Addiction (Abingdon, England) 2017;112:1952–1960. doi: 10.1111/add.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Goldschmidt L, Leech S, Willford J. Prenatal cocaine exposure: Effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol Teratol. 2011;33:69–77. doi: 10.1016/j.ntt.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicology and teratology. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Richardson KA, Hester AK, McLemore GL. Prenatal cannabis exposure - The "first hit" to the endocannabinoid system. Neurotoxicol Teratol. 2016;58:5–14. doi: 10.1016/j.ntt.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil. Neural Repair. 2009;23:600–608. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology. 2012;222:1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Chudzynski J, Mooney LJ, Rawson RA, Dolezal BA, et al. Effect of Exercise Training on Striatal Dopamine D2/D3 Receptors in Methamphetamine Users during Behavioral Treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:1629–1636. doi: 10.1038/npp.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, Audrain-McGovern J. Team sport participation and smoking: analysis with general growth mixture modeling. J Pediatr. Psychol. 2004;29:299–308. doi: 10.1093/jpepsy/jsh031. [DOI] [PubMed] [Google Scholar]
- Rojas VS, Knicker A, Hollmann W, Bloch W, Struder HK. Effect of resistance exercise on serum levels of growth factors in humans. Horm. Metab Res. 2010;42:982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Rosenbaum S, Richards J, Ward PB, Veronese N, et al. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Rev Bras. Psiquiatr. 2016;38:247–254. doi: 10.1590/1516-4446-2016-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Selzler AM, Rodgers WM, Berry TR, Stickland MK. The importance of exercise self-efficacy for clinical outcomes in pulmonary rehabilitation. Rehabil. Psychol. 2016;61:380–388. doi: 10.1037/rep0000106. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Caldu X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav. Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, et al. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188:228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, et al. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade EP, Stuart EA, Salkever DS, Karakus M, Green KM, Ialongo N. Impacts of age of onset of substance use disorders on risk of adult incarceration among disadvantaged urban youth: a propensity score matching approach. Drug and alcohol dependence. 2008;95:1–13. doi: 10.1016/j.drugalcdep.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, et al. The Efficacy of Vigorous-Intensity Exercise as an Aid to Smoking Cessation in Adults With High Anxiety Sensitivity: A Randomized Controlled Trial. Psychosom. Med. 2016;78:354–364. doi: 10.1097/PSY.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Lauseng D, Lee S, Nordstrom M, Katch V. Enhanced Physical Activity Improves Selected Outcomes in Children With ADHD: Systematic Review. West J Nurs. Res. 2016;38:1155–1184. doi: 10.1177/0193945916649954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clinical EEG and neuroscience. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. NSDUH Series H-48, HHS Publication No. SMA 14-4863. [Google Scholar]
- Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak I, Mazur J, Zawadzka D. Physical activity as a factor protecting teenage boys from tobacco and marihuana use. Przegl. Epidemiol. 2015;69:795–22. [PubMed] [Google Scholar]
- Tarumi T, Zhang R. Cerebral Blood Flow in Normal Aging Adults: Cardiovascular Determinants, Clinical Implications, and Aerobic Fitness. J Neurochem. 2017 doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Dev Cogn Neurosci. 2016;17:57–67. doi: 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NT, Qu Y. Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Soc Cogn Affect. Neurosci. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction (Abingdon, England) 2011;106:1855–1865. doi: 10.1111/j.1360-0443.2011.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, et al. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav. Brain Res. 2010;215:77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nature reviews. Neuroscience. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thylstrup B, Clausen T, Hesse M. Cardiovascular disease among people with drug use disorders. Int J Public Health. 2015;60:659–668. doi: 10.1007/s00038-015-0698-3. [DOI] [PubMed] [Google Scholar]
- Todd G, Noyes C, Flavel SC, Della Vedova CB, Spyropoulos P, Chatterton B, et al. Illicit stimulant use is associated with abnormal substantia nigra morphology in humans. PloS one. 2013;8:e56438. doi: 10.1371/journal.pone.0056438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Rethorst CD, Carmody T, Grannemann BD, Walker R, et al. Randomized Controlled Trial Comparing Exercise to Health Education for Stimulant Use Disorder: Results From the CTN-0037 STimulant Reduction Intervention Using Dosed Exercise (STRIDE) Study. J Clin Psychiatry. 2017 doi: 10.4088/JCP.15m10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Pollard MS, de la Haye K, Kennedy DP, Green HD., Jr Neighborhood characteristics and the initiation of marijuana use and binge drinking. Drug and alcohol dependence. 2012 doi: 10.1016/j.drugalcdep.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Healthy people 2020 topics and objectives. Web page. Ref Type: Internet Communication. [Google Scholar]
- Urosevic S, Collins P, Muetzel R, Schissel A, Lim KO, Luciana M. Effects of reward sensitivity and regional brain volumes on substance use initiation in adolescence. Soc Cogn Affect. Neurosci. 2015;10:106–113. doi: 10.1093/scan/nsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher MH, Taylor AH, Faulkner GE. Exercise interventions for smoking cessation. Cochrane. Database. Syst. Rev. 2014 doi: 10.1002/14651858.CD002295.pub5. CD002295. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde AC, Jansen BR, Visser I, Huizenga HM. Affective and cognitive decision-making in adolescents. Dev Neuropsychol. 2010;35:539–554. doi: 10.1080/87565641.2010.494749. [DOI] [PubMed] [Google Scholar]
- Van Rensburg KJ, Taylor A, Hodgson T. The effects of acute exercise on attentional bias towards smoking-related stimuli during temporary abstinence from smoking. Addiction (Abingdon, England) 2009;104:1910–1917. doi: 10.1111/j.1360-0443.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- van PH, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkooijen KT, Nielsen GA, Kremers SP. The Association between leisure time physical activity and smoking in adolescence: an examination of potential mediating and moderating factors. Int J Behav. Med. 2008;15:157–163. doi: 10.1080/10705500801929833. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014;76(Pt B):235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci. 2015;38:345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2017 doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, et al. Predominance of D2 receptors in mediating dopamine's effects in brain metabolism: effects of alcoholism. J Neurosci. 2013;33:4527–4535. doi: 10.1523/JNEUROSCI.5261-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu. Rev Pharmacol. Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav. Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li F, Zhang X, Li Z, Li H. Smoking increases risks of all-cause and breast cancer specific mortality in breast cancer individuals: a dose-response meta-analysis of prospective cohort studies involving 39725 breast cancer cases. Oncotarget. 2016;7:83134–83147. doi: 10.18632/oncotarget.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Korycinski ST, Soules M, Zubieta JK, Zucker RA, Heitzeg MM. Substance abuse risk in emerging adults associated with smaller frontal gray matter volumes and higher externalizing behaviors. Drug and alcohol dependence. 2014;137:68–75. doi: 10.1016/j.drugalcdep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby T, Good M, Adachi PJ, Hamza C, Tavernier R. Examining the link between adolescent brain development and risk taking from a social-developmental perspective. Brain Cogn. 2013;83:315–323. doi: 10.1016/j.bandc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Wilson MG, Ellison GM, Cable NT. Basic science behind the cardiovascular benefits of exercise. Br. J Sports Med. 2016;50:93–99. doi: 10.1136/bjsports-2014-306596rep. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2016 Ref Type: Pamphlet. [Google Scholar]
- Young AM, Glover N, Havens JR. Nonmedical use of prescription medications among adolescents in the United States: a systematic review. J Adolesc Health. 2012;51:6–17. doi: 10.1016/j.jadohealth.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane. Database. Syst. Rev. 2015 doi: 10.1002/14651858.CD005381.pub4. CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wu LT. Trends and Correlates of Cannabis-involved Emergency Department Visits: 2004 to 2011. J Addict. Med. 2016;10:429–436. doi: 10.1097/ADM.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschucke E, Heinz A, Strohle A. Exercise and physical activity in the therapy of substance use disorders. Scientific World Journal. 2012;2012:901741. doi: 10.1100/2012/901741. [DOI] [PMC free article] [PubMed] [Google Scholar]