Abstract
Objective
Our goal is to develop an interface that integrates chronic monitoring of lower urinary tract (LUT) activity with stimulation of peripheral pathways.
Approach
Penetrating microelectrodes were implanted in sacral dorsal root ganglia (DRG) of adult male felines. Peripheral electrodes were placed on or in the pudendal nerve, bladder neck and near the external urethral sphincter. Supra-pubic bladder catheters were implanted for saline infusion and pressure monitoring. Electrode and catheter leads were enclosed in an external housing on the back. Neural signals from microelectrodes and bladder pressure of sedated or awake-behaving felines were recorded under various test conditions in weekly sessions. Electrodes were also stimulated to drive activity.
Main results
LUT single- and multi-unit activity was recorded for 4 to 11 weeks in four felines. As many as 18 unique bladder pressure single-units were identified in each experiment. Some channels consistently recorded bladder afferent activity for up to 41 days, and we tracked individual single-units for up to 23 days continuously. Distension-evoked and stimulation-driven (DRG and pudendal) bladder emptying was observed, during which LUT sensory activity was recorded.
Significance
This chronic implant animal model allows for behavioral studies of LUT neurophysiology and will allow for continued development of a closed-loop neuroprosthesis for bladder control.
1. Introduction
There is a growing effort to understand the neural control of internal organs. The National Institutes of Health, particularly through their SPARC program, DARPA, and GlaxoSmithKline are supporting initiatives to examine the neural control of autonomic organs, towards developing novel bioelectrical treatments. There is a specific focus on the peripheral nervous system to map neural signals controlling individual functions of an organ with eventual progression to closed-loop devices [1,2]. The bladder is one such organ where closed-loop control is expected to remedy dysfunctions.
Understanding lower urinary tract (LUT) neurophysiology is important for improved neuromodulation applications, such as a closed-loop bladder neuroprosthesis in which information about the bladder state is used to control and improve stimulation [3]. Historically, the majority of studies examining LUT function have been performed using acute, anesthetized animal preparations. However, long-term studies of peripheral organs are required to derive maximal clinical benefit. Recently, a few chronic studies have been performed in conscious animals to examine and modulate LUT function. Zvara et al. (2010) recorded bladder multifiber sensory activity in a conscious mouse model using wire hook electrodes, which provide only a limited amount of information [4]. Recently, Brink et al. (2015) performed sacral neuromodulation in conscious sheep using the Medtronic Interstim device, but their study focused on acute or continuous stimulation without feedback from the bladder [5]. Other studies have used animals with chronic implants or chronic spinal cord injury (SCI) but in terminal procedures, without analyzing the long-term effects of chronic interfaces or SCI on LUT neurophysiology [6,7]. A recent study showed chronic bladder recordings for up to 3 months from rats with teased dorsal rootlets placed in microchannels [8]. However, this procedure is not readily translatable to clinical practice, and the technique suffers from a limited number of channels available to record bladder sensory information.
Penetrating microelectrode arrays implanted in the pudendal nerve have yielded a greater number of LUT neural signals [9]. Penetrating arrays have also successfully been implanted in the feline dorsal root ganglia (DRG) to record LUT activity and evoke reflex responses [10–12]. There are several reasons why the sacral DRG are an ideal location for accessing information about the LUT. The DRG contain large cell bodies (20–150 μm diameter [13]), which allow for recording of large-amplitude single-units [14]. Peripheral nerves converge upon the spinal cord at the level of the DRG, which serve as a convenient location to record afferent activity from multiple peripheral sources rather than interfacing at multiple distal nerves, particularly for fine autonomic sensory fibers that can be challenging to access. Additionally, DRG are a stable anatomical location for an interface due to their protected location within vertebrae.
Our study aim is to chronically record afferent activity of the LUT as a first step towards closed-loop control of the bladder. We have developed an integrated interface that allows for chronic monitoring of afferent activity of the LUT via the DRG while stimulating peripheral pathways in a feline model. We also demonstrated the feasibility of our setup in awake-behaving felines. From these data, we hope to gain a better understanding of LUT afferent neurophysiology, which will be used to develop improved stimulation patterns and closed-loop control algorithms in a DRG interface or at other anatomical locations. Our use of a feline model is consistent with their applicability to humans and with extensive prior use in the field [15].
2. Methods
2.1. Implant Surgery
All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee in accordance with the National Institute of Health’s guidelines for the care and use of laboratory animals. Aseptic techniques were used for all surgical procedures. Four male, domestic, short-hair felines (age 0.8–1.2 years, avg. 1.0 years; 4.4–5.6 kg, avg. 5.1 kg; Liberty Research, Inc, Waverly, NY, USA) were induced with an intramuscular injection of a Dexdomitor-butorphanol-ketamine mixture (Dexdomitor 0.030–0.033 mg/kg; butorphanol 0.66 mg/kg; ketamine 6.6 mg/kg). After induction, the animal was intubated and connected to a ventilator. Fluid infusion of 1:1 ratio of lactated Ringers solution and 5% dextrose (5–10 mL/kg/h; rate increased up to 30 mL/kg/h during surgery as needed) was maintained throughout surgery via a cephalic intravenous line. Surgical anesthesia was maintained with isoflurane (1–3% gas). Vital signs (heart rate, blood pressure, electrocardiogram, oxygen saturation, and exhaled carbon dioxide) were monitored every 15 minutes during the procedure to ensure a stable anesthetic plane.
In Experiment 1 after a laparotomy, a dual-lumen catheter (DLC-6D, Laborie, Mississauga, Ontario, Canada) was inserted into the bladder and secured in place with purse-string sutures. The catheter and ports were tunnelled subcutaneously to the base of the neck, externalized, and sutured in place. The constant motion at the shoulder joints led to failure of the catheter wye. Surgical revision was performed to re-attach the wye. In all subsequent experiments two catheters (1 mm outer diameter, Silastic 508-005, Dow Corning, Auburn, MI, USA) with injection ports on the end were inserted into the bladder dome and secured in place with purse-string sutures. These catheters were tunnelled subcutaneously to a midline incision on the dorsal side of the feline.
EMG electrodes (Microprobes for Life Science, Gaithersburg, MD, USA) were inserted into the bladder muscle, bladder neck, and external urethral sphincter for electrical stimulation. A bipolar nerve cuff (2.0 mm inner diameter Silastic 508-009 tubing; 0.4 mm stainless steel Cooner wire contacts) was placed on the pudendal nerve on the left side after a postero-lateral gluteal incision and access. EMG and nerve cuff wires were tunnelled subcutaneously to the dorsal midline incision. After a partial sacral laminectomy leaving vertebral processes intact, Utah penetrating microelectrode arrays with 4×8 layout, 1 mm shank length, 400 μm shank spacing, Ir/Ox coated tips, and Parylene-C insulation (Blackrock Microsystems, UT, USA) were inserted unilaterally in DRG at sacral levels S1 and S2 (figure 1A) with a pneumatic inserter (Blackrock Microsystems, UT, USA). The wired bundles were secured to the dura or other nearby tissue with 8-0 ophthalmic silk suture. A sterilized piece of Saran wrap was placed over inserted arrays in Exp. 1–3 to reduce tissue overgrowth. To ensure that the electrodes were functioning after implantation, impedance at 1 kHz was measured and neural signals were viewed in the Trellis Software Suite (Ripple, Salt Lake City, UT, USA). Cutaneous dermatome stimulation served as another check for spinal root implant level.
Figure 1.
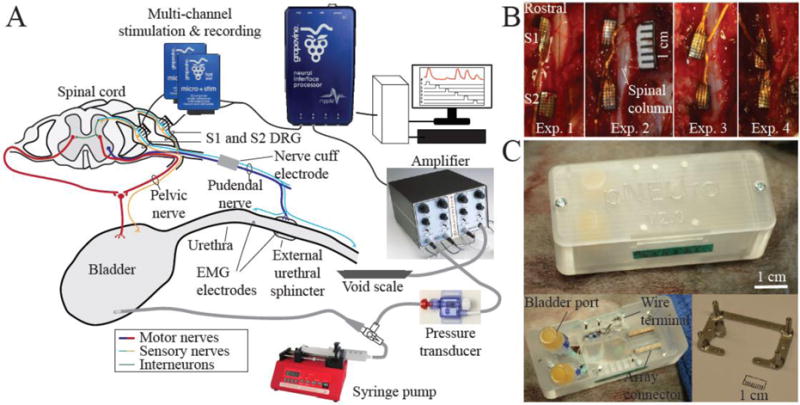
A. Experimental setup adapted from Bruns et al. 2015 [12]. B. Dorsal view of penetrating microelectrode arrays implanted unilaterally in left S1 and S2 DRG in four felines. C. Custom acrylic backpack housing (top) based on previous designs [42,43]. The backpack with cover removed (bottom left). Bladder catheter ports, array connectors, and cuff and other wires are housed inside the backpack. The stainless steel base plate (bottom right) is mounted on the iliac crest of the animal. The plastic housing is screwed into the four transcutaneous posts of the base plate.
The array connectors, bladder catheter ports, nerve cuff wires, and EMG electrode wires were placed in a custom-built polycarbonate backpack (Proto Labs, Inc., Maple Plain, MN, USA) for easy access (figure 1C). To mount the backpack, a stainless steel base plate was first secured to the iliac crests with stainless steel wire wrapped around embedded bone screws (1/4 or 5/16 inch). The plastic housing of the backpack was screwed into four transcutaneous posts of the base plate after all incisions were closed. After surgery completion, each feline was weaned off of anaesthesia and ventilation and allowed to wake up on its own.
Each feline was monitored for at least 5 days after surgery before testing sessions were started. A feline was considered recovered from surgery when it was walking, eating, and behaving normally. Analgesics were administered for 3 days during the post-surgery period. Buprenorphine (0.005–0.017 mg/kg; IM) was administered every 6–8 hours for the first 24 hours after surgery. Subsequently a smaller dose (0.005 mg/kg; IM) of buprenorphine was administered every 12 hours for the next 2 days. Ketoprofen (1.8–2.0 mg/kg; SQ) was given with the first post-op buprenorphine dose and then administered daily for 2 days as needed. Antibiotics were administered every 24 hours for 3 days or as recommended by the veterinary staff (Feline 1: Chlorpromazine, 0.01–0.025 mg/kg, IM. Feline 2: cefazolin, 19.4–20.6 mg/kg, Convenia, 7.5–8.5 mg/kg, SQ or IM; Enrofloxacin, 1.25 tablet, oral; Clavamox, 1 mL, oral. Feline 3: Cerenia, 0.5 mg/kg SQ; Cyproheptadine, 0.36 mg/kg, oral; Convenia, 8 mg/kg, IM. Feline 4: Convenia, 8 mg/kg, SQ). Vetericyn Plus Wound and Skin Care (Vetericyn, Rialto, CA, USA) was sprayed periodically on any transcutaneous components throughout the chronic study period. Animals were free-range housed with 0–3 fellow felines in a 413 ft2 room with controlled temperature (19–21 °C) and relative humidity (35–60%), food and water available ad lib, and a 12 h light/dark cycle. Enrichment was provided by toys and daily staff interaction.
2.2. Sedated Testing Sessions
Testing sessions lasting less than four hours were held up to three times a week. The animal was administered dexmeditomidine (0.019–0.039 mg/kg IM, Dexdomitor, MWI Veterinary Supply, Boise, ID, USA) for sedation, with one additional dose given during a testing session. Once sedated, the animal was placed on a custom platform (80/20 Inc., Columbia City, IN, USA) to elevate the animal off the testing bench and connections were made to the recording system as shown in figure 1. In most sessions, a custom-made void scale was placed under the perineal region of the animal to measure any expelled urine. Bladder catheters, array connectors, and other wires were accessed via the housing on the dorsal side of the animal. A disposable pressure transducer (Deltran Model 6069, Utah Medical Products, Inc., Midvale, UT, USA) and a fluid infusion line were connected to the bladder catheters. Sterile, warm saline (41 °C, HOTLINE Fluid Warmer, Smiths Medical, Dublin, OH, USA) was infused into the bladder at a fixed flow rate (2 mL/min) with a programmable syringe pump (Model NE-1000, New Era Pump Systems, Inc., Farmingdale, NY, USA) or manually infused with a syringe. The array connectors were attached to headstages (Ripple, Salt Lake City, UT, USA) and connected to a Grapevine Neural Interface Processor (Ripple, Salt Lake City, UT, USA). The pudendal cuff and EMG wires were connected to an Isolated Pulse Stimulator (Model 2100, A-M Systems, Carlsborg, WA, USA). In some experiments, DRG unit activity was driven with peripheral stimulation through the pudendal cuff or EMG wires.
During test sessions, after performing impedance measurements at 1 kHz to check electrode viability, various stimuli were applied to the bladder and perineal region while recording bladder pressure and neural activity in order to identify specific LUT responses offline. A series of trials were performed to drive LUT neural activity: fluid infusion into the bladder until leakage or voiding, perineal region cutaneous brushing, and electrical stimulation (1–30 Hz; variable amplitude) on implanted electrodes.
At the end of a sedated test session, felines were administered Antisedan (0.19–0.38 mg/kg IM, MWI Veterinary Supply, Boise, ID, USA) to reverse the effects of dexmeditomidine.
2.3. Awake Testing Sessions
We performed awake testing in two felines (Exp. 2, Exp. 4). Twice a week prior to surgery each animal was conditioned to a test enclosure, starting at a few minutes in duration and extending up to two hours as the animal acclimatized. The enclosure is designed to allow the animal to change posture and move around while being tethered (figure 2). During a test session the backpack cover was removed and the animal was tethered to a fluid infusion line and a pressure sensor, as during sedated testing, and headstages were attached to the array connectors. The bladder was infused with warm (41 °C) sterile saline at a constant rate of 2 mL/min. Bladder pressure was recorded continuously throughout a given trial. Throughout testing, the animal could move freely inside the enclosure and void naturally through grating in the floor.
Figure 2.
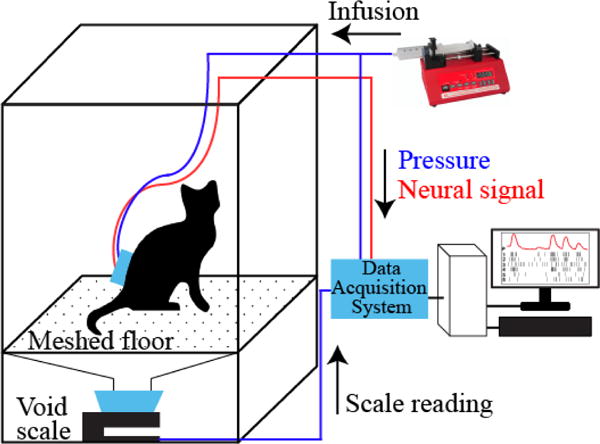
Awake-testing setup. The animal was placed in a clear plexiglass enclosure and connected to the infusion pump and the data acquisition system for real-time neural and bladder pressure recording.
2.4. Terminal Procedure
Decisions to terminate experiments were made based on animal health and neural signal viability. In Experiment 1, human error led to the loss of array connectors and bladder catheters at 28 days. All other chronic procedures lasted for at least 9 weeks. Animals were euthanized with sodium-pentobarbital solution (36 mg/kg, IV or IC) followed by transcardial perfusion with phosphate buffered saline and then paraformaldehyde (4%). A post-mortem dissection was performed to explant arrays, DRG, and nerve sections as needed, and to measure conduction distances between peripheral electrodes and the DRG for conduction velocity calculations. Explanted tissues were soaked in paraformaldehyde fixative for preservation and gross tissue analysis.
2.5. Data Analysis
Neural data was imported into Offline Sorter (Plexon, Dallas, TX, USA) to discriminate neural activity from noise with automated clustering using principal component analysis followed by manual review. Unit firing rates were calculated in MATLAB using a triangle kernel method as described previously [16]. Bladder pressure afferents were identified by correlating unit firing rate with bladder pressure in MATLAB. Units with correlation coefficient ρ ≥ 0.2 were considered bladder pressure afferents [16]. A manual, qualitative check was performed to eliminate any movement artifact or other electrical noise that correlated with bladder pressure. Other LUT units were identified based on their increased firing rate response during cutaneous brushing or electrical stimulation periods. Sorted units fell into two categories: single-unit (SU) or multi-unit (MU) activity. Units were classified as SU if less than 1% of the interspike intervals (ISI) were below 2.5 ms [17]. Otherwise, units were classified as MU. A qualitative, manual review across test sessions was performed to identify units that could be tracked over time. Units on the same electrode channel that had 1) a consistent waveform shape, 2) a similar peak-to-peak amplitude, and 3) were driven by the same underlying physiological activity (e.g. bladder pressure) were considered to be the same.
3. Results
Four adult male felines were used in this study (table 1). Raw and analyzed data, and MATLAB scripts used in data analysis can be found online at [18]. Experiments ranged in duration from 4–12 weeks with each feline undergoing varying number of test sessions. After recovery from surgery, felines did not vocalize abnormally and maintained a normal appetite. Also, they did not withdraw or otherwise react negatively to palpation of the sacral dermatomes. In each experiment, the number of functional DRG microelectrode channels was 81% or higher when averaged over the duration of the experiment. A channel was considered functional and included in analyses if its impedance measurement was less than 1 MΩ at 1 kHz. The impedance measurements for all functional channels for the duration of each experiment are shown in figure 3A. The S1 array from Exp. 1 and the S2 array from Exp. 3 were excluded, regardless of their impedance because no LUT activity was observed on these arrays (discussed in further detail below). The average impedance for functional channels across all experiments remained less than 400 kΩ. For Experiments 1–3, the general trend was a slow decline in impedance.
Table 1.
Experiment summary. Each experiment represents a separate feline. The percentage of functional channels (impedance less than 1 MΩ at 1 kHz, averaged across test sessions) is given as avg. ± sd.
| Experiment Number | Implant Surgery Age (years) | Implant Surgery Weight (kg) | Terminal Surgery Weight (kg) | # Test Sessions | Experiment Duration (days) | % Functional Channels |
|---|---|---|---|---|---|---|
| 1 (S2: 32 ch.) | 1.0 | 5.2 | 5.2 | 4 | 28 | 97% ± 7% |
| 2 (S1, S2: 64 ch.) | 1.2 | 5.1 | 5.2 | 11 | 76 | 81% ± 5% |
| 3 (S1: 32 ch.) | 1.0 | 5.6 | 5.4 | 13 | 85 | 100% ± 1% |
| 4 (S1, S2: 64 ch.) | 0.8 | 4.4 | 4.8 | 18 | 68 | 95% ± 4% |
Figure 3.
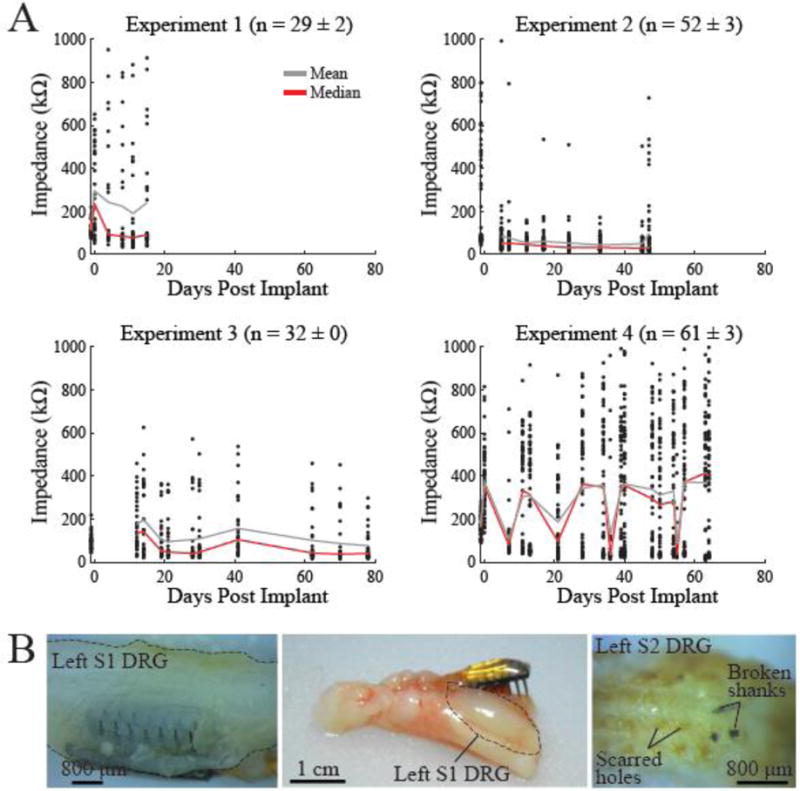
A. Impedance at 1 kHz of each channel of the implanted arrays over time for the four experiments. Only channels with impedance under 1 MΩ were considered functional and are shown here. The average impedance (gray line) was approximately 400 kΩ or lower across the duration of all experiments. Median impedance shown with red line. Number of functional electrodes for each experiment (average ± standard deviation) across sessions given in header for each sub-figure. B. Explanted arrays after experiment termination. While some arrays were well-embedded in DRG tissue upon explant (left-Exp. 2 viewed from ventral surface), other arrays may have pulled out of DRG before, or at explant (middle-Exp. 1). Scarred electrode holes (right-Exp. 3) and loss of signals on one array during the experiment suggest that this array pulled out of the DRG in vivo in Exp. 3. Some broken shanks can be seen lodged in the DRG tissue.
Figure 3B shows typical explanted DRG tissue from several experiments. Most arrays were found to be well-embedded at explant while some were not properly situated in the DRG. Out of the eight total implanted arrays, three may not have been well-embedded in tissue. Two of these arrays did not record any LUT activity (Exp. 1–Left S1 and Exp. 3–Left S2). Both arrays were found to be dislodged from tissue upon explant. Alternatively, removal of scar tissue during explant may have led to arrays becoming dislodged. This was likely the case with the left S1 array in Exp. 1 since it did record neural signals during the experiment. None of the recorded activity was LUT-related, however. Instead, the electrodes responded to cutaneous brushing of the hip, knee, and calf. Stimulating all channels simultaneously caused hind limb movement. This led us to conclude that the array may have been implanted in the L7 DRG instead of S1. Array movement may even occur in vivo as illustrated by the electrode holes that were found to be scarred over upon explant in Experiment 3. While the impedance of this array was within the functional range (S2: 95.2 ± 2.6%), none of the electrodes recorded a viable neural signal for the duration of the experiment (figure 4–Left S2), indicating that the array may have been dislodged soon after implantation during the 5-day recovery period. We also lost signals on the left S1 array in Exp. 4 after the first test session, possibly due to movement of the array in-vivo. There was a sharp rise in impedance of this array after the first test session, with an average impedance increase from 124 kΩ (Day 7) to 519 kΩ (Day 11).
Figure 4.
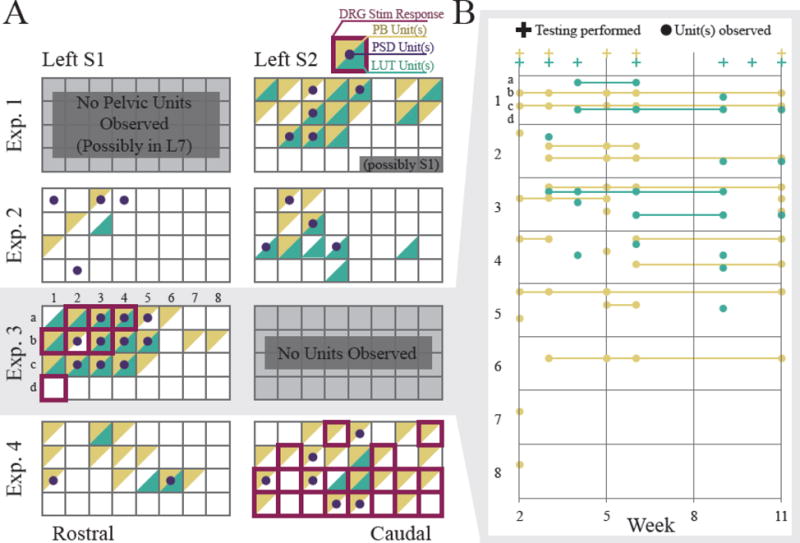
A. Array maps showing channels with recorded LUT activity at any point during an experiment. Each square represents a channel on a 4×8 array. Channels are highlighted based on whether they recorded LUT units (green) or perineal brushing (PB) units (yellow). Note that multiple afferents were often recorded on one channel. White channels include spontaneously firing units, units with no response to bladder pressure or perineal brushing, and channels with no activity or non-functional impedance > 1 MΩ). For Exp. 3 and 4, red outlines indicate electrode channels at which DRG microstimulation elicited an observed response (e.g. bladder contractions, tail twitch, anal winking, etc.). Dots indicate channels that were driven by peripheral stimulation (PSD Units) on the pudendal nerve or urethra. B. Activity of the S1 array in Exp. 3 across experiment duration. Plus signs (+) indicate when testing for bladder pressure (green) or perineal brushing (yellow) units took place. Some channels drifted in and out of recording units. A part of the array (most of columns 6–8) did not record signals after the first test session. This may have been due to encapsulation around electrode tips or a shift in the array position [20].
Out of 8 total arrays implanted (2 per feline), 6 arrays recorded LUT signals after implant (figure 4A). In Exp. 1, while both arrays recorded neural signals, only one recorded activity responding to bladder pressure and cutaneous brushing in the perineal region. In Exp. 3, the array in left S2 did not record any signals. This was the same array that seemed to have been dislodged from the DRG, allowing scar tissue to form in electrode holes. The footprint of this array is shown in figure 3B-right. Out of the 6 viable arrays, unit activity responding to bladder pressure was recorded on 36 channels while activity responding to cutaneous brushing in the perineal region was recorded on 75 channels. Activity from the single functioning array in Exp. 3 is displayed across several weeks in figure 4B. For some responding channels there were gaps in activity in some test sessions as the channels drifted in and out of recording relevant LUT units.
For each feline, varying numbers of bladder afferents were identified from the slow bladder infusions across test sessions (figure 5A). The largest number of unique bladder pressure units recorded was 18, on day 62 of Exp. 3. All 18 of these units were well-isolated and were classified as SU. In general, most LUT units that we found were well-isolated SUs. A few LUT MUs were found in Exp. 2–4, but the contribution of these units to the total unit count was minimal. The largest number of MUs were found on Day 21 of Exp. 4. Three out of six total units (50%) were classified as MU. Taken together, the single- and multi-unit bladder activity data show a general trend where the number of units increased after the first week of implant and then declined. A representative bladder afferent with strong correlation to bladder pressure (ρ = 0.9) is shown in figure 5B. The firing rate of this unit rises sharply as the bladder pressure increases and then falls steadily with bladder pressure.
Figure 5.
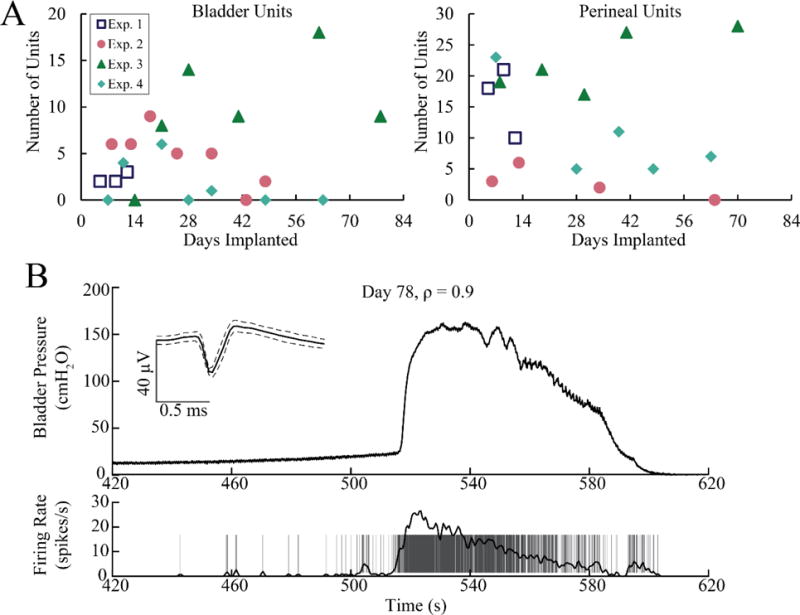
A. The number of bladder pressure and perineal brushing units observed during sedated test sessions varied across the duration of each experiment. Left: single- and multi-unit bladder activity for all four experiments. Right: single- and multi-unit perineal brushing activity for all four experiments. B. An Exp. 3 bladder pressure afferent with a high linear correlation to bladder pressure.
Units responding to cutaneous brushing in the perineal region were also recorded in all four felines. The largest number of unique units responding to brushing was 28 on Day 70 of Exp. 3. These units responded to brushing of the scrotum and anus. Out of these 28 units, two units responded exclusively to scrotal brushing while one unit responded exclusively to anal brushing. The remaining 25 units responded to both types of brushing. From one test session to the next, the largest number of cutaneous brushing units was found in Exp. 3, on average (avg. 22 units per test session). The lowest number was found in Exp. 2 (avg. 3 units per test session).
Some single-units responding to bladder pressure were tracked consistently for several days. A total of 11 single-units were tracked in all four experiments for durations of 5–23 days with a median of 16 days. The longest-tracked unit, recorded for 23 days, was observed in Exp. 2. Figure 6 displays this unit along with another bladder pressure afferent tracked for two weeks. The correlation coefficient ρ for these two tracked units changed only slightly across the testing span (largest ρ difference = 0.19). For one of the tracked units in Exp. 3, the largest ρ difference of 0.41 was observed. All other tracked units displayed ρ differences of 0.19 or less (0.1 avg. ± 0.06 s.d.). The longest-tracked bladder pressure afferent was also recorded during awake testing in Exp. 2 (figure 7). The relative pressure changes due to distension-evoked contractions were easily identifiable and the strong correlation between the afferent and bladder pressure (ρ = 0.66) was still observed in this awake paradigm.
Figure 6.
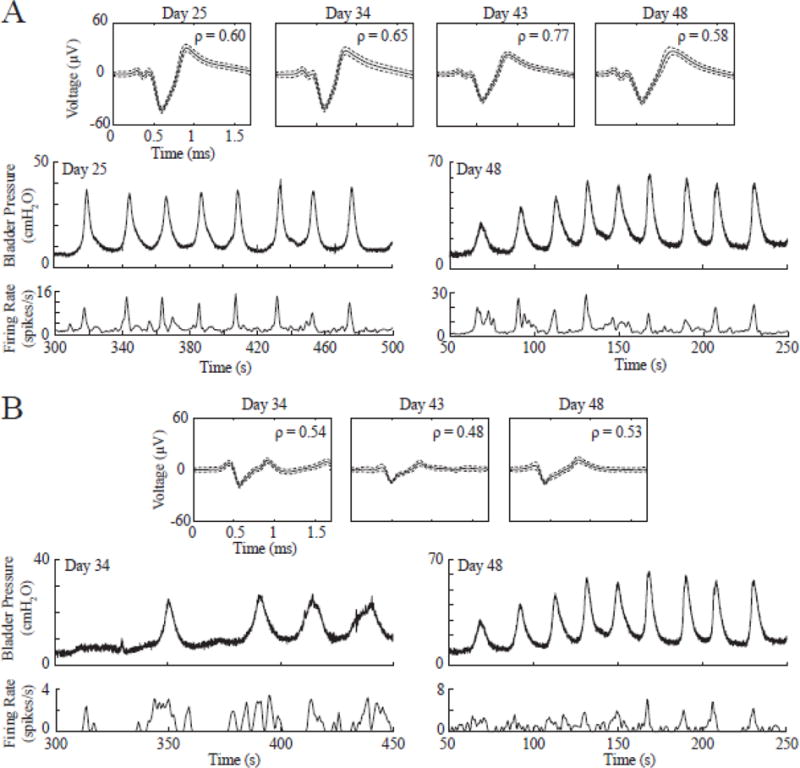
A. The longest continuously-tracked bladder afferent (observed in Exp. 2) is shown across several days along with bladder pressure and firing rate on Day 25 (first day unit was observed) and Day 48 (last day unit was observed). B. Another bladder pressure afferent (also from Exp. 2) consistently tracked for two weeks. Bladder pressure and firing rate are shown on Day 34 (first day unit was observed) and Day 48 (last day unit was observed).
Figure 7.
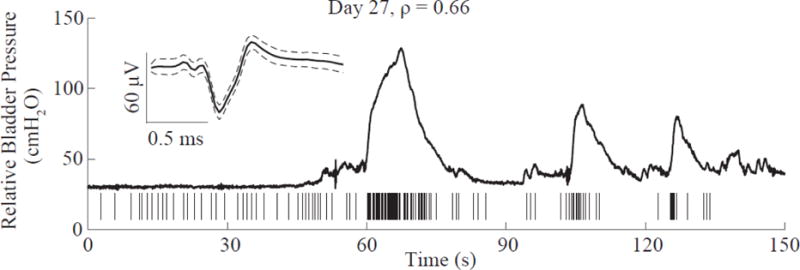
Same bladder pressure afferent as in Figure 6A shown here during awake testing on Day 27 of Experiment 2. The strong correlation to bladder pressure was maintained in this awake-behaving session with ρ = 0.66. The bladder pressure trace has some small motion artifacts due to occasional shifts in the feline’s position. Here, the bladder pressure is a relative value because feline movement made it challenging to establish an accurate baseline pressure.
Along with bladder pressure and perineal afferents, other types of LUT activity were also recorded in our experiments. For all four felines, we observed voiding at least once a week in a test session. Voiding was either natural (resulting from slow saline infusion into the bladder) or stimulation driven. Figure 8A shows a unit with a bursting firing pattern that was only active during urine flow. In figure 8B, one unit displays a negative correlation to bladder pressure as its firing rate drops below a steady level with a distension-evoked contraction and then recovers. This may be a stretch receptor in the bladder neck providing feedback to a spinal circuit to maintain muscle tone. The other unit responded to pelvic floor movement that we observed during some sedated test sessions at the beginning of distension-evoked contractions. This pelvic floor movement often consisted of contraction of the perineal region and lifting of the hind legs, even during sedation.
Figure 8.
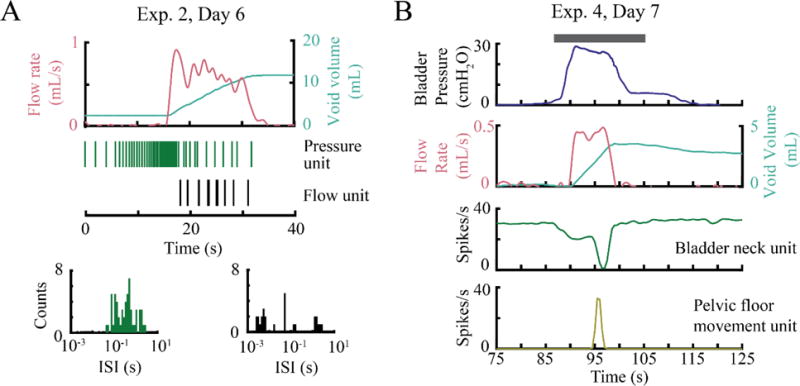
Different types of LUT activity recorded in chronic experiments, including possible urethral flow and pelvic floor movement afferents. A. While the pressure waveform was not available for this trial, the unit shown (green) is most likely a pressure unit due to its recognizable firing pattern, similar to Figure 5B. The flow unit fires only during the voiding period and displays bursts of action potentials, as shown by the corresponding interspikeinterval (ISI) histogram. B. Firing rates of a possible bladder neck unit that shows an inverse relationship to bladder pressure and a pelvic floor movement unit are shown. Note that these units are not driven by bladder pressure, urine flow, or stimulation (gray line, pudendal nerve stimulation, 5 Hz, 5 V).
We were also able to drive voiding with stimulation at the DRG and the pudendal nerve. Stimulation on four channels of the array implanted in the S2 DRG in Exp. 4 caused a voiding contraction with almost 10 mL expelled out of approximately 20 mL infused (figure 9A). Stimulation of the pudendal cuff at 5 Hz caused voiding in Exp. 1 (figure 9B). The bladder was infused with 20 mL of saline, and 16 mL was voided during stimulation (80% voiding efficiency). In this trial, we observed bladder pressure units that increased in firing rate while the bladder pressure was high and stimulation-driven units that fired in synchrony with the 5 Hz stimulation pulse. We also observed a urethral flow unit that fired only during voiding and displayed a bursting pattern. The interval between firing bursts of this unit was large at the beginning as leakage started with drips. As the voiding transitioned into a steady stream, the interval between bursts decreased.
Figure 9.
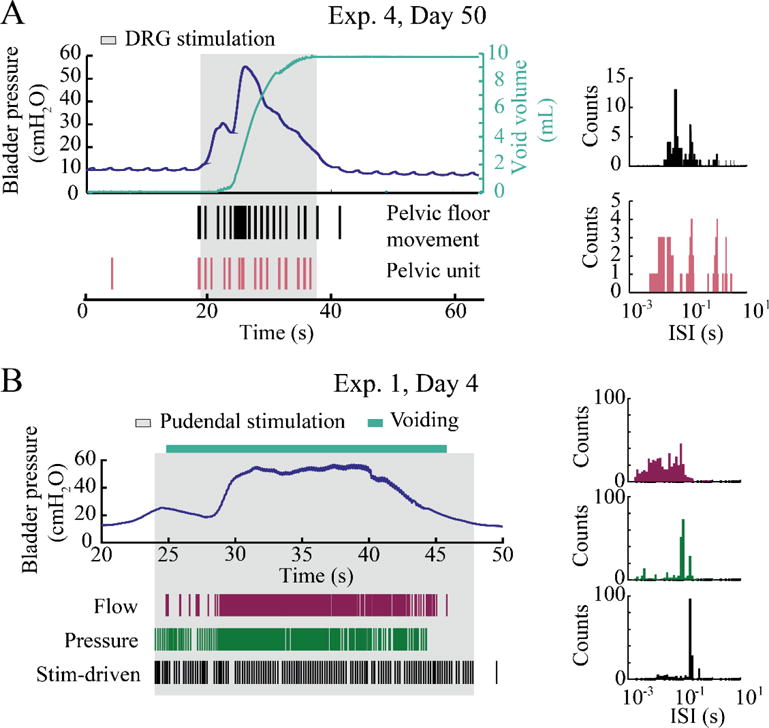
Stimulation-driven bladder emptying under sedation. A. DRG stimulation (1 Hz, 15 μA, 4 channels in S1) produced a voiding contraction on Day 50 of Exp. 4. The raster and ISI histogram for two units that fired during pelvic floor movement are shown. B. Pudendal stimulation (5 Hz, 0.75 V) elicited a robust bladder contraction and voiding of 16 mL (no void scale trace; video analysis provided voiding duration) in Exp. 1. Three representative units are shown from this trial: i) a flow unit that responded only while urine was being expelled, with a bursting firing pattern, ii) a bladder pressure unit, and iii) a stimulation-driven unit that was synchronized with the stimulation pulses (conduction velocity = 1.9 m/s).
We also applied electrical stimulation on the pudendal nerve and EMG electrodes to determine the conduction velocities of some DRG neurons. Figure 10 shows that a unit that responded to brushing of the scrotum was also driven by pudendal stimulation in Exp. 1 (conduction velocity estimate: 1.6 m/s = unmyelinated C-fiber). Units with conduction velocity ≤ 2.5 m/s were classified as unmyelinated C-fibers while units above this threshold were classified as myelinated A-fibers [19]. Figure 10C shows the distribution of identified A- and C-fibers in each of the experiments based on conduction velocity. More fibers were driven by pudendal stimulation as compared to urethral stimulation in all experiments except Exp. 1. The calculated conduction velocities had a range of 0.6–59.5 m/s. High velocity A-fibers (>20 m/s) were only activated in Exp. 2 and 3.
Figure 10.
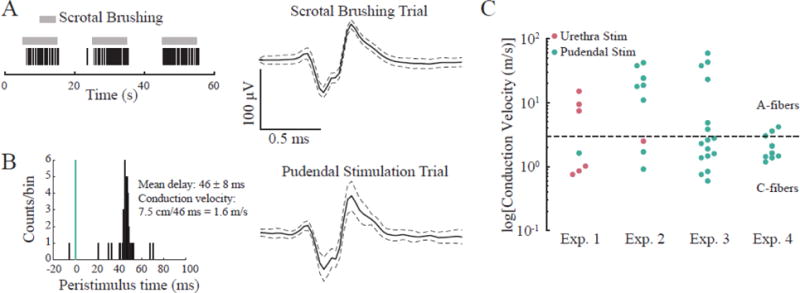
Some DRG units responding to perineal brushing were also driven by peripheral electrical stimulation. Example unit that A. fires in response to brushing of the scrotum and B. was driven by electrical stimulation of the pudendal nerve, with a mean conduction delay after each stimulus pulse of 46 ms. This unit was classified as a C-fiber with a conduction velocity of 1.6 m/s. C. Conduction velocities for DRG units driven by pudendal or urethra stimulation.
4. Discussion
This is the first study showing chronic recordings of LUT activity via the DRG, in both sedated and awake-behaving animals. We identified and tracked bladder pressure afferents over time in sedated animals (figures 4–6). We also demonstrated recording of bladder pressure afferents in awake-behaving animals (figure 7). We observed various kinds of LUT activity, such as single-units responding to urine flow and pelvic floor movement during voiding (figure 8). Distension-evoked bladder emptying was often observed in our experiments (figure 8A), and we were able to drive voiding by stimulating the DRG or pudendal nerve (figure 9). Finally, we were able to identify fiber types of stimulation-driven DRG neurons (figure 10).
The microelectrode quality remained relatively stable for the duration of the experiments (figure 3). In three experiments, there was a slight decrease in impedance over time. This steady decrease in impedance of chronically implanted silicon microelectrode arrays has been reported before and is likely due to delamination of the Parylene-C coating [20,21]. Based on loss of neural signals during the experiments and observations of explanted electrodes, we concluded that at least two out of eight total implanted arrays may have moved significantly in-vivo. When explanted, we noticed that arrays were often lifted up from the DRG on the opposite side of the wire bundle. Scar tissue forming around the wire bundle may cause the opposite side of the array to lift up from the DRG. The Saran wrap layer we placed at implant remained in place but did not minimize the tissue in-growth in Exp. 1–3 as compared to Exp. 4 which did not have Saran wrap. We are investigating approaches for providing better array stability, such as an overlay of cyanoacrylate. A metal mesh encapsulated with a silicone elastomer like Kwik-Cast (World Precision Instruments, Sarasota, FL, USA) may also be a viable means of stabilizing the arrays [22].
While we were able to track DRG neurons over time, individual neurons regularly drifted from one test session to the next, particularly during the first few weeks of each implant. Small micromovements of penetrating arrays in-vivo have been reported in acute experiments [9]. In chronic experiments, scar tissue forming around the array over time may cause these micromovements, resulting in waveform shape changes or the disappearance or appearance of units on different test days. Implementing an approach for improved array stability may mitigate these signal changes.
We observed units responding to bladder pressure and cutaneous stimuli in all four of our felines. This is in contrast to other studies with nerve recordings, in which neural responses to bladder and tactile stimuli were observed in only a subset of felines [9]. In that pudendal nerve interfacing study, 10 total units were recorded in the 4 out of 7 felines that displayed neural activity responsive to bladder pressure or filling. In contrast, we recorded 18 unique bladder units in a single trial in one animal. In almost all cases, we recorded more bladder and tactile units per animal. Our total unit observations are comparable to our prior acute study [11]. However, it is difficult to directly compare our chronic study results with previous acute studies as the number of bladder or tactile units we observed changed from one test session to the next.
It has been shown that sedation agents like Dexdomitor can affect the micturition reflex [23–25]. Stimulation-driven voiding in our experiments lasted for approximately 20 seconds (Fig. 9). A cross-species analysis has shown that this is a typical voiding duration [26], suggesting that we were observing a physiological process, even under sedation. This is in contrast to anesthetized voiding sessions which have reported similar voided amounts across longer durations (1–3 minutes) [27,28].
The range of observed conduction velocities (figure 10) indicates that we are sampling from multiple fiber types: myelinated Aδ fibers (Type III; 5–30 m/s typical conduction velocities) and unmyelinated C-fibers (Type IV; 0.5–2 m/s typical conduction velocities), though myelinated Aβ fibers (Type II; 35–75 m/s typical conduction velocities) [29] may also have been activated in Exp. 2 and Exp. 3. These conduction velocity ranges match those reported previously for LUT studies [30,31]. Pudendal stimulation was more effective at driving peripheral fibers in all experiments except Exp. 1. This may be because the pudendal cuff was more securely placed on the pudendal nerve than the wire-hook electrodes were in the urethra.
Performing LUT studies with awake, behaving animals provides greater physiological and clinical relevance over studies utilizing acute preparations [32]. In our awake testing we were successfully able to identify bladder pressure afferents. The strong correlation between these units and bladder pressure during sedated sessions was maintained in the awake setting despite the presence of movement artifacts on the bladder pressure signal. Even in this awake, unrestrained setting, it is possible that the enclosure or use of a tether to connect to the backpack limited the animals’ behavior.
In these experiments we used Utah penetrating microelectrode arrays. These arrays are an inefficient DRG interface due to the mismatch between the planar substrate and the curved DRG tissue surface (figure 3B). This shape mismatch may have contributed to some of our arrays slowly pulling out of DRG tissue over the duration of the implant. If appropriately sized for DRG, penetrating arrays would allow us to sample from a larger number of neurons in the DRG. Furthermore, our use of equal-length electrode shanks limited our ability to sample from superficial neurons. Use of a variable depth array, such as a Utah slant array [9,33], would allow for neuron sampling across a DRG cross-section.
As mentioned above, penetrating Utah arrays often show a progressive decline in signal quality due to delamination of the parylene coating. An electrode array on the surface of DRG may allow for improved signal longevity. Previously we have demonstrated single-units with a non-penetrating DRG interface [17], and we are developing new DRG surface electrode arrays through the NIH SPARC program [34,35]. These surface arrays will be less invasive than penetrating approaches, and may have significantly reduced tissue responses.
Our animals were implanted with indwelling bladder catheters. This is a standard clinical practice but can induce complications or affect bladder activity due to occlusion or other mechanical issues [36]. In terms of identifying bladder pressure afferents, the relationship between bladder pressure neuron firing rates and the bladder pressure is not purely linear but exhibits hysteresis [16]. Although convenient, a simple correlation coefficient value may not be the optimal measure for identifying bladder pressure afferents.
As demonstrated here, a DRG interface can be particularly useful for studying the neurophysiology of peripheral organs such as the bladder. At DRG, many peripheral sources can be sampled simultaneously (figures 8 & 9), allowing for a full concert of neural signals to be observed during an activity. Furthermore, DRG may serve as a stable location for sensory feedback signals for a closed-loop neuroprosthesis. This approach has been investigated by several groups pursuing closed-loop control over limb position [37,38]. Our interests lie in developing a closed-loop DRG interface for bladder control. Electrodes at one or two sacral DRG allow for the bladder state (pressure) to be observed, via pelvic nerve afferents (figures 4–8) [10,11], and for the bladder state to be driven, by activating pudendal nerve afferents (figure 9) [12]. A single location for both state detection and state control offers significant benefits over approaches that require interfaces at multiple locations. Direct estimation of the bladder volume using bladder pressure units (figures 5–9) may be challenging as bladder units generally start firing after a threshold pressure is reached [39]. Other LUT neurons such as those activated by urine flow (figure 8A, 9B) or the strain in the bladder neck (figure 8B) provide additional state information. A closed-loop neuroprosthesis using current technology will need regular recalibration to account for fluctuations in signal quality over time. This may involve brief catheterizations during a monthly doctor’s visit or may be possible at home [40]. As electrode interface technology and our field’s ability to mitigate inflammation and neural signal loss over time are improved, an annual recalibration that aligns with the typical interval for SCI patient urodynamic evaluations [41] may be ideal.
5. Conclusions
We have demonstrated the potential utility of a feline chronic DRG implant model for monitoring bladder activity over time. Furthermore, this animal model may be useful for evaluating the chronic effects and stability of DRG stimulation for LUT control. It may also be useful for observing long-term changes in LUT neurophysiology after system perturbations caused by induced LUT dysfunction, such as overactive bladder. Chronic implant models such as this will lead to a greater understanding of LUT neurophysiology and drive improved neuroprostheses.
Acknowledgments
We thank other current and prior members of the Peripheral Neural Engineering and Urodynamics Lab (Kaile Bennett, Eric Kennedy, John Bentley, Lauren Zimmerman, Matthew Biggers, Colin M. Mahar, Indie Rice, Micah Levy, Grant Kulik, and Seth Ringel) for their assistance during experiments and/or with data analysis. We also thank the University of Michigan Unit for Laboratory Animal Medicine for their services. Research reported in this publication was supported by the Craig H. Neilsen Foundation (Grant # 314980) and by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number U18EB021760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Craig H. Neilsen Foundation or the National Institutes of Health.
References
- 1.Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: A jump-start for electroceuticals. Nature. 2013;496:159–61. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, Pasricha P, Weber DJ, Ludwig K, Famm K. Bioelectronic medicines: a research roadmap. Nat Rev Drug Discov. 2014 May 30;13(6):399–400. doi: 10.1038/nrd4351. [DOI] [PubMed] [Google Scholar]
- 3.Tennyson LE, Tai C, Chermansky CJ. Using the Native Afferent Nervous System to Sense Bladder Fullness: State of the Art. Curr Bladder Dysfunct Rep. 2016:1–4. doi: 10.1007/s11884-016-0391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zvara P, Wright AJ, Roach K, Ursiny M, Shapiro B, Dagrosa LM, Nelson MT, Heppner TJ. A non-anesthetized mouse model for recording sensory urinary bladder activity. Front Neurol. 2010 Jan;1:127. doi: 10.3389/fneur.2010.00127. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brink TS, Zimmerman PL, Mattson Ma, Su X, Nelson DE. A chronic, conscious large animal platform to quantify therapeutic effects of sacral neuromodulation on bladder function. J Urol. 2015;194(1):252–8. doi: 10.1016/j.juro.2015.01.109. [DOI] [PubMed] [Google Scholar]
- 6.Tai C, Chen M, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC. Plasticity of urinary bladder reflexes evoked by stimulation of pudendal afferent nerves after chronic spinal cord injury in cats. Exp Neurol. 2011 Mar;228(1):109–17. doi: 10.1016/j.expneurol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCoin JL, Bhadra N, Gustafson KJ. Electrical stimulation of sacral dermatomes can suppress aberrant urethral reflexes in felines with chronic spinal cord injury. Neurourol Urodyn. 2013;32(1):92–7. doi: 10.1002/nau.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chew DJ, Zhu L, Delivopoulos E, Minev IR, Musick KM, Mosse CA, Craggs MD, Donaldson N, Lacour SP, McMahon SB, Fawcett JW. A Microchannel Neuroprosthesis for Bladder Control After Spinal Cord Injury in Rat. Sci Transl Med. 2013 Nov 6;5(210):210ra155. doi: 10.1126/scitranslmed.3007186. [DOI] [PubMed] [Google Scholar]
- 9.Mathews KS, Wark HaC, Warren DJ, Christensen MB, Nolta NF, Cartwright PC, Normann Ra. Acute monitoring of genitourinary function using intrafascicular electrodes: selective pudendal nerve activity corresponding to bladder filling, bladder fullness, and genital stimulation. Urology. 2014 Sep;84(3):722–9. doi: 10.1016/j.urology.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Bruns TM, Gaunt RA, Weber DJ. 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Boston, MA, USA: IEEE; 2011. Estimating bladder pressure from sacral dorsal root ganglia recordings; pp. 4239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruns TM, Gaunt RA, Weber DJ. Multielectrode array recordings of bladder and perineal primary afferent activity from the sacral dorsal root ganglia. J Neural Eng. 2011 Aug 30;8(5):56010. doi: 10.1088/1741-2560/8/5/056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruns TM, Weber DJ, Gaunt RA. Microstimulation of afferents in the sacral dorsal root ganglia can evoke reflex bladder activity. Neurourol Urodyn. 2015;34(1):65–71. doi: 10.1002/nau.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999 Aug;6:S27–35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- 14.Aoyagi Y, Pearson KG, Stein RB, Branner A, Normann RA. Capabilities of a penetrating microelectrode array for recording single units in dorsal root ganglia of the cat. J Neurosci Methods. 2003;128:9–20. doi: 10.1016/s0165-0270(03)00143-2. [DOI] [PubMed] [Google Scholar]
- 15.Fry CH, Daneshgari F, Thor K, Drake MJ, Eccles R, Kanai A, Birder L. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn. 2010;29(4):603–608. doi: 10.1002/nau.20903. [DOI] [PubMed] [Google Scholar]
- 16.Ross SE, Sperry ZJ, Mahar CM, Bruns TM. Hysteretic behavior of bladder afferent neurons in response to changes in bladder pressure. BMC Neurosci. 2016;17:57. doi: 10.1186/s12868-016-0292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaunt RA, Bruns TM, Crammond D, Tomycz N, Moossy JJ, Weber DJ. 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Boston, MA, USA: IEEE; 2011. Single- and multi-unit activity recorded from the surface of the dorsal root ganglia with non-penetrating electrode arrays; pp. 6713–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy M, Khurram A, Ross SE, Sperry ZJ, Ouyang A, Jiman AA, Stephan CJ, Bruns TM. Chronic monitoring of lower urinary tract activity via a sacral dorsal root ganglia interface [Internet] Open Science Framework. 2017 doi: 10.17605/OSF.IO/WSBR6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KH, Chung K, Chung JM, Coggeshall RE. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol. 1986;243:335–46. doi: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- 20.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013 Dec;10(6):66014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane SR, Cogan SF, Ehrlich J, Plante TD, McCreery DB, Troyk PR. Electrical performance of penetrating microelectrodes chronically implanted in cat cortex. IEEE Trans Biomed Eng. 2013 Aug;60(8):2153–60. doi: 10.1109/TBME.2013.2248152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledbetter NM, Ethier C, Oby ER, Hiatt SD, Wilder AM, Ko JH, Agnew SP, Miller LE, Clark GA. Intrafascicular stimulation of monkey arm nerves evokes coordinated grasp and sensory responses. J Neurophysiol. 2013 Oct 17;109(2):580–90. doi: 10.1152/jn.00688.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horváth G, Morvay Z, Kovács M, Szikszay M, Benedek G. An ultrasonographic method for the evaluation of dexmedetomidine on micturition in intact rats. J Pharmacol Toxicol Methods. 1994;32(4):215–8. doi: 10.1016/1056-8719(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Ansah OB, Raekallio M, Vainio O. Comparison of three doses of dexmedetomidine with medetomidine in cats following intramuscular administration. J Vet Pharmacol Ther. 1998;21(5):380–7. doi: 10.1046/j.1365-2885.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- 25.Cullen LK. Medetomidine sedation in dogs and cats: a review of its pharmacology, antagonism and dose. Br Vet J. 1996;152(5):519–35. doi: 10.1016/s0007-1935(96)80005-4. [DOI] [PubMed] [Google Scholar]
- 26.Yang PJ, Pham J, Choo J, Hu DL. Duration of urination does not change with body size. Proc Natl Acad Sci. 2014;111(33):11932–7. doi: 10.1073/pnas.1402289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruns TM, Bhadra N, Gustafson KJ. Bursting stimulation of proximal urethral afferents improves bladder pressures and voiding. J Neural Eng. 2009;6:6606. doi: 10.1088/1741-2560/6/6/066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008 Jul;212(1):218–25. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren S, Capra N, Yezierski R. The Somatosensory System I: Tactile Discrimination and Position Sense. In: Haines DE, editor. Fundamental Neuroscience for Basic and Clinical Applications. 4th. Elsevier; Health Sciences: 2013. p. 492. [Google Scholar]
- 30.Bahns E, Halsband U, Jänig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflügers Arch Eur J Physiol. 1987 Oct;410(3):296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- 31.Kawatani M, Tanowitz M, de Groat WC. Morphological and electrophysiological analysis of the peripheral and central afferent pathways from the clitoris of the cat. Brain Res. 1994;646:26–36. doi: 10.1016/0006-8993(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 32.Comiter CV. Conscious neuromodulation of the bladder before clinical use. J Urol. 2015;194(1):16–7. doi: 10.1016/j.juro.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51(1):146–51. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- 34.Sperry ZJ, Seymour JP, Wu F, Ross SE, Kim K, Bentley JT, Yoon E, Bruns TM. Society for Neuroscience Annual Meeting. Chicago, IL: 2015. Dorsal root ganglia neural recordings with a novel non-penetrating thin-film microelectrode array; p. 540.04. [Google Scholar]
- 35.Sperry ZJ, Chiel HJ, Seymour JP, Lu H, Kehl CE, Na K, Yoon E, Bruns TM. High-density extracellular recording from the buccal ganglia of Aplysia californica using a novel thin-film electrode array. Soc Neurosci Annu Meet [Abstract] 2016;848:10. [Google Scholar]
- 36.Yaksh TL, Durant PA, Brent CR. Micturition in rats: a chronic model for study of bladder function and effect of anesthetics. Am J Physiol. 1986 Dec;251(6):R1177–85. doi: 10.1152/ajpregu.1986.251.6.R1177. [DOI] [PubMed] [Google Scholar]
- 37.Bruns TM, Wagenaar JB, Bauman MJ, Gaunt RA, Weber DJ. Real-time control of hind limb functional electrical stimulation using feedback from dorsal root ganglia recordings. J Neural Eng. 2013;10(2):26020. doi: 10.1088/1741-2560/10/2/026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holinski BJ, Everaert DG, Mushahwar VK, Stein RB. Real-time control of walking using recordings from dorsal root ganglia. J Neural Eng. 2013 Aug 8;10(5):56008. doi: 10.1088/1741-2560/10/5/056008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Häbler HJ, Jänig W, Koltzenburg M, Janig W. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol. 1993;463:449–60. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damaser MS, Brzezinski K, Walter JS, Wheeler JS, Schroeder LS, Hatch DA. Estimating detrusor pressure at home in pediatric patients with myelomeningocele. J Urol. 1999;162(4):1410–4. [PubMed] [Google Scholar]
- 41.Linsenmeyer TA, Linsenmeyer MA. Impact of annual urodynamic evaluations on guiding bladder management in individuals with spinal cord injuries. J Spinal Cord Med. 2013;36(5):420–6. doi: 10.1179/2045772313Y.0000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debnath S, Bauman MJ, Fisher LE, Weber DJ, Gaunt RA. Microelectrode array recordings from the ventral roots in chronically implanted cats. Front Neurol. 2014;5:104. doi: 10.3389/fneur.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher LE, Ayers CA, Ciollaro M, Ventura V, Weber DJ, Gaunt RA. Chronic recruitment of primary afferent neurons by microstimulation in the feline dorsal root ganglia. J Neural Eng. 2014 Jun 1;11(3):36007. doi: 10.1088/1741-2560/11/3/036007. [DOI] [PubMed] [Google Scholar]


