Abstract
Background
Ferns are large and underexplored group of vascular plants (~ 11 thousands species). The genomic data available by now include low coverage nuclear genomes sequences and partial sequences of mitochondrial genomes for six species and several plastid genomes.
Results
We characterized plastid genomes of three species of Dryopteris, which is one of the largest fern genera, using sequencing of chloroplast DNA enriched samples and performed comparative analysis with available plastomes of Polypodiales, the most species-rich group of ferns. We also sequenced the plastome of Adianthum hispidulum (Pteridaceae). Unexpectedly, we found high variability in the IR region, including duplication of rrn16 in D. blanfordii, complete loss of trnI-GAU in D. filix-mas, its pseudogenization due to the loss of an exon in D. blanfordii. Analysis of previously reported plastomes of Polypodiales demonstrated that Woodwardia unigemmata and Lepisorus clathratus have unusual insertions in the IR region. The sequence of these inserted regions has high similarity to several LSC fragments of ferns outside of Polypodiales and to spacer between tRNA-CGA and tRNA-TTT genes of mitochondrial genome of Asplenium nidus. We suggest that this reflects the ancient DNA transfer from mitochondrial to plastid genome occurred in a common ancestor of ferns. We determined the marked conservation of gene content and relative evolution rate of genes and intergenic spacers in the IRs of Polypodiales. Faster evolution of the four intergenic regions had been demonstrated (trnA- orf42, rrn16-rps12, rps7-psbA and ycf2-trnN).
Conclusions
IRs of Polypodiales plastomes are dynamic, driven by such events as gene loss, duplication and putative lateral transfer from mitochondria.
Electronic supplementary material
The online version of this article (doi: 10.1186/s12870-017-1195-z) contains supplementary material, which is available to authorized users.
Background
Сhloroplast genomes (plastomes) of land plants are generally conserved in their size, gene content and order. The evolutionary origin of chloroplasts, as well as mitochondria, traces back to ancient endosymbiotic bacteria that consequently greatly reduced the genome, and contain only a small proportion of the ancestor’s genes [1, 2].
Plant plastomеs possess a quadripartite structure composed of large single-copy (LSC) and small single-copy (SSC) regions divided by two parts of inverted repeat (IR) [2, 3]. Plastome size of higher plants is usually around 150,000 bp in length and comprise approximately 120–130 genes, among which about 75 genes encode proteins of photosystem I and II, as well as for other proteins, involved in photosynthesis [see, for example, [4]], while other genes encode ribosomal RNA and proteins and transfer RNA. The highest deviations of the gene order and content in plastomes of land plants have been reported in non-photosynthetic species, in which extremely reduced plastomes were found - up to 11 Kbp [5]. However, in photosynthetic plants lineage-specific gene losses, as well as translocations that change the gene order were also observed [6–8].
IRs are usually regarded as the most stable part of the plastome. Indeed, the substitution rate of the IR sequences is the lowest compared to single copy regions [3]; the plastomes where IRs are absent (e.g. IRLC clade of Fabaceae) exhibit elevated substitution rates [9] and vice versa, genes that were translocated from SC to IR slow down their substitution rate [10]. IRs typically range in size from 15 to 30 kbp and contain a core set of genes consisting of four rRNA genes (4.5S, 5S, 16S and 23S rRNA), tRNA genes (trnAUG, trnI-GAU, trnN-GUU, trnR-ACG and trnV-GAC). The IRs of many land plants also contain a number of other genes as a result of lineage-specific expansions and contractions [3]. Few evolutionary lineages demonstrate large-scale expansions (exceeding several kbp and containing numerous genes) of the IR [3, 11]. In particular, several overlapping inversions affect size, gene content and order of the IR in leptosporangiate ferns, the clade that includes most fern species [8, 10].
Another evolutionary event that can affect plastome structure is horizontal gene transfer (HGT). HGT between nucleus, mitochondria and plastids has been shown to occur with a high rate and contributed significantly to the plant genome evolution by relocating and refashioning of the genes and consequently contributing to genetic diversity. Transfers of DNA fragments from the mitochondria or plastids to the nucleus are the most common reported ones [12–16]. The mitochondrial genomes are also often invaded by plastome-derived sequences; the presence of DNA from nuclear genomes also has been shown in a number of lineages of flowering plants [17–24] and ferns [25]. Translocations of mtDNA fragments to plastid genome are much rarer. Currently, only two cases, all from flowering plants, are known. In Daucus carota (order Apiales, Apiaceae) plastome a ~1.5 Kb region with high similarity to Vitis vinifera (order Vitales) mitochondrial sequence was found [26]. This region did not contain any typical plastid genes. Characterization of Daucus mitogenome and screening of plastomes of other Apiaceae suggest that it was inserted from the mitochondrial genome to the plastome in a common ancestor of the genus Daucus [27]. Another example is the horizontal gene transfer is a 2.4-kb segment of mitochondrial DNA into the rps2–rpoC2 intergenic spacer of the plastome of Asclepias syriaca (Apocynaceae) [28]. Thus, unlike the mitochondrial genomes, which are affected by insertions of plastid and nuclear sequences, the plastomes of flowering plants are infrequently profited by DNA transfer from the other cell compartments [22, 29, 30].
Extant ferns are non-seed vascular plants for which 45 families are currently known (with approx. 280 genera), of which more than half belong to the order Polypodiales, in line with the classification of [31]. The majority of Polypodiales species fall into two large sister clades - Eupolypods I and Eupolypods II, and the remaining to families Pteridaceae, Dennstaedtiaceae, Saccolomataceae, Lindsaeaceae (basal clade) [32]. Wolf et al. [8] for the first time examined structure of the plastome across few widely ranged representative fern taxa but no one has represented the Polypodiales. Zhu et al. [3] analyzed the evolutionary rate and shifts in IR boundaries of land plants, including seven ferns but also no Polypodiales were considered. Raman et al. [33] characterized Cyrtomium falcatum plastid genome and found some differences with congeneric species C. devexiscapulae in tRNA gene content and start codons. For 24 fern samples, five of which were Polypodiales species (other represented ten extant orders), the part of LSC region (rpoB-psbZ) was analyzed and considerable genomic changes for distant species (belonging to different orders) were found [34]. A comparison of a few taxon-wide fern plastomes (of Lycopodiophyta, Psilotopsida, Equisetopsida, Marattiopsida and Polypodiopsida) showed that some lineages have experienced multiple IR changes including expansions and inversions while others demonstrated the stasis [35]. Recently many new sequences of fern plastomes (including Polypodiales species) were released. However, the corresponding study reports only the results of phylogenetic analysis of these sequences, without detailed analysis of their gene content and structure [36].
This clearly indicates that the diversity of plastome structures in ferns is insufficiently explored. With this premise, we characterized four additional plastome sequences from Polypodiales, three from Dryopteris and one from Adianthum, and performed comparative analysis of all available fern plastomes.
Results
We sequenced and assembled de novo new plastome sequences for three Eupolypods I species: Dryopteris filix-mas, Dryopteris blanfordii and Dryopteris villarii. The plastomes have typical quadripartite structure, are similar in their size (148,568, 152,945 and 148,727 bp, respectively). A total of 130 genes were annotated by DOGMA for D. filix-mas, including 91 protein-coding genes (5 of them are duplicated in IRs), 25 tRNA genes (5 of them are duplicated in IRs) and 4–5 rRNA genes (all of them are duplicated in IRs). Plastomes of Dryopteris species, including D. blanfordii, D. villarii, D. filix-mas and previously reported D. decipiens were identical to each other in gene content of LSC and SSC, but differed in IRs (Fig. 1).
Fig. 1.
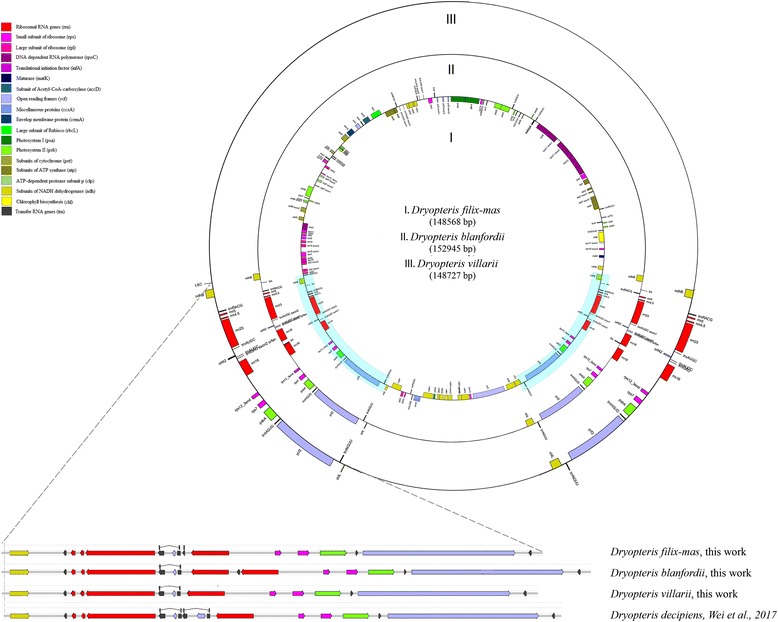
Gene maps of the Dryopteris plastomes. Genes drawn inside the circle are transcribed clockwise, and those outside the circle are transcribed counter clockwise
We also sequenced one the plastome of Adianthum hispidulum, from Pteridaceae, the basal group relative to eupolypods. The complete plastome sequence of A. hispidulum was 151,327 bp in length, consisted of an LSC (83,188 bp), SSC (21,459 bp), and IRs (23,340 bp). The gene content in the plastome of A. hispidulum slightly differs from that of congeneric species A. capillus-veneris (published in [37]) – in A. hispidulum trnT-UGU gene (located in IR) is completely absent while in A. capillus-veneris it is represented by a pseudogene (Fig. 2).
Fig. 2.
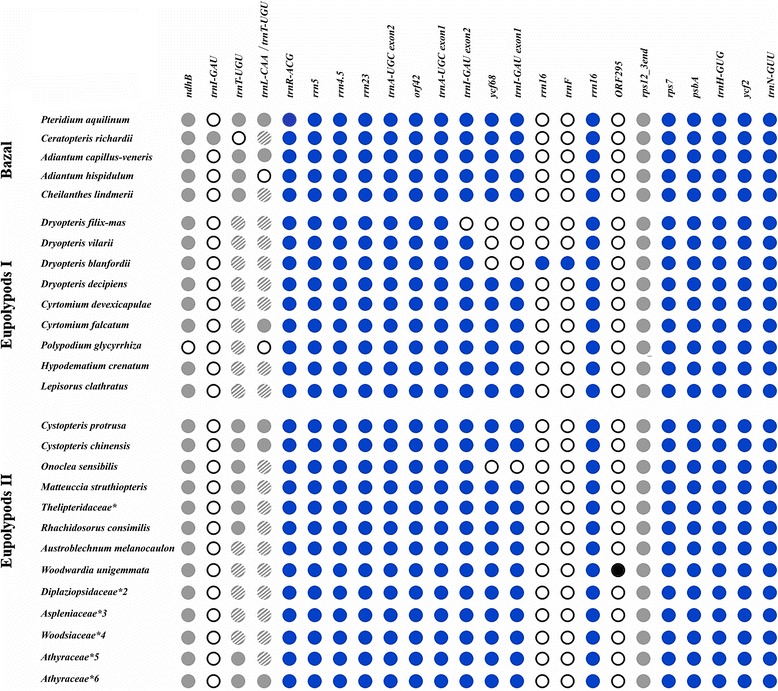
The IR gene content among Polypodiales ferns. The blue filled and open circles indicate the genes present and absent, respectively, in the corresponding species. The light-grey filled circles denote sequences that were marked as pseudogenes by DOGMA and shaded light-grey – pseudogenes that were not found by DOGMA, only by manual check using BLAST. * – Macrothelypteris torresiana, Thelypteris aurita, Stegnogramma sagittifolia, Cyclosorus procerus, Ampelopteris prolifera; *2 – Homalosorus pycnocarpos, Diplaziopsis javanica, Diplaziopsis cavaleria; *3 – Asplenium prolongatum, Asplenium pekinense, *4 – Woodsia macrochlaena, Woodsia polystichoides; *5 – Conopteris opaca (syn. Athyrium opacum) and all Deparia species of Athyraceae, *6 – Anisocampium sheareri (syn. Athyrium sheareri) and all other Athyrium species of Athyraceae
Comparative dataset of IR sequences which includes all plastomes for Polypodiales available in the public sequences databases and our new data comprises 45 sequences: 31 belong to Eupolypods I, 9 – to Eupolypods II and the remaining 5 - to Pteridaceae or Dennstaedtiaceae. We have made re-annotation of published Polypodiales plastomes. The IR/SSC border of all species lies within the ndhF gene and IR/LSC - within ndhB gene. The similar IR borders were previously defined for many groups of monilophytes (Psilotales, Ophioglossales, Equisetales, Marattiales and Polypodiopsida) [35]. Commonly, chloroplast genomes of Polypodiales carry sequences (~ 246 bp) with high similarity to ycf68 ORF in the IR regions, though they were not previously annotated by the authors. The ycf68 is a putatively functional gene located in the trnI-GAU intron, which is present in many land plant chloroplast genomes but often is not annotated as its function is still unknown [38, 39]. Another putative gene, ORF42, was annotated in the trnI-GAU intron for the all species included in the analysis. ORF42 was found previously in the intron region of trnA-UGC plastid gene of some flowering plant species, for example, Veratrum patulum O. Loes. (Melanthiaceae) [40] and Pelargonium × hortorum L. H. Bailey (Geraniaceae) [11]. The sequence with high similarity to ORF42 was found in the mitochondrial genome of Phaseolus; presumably as a result of plastid-to-mitochondrion lateral gene transfer [19].
The IR structures of Polypodiales are shown in Fig. 2 and Additional file 1. The number of genes normally varies from 14 to 16. The plastomes of Polypodiales mainly accumulated the gene number variability within IR in two areas: from 0 to ~3 Kbp and from ~7 to ~11,5 Kbp regions of IR. These regions also demonstrate lower sequence similarity. The other two regions - from ~3 to ~7 Kbp and from ~11,5 Kbp till the end of IR - were largely conservative in gene number and sequence.
The 0 to ~3 Kbp region contains tRNA (pseudo)gene (Fig. 2). Though it is annotated as functional two-exon trnT-UGU gene in many species (e.g. 33, 37), the tRNA structure prediction with tRNAscan-SE does not support its functionality. In other species it is annotated as pseudogene (trnT-UGU or trnL-CAA) or completely missing. Manual check however shows that pseudogene is present in all species analyzed (Fig. 2) except for Adianthum hispidulum where it was completely lost.
In the ~7 to ~11,5 Kbp variable region three genes show partial or full deletions or duplications (trnI-GAU, ycf68, rrn16) (Fig. 2) (Additional file 1). Some species of Polypodiales have partially or completely lost trnI-GAU and ycf68 (located in intron of trnI-GAU) namely: O. sensibilis (Onocleaceae) and three of four Dryopteris species (D. blanfordii, D. villarii, D. filix-mas, Dryopteridaceae). In D. filix-mas trnI-GAU and ycf68 are completely lost. D. blanfordii has partially lost the trnI-GAU gene (the intron and one of exons were deleted), but has a duplication of the large part of rrn16 gene. D. villarii has lost intron and one exon of trnI-GAU but no rrn16 duplication. As result of deletions/duplications the IR size of Dryopteris species varied: IR of D. filix-mas and D. villarii were ~1450–1570 bp shorter but D. blanfordii, on the contrary, had IRs 647 bp longer (Cyrtomium species were used as reference). Surprisingly D. decipiens, reported in [36], has no deletions in this region. O. sensibilis (Onocleaceae) plastome has a deletion of ycf68-trnI-GAU region. Onoclea and Dryopteris species belong to different clades: Eupolypod II and Eupolypod I. Therefore, the losses of ycf68-trnI-GAU regions are independent events.
Due to the deletions and duplications IR size in Polypodiales varies from 22 Kbp (Ceratopteris richardii [KM052729]) to 26,9 Kbp (Cystopteris protrusa, [KP136830]. In particular, we found the most of the large insertions (370 bp and more) in the highly variable intergenic spacers mentioned above: intergene 14 (between rrn16 and rps12), intergene 16 (between rps7 and psbA) and intergene 19 (between ycf2 and trnN-GUU), see Fig. 3. For the insertion’s length, localization and similarity to known high plant sequences see Table 1.
Fig. 3.
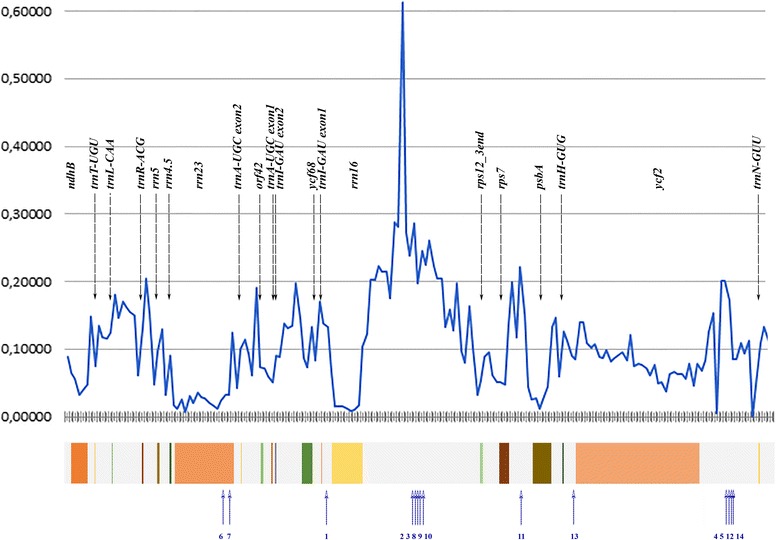
Nucleotide diversity and position of insertions among IRs of the Polypodiales. Horizontal axis indicates the coordinates within the aligned IRs of all Polypodiales. Vertical axis indicate Sliding window analysis (window = 200 b.p.) of p-distances, i.e. the proportion of nucleotide differences per site between sequences calculated by perl script made by Masafumi Nozawa. Colored boxes below marked the coding regions of genes (in the IR of averaged Polypodiales). Blue numbered arrows indicate position of insertions in IR sequences of different fern species (see also Table 2)
Table 1.
Relative evolution rate (ERaBLE output was normalized by rate of evolution in concatenated RNA- and protein-coding sequences) in different regions of IR
| Region | All Polypodiales | Eupolypods 1 | Eupolypods 2 | Dennstaedtiaceae + Pteridium |
|---|---|---|---|---|
| Intergene1 | 1,27 | 1,43 | 1,22 | 1,65 |
| ndhB | 2,46 | 3,50 | 2,78 | 2,83 |
| Intergene2 | 11,52 | 12,55 | 10,86 | 14,59 |
| trnT-UGU | 16,59 | 26,18 | 3,11 | 31,63 |
| Intergene3 | 2,68 | 3,32 | 2,20 | 3,45 |
| trnL-CAA | 2,20 | 3,24 | 2,44 | 2,53 |
| Intergene3p | 5,43 | 8,53 | 6,47 | 5,13 |
| trnR-ACG | 85,25 | 0,00 | 138,59 | 0,00 |
| Intergene4 | 10,11 | 13,42 | 12,65 | 10,99 |
| rrn5 | 0,15 | 0,22 | 0,27 | 0,11 |
| Intergene5 | 7,25 | 0,46 | 8,02 | 7,12 |
| Intergene6 | 2,35 | 2,49 | 3,46 | 0,93 |
| rrn23 | 0,78 | 9,81 | 1,77 | 0,30 |
| Intergene7 | 2,97 | 2,51 | 4,30 | 2,26 |
| trnA-UGC | 0,00 | 0,00 | 0,00 | 0,00 |
| Intergene8 | 22,93 | 10,48 | 59,98 | 11,34 |
| orf42 | 530,31 | 1221,25 | 3,38 | 3,77 |
| Intergene9 | 0,74 | 1,05 | 0,86 | 0,73 |
| trnA-UGC2 | 0,02 | 0,04 | 0,00 | 0,00 |
| Intergene10 | 2,67 | 16,92 | 1,71 | 1,70 |
| trnI-GAU | 0,00 | 0,00 | 0,00 | 0,00 |
| Intergene11 | 2,07 | 3,40 | 2,61 | 2,22 |
| ycf68 | 2,87 | 2,55 | 8,39 | 1,07 |
| Intergene12 | 68,77 | 41,48 | 245,28 | 15,63 |
| trnI-GAU2 | 2,31 | 5,57 | 0,00 | 0,00 |
| Intergene13 | 3,01 | 4,05 | 3,76 | 3,10 |
| rrn16 | 0,42 | 0,18 | 0,97 | 0,20 |
| Intergene14 | 9,37 | 27,47 | 11,23 | 8,26 |
| rps12 | 1,88 | 2,13 | 2,77 | 1,44 |
| Intergene15 | 1,43 | 1,48 | 1,24 | 1,59 |
| rps7 | 2,20 | 2,32 | 2,65 | 1,83 |
| Intergene16 | 7,31 | 7,69 | 7,79 | 7,57 |
| psbA | 1,42 | 1,89 | 1,78 | 1,30 |
| Intergene17 | 2,57 | 4,21 | 2,63 | 2,91 |
| trnH-GUG | 0,35 | 0,86 | 0,00 | 0,01 |
| Intergene18 | 3,14 | 1,92 | 3,36 | 4,20 |
| Intergene19 | 5,47 | 5,07 | 4,97 | 6,56 |
| trnN-GUU | 0,00 | 0,00 | 0,00 | 0,00 |
| Intergene20 | 3,02 | 5,42 | 3,27 | 4,03 |
The bolded rows marked intergenic spacers there LRT for the branch site model A was significantly better than the model B. (Model A was used for the ribosomal RNA and non-coding regions and supposes that substitutions in a DNA sequences of different tree branches are homogenous, model B – the same but supposes non-homogenous substitutions, see also materials and methods)
An unusual 1663 bp insertion was found rrn16 and rps12 genes of Woodwardia unigemmata (coordinates 93,404...95067). W. unigemmata is a fern of family Blechnaceae (Eupolypods II), whose plastome was sequenced by Lu et al., 2015 [41], and no genes were annotated in this region previously. The large part of W. unigemmata insertion has high sequence similarity to the insertion of Lepisorus clathratus (Polypodiaceae, Eupolipods I) located in the same region of IR, between rrn16 and rps12 genes. About 1160 bp of W. unigemmata and L. clathratus insertions demonstrated 65–78% similarity to each other. No similar sequences were found in IR of other Polypodiales, except for small (about 150 bp) sequence in Matteuccia struthiopteris (Onocleaceae, Eupolypods II) which has 62–66% similarity to L. clathratus and W. unigemmata insertions (further called WL-sequences).
Surprisingly the WL-like sequences were found in LSC regions of species from distant taxonomic groups of ferns, outside Polypodiales (Table 2). Firstly, small part of WL sequence has similarity to the fragment of LSC plastome of Plagiogyria species (Plagiogyria is a single genus in monotypic family Plagiogyriaceae, Cyatheales, see for example «Flora of China» [42]). To be more precise, the 772 bp part of WL sequence has 67% identity to the region of the P. glauca plastome (coordinates 29,128...29895, KP136831) and to P. japonica plastome (partial sequence, coordinates 4503–5273, HQ658099). In both species sequences with homology to WL lie within trnD-GUC-psbM intergenic spacer. Other ferns that also contain WL-like sequences in LSC are Ophioglossum californicum (KC117178) [34] and Mankyua chejuensis (KP205433). Both these species belong to Ophioglossales (basal ferns); they are distant from Polypodiales (core leptosporangiate ferns) and from Cyatheales. The fragments with similarity to WL sequences in Ophioglossales ferns have about 255–275 bp length and are located in the different regions of LSC. In O. californicum (KC117178) it is found in intergenic spacer between the trnT-GGU and trnfM-CAU genes, in M. chejuensis (KP205433, JF343520) it is also located in LSC region but in the other intergenic spacer (between trnL-UAA and rps4). It is annotated as ORF295 (Fig. 4). Altogether, this suggests that there is a translocation of DNA fragments from LSC to IR (or vice versa) during evolution of fern plastomes.
Table 2.
Coordinates of insertions with size 45 bp more inside Polypodiales IRs
| № | Clades | Species | Insertion IR coordinates | Insertion size | Location | Genbank accession number | Description | |
|---|---|---|---|---|---|---|---|---|
| start | end | |||||||
| 1 | 1Eu* | Dryopteris blanfordii | 7615 | 10,000 | 2385 | Large insertion between trnI-GAU and rrn16 | This work | Contains duplicated rrn16 and trnF-GAA genes |
| 2 | 1Eu | Polypodium glycyrrhiza | 9546 | 11,764 | 2218 | intergene14 ( rrn16-rps12) | KP136832 | ~ 700 bp part of insertion has homology to insertion of L. clathratus |
| 3 | 1Eu | Lepisorus clathratus | 10,863 | 14,073 | 3210 | intergene14 (rrn16-rps12) | KY419704 | Part if insertion has homology to part of WL and another to insertion of P. glycyrrhiza |
| 4 | 1Eu | Cyrtomium devexiscapulae | 22,596 | 22,968 | 372 | intergene19 (ycf2-trnN-GUU) | KT599100 | No significant homology to known sequences was found |
| 5 | 1Eu | Cyrtomium falcatum | 22,570 | 22,942 | 372 | intergene19 (ycf2-trnN-GUU) | KP189363 | No significant homology to known sequences was found |
| 6 | 2Eu | Cystopteris protrusa | 6100 | 6286 | 186 | rrn23 | KP136830 | Duplication inside IR (homologous to 6403–6560) |
| 7 | 2Eu | Cystopteris protrusa | 6897 | 9498 | 2631 | rrn23 | KP136830 | ~ 950 bp fragment of insertion has 75–76% homology to mitochondrion of different mosses |
| 8 | 2Eu | Asplenium pekinense | 10,447 | 12,688 | 2316 | intergene14 (rrn16-rps12) | KY427331 | ~ 200 bp of insertion has homology to rrn16-rps12 spacer of L. clathratus |
| 9 | 2Eu | Rhachidosorus consimilis | 10,780 | 11,683 | 903 | intergene14 (rrn16-rps12) | KY427356 | No significant homology to known sequences was found |
| 10 | 2Eu | Woodwardia unigemmata | 11,015 | 12,678 | 1663 | intergene14 (rrn16-rps12) | KT599101 | WL sequence |
| 11 | 2Eu | Matteuccia struthiopteris | 13,885 | 14,255 | 370 | intergene16 (rps7-psbA) | KY427353 | Part if insertion has homology to part of WL sequence |
| 12 | 2Eu | Asplenium prolongatum | 22,083 | 23,414 | 1331 | intergene19 (ycf2-trnN-GUU) | KY427332 | The most of insertion has homology to part of rrn16-rps12 spacer of Polypodium glycyrrhiza |
| 13 | Pt | Ceratopteris richardii | 14,185 | 14,499 | 314 | intergene18 (trnH-ycf2) | KM052729 | No significant homology to known sequences was found |
| 14 | Pt | Cheilanthes lindheimeri | 22,714 | 25,096 | 2382 | intergene19 (ycf2-trnN-GUU) | HM778032 | No significant homology to known sequences was found |
* 1Eu Eupolypods I, 2Eu Eupolypods II, Pt Pteridaceae
Fig. 4.
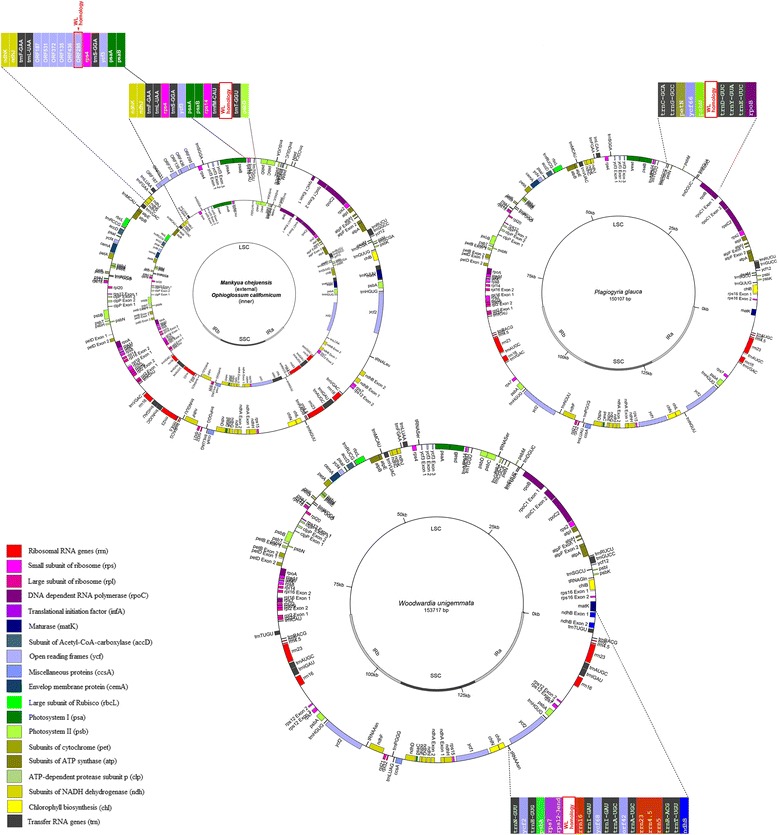
Location of WL-homologous fragments in plastomes of Ophioglossales, Cyatheales and Polypodiales ferns
Interestingly, the part of the WL sequence has high identity (67%) to the 203-bp fragment of about 5,9 Kb intergenic spacer between tRNA-CGA and tRNA-TTT genes of mitochondrial genome of Asplenium nidus (partial sequence, coordinates 3188…2986, AM600641) (Table 2). Asplenium is a genus that belongs to the same clade of Polypodiales - eupolypods II as Woodwardia but to the other family - Aspleniaceae. We performed similarity search of WL-sequence against fern mitochondrial contigs available in Utah State University Repository [45; http://digitalcommons.usu.edu/fern_genome/]. Two hits were found in Plagiogyria formosana - 244 bp (identity 72%, contig №439, coordinates 5336–5585) and 277 bp (identity 72%, contig №93, coordinates 1–277). No hits were detected in the contigs of another available fern species (D. conjugata, Pteridium aquilinum, C. richardii, Polypodium glycyrrhiza, C. protrusa).
We have estimated relative evolution rate of genes and intergenic spacers of Polypodiales IR. For protein-coding genes we analyzed dN/dS using branch-site model, for non-coding regions two models: homogenous substitution parameters and non-homogenous were used (see materials and methods for details). We found that for four non-coding (intergenic) regions the model, which has different substitution parameters for different branches, had significantly higher likelihood (LRT) compared to model based on assumption of identical substitution parameters in different lineages (see Table 3). All these regions are spacers: between trnA-UGC and orf42 (intergene8), between rrn16 and rps12 (intergene14), between rps7 and psbA (intergene16), and the last one between ycf2 and trnN-GUU (intergene19).
Table 3.
Coordinates, size and identity of WL-homologous regions in different species
| Species | GenBank accession number | Region | Coordinates | Annotation | % Identity to WI-homologous fragment of W. unigemmata | b.p. |
|---|---|---|---|---|---|---|
| Plagiogyria glauca plastid, partial | KP136831 | LSC | 29,128...29895 | intergenic spacer | 67% | 768 |
| Plagiogyria japonica plastid, partial | HQ658099 | LSC | 4503…5273 | intergenic spacer | 67% | 770 |
| Mankyua chejuensis plastid, complete genome | KP205433 | LSC | 62,408...62158 | ORF295 | 66% | 255 |
| Ophioglossum californicum plastid, complete genome | KC117178 | LSC | 49,930...49660 | intergenic spacer | 65% | 270 |
| Asplenium nidus mitochondrion, partial | AM600641 | Mitochondrion | 3188…2986 | intergenic spacer | 67% | 202 |
Discussion
In contrast to previous observations on the stability of the IR region, we found high variability in IR sequence and gene content in Polypodiales ferns. There are two hypervariable regions – one located at the beginning of IR, 0–3 Kb, and the second is 7–11 Kb region. These regions are the subject to the similar evolutionary changes occurred independently in the different clades. The first region in most species contains tRNA pseudogene. The members of both Eupolypods I and Eupolypods II demonstrated independent deletion of trnI-GAU-ycf68 region (i.e. Dryopteris and Onoclea). Polypodiales (together with Salviniales and Cyatheales) belong to a clade called core leptosporangiates [43]. Their plastomes acutely differ from those of eusporangiate ferns (Psilotales or whisk ferns, Ophioglossales, Marattiales, Equisetales) [8, 33]. It should be noted however that comparative analysis of fern plastomes is obfuscated by the uncertainty of the annotation of tRNA genes. This concerns, in particular, the intron-containing trnT-UGU, which was reported in IR (between ndhB and trnR-ACG) of several fern plastomes [37, 43, 44] and thought to be specific feature of core leptosporangiates. But intron-containing trnT-UGU was not found in other fern lineages [45, 46] or in any other plants outside ferns; only intronless trnT-UGU is present. This is unusual given that plastid tRNA genes, in contrast to protein-coding genes, have highly conserved exon-intron structure. Gao and coworkers supposed that tRNA genes may be lost repeatedly independently during evolution of ferns and probably the loss of trnT-UGU is the one of those events [45]. Our analysis which included manual reannotation and check using tRNA prediction program tRNAscan-SE however does not support the functionality of intron-containing IR-located trnT-UGU in any Polypodiales species where it was reported. In contrast, we found the trnT-UGU pseudogene in the IR of almost all Polypodiales. Most likely, this pseudogene is difficult to be recognized and therefore results of automatic annotation could be interpreted as gene loss. Notably, Gao and co-workers [45] compared the sequences of putative intron-containing and intronless trnT-UGU and it can be seen that the former are unusually divergent, much higher than expected for a functional tRNA gene. We conclude that intron-containing IR-located trnT-UGU is an artifact caused by the shortcomings of the automatic annotation. Moreover, two parts (“exons”) of this pseudogenes can be recognized by automation annotation programs, such as DOGMA, as different tRNAs - trnT-UGU and trnL-CAA.
In the second hypervariable region, 7–11 Kb, we found an unusual insertion (the WL-sequence) in two unrelated Polypodiales – Woodwartia unigemmata and Lepisorus clathrathus. Smaller insertion with high similarity to the WL-sequence was found in the same region in Mattheucia sthruttiopteris. In addition, the insertions with high similarity to WL-sequence were found in plastomes of Ophioglossales (basal ferns, distant from Polypodiales) but in different position – in LSC region.
The WL-sequence has high similarity with the region of mitochondrial genome of Asplenium nidus. This has two possible explanations: that it is either the sequence of mitochondrial origin, which was integrated in the plastome, or the sequence of plastid origin, which was integrated into mitochondrial genome and lost from the plastid genomes of most ferns, with exception of W. unigemmata and L. clathrathus. By now we can’t make a conclusion about which of these two hypotheses is true, due to the unavailability of fern complete mitochondrial genome sequences.
In any case, it is a result of the horizontal genome fragment transfer between mitochondria and plastids. Horizontal genome fragment transfer is a phenomenon, commonly observed in the pro- and eukaryotes. In plants, the presence of three genomes within a cell compartments (mitochondria, chloroplast and nucleus) leads to different possible types of intracellular genome fragments exchange: between organelles and nucleus and between mitochondria and chloroplasts, bidirectional [47]. The transfer of genetic material from organelles to the nucleus seems to be a continuing evolutionary process of the prokaryotic ancestors’ genome reduction [48, 49]. Many reports asserted that plant mitochondrial genomes are unusually prone to the introgression of alien sequences compared to chloroplast and nuclear genomes [47, 50]. There are only few data on mitochondria of ferns. No complete mitochondrial genome assemblies are available, only contigs. Multiple regions with strong sequence similarity to plastid DNA were detected by [51] but they didn’t relate to the plastome sequences in the total genomic contigs of six ferns species Dipteris conjugata (Gleicheniales), Plagiogyria formosana (Cyatheales), Pteridium aquilinum (Dennstaedtiaceae), Ceratopteris richardii (Pteridaceae), Polypodium glycyrrhiza (eupolypods) and Cystopteris protrusa (eupolypods). Authors speculated that the plastome-like sequences reside within the nuclear or mitochondrial genomes [42]. Assuming this is the case, it implies that the horizontal transfer of organelle genome fragments are not rare events in the evolution of ferns.
Conclusions
In this study we investigated the structure and evolutionary stability of IRs of plastomes in Polypodiales ferns. The two regions of IRs were found to be highly variable: (i) the sequences between ndhB and trnR-ACG genes (~3 Kbp) and (ii) the fragment including the rrn16 gene and flanking vicinity regions (~4,5 Kbp). This blinking of trnI-CAU, trnT-UGU, ndhB and rps12, trnI-GAU, ycf68, rrn16 genes related to these regions was observed in different species. The plastomes of three Dryopteris species demonstrate dynamic process of trnI-GAU elimination/rrn16 duplication.
Two Polypodiales species - W. unigemmata and L. clathratus - have an unusual sequence in the IR region. It demonstrates similarity to LSC spacers trnL-rps4 of Ophioglossales and pbsM-trnD of Cyatheales and with the part of mitochondrial genome of Asplenium (Polypodiales). We suppose these features are a consequence of intraplastomic rearrangements as well as of the transfer between the chloroplast and mitochondrial genomes during the evolution of ferns.
Methods
Plant material
Mature fronds of both Dryopteris filix-mas (L.) Schott, Dryopteris blanfordii (C. Hope) C. Christensen and Dryopteris villarii (Bellardi) Woyn. ex Schinz & Thell were sampled from outdoor section of the Moscow State University Botanical Garden.
Dryopteris filix-mas (L.) Schott is a common fern species in the Russian forests, therefore the specimen’s collection locality was stated only approximately as “in the vicinity of Moscow”.
Dryopteris blanfordii (C. Hope) C. Christensen grows in Picea or Abies forests at 2900–3500 m AMSL in China (Gansu, Sichuan, Xizang, Yunnan), Afghanistan, India, Kashmir, Nepal, and Pakistan [52–54]. The parent plant was collected in 2003 in India. Spores of the specimen were germinated under artificial conditions of the greenhouse of Botanical Garden of the Moscow State University. Developed sporophytes were then transplanted to the outdoor section of the Botanical Garden.
Dryopteris villarii (Bellardi) Woyn. ex Schinz & Thell. - subalpine species, grows on outcrops of hills, limestone cliffs, including high-mountain in Central and SouthEurope [55]. The spores, courtesy of Zürich Botanical Garden seed department (collected in natural habitat of Swiss Confederation), was germinated and specimen was germinated and grown in small greenhouse of Moscow State University Botanical Garden during 2013–2017.
Adiantum hispidulum Sw. pantropical, paleotropical species, it is distributed from eastern Africa through southern India, Thailand and the Ceylon to Pacific islands, Polynesia, New Zealand and Australasia [56–59]. The adult frond of Adiantum hispidulum was collected from greenhouse of Botanical Garden of Moscow State University. The voucher specimen was kept in Herbarium of Biology Department of Moscow State University.
Chloroplast genome sequencing, de novo assembly and annotation
The chloroplast DNA (cpDNA) were sequenced using the Illumina MiSeq high-throughput sequencing platform. For a sample preparation, the adult live plants were taken from the collection of the Moscow State University Botanical Garden. cpDNA was extracted from 2,6 g. (fresh weight) of fronds using the cpDNA extraction protocol [60, 61] with small modifications: after cleaning with a distilled water, the fronds were homogenized in 35 ml isolation buffer at +4 °C (Tris-HCl (pH 8,0) 50 mM, EDTA 7 mM, 1% PVP-40, NaCl 1,25 M, ascorbic acid 0,25 M, sodium metabisulfite 10 mM, Borax 0,0124 M) and the homogenate was filtered using soft wipes. The homogenate was then successively centrifuged at 200 g for 15 min at 4 °C (cell wall debris was discarded), at 1000 g for 20 min at +4 °C (the precipitate was discarded) and finally at 2000 g for 20 min at +4 °C. In the latter case, the precipitate was resuspended in 3 ml of wash buffer (Tris-HCl (pH 8,0) 50 mM, EDTA 25 mM) and carefully loaded into a 15 ml tube containing sucrose gradient consisting of 7 ml of 52% sucrose in wash buffer and overlaid 4 ml of 52% sucrose in wash buffer. The tube with the sample and sucrose gradient was centrifuged at 3500 g for 60 min at 4 °C. The interface between 52% and 30% sucrose (about 1 ml) was collected, centrifuged at 12,000 g. The pellet was resuspended in 900 μl of wash buffer and 100 μl of 10% CTAB was added for lysis (1 h, 55 °C). Then the DNA purification step was carried out using the protocol described in [62].
The TruSeq protocol (NEBNext® DNA Library Prep Master Mix Set for Illumina, E6040, NEB reagents) was used for preparing the genomic libraries. We made PE sequence (2 × 300 bp.) with a double number of each library reads about 1.2–1.97 M. After the quality trimming with Trimmomatic [63], sequencing reads were filtered using 13 complete and 5 partial fern chloroplast genome sequences from RefSeq database and Bowtie2 [64]. Then the two contig sets were produced for both filtered and unfiltered reads sets using the Velvet Assembler [65] and MIRA4 [66]. Assembled contigs and scaffolds were selected for the next assembly if they showed similarity to the published fern chloroplast genomes. The final de novo assembly was finished through a few iterative steps. The draft sequence was manually corrected by the PE reads mapping.
We have obtained the reads of complete circulated chloroplast genomes comprising the large single-copy region (LSC), small single-copy region (SSC) and the two inverted repeat (IR) regions. Finally, mapping of the initial reads was performed to the assembly in order to check for the potential assembly artefacts. Protein-coding gene annotation in the assembled chloroplast genome was annotated by DOGMA [67]. Bowtie2, VarScan (v.2.3.7) and SAMtools/BCFtools software packages were used for mapping of the reads and variant calling [64, 68, 69].
Chloroplast genomes analysis
Genbank or ENA accession numbers of sequences included in this study are listed in Table 2.
Analysis of the complete chloroplast genomes was carried out on species sequenced in this study together with previously reported species. Nine plastomes were downloaded from the GenBank. A complete list of the analyzed species can be found in the Table 1. Firstly, sequences of all the chloroplast sequences were pair-wise aligned against each other by Kalign (www.ebi.ac.uk/Tools/msa/kalign). Phylogenetic analysis was carried out by a maximum likelihood (ML) using Mega 6.0 [70]. Comparative analysis of chloroplast genome sequences was performed by the mVista web-tool (http://genome.lbl.gov/vista/mvista/submit.shtml).
For evolution rate analysis for the each region of IRs (genes and intergenic regions separately), as well as for the concatenate of all coding sequences, the alignments was built using MUSCLE [71]. An ML tree was constructed using concatenate alignment. Substitution model with lowest BIC score was chosen using modelTest function from phangorn package [72]. The tree topology was optimized using follow parameters: the nucleotide substitution matrix, gamma, the proportion of invariant sites and gamma distribution parameter. For non-protein coding regions the tree branch lengths were calculated by two models (homogenous substitution parameters - nhomo = 1, non-homogenous - nhomo = 4) using baseml [73], then models were compared by LRT. For protein-coding regions, dN and dS were estimated for each gene using codeml from PAML package [73], dN/dS ratio in each lineage was estimated by branch and M0 model. For both baseml and codeml analysis phagorn concatenate tree with nearest neighbour interchange was used. Distance matrices were calculated using baseml/codeml trees in the ape package [74]. Then relative evolution rate for each region (coding and non-coding) was calculated using ERaBLE [75]. Sliding window analysis (window = 200 b.p.) of p-distances, i.e. the proportion of nucleotide differences per site between sequences was calculated by perl script made by Masafumi Nozawa [76].
Acknowledgements
We are very thankful Dr. Christopher R. Fraser-Jenkins for correction of our definition of the Dryopteris blanfordii specimen and Botanical Garden of Zürich for providing spores of D. villarii.
Funding
This work was supported by the Russian Foundation for Basic Research grant no. 14–04-01852 (except sequencing work). The sequencing works were supported by Russian Science Foundation grant no. 14–50-00029. Publication costs were funded by the corresponding author.
Availability of data and materials
The sequences of full chloroplast genomes have been deposited in the European Nucleotide Archive (ENA). Other data used in the analysis are included within the article and the additional files.
About this supplement
This article has been published as part of BMC Plant Biology Volume 17 Supplement 2, 2017: Selected articles from Belyaev Conference 2017: plant biology. The full contents of the supplement are available online at https://bmcplantbiol.biomedcentral.com/articles/supplements/volume-17-supplement-2.
Additional file
List of genes annotated for D. filix-mas and D. blanfordii chloroplast genome by DOGMA. (XLSX 46 kb)
Authors’ contributions
SK selected and provided the plant material for this study; AK isolated chloroplasts and extracted cpDNA, performed phylogenetic analysis; AS and ML prepared library and performed sequencing work; AS, ML and AK performed comparative analysis of genomic data; KK and MB performed the bioinformatics analysis, assembled chloroplast genome; MB and AS prepared genomes annotation; EK – conducted evolution rate analysis; AS and ML conceived the study and wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi: 10.1186/s12870-017-1195-z) contains supplementary material, which is available to authorized users.
References
- 1.Gray MW. Origin and evolution of organelle genomes. Curr Opin Genet Dev. 1993;3(6):884–890. doi: 10.1016/0959-437X(93)90009-E. [DOI] [PubMed] [Google Scholar]
- 2.Olejniczak SA, Lojewska E, Kowalczyk T, Sakowicz T. Chloroplasts: state of research and practical applications of plastome sequencing. Planta. 2016;244(3):517–527. doi: 10.1007/s00425-016-2551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu A, Guo W, Gupta S, Fan W, Mower JP. Evolutionary dynamics of the plastid inverted repeat: the effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016;209(4):1747–1756. doi: 10.1111/nph.13743. [DOI] [PubMed] [Google Scholar]
- 4.Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17(1):134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellot S, Renner SS. The Plastomes of two species in the Endoparasite genus Pilostyles (Apodanthaceae) each retain just five or six possibly functional genes. Genome Biol Evol. 2015;8(1):189–201. doi: 10.1093/gbe/evv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haberle RC, Fourcade HM, Boore JL, Jansen RK. Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. J Mol Evol. 2008;66(4):350–361. doi: 10.1007/s00239-008-9086-4. [DOI] [PubMed] [Google Scholar]
- 7.Guisinger MM, Kuehl JV, Boore JL, Jansen RK. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: rearrangements, repeats, and codon usage. Mol Biol Evol. 2011;28(1):583–600. doi: 10.1093/molbev/msq229. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PG, Roper JM, Duffy AM. The evolution of chloroplast genome structure in ferns. Genome. 2010;53(9):731–738. doi: 10.1139/G10-061. [DOI] [PubMed] [Google Scholar]
- 9.Perry AS, Wolfe KH. Nucleotide substitution rates in legume chloroplast DNA depend on the presence of the inverted repeat. J Mol Evol. 2002;55(5):501–508. doi: 10.1007/s00239-002-2333-y. [DOI] [PubMed] [Google Scholar]
- 10.Li FW, Kuo LY, Pryer KM, Rothfels CJ. Genes Translocated into the plastid inverted repeat show decelerated substitution rates and elevated GC content. Genome Biol Evol. 2016;8(8):2452–2458. doi: 10.1093/gbe/evw167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chumley TW, Palmer JD, Mower JP, Fourcade HM, Calie PJ, Boore JL, Jansen RK. The complete chloroplast genome sequence of pelargonium x hortorum: organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol Biol Evol. 2006;23(11):2175–2190. doi: 10.1093/molbev/msl089. [DOI] [PubMed] [Google Scholar]
- 12.Gantt JS, Baldauf SL, Calie PJ, Weeden NF, Palmer JD. Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron. EMBO J. 1991;10(10):3073–3078. doi: 10.1002/j.1460-2075.1991.tb07859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice DW, Palmer JD. An exceptional horizontal gene transfer in plastids: gene replacement by a distant bacterial paralog and evidence that haptophyte and cryptophyte plastids are sisters. BMC Biol. 2006;4:31. doi: 10.1186/1741-7007-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bock R. The give-and-take of DNA: horizontal gene transfer in plants. Trends Plant Sci. 2010;15(1):11–22. doi: 10.1016/j.tplants.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Smith DR. Extending the limited transfer window hypothesis to inter-organelle DNA migration. Genome Biol Evol. 2011;3:743–748. doi: 10.1093/gbe/evr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cusimano N, Wicke S. Massive intracellular gene transfer during plastid genome reduction in nongreen Orobanchaceae. New Phytol. 2016;210(2):680–693. doi: 10.1111/nph.13784. [DOI] [PubMed] [Google Scholar]
- 17.Bergthorsson U, Adams KL, Thomason B, Palmer JD. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature. 2003;424(6945):197–201. doi: 10.1038/nature01743. [DOI] [PubMed] [Google Scholar]
- 18.Bergthorsson U, Richardson AO, Young GJ, Goertzen LR, Palmer JD. Massive horizontal transfer of mitochondrial genes from diverse land plant donors to the basal angiosperm Amborella. Proc Natl Acad Sci U S A. 2004;101(51):17747–17752. doi: 10.1073/pnas.0408336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woloszynska M, Bocer T, Mackiewicz P, Janska H. A fragment of chloroplast DNA was transferred horizontally, probably from non-eudicots, to mitochondrial genome of Phaseolus. Plant Mol Biol. 2004;56(5):811–820. doi: 10.1007/s11103-004-5183-y. [DOI] [PubMed] [Google Scholar]
- 20.Xi Z, Wang Y, Bradley RK, Sugumaran M, Marx CJ, Rest JS, Davis CC. Massive mitochondrial gene transfer in a parasitic flowering plant clade. PLoS Genet. 2013;9(2):e1003265. doi: 10.1371/journal.pgen.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hepburn NJ, Schmidt DW, Mower JP. Loss of two introns from the Magnolia Tripetala mitochondrial cox2 gene implicates horizontal gene transfer and gene conversion as a novel mechanism of intron loss. Mol Biol Evol. 2012;29(10):3111–3120. doi: 10.1093/molbev/mss130. [DOI] [PubMed] [Google Scholar]
- 22.Rice DW, Alverson AJ, Richardson AO, Young GJ, Sanchez-Puerta MV, Munzinger J, Barry K, Boore JL, Zhang Y, dePamphilis CW, et al. Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science. 2013;342(6165):1468–1473. doi: 10.1126/science.1246275. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Grewe F, Zhu A, Ruhlman TA, Sabir J, Mower JP, Jansen RK. Dynamic evolution of geranium mitochondrial genomes through multiple horizontal and intracellular gene transfers. New Phytol. 2015;208(2):570–583. doi: 10.1111/nph.13467. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Wu YW, Shih AC, Wu CS, Wang YN, Chaw SM. Transfer of chloroplast genomic DNA to mitochondrial genome occurred at least 300 MYA. Mol Biol Evol. 2007;24(9):2040–2048. doi: 10.1093/molbev/msm133. [DOI] [PubMed] [Google Scholar]
- 25.Davis CC, Anderson WR, Wurdack KJ. Gene transfer from a parasitic flowering plant to a fern. Proc Biol Sci. 2005;272(1578):2237–2242. doi: 10.1098/rspb.2005.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goremykin VV, Salamini F, Velasco R, Viola R. Mitochondrial DNA of Vitis Vinifera and the issue of rampant horizontal gene transfer. Mol Biol Evol. 2009;26(1):99–110. doi: 10.1093/molbev/msn226. [DOI] [PubMed] [Google Scholar]
- 27.Iorizzo M, Grzebelus D, Senalik D, Szklarczyk M, Spooner D, Simon P. Against the traffic: the first evidence for mitochondrial DNA transfer into the plastid genome. Mobile Genet Elem. 2012;2(6):261–266. doi: 10.4161/mge.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straub SC, Cronn RC, Edwards C, Fishbein M, Liston A. Horizontal transfer of DNA from the mitochondrial to the plastid genome and its subsequent evolution in milkweeds (apocynaceae) Genome Biol Evol. 2013;5(10):1872–1885. doi: 10.1093/gbe/evt140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koulintchenko M, Konstantinov Y, Dietrich A. Plant mitochondria actively import DNA via the permeability transition pore complex. EMBO J. 2003;22(6):1245–1254. doi: 10.1093/emboj/cdg128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knoop V. The mitochondrial DNA of land plants: peculiarities in phylogenetic perspective. Curr Genet. 2004;46(3):123–139. doi: 10.1007/s00294-004-0522-8. [DOI] [PubMed] [Google Scholar]
- 31.Christenhusz MJM, Zhang XC, Schneider H. A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa. 2011;19:7–54. doi: 10.11646/phytotaxa.19.1.2. [DOI] [Google Scholar]
- 32.Rothfels CJ, Sundue MA, Kuo LY, Larsson A, Kato M, Schuettpelz E, Pryer KM. A revised family-level classification for eupolypod II ferns (Polypodiidae: Polypodiales) Taxon. 2012;61(3):515–533. [Google Scholar]
- 33.Raman G, Choi KS, Park S. Phylogenetic relationships of the Fern Cyrtomium Falcatum (Dryopteridaceae) from Dokdo Island based on chloroplast genome sequencing. Genes. 2016;7(12):115. doi:10.3390/genes7120115. http://www.mdpi.com/2073-4425/7/12/115. [DOI] [PMC free article] [PubMed]
- 34.Gao L, Zhou Y, Wang ZW, Su YJ, Wang T. Evolution of the rpoB-psbZ region in fern plastid genomes: notable structural rearrangements and highly variable intergenic spacers. BMC Plant Biol. 2011;11:64. doi: 10.1186/1471-2229-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grewe F, Guo W, Gubbels EA, Hansen AK, Mower JP. Complete plastid genomes from Ophioglossum Californicum, Psilotum Nudum, and Equisetum Hyemale reveal an ancestral land plant genome structure and resolve the position of Equisetales among monilophytes. BMC Evol Biol. 2013;13:8. doi: 10.1186/1471-2148-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei R, Yan Y-H, Harris AJ, Kang J-S, Shen H, Xiang Q-P. Plastid Phylogenomics resolve deep relationships among Eupolypod II ferns with rapid radiation and rate heterogeneity. Genome Biol Evol. 2017;9(6):1646–1657. doi: 10.1093/gbe/evx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolf PG, Rowe CA, Sinclair RB, Hasebe M. Complete nucleotide sequence of the chloroplast genome from a leptosporangiate fern, Adiantum capillus-veneris L. DNA Res. 2003;10:59–65. doi: 10.1093/dnares/10.2.59. [DOI] [PubMed] [Google Scholar]
- 38.Logacheva MD, Shipunov AB. Phylogenomic analysis of Picramnia, Alvaradoa, and Leitneria supports the independent Picramniales. J Syst Evol. 2017;55(3):171–176. doi: 10.1111/jse.12246. [DOI] [Google Scholar]
- 39.Roper JM, Hansen SK, Wolf PG, Karol KG, Mandoli DF, Everett KDE, Kuehl J, Boore JL. The complete plastid genome sequence of Angiopteris Evecta (G. Forst.) Hoffm. (Marattiaceae) Am Fern J. 2007;97(2):95–106. doi: 10.1640/0002-8444(2007)97[95:TCPGSO]2.0.CO;2. [DOI] [Google Scholar]
- 40.Do HD, Kim JS, Kim JH. Comparative genomics of four Liliales families inferred from the complete chloroplast genome sequence of Veratrum Patulum O. Loes. (Melanthiaceae) Gene. 2013;530(2):229–235. doi: 10.1016/j.gene.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 41.Lu JM, Zhang N, Du XY, Wen J, Li DZ. Chloroplast phylogenomics resolves key relationships in ferns. J Syst Evol. 2015;53(5):448–457. doi: 10.1111/jse.12180. [DOI] [Google Scholar]
- 42.Lycophytes & ferns. In: Zhengyi W, Raven PH, Deyuan H, eds. Flora of China vol. 2–3. Beijing and St. Louis: Science Press and Missouri Botanical Garden Press.
- 43.Wolf PG, Der JP, Duffy AM, Davidson JB, Grusz AL, Pryer KM. The evolution of chloroplast genes and genomes in ferns. Plant Mol Biol. 2011;76(3–5):251–261. doi: 10.1007/s11103-010-9706-4. [DOI] [PubMed] [Google Scholar]
- 44.Gao L, Yi X, Yang YX, Su YJ, Wang T. Complete chloroplast genome sequence of a tree fern Alsophila Spinulosa: insights into evolutionary changes in fern chloroplast genomes. BMC Evol Biol. 2009;9:130. doi: 10.1186/1471-2148-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao L, Wang B, Wang ZW, Zhou Y, Su YJ, Wang T. Plastome sequences of Lygodium Japonicum and Marsilea Crenata reveal the genome organization transformation from basal ferns to core leptosporangiates. Genome Biol Evol. 2013;5(7):1403–1407. doi: 10.1093/gbe/evt099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HT, Chung MG, Kim KJ. Chloroplast genome evolution in early diverged leptosporangiate ferns. Mol Cells. 2014;37(5):372–382. doi: 10.14348/molcells.2014.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao C, Ren X, Mason AS, Liu H, Xiao M, Li J, Fu D. Horizontal gene transfer in plants. Funct Integr Genomics. 2014;14(1):23–29. doi: 10.1007/s10142-013-0345-0. [DOI] [PubMed] [Google Scholar]
- 48.Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 2004;5(2):123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 49.Soucy SM, Huang J, Gogarten JP. Horizontal gene transfer: building the web of life. Nat Rev Genet. 2015;16(8):472–482. doi: 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]
- 50.Archibald JM, Richards TA. Gene transfer: anything goes in plant mitochondria. BMC Biol. 2010;8:147. doi: 10.1186/1741-7007-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolf PG, Sessa EB, Marchant DB, Li FW, Rothfels CJ, Sigel EM, Gitzendanner MA, Visger CJ, Banks JA, Soltis DE, Soltis PS, Pryer KM, Der JP. An exploration into Fern genome space. Genome Biol Evol. 2015;7(9):2533–2544. doi: 10.1093/gbe/evv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mir SA, Mishra AK, Reshi ZA, Sharma MP. Four newly recorded species of Dryopteridaceae from Kashmir valley, India. Biodiversitas. 2014;15(1):6–11. doi: 10.13057/biodiv/d150102. [DOI] [Google Scholar]
- 53.Mir SA, Mishra AK, Pala SA, Reshi ZA, Sharma MP. Ferns and fern allies of district Shopian, Kashmir Valley, India. Biodiversitas. 2015;16(1):27–43. doi: 10.13057/biodiv/d160105. [DOI] [Google Scholar]
- 54.Fraser-Jenkins CR. A monograph of Dryopteris (Pteridophyta: Dryopteridaceae) in the Indian subcontinent, Bulletin of the British museum (natural history). London: British Museum (Natural History); 1989. p. 386–9.
- 55.Olsen S: Dryopteris villarii. In: Encyclopedia of Garden Ferns. China: Timber Press Inc.; 2007.
- 56.Parris BS. Adiantum Hispidulum Swartz and A. Pubescens Schkuhr (Adiantaceae: Filicales) in New Zealand. N Z J Bot. 1980;18:503–506. doi: 10.1080/0028825X.1980.10425173. [DOI] [Google Scholar]
- 57.Hemp A. Ecology of the pteridophytes on the southern slopes of Mt. Kilimanjaro. Plant Ecol. 2002;159:211–239. doi: 10.1023/A:1015569125417. [DOI] [Google Scholar]
- 58.Lu JM, Wen J, Lutz S, Wang YP, Li DZ. Phylogenetic relationships of Chinese Adiantum based on five plastid markers. J Plant Res. 2012;125(2):237–249. doi: 10.1007/s10265-011-0441-y. [DOI] [PubMed] [Google Scholar]
- 59.Boonkerd T, Pollawatn R. Note on Adiantum Hispidulum (Pteridaceae), a new record species to Fern Flora of Thailand Songklanakarin. J Sci Technol. 2013;35(5):513–516. [Google Scholar]
- 60.Shi C, Hu N, Huang H, Gao J, Zhao YJ, Gao LZ. An improved chloroplast DNA extraction procedure for whole plastid genome sequencing. PLoS One. 2012;7(2):e31468. doi: 10.1371/journal.pone.0031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieira LD, Faoro H, Fraga HPD, Rogalski M, de Souza EM, Pedrosa FD, Nodari RO, Guerra MP. An improved protocol for intact chloroplasts and cpDNA isolation in conifers. PLoS One. 2014;9(1):e84792. doi: 10.1371/journal.pone.0084792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krinitsina AA, Sizova TV, Zaika MA, Speranskaya AS, Sukhorukov AP. A rapid and cost-effective method for DNA extraction from archival herbarium specimens. Biochemistry(Mosc) 2015;80(11):1478–1484. doi: 10.1134/S0006297915110097. [DOI] [PubMed] [Google Scholar]
- 63.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–U354. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chevreux B, Pfisterer T, Drescher B, Driesel AJ, Muller WEG, Wetter T, Suhai S. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res. 2004;14(6):1147–1159. doi: 10.1101/gr.1917404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- 68.Koboldt DC, Zhang QY, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22(3):568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Proc GPD. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schliep KP. Phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27(4):592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24(8):1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 74.Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20(2):289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 75.Binet M, Gascuel O, Scornavacca C, Douzery EJ, Pardi F. Fast and accurate branch lengths estimation for phylogenomic trees. BMC Bioinformatics. 2016;17:23. doi: 10.1186/s12859-015-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nozawa M, Miura S, Nei M. Origins and evolution of microRNA genes in drosophila species. Genome Biol Evol. 2010;2:180–189. doi: 10.1093/gbe/evq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences of full chloroplast genomes have been deposited in the European Nucleotide Archive (ENA). Other data used in the analysis are included within the article and the additional files.


