Abstract
Background
Structural component of plant biomass, lignocellulose, is the most abundant renewable resource in nature. Lignin is the most recalcitrant natural aromatic polymer and its degradation presents great challenge. Nowadays, the special attention is given to biological delignification, the process where white-rot fungi take the crucial place owing to strong ligninolytic enzyme system. However, fungal species, even strains, differ in potential to produce high active ligninolytic enzymes and consequently to delignify plant biomass. Therefore, the goals of the study were characterization of Mn-oxidizing peroxidases and laccases of numerous mushrooms as well as determination of their potential to delignify wheat straw, the plant raw material that, according to annual yield, takes the first place in Europe and the second one in the world.
Results
During wheat straw fermentation, Lentinus edodes HAI 858 produced the most active Mn-dependent and Mn-independent peroxidases (1443.2 U L−1 and 1045.5 U L−1, respectively), while Pleurotus eryngii HAI 711 was the best laccase producer (7804.3 U L−1). Visualized bends on zymogram confirmed these activities and demonstrated that laccases were the dominant ligninolytic enzymes in the studied species. Ganoderma lucidum BEOFB 435 showed considerable ability to degrade lignin (58.5%) and especially hemicellulose (74.8%), while the cellulose remained almost intact (0.7%). Remarkable selectivity in lignocellulose degradation was also noted in Pleurotus pulmonarius HAI 573 where degraded amounts of lignin, hemicellulose and cellulose were in ratio of 50.4%:15.3%:3.8%.
Conclusions
According to the presented results, it can be concluded that white-rot fungi, due to ligninolytic enzymes features and degradation potential, could be important participants in various biotechnological processes including biotransformation of lignocellulose residues/wastes in food, feed, paper and biofuels.
Keywords: Delignification, Laccases, Mn-oxidizing peroxidases, Mushrooms, Wheat straw
Background
Lignocellulose, a structural component of plant biomass, is considered to be the most abundant renewable organic resource in terrestrial environments. It consists of a cellulose-hemicellulose matrix immersed in net of lignin, the most recalcitrant natural aromatic polymer [1]. Although various methodologies for the biomass delignification are available, they are commonly unsatisfactory due to high process cost, significant energy consumption and release of non-enviromentally friendly by-products [2]. Since chemical, physical, and physico-chemical biomass pre-treatments are characterized with all mentioned shortages, recently there is a great deal of interest for biological delignification [3]. The advantages of biological methods are significant delignification selectivity, low energy consumption, absence of toxic by-products and economical justification [4]. White-rot fungi take the crucial place in the biological biomass pre-treatment as they possess strong ligninolytic enzyme system containing lignin- and Mn-oxidizing peroxidases, laccases and some auxiliary enzymes [5]. The high efficiency of the enzymes is based on their strong oxidative activity and low substrate specificity and depends on fungal species/strain capacity, oxidative mechanism, lignocellulose nature and cultivation conditions [6]. The remarkable ligninolytic potential makes the white-rot mushrooms the main actors in various biotechnological processes, such as production of food, feed, paper, biofuel, textile, as well as soil and water remediation [7].
According to abundance, wheat straw takes the first place in Europe, with annual yield of 170 million tons, and the second one in the world [8, 9]. Therefore, it presents a promising, one of the cheapest and the most useful raw material for biotransformation in various products [6]. Since, bioconversion capacity varies depending on fungal species and/or strain, the objectives of this research were profiling of Mn-oxidizing peroxidases and laccases of selected mushrooms as well as determination of their wheat straw delignification potential.
Methods
Organisms and cultivation conditions
The cultures of studied species/strains were isolated from fruiting bodies collected in Serbia, Israel, Russia, Ukraine and England (Table 1), and maintained on Malt agar medium in culture collection of University of Belgrade - Faculty of Biology (BEOFB).
Table 1.
The studied species/strains
| Studied species | Strain code | Origin of strains |
|---|---|---|
| Ganoderma lucidum | BEOFB 435 | Belgrade, Serbia |
| Hypsizygus tessulatus | BEOFB 910 | from Aesculus hippocastanum, Serbia |
| Lenzites betulinus | BEOFB 500 | from Populus tremula, Russia |
| Lentinus edodes | BEOFB 858 | Poznan, Poland |
| Pleurotus citrinopileatus | HAI 435 | Cultivated strain, England |
| Pleurotus eryngii | HAI 193 | from Stipa sp., Kherson region, Ukraine |
| HAI 711 | from Ferula sp., Tabor, Izrael | |
| Pleurotus ostreatus | HAI 592 | Russia |
| Pleutorus pulmonarius | HAI 573 | Sochi, Russia |
| Xylaria polymorpha | BEOFB 1110 | Botanical Garden, Belgrade, Serbia |
The inoculation of 100 mL of synthetic medium (glucose, 10.0 g L−1; NH4NO3, 2.0 g L−1; K2HPO4, 1.0 g L−1; NaH2PO4 × H2O, 0.4 g L−1; MgSO4 × 7H2O, 0.5 g L−1; yeast extract, 2.0 g L−1; pH 6.5) with mycelial agar discs of 7-day old culture, incubation at room temperature (22 ± 2 °C) on rotary shaker (100 rpm), washing of obtained biomass by sterilized distilled water (three times) and its homogenization in laboratory blender were the main steps of inoculum preparation [10]. Solid-state cultivation was performed during 21 days, at 25 °C, in 100 mL flasks containing substrate composed of 2.0 g of wheat straw, as the carbon source, and 10.0 mL of the modified synthetic medium (without glucose) inoculated with 3.0 mL of the homogenized inoculum.
Determination of enzyme activity and total protein content
According to the method of Stajić et al. [10], the produced extracellular enzymes were extracted by sample stirring with 50.0 mL dH2O on a magnetic stirrer at 4 °C for 10 min. The obtained extracts were centrifugated (4 °C, 3000 rpm, 15 min) and resulting supernatants were used for measurement of activities of Mn-oxidizing peroxidases [Mn-dependent peroxidase (MnP; EC 1.11.1.13) and Mn-independent peroxidase (MnIP; EC 1.11.1.16)] and laccases (EC 1.10.3.2), as well as the protein content, spectrophotometrically [CECIL CE2501 (BioQuest)]. Activities of Mn-oxidizing peroxidases and laccases were determined using phenol red (ε610 = 22,000 M−1 cm−1) and 2,2′-azino-bis-[3-ethylbenzothiazoline-6-sulfonate (ABTS) (ε436 = 29,300 M−1 cm−1), respectively, as the substrates. The amount of enzyme which transformed 1.0 μmol of substrate per minute was defined as the enzymatic activity of 1 U.
The content of total proteins (mg mL−1) was determined in the reaction mixture composed of Bradford reagent and sample, at λ = 595 nm, by standard curve obtained from known concentrations of bovine serum albumin [11].
All assays were carried out in quadruplicate and the results are expressed as the mean ± standard error.
Electrophoresis
Mini IEF Cell-Model 111 (BIO-RAD) was used for isoelectric focusing (IEF) and defining isoelectric points (pI) of enzyme isoforms. IEF was carried out in 7.5% polyacrylamide gel with 5.0% ampholyte on a pH gradient from 3.0 to 10.0 using IEF marker (pI range from 3.6 to 9.3; Sigma-Aldrich) [6]. MnP and MnIP isoenzymes were visualized by gel incubation in 4-Cl-1-naphtol/H2O2/potassium phosphate buffer solution with or without MnSO4, respectively, at room temperature till appearance of dark-brown bands. The laccase bands were located by dyeing into ABTS/phosphate buffer solution. After focusing, the gel was fixed in 12.0% trichloroacetic acid and protein bands were detected by staining with 0.1% Coomassie Brilliant Blue R (CBB R) in fixative (methanol, acetic acid, and H2O in ratio 45:10:45).
Determination of hemicellulose, cellulose and lignin contents
Determination of hemicellulose, cellulose and lignin contents in untreated and fungal-treated wheat straw was carried out using modified methods of Kirk and Obst [12] and Van Soest et al. [13]. The dried (at 50 °C) and finely ground samples were treated by neutral-detergent solution (NDS), boiled and refluxed in order to remove the soluble sugars, proteins, pectin, lipids and vitamins from them. Acid-detergent solution (ADS) and 72% H2SO4 were used for removal the hemicellulose and cellulose from the residues and determination of lignin content. The difference in weight of the samples treated with NDS and ADS represents the hemicellulose content. Lignin content (LC) was defined by Klason method by incubation of sample obtained after ADS treatment with 72% H2SO4 at 30 °C and hydrolysis at 120 °C, and expressed as percentage of that present in fungal-untreted sample. Cellulose amount presented difference in mass of the ADS-treated sample and LC. The assays were carried out in four replications and the results are expressed as a mean ± standard error.
Results
The selected fungal species have shown the ability to secrete Mn-oxidizing peroxidases and laccases during solid-state fermentation of wheat straw. The obtained results demonstrated a significant inter- and intraspecific diversity in enzymes production among studied fungal species/strains (Fig. 1).
Fig. 1.
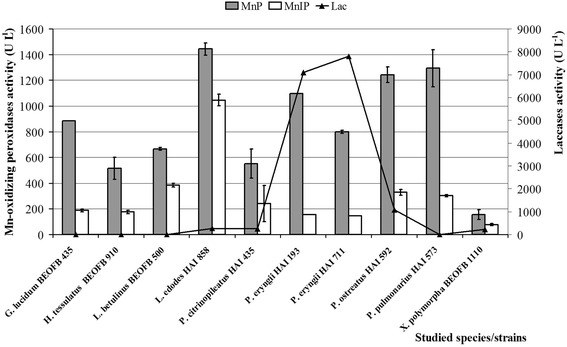
The activity of Mn-oxidizing peroxidases and laccases produced by selected fungal species after 21 days of solid-state fermentation of wheat straw
The peak of MnP activity (1443.2 U L−1) was observed in Lentinus edodes HAI 858 and slightly lower values were noted in Pleurotus pulmonarius HAI 573 (1294.2 U L−1), P. ostreatus HAI 592 (1243.7 U L−1) and P. eryngii HAI 193 (1096.0 U L−1). The level of MnIP activity was slightly lower and the maximum was also noted in L. edodes HAI 858 (1045.5 U L−1). Generally, levels of laccase activity were remarkable higher than levels of Mn-oxidizing peroxidases activity, and the maximum value was obtained for P. eryngii HAI 711 (7804.3 U L−1) (Fig. 1).
The total protein content affected profiles of specific enzyme activities. The maximum MnP and MnIP specific activities were detected during wheat straw fermentation by Trametes hirsuta HAI 300 (110.4 U mg−1) and L. edodes HAI 858 (48.7 U mg−1), respectively, while the maximum specific laccase activity was noted in P. ostreatus HAI 592 (47.4 U mg−1).
The IEF profiles of MnP, MnIP and laccase isoforms were variable depending on species/strain (Fig. 2). In the case of MnP, one very strong (pI about 4.6) and a few weak isoforms were visualized in L. edodes HAI 858 and P. citrinopileatus HAI 435, while in P. ostreatus HAI 592 two bends of pI 5.9 were observed. The intraspecific diversity was demonstrated in P. eryngii, i.e. in the starin HAI 193 only one weak bend of pI about 5.9 was noted while in the strain HAI 711 three isoforms of pI between 4.6 and 5.9 were separated (Fig. 2a). One MnIP bend of pI in the range between 3.6 and 5.9 was visualized in all tested species/strains, except P. eryngii HAI 711 where a few isoforms of pI about 5.9 appeared (Fig. 2b). Laccase zymogram has confirmed that the enzyme was the dominant ligninolytic enzyme in studied species, i.e. the most numerous strong isoforms were separated (Fig. 2c). Three laccase bends, one of pI 3.6 and two of pI about 4.6, were visualized in P. citrinopileatus HAI 435, two bends (pI of 3.6 and 4.6) were observed on Ganoderma lucidum BEOFB 435 and P. eryngii HAI 193 and HAI 711 zymograms, while other studied species and strains produced this enzyme in only one isoform.
Fig. 2.
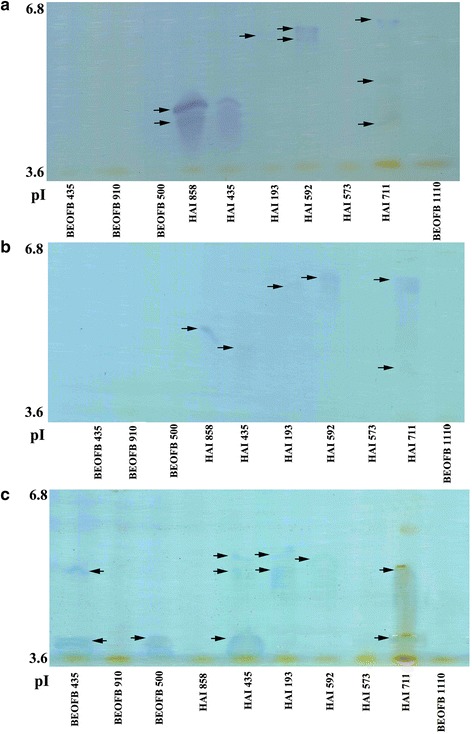
Isoelectric focusing pattern of Mn-dependent peroxidases (a), Mn-independent peroxidases (b) and laccases (c) obtained after 21 days of solid-state fermentation of wheat straw by selected fungal species
Lignocellulose degradation
The selected fungal species showed variable capacity of wheat straw delignification, as well as different levels of selectivity in polymers’ degradation (Table 2). G. lucidum BEOFB 435, P. citrinopileatus HAI 435 and L. edodes HAI 858 were the best delignifiers (58.5%, 56.0%, and 55.7%, respectively), while Lenzites betulinus BEOFB 500 was the weakest one which broke down only 33.5% of wheat straw lignin. G. lucidum BEOFB 435 was also the most effective hemicellulose degrader (74.8%) and the most selective degrader because it decayed only 0.7% of cellulose. Significant degradation selectivity was also obtained in wheat straw fermentation with P. eryngii HAI 711, P. ostreatus HAI 592, P. pulmonarius HAI 573 and L. betulinus BEOFB 500, while the lowest selectivity was noted for Xylaria polymorpha BEOFB 1110 which degraded lignin, hemicellulose and cellulose in the ratio of 42.9%:54.9%:44.0% (Table 2).
Table 2.
The level of lignocellulose degradation by studied species/strains
| Studied species | Strain code | Level of polymers degradation [%] | ||
|---|---|---|---|---|
| Lignin | Hemicellulose | Celullose | ||
| Ganoderma lucidum | BEOFB 435 | 58.5 | 74.8 | 0.7 |
| Hypsizygus. tessulatus | BEOFB 910 | 49.4 | 30.6 | 20.0 |
| Lentinus edodes | HAI 858 | 55.7 | 12.4 | 16.6 |
| Lenzites betulinus | BEOFB 500 | 33.5 | 56.4 | 2.9 |
| Pleurotus eryngii | HAI 193 | 36.7 | 2.1 | 1.4 |
| HAI 711 | 34.2 | 51.6 | 0.5 | |
| Pleurotus pulmonarius | HAI 573 | 50.4 | 15.3 | 3.8 |
| Pleurotus ostreatus | HAI 592 | 40.3 | 35.8 | 0.8 |
| Pleurotus citrinopileatus | HAI 435 | 56.0 | 30.7 | 29.2 |
| Xylaria polymorpha | BEOFB 1110 | 42.9 | 54.9 | 44.0 |
Discussion
Profiling ligninolytic potential of numerous insufficient studied fungal species, focused on the correlation between enzymes’ activity and polymers’ degradation extent, is the main contribution of this study. Previous results showed that important factors such as type of cultivation, composition of the plant material (especially the content of lignin in relation to hemicellulose and celullose), as well as cultivation conditions determine expression of ligninolytic enzymes [14–18]. However, the genetic basis of fungal species/strains has the essential role in ligninolytic enzyme synthesis. The existence of significant inter- and intraspecific variabilities in activities of studied ligninolytic enzymes as well as in the rate of lignin mineralization were in accordance with previous results. Thus, Camarero et al. [19] Stajić et al. [20] and Ćilerdžić et al. [21] showed interspecific diversity in MnP and laccase activity between Coriolus hirsutus and C. pubescens, G. lucidum and G. carnosum, and among numerous Pleurotus species, as well as in the delignification extent during solid-state fermentation of wheat straw with four Pleurotus species (P. pulmonarius, P. floridanus, P. ostreatus and P. sajor-caju). Silva et al. [11], Camarero et al. [19], Stajić et al. [20], Manavalan et al. [22] and Ćilerdžić et al. [23] also reported remarkable level of intraspecific diversity in laccase and MnP activities into Cerrena maxima, G. lucidum, and numerous Pleurotus species. Thus, Silva et al. [11] noted considerable diversity in activity of laccase produced during submerged wheat bran fermentation with four G. lucidum strains, i.e. activities were ranged from 0.58 U L−1 to 49,519 U L−1. After 14 days of wheat straw submerged fermentation with four G. lucidum strains studied by Ćilerdžić et al. [23] laccase activity was in the range from 153.5 U L−1 in strain BEOFB 432 to 5921.5 U L−1 in strain BEOFB 434. Likewise, activity of MnP produced during 21-old day solid-state fermentation of sugarcane bagasse by G. lucidum strain studied by Manavalan et al. [22] was significantly lower in comparison with value reported for G. lucidum BEOFB 435 (70 U g−1 substrate vs. ~900 U 2 g−1 of substrate). Intraspecific variability in ligninolytic enzymes activities during submerged fermentation of mandarin peels with G. applanatum and Trametes versicolor, as well as solid-state fermentation of grapevine sawdust with numerous strains of Pleurotus species and wheat straw with L. edodes and T. versicolor was also demonstrated by Stajić et al. [20] and Songulashvili et al. [24]. Numerous results also demonstrated significant differences in MnP and laccase activity among P. ostreatus strains originated from various areas and cultivated on various plant raw materials under submerged or solid-state conditions [1, 25, 26]. Thus, in submerged fermentation of sugarcane bagasse, Dong et al. [25] noted MnP activity of even 150,000 U L−1 and absence of laccase production, while in cultivation on wheat bran under the same conditions strain studied by Sergentani et al. [1] synthetized highly active laccase (4730 U L−1) but not MnP. On the other hand, solid-state cultivation induced well secretion of the enzymes independently on substrate. Namely, during potato peels fermentation Ergun and Urek [26] obtained remarkable activities of both MnP and laccase (about 1000 U L−1 and 2000 U L−1, respectively) which were similar to values obtained after wheat straw fermentation by strain HAI 592 (1243.7 and 1085.9 U L−1, respectively). However, in the case of Xylaria polymorpha diversity in laccase activity between strain studied by Liers et al. [27] and strain BEOFB 1110 was remarkable, i.e. value obtained after submerged tomato juice fermentation was almost 5-fold higher than after solid-state wheat straw fermentation.
Species/strain genetical basis as well as cultivation conditions also affected isoform profile of the ligninolytic enzymes. Contrary to only two laccase isoforms produced during cultivation of G. lucidum in malt medium [28] and three isoenzymes in Korean strain grown in liquid glucose/peptone/yeast extract medium [29], Manavalan et al. [22] visualized even five isoforms in strain cultivated on solid sugarcane bagasse substrate. However, Stajić et al. [10] and Ćilerdžić et al. [23] demonstrated higher effect of genetic on isoenzyme profile. Namely, two G. lucidum strains of different origin, HAI 447 and BEOFB 431, synthetized four and three isoforms, respectively, in cultivation under the same conditions, in solid-state wheat straw/NH4NO3 medium. In the case of MnP, strain studied by Manavalan et al. [22] produced one isoform of pI 4.19 on sugarcane bagasse, strain BEOFB 431 synthetized two isoforms of pI 3.6 and 3.7 after submerged fermentation of wheat straw [6], while any isoform no visualized after 21-day old solid-state fermentation of that raw material with strain BEOFB 435. Inter- and intraspecific diversity in isoenzyme profile was also demonstrated within genus Pleurotus. Namely, Muñoz et al. [30, 31] reported two laccase bands in P. eryngii cultivated in glucose/ammonium-tartrate medium, two isoforms were separated after submerged fermentation of mandarine peels with strain HAI 616 [32], while strains studied in that study (HAI 193 and HAI 711) produced three and two isoenyzmes, respectively, during growth on solid wheat straw based medium. In the case of P. ostreatus, contrary to four laccase isoforms (POXA1b, POXA1w, POXA2, and POXC) separated by Guardina et al. [33] and Palmieri et al. [34], three ones were sinthetized by strains HAI 493 and HAI 494 after cultivation on grapevine sawdust [32], while any isoform was visualized after solid-state wheat straw fermentation with strain HAI 592.
The numerous reports also showed considerable diversity among species and strains in delignification capacity and polymer degradation selectivity [6, 25, 35]. Species studied in this study were better delignifiers than numerous other previously tested. Thus, G. lucidum BEOFB 435 degraded in approximatelly 38% more lignin than strain BEOGB 432 and about 35% more than G. applanatum, while the difference was higher within P. eryngii, i.e. strains HAI 193 and HAI 711 were more than twice better delignifiers than strain HAI 507 [6, 35]. In the case of P. ostreatus, strain HAI 592 was more selective degrader of wheat straw under solid-state cultivation than strain studied by Dong et al. [25] which submergedly fermented sugarcane bagasse (40.3% of lignin, 35.8% of hemicellulose and 0.8% of cellulose vs. 50%:35%:5%).
Conclusion
During wheat straw fermentation, Lentinus edodes HAI 858 produced the most active Mn-oxidizing peroxidases and Pleurotus eryngii HAI 711 the most active laccase, which was confirmed by obtained zymograms. Visualized enzymes’ isoforms also demonstrated that laccases were the dominant ligninolytic enzymes in studied species. Ganoderma lucidum BEOFB 435 and Pleurotus pulmonarius HAI 573 were the most selective lignocellulose degraders. The studied white-rot fungal species have high capacity to degrade selectively lignocellulose and owing to that ability could be important participants in the processes of bioconversion of plant biomas in food, feed, paper and biofuels.
Acknowledgments
Not applicable.
Funding
This study was carried out under Project No. 173032, which is financially supported by the Ministry of Education, Science and Technological Development of Republic of Serbia. Publication costs were funded by the same Ministry.
Availability of data and materials
Not applicable
About this supplement
This article has been published as part of BMC Plant Biology Volume 17 Supplement 2, 2017: Selected articles from Belyaev Conference 2017: plant biology. The full contents of the supplement are available online at https://bmcplantbiol.biomedcentral.com/articles/supplements/volume-17-supplement-2.
Authors’ contributions
Conceptualization: MS, JĆ. Formal analysis and investigation: MG, IB. Project administration, supervision: JV. Writing - original draft preparation: MS. Writing – review and editing: MS, JĆ. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sergentani AG, Gonou-Zagou Z, Kapsanaki-Gotsi E, Hatzinikolaou DG. Lignocellulose degradation potential of Basidiomycota from Thrace (NE Greece) Int Biodeter Biodegr. 2016;114:268–277. doi: 10.1016/j.ibiod.2016.07.004. [DOI] [Google Scholar]
- 2.Devendra LP, Kiran KM, Ashok P. Evaluation of hydrotropic pretreatment on lignocellulosic biomass. Bioresour Technol. 2016;213:350–358. doi: 10.1016/j.biortech.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez A, Rencoret J, Cadena EM, Rico A, Barth D, del Río JC, Martínez AT. Demonstration of laccase-mediator removal of lignin from wood and non-wood plant feedstocks. Bioresour Technol. 2012;119:114–122. doi: 10.1016/j.biortech.2012.05.112. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Bioetchnol Adv. 2009;27:185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Stajić M, Vukojević J, Milovanović I, Ćilerdžić J, Knežević A. Role of mushroom Mn-oxidizing peroxidases in biomass conversion. In: Gupta VK, editor. Microbial Enzymes in Bioconversion of Biomass. Switzerland: Springer International Publishing AG; 2016. p. 251–269.
- 6.Ćilerdžić J, Stajić M, Vukojević J. Degradation of wheat straw and oak sawdust by Ganoderma applanatum. Int Biodeter Biodegr. 2016;114:39–44. doi: 10.1016/j.ibiod.2016.05.024. [DOI] [Google Scholar]
- 7.Stajić M, Vukojević J, Duletić-Laušević S. Biology of Pleurotus eryngii and the role in biotechnological processes: a review. Crit Rev Biotechnol. 2009;29:55–66. doi: 10.1080/07388550802688821. [DOI] [PubMed] [Google Scholar]
- 8.Dias AA, Freitas GS, Marques GSM, Sampaio A, Fraga IS, Rodrigues MAM, Evtugin DV, Bezerra RMF. Enzymatic saccharification of biologically pre-treated wheat straw with white-rot fungi. Bioresour Technol. 2010;101:6045–6050. doi: 10.1016/j.biortech.2010.02.110. [DOI] [PubMed] [Google Scholar]
- 9.Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: an overview of pretreatment, hydrolysis and fermentation. Bioresour Technol. 2010;101:4744–4753. doi: 10.1016/j.biortech.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 10.Stajić M, Kukavica B, Vukojević J, Simonić J, Veljović-Jovanović S, Duletić-Laušević S. Wheat straw conversion by enzymatic system of Ganoderma lucidum. Bioresources. 2010;5:2362–2373. [Google Scholar]
- 11.Silva CMMS, Melo SI, Oliveira RP. Ligninolytic enzyme production by Ganoderma spp. Enzyme Microb Technol. 2005;37:324–329. doi: 10.1016/j.enzmictec.2004.12.007. [DOI] [Google Scholar]
- 12.Kirk TK, Obst JR. Lignin determination. In: Colowick SP, Kaplan NO, editors. Methods in Enzymology 161. Academic Press Inc.: San Diego; 1988. pp. 87–101. [Google Scholar]
- 13.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 14.Arora DS, Gill PK. Laccase production by some white rot fungi under different nutritional conditions. Bioresour Technol. 2000;73:283–285. doi: 10.1016/S0960-8524(99)00141-8. [DOI] [Google Scholar]
- 15.Fenice M, Sermanni GG, Federici F, D’Annibale A. Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media. J Biotechnol. 2003;100:77–85. doi: 10.1016/S0168-1656(02)00241-9. [DOI] [PubMed] [Google Scholar]
- 16.Songulashvili G, Elisashvili V, Wasser S, Nevo E, Hadar Y. Laccase and manganese peroxidase activities od Phellinus robustus and Ganoderma adspersum grown on food industry wastes in submerged fermentation. Biotechnol Lett. 2006;28:1425–1429. doi: 10.1007/s10529-006-9109-4. [DOI] [PubMed] [Google Scholar]
- 17.Simonić J, Vukojević J, Stajić M, Glamočlija J. Intraspecific diversity within Ganoderma lucidum in laccase and Mn-dependent peroxidases production during plant residues fermentation. Appl Biochem Biotechnol. 2010;162:408–415. doi: 10.1007/s12010-009-8833-3. [DOI] [PubMed] [Google Scholar]
- 18.Stajić M, Ćilerdžić J, Galić M, Ivanović Ž, Vukojević J. Lignocellulose degradation by Daedaleopsis confragosa and D. tricolor. Bioresources. 2017;12:7195–7204. doi: 10.1007/s12010-018-2884-2. [DOI] [PubMed] [Google Scholar]
- 19.Camarero S, Böckle B, Martinez JM, Martinez TA. Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl Environ Microbiol. 1996;62:1070–1072. doi: 10.1128/aem.62.3.1070-1072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stajić M, Persky L, Cohen E, Hadar Y, Brčeski I, Wasser SP, Nevo E. Screening of the laccase, manganese peroxidase, and versatile peroxidase activities of the genus Pleurotus in media with some raw plant materials as carbon sources. Appl Biochem Biotechnol. 2004;117:155–164. doi: 10.1385/ABAB:117:3:155. [DOI] [PubMed] [Google Scholar]
- 21.Ćilerdžić J, Stajić M, Duletić-Laušević S, Vukojević J, Knežević A. Potential of Trametes hirsuta to produce ligninolytic enzymes during degradation of agricultural residues. Bioresources. 2011;6:2885–2895. [Google Scholar]
- 22.Manavalan T, Manavalan A, Thangavelu KP, Heese K. Secretome analysis of Ganoderma lucidum cultivated in sugarcane bagasse. J Proteomics. 2012;77:298–309. doi: 10.1016/j.jprot.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Ćilerdžić J, Stajić M, Vukojević J, Lončar N. Intraspecific diversity in the production and characterization of laccase within Ganoderma lucidum. Bioresources. 2014;9:5577–5587. [Google Scholar]
- 24.Songulashviili G, Elisashvili V, Wasser SP, Nevo E, Hadar Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb Technol. 2007;41:57–61. doi: 10.1016/j.enzmictec.2006.11.024. [DOI] [Google Scholar]
- 25.Dong XQ, Yang JS, Zhu N, Wang ET, Yuan HL. Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour Technol. 2013;131:443–451. doi: 10.1016/j.biortech.2012.12.182. [DOI] [PubMed] [Google Scholar]
- 26.Ergun SO, Urek RO. Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann Agr Sci. 2017;15:273–277. doi: 10.1016/j.aasci.2017.04.003. [DOI] [Google Scholar]
- 27.Liers C, Ullrich R, Pecyna M, Schlosser D, Hofrichter M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzyme Microb Technol. 2007;41:785–793. doi: 10.1016/j.enzmictec.2007.07.002. [DOI] [Google Scholar]
- 28.D’Souza TM, Boominathan K, Reddy CA. Isolation of laccase gene – specific sequences from white rot and brown fungi by PCR. Appl Environ Microbiol. 1996;62:3739–3744. doi: 10.1128/aem.62.10.3739-3744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko EM, Leem YE, Choi HT. Purification and characterization of laccase isozymes from the white – rot basidiomycete Ganoderma lucidum. Berlin: Springer-Verlag; 2001. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz C, Guillen F, Martínez TA, Martínez JM. Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol. 1997;34:1–5. doi: 10.1007/s002849900134. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz C, Guillen F, Martínez TA, Martínez JM. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2+ oxidation. Appl Environ Microbiol. 1997;63:2166–2174. doi: 10.1128/aem.63.6.2166-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stajić M, Persky LD, Friesem D, Hadar Y, Wasser SP, Nevo E, Vukojević J. Effect of different carbon and nitrogen sources on laccase and peroxidases activity by selected Pleurotus species. Enzyme Microb Technol. 2006;38:65–73. doi: 10.1016/j.enzmictec.2005.03.026. [DOI] [Google Scholar]
- 33.Guardina P, Aurilia V, Cannio R, Marzullo L, Amoresano A, Siciliano R, Pucci P, Sannia G. The gene, protein and glycin structures of laccase from Pleurotus ostreatus. Eur J Biochem. 1996;235:508–515. doi: 10.1111/j.1432-1033.1996.00508.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G. A novel white laccase from Pleurotus ostreatus. J Biol Chem. 1997;272:31301–31307. doi: 10.1074/jbc.272.50.31301. [DOI] [PubMed] [Google Scholar]
- 35.Knežević A, Milovanović I, Stajić M, Lončar N, Brčeski I, Vukojević J, Ćilerdžić J. Lignin degradation by selected fungal species. Bioresour Technol. 2013;138:117–123. doi: 10.1016/j.biortech.2013.03.182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


