Abstract
Aims:
Despite treatment recommended by guidelines, many patients with chronic heart failure remain symptomatic. Evidence is accumulating that mindfulness-based interventions (MBIs) have beneficial psychological and physiological effects. The aim of this study was to explore the feasibility of MBI on symptoms and signs in patients with chronic heart failure in outpatient clinical settings.
Methods:
A prospective feasibility study. Fifty stable but symptomatic patients with chronic heart failure, despite optimized guideline-recommended treatment, were enrolled at baseline. In total, 40 participants (median age 76 years; New York Heart Association (NYHA) classification II−III) adhered to the study. Most patients (n=17) were randomized into MBI, a structured eight-week mindfulness-based educational and training programme, or controls with usual care (n=16). Primary outcome was self-reported fatigue on the Fatigue severity scale. Secondary outcomes were self-reported sleep quality, unsteadiness/dizziness, NYHA functional classification, walking distance in the six-minute walk test, and heart and respiratory rates. The Mann–Whitney U test was used to analyse median sum changes from baseline to follow-up (week 10±1).
Results:
Compared with usual care (zero change), MBI significantly reduced the self-reported impact of fatigue (effect size −8.0; p=0.0165), symptoms of unsteadiness/dizziness (p=0.0390) and breathlessness/tiredness related to physical functioning (NYHA class) (p=0.0087). No adverse effects were found.
Conclusions:
In stable but symptomatic outpatients with chronic heart failure, MBI alleviated self-reported symptoms in addition to conventional treatment. The sample size is small and further studies are needed, but findings support the role of MBI as a feasible complementary option, both clinically and as home-based treatment, which might contribute to reduction of the symptom burden in patients diagnosed with chronic heart failure.
Keywords: Feasibility study, heart failure, mindfulness-based intervention (MBI), patient-reported outcome, Fatigue severity scale
Introduction
Despite treatment recommended by guidelines, many patients with chronic heart failure (CHF) remain symptomatic. Fatigue, breathlessness, difficulty sleeping, lack of energy, and feeling sad, nervous and depressed are dominant among the most prevalent and distressing symptoms reported in CHF.1–5 The prevalence and burden of these symptoms are high in outpatients with CHF2,6 and contribute to poor health-related quality of life (HRQoL),7 some patients even preferring symptom relief over prolonged life.8 Moreover, fatigue has been shown to have significant and independent prognostic implications for patients with CHF.9,10 However, limited research has focused on symptom relief, and patients’ self-reported symptoms have rarely been selected as primary outcomes.11–13 Meanwhile little is known about the underlying pathogenesis of why stable patients with CHF experience fatigue and breathlessness.14 Currently available symptom relief is insufficient and health care providers’ knowledge of the management, control and palliation of symptoms is limited.6,15
The pathological role of stress and increased sympathetic activity in the development and progression of heart failure is well established.11,16,17 Heart rate has been associated with long-term survival in cardiovascular disease11 and HRQoL in CHF.18 Drug treatment prescribed to achieve heart-rate reduction in selected patients with symptomatic CHF has shown improved outcomes and HRQoL.11,18 An increase in respiratory rate is associated with worsening heart failure, but breathing as a vital function differs from others because it is regulated both automatically and voluntarily,19 opening up the possibility also to intervene non-pharmacologically. A study on deep slow breathing rate for relief of breathlessness showed promising results.20 Mindfulness-based intervention (MBI) includes meditative exercises using focused breathing as a tool, and has been effective in reducing stress, anxiety and depressive symptoms21,22 as well as improving physical functioning21,23,24 and signs of decreased cardiovascular sympathetic activity in randomized controlled trials (RCTs).23,24
Mindfulness is described as the ability to pay attention in a particular way: on purpose, in the present moment and non-judgmentally.25 The two main programmes of MBI are mindfulness-based stress reduction (MBSR)25 and mindfulness-based cognitive therapy (MBCT).26 MBSR as a mainstream meditative method was developed at the University of Massachusetts in 1979 by John Kabat-Zinn for the management of chronic pain in mixed groups of outpatients. The original MBSR programme required a minimum of 45 minutes of meditation (formal training) per day, six days per week.25 MBCT, derived from MBSR, was originally designed to prevent the recurrence of depression.26 The MBI programme used in the present study is based on MBSR and MBCT, and has been previously shown to be non-inferior to conventional treatment in outpatients with depressive, anxiety, stress and adjustment disorders in primary care in Sweden.22 Hence, patients with CHF suffering from distressing symptoms might benefit from MBI training.
The rationales behind outcomes selected for this study were several. Fatigue as primary outcome is a prevalent and distressing symptom1–6 with a considerable disabling impact on daily living, a lack of effective additional treatment,3–6 and prognostic implications in patients diagnosed with CHF.9,10 Life with CHF is also problematic because of other commonly reported symptoms such as ‘impaired sleep’ (sleep quality), known to co-variate with fatigue.27 Another less studied symptom, but frequently reported in this study and at follow-up in the outpatient clinical setting, is unsteadiness/dizziness. Core symptoms in CHF are breathlessness and tiredness related to physical functioning reported by patients themselves, and their ‘degree of discomfort’ interpreted by professionals and documented as New York Heart Association (NYHA) functional classification.9,28 Functional capacity can be feasibly measured in patients with CHF by measuring the walking distance completed in the six-minute walk test (6MWT).29 Heart rate, linked with stress and sympathetic activity,16,17 is also associated with long-term survival and HRQoL.18 Respiratory rate is less studied in patients with CHF20 but has the potential to be significantly affected by mindfulness-based training.
Patients with CHF have been shown to use involuntary attention similar to mindfulness meditation to relieve fatigue,4 and focused breathing reduced breathlessness in patients with CHF.20 Over the last decade evidence on the effectiveness of MBI in several chronic conditions has been rapidly accumulating in RCTs, although few studies have been conducted in patients with CHF.21,24 The aim of this study was to explore the feasibility of MBI on symptoms and signs in outpatients diagnosed with CHF.
Methods
Design
A prospective feasibility study was carried out in two phases, the first of which lasted from 2010 to 2011. The second phase was conducted in 2013 and was planned and initiated as an RCT, called the ‘Main study’. However, for feasibility reasons (mainly the slow recruitment rate), non-randomized participants were included and the planned interventional study was transformed into a feasibility study. No power analysis or sample size calculation was performed because no cut-off point could be suggested for the main outcome fatigue, and also because the current work was a feasibility study.
Training of instructor
A heart failure Registered Nurse (RN) specialist completed the training programme to become an instructor in mindfulness over a total of six days between May 2009 and January 2010, directed by Mindfulnesscenter (Mfc) and Ola Schenstrom (MD).30 The instructor programme included how to guide individuals and groups in mindfulness training, following structured manuals. The programme has been used to train professionals in Sweden since 2005, and also recently in a primary care setting RCT.22
Patient recruitment
Stable but symptomatic patients with CHF despite optimized treatment recommended by guidelines28 were enrolled at a heart failure outpatient clinic at a university hospital in Gothenburg, Sweden between 2010 and 2013. Optimal treatment according to guidelines28 was assessed by a heart failure specialist. Most of the patients (n=43) were recruited from the heart failure clinic, while some in 2013 (n=7) were enrolled from a larger study on follow-up conducted at the same hospital two years after myocardial infarction (MI).
Inclusion criteria
The following four criteria had to be fulfilled for inclusion in the study: (i) diagnosed CHF by echocardiography; (ii) NYHA functional class II–IV; (iii) symptoms of breathlessness and/or tiredness rated 2–5 by the patient using five-point scales ranking from asymptomatic (1) to symptoms at rest (5)9,28; (iv) ‘stable conditions’, that is, no deterioration of heart failure symptoms or new CHF drug or hospitalization because of decompensated heart failure within the last four weeks.
Exclusion criteria
(i) Severe psychiatric diagnosis (requiring treatment); (ii) severe substance abuse (documented in journal); (iii) severe somatic disease with short expected survival (i.e. malignancy); (iv) communication difficulties (i.e. impaired vision or hearing, need of an interpreter to understand Swedish); (v) cognitive or adherence difficulties (documented in journal); (vi) unstable angina pectoris; (vii) post-partum cardiomyopathy; (viii) ongoing participation in any other interventional study; or (ix) unwillingness to participate.
Randomization
Most patients were randomized (1:1) using sealed and numbered envelopes (opened after the first visit was completed) to either a structured eight-week mindfulness-based educational and training programme (MBI) or a control group undergoing usual care.
Intervention group
Participants in the intervention group received MBI in addition to usual care. The Swedish MBI programme used in the present study is based on MBSR25 and MBCT,26 further developed in a simplified version by Ola Schenstrom (OS) as a manualized, structured eight-week educational and training programme. OS was trained at the Center for Mindfulness in Medicine, Health Care, and Society, founded by Jon Kabat-Zinn at the University of Massachusetts, USA. OS is a renowned expert in mindfulness education in Sweden.30 The course material consisted of: facts booklet; weekly training manuals; a compact disc (CD) with the five formal guided exercises (body scan, breathing anchor, breathing space, mindful yoga and sitting meditation); and a diary. The weekly homework also comprised informal training of being present in daily life activities. Participants met weekly in two-hour instructor-led group sessions at the heart failure outpatient clinic. The first session included an introduction to the MBI programme, after which the formal exercises were gradually introduced (Table 1), then learned and reflected upon week by week, aiming to be performed in home practices 20−30 min per day, six days per week. Adherence to the intervention was followed by participants’ self-reported weekly training in manuals asked to be submitted at group sessions. Participants were allocated to six different groups to be followed during their eight-week study period. The first instructor-led MBI group session took place in April 2010 and the MBI group participants attended their last group session (week 8) in November 2013.
Table 1.
Description of the weekly teams and formal training and homework in the eight-week MBI programme.
| Period | Team of the week | Formal training and homework |
|---|---|---|
| Week 1 | LEAVE AUTO PILOT − feel your body | Body scanning, guided on CD track 1 |
| Week 2 | OBSERVE YOUR BREATHING − train your breathing anchor | Breathing anchor, guided on CD track 2 |
| Week 3 | BE PRESENT IN BREATHING AND BODY MOMENT | Breathing space, guided on CD track 3 + Mindful yoga (sitting position), guided on CD track 4 |
| Week 4 | JUST SIT − here and now | Sitting Meditation, guided on CD track 5 |
| Week 5 | ACCEPT AND LETTING GO Walking meditation additional at group session |
Sitting Meditation + Breathing space Stop-Observe-Accept → (Solve) or Letting go |
| Week 6 | DEALING WITH DIFFICULTIES Love and kindness meditation additional at group session |
Sitting meditation + Breathing space dealing with difficulties |
| Week 7 | THOUGHTS ARE NO FACTS – taking care of yourself in the best way |
Building your own training programme Body scanning/Mindful yoga/Sitting meditation +Breathing anchor/Breathing space (free choice) guided on CD tracks 1−5. Confirm and consolidate your own mindfulness workout practice |
| Week 8 | ACCEPT AND CHANGE − HERE AND NOW – possibilities and the future mindfulness workout perspectives |
Practise/develop your own programme Body scanning/Mindful yoga/Sitting meditation + Breathing anchor/Breathing space (free choice) guided on CD tracks 1−5. Confirm and consolidate your own mindfulness workout practice |
MBI: mindfulness-based intervention; CD: compact disc.
Control group
The participants in the control group received usual care comprising standard health care for patients with CHF. The patients met RN heart failure specialists, cardiology specialists and physiotherapists and were referred to occupational therapists, psychologists or social workers if needed.
Follow-up
Patients were examined at baseline and at follow-up (week 10±1) with repeated measures.
Definitions
The definition of heart failure was based on the presence of typical symptoms (e.g. breathlessness and/or fatigue) and signs of structural and functional cardiac abnormalities (e.g. systolic and/or diastolic left ventricular (LV) dysfunction). Heart failure with reduced ejection fraction (HFrEF) defined by an LVEF of <50%, while heart failure with preserved EF (HFpEF) was defined by LVEF ≥50%.28 Signs of diastolic dysfunction were defined as at least two signs out of LV hypertrophy, abnormal myocardial relaxation, left atrial dilation, and increased LV filling pressure and/or pulmonary hypertension.28 NYHA functional classification was termed 1−5 (1, I; 2, II; 3, IIIa; 4, IIIb; 5, IV).
Outcome measures
Fatigue severity scale
The primary outcome fatigue was measured using the Fatigue severity scale (FSS),31 a self-administered questionnaire including nine items investigating the impact of disabling fatigue on daily functioning during the past week.31 Grading of each item ranges from 1 to 7, with the total possible fatigue sum score ranging from 9 to 63, higher scores indicating higher levels of indexed impact of fatigue.31 FSS has not previously been tested in a CHF population.32
Karolinska Sleep Questionnaire
The secondary outcome on symptoms of ‘impaired sleep’ was assessed using the Karolinska Sleep Questionnaire sleep quality index (KSQ-sqi), which includes four questions regarding ‘difficulty falling asleep’, ‘repeated awakening with difficulty falling back to sleep’, ‘premature awakening’ and ‘disturbed sleep’.33 There were six response alternatives ranging from 1 (always/five times or more per week) to 6 (never). A high score indicates good sleep quality, and total index range from 4 to a maximum 24 sum-score. Based on participants’ self-reported KSQ-sqi sum score, three categories of sleep quality were created: (1) ‘Poor sleep’ (score 4−10); (2) ‘Average sleep’ (score 11−17); and (3) ‘Good sleep’ (score 18−24).
NYHA classification based on self-reported symptoms on five-point Likert scale
Secondary outcome professional assessments of NYHA functional classification (NYHA class)28 was based on patients’ self-reported symptoms of breathlessness and tiredness related to physical exertion, using a five-point Likert scale.9 Patient response alternatives ranged from 1, asymptomatic (‘I am never breathless/tired’) to 5 (‘when I am at rest’), with each symptom reported separately (Table S1 in Supplementary Material online).
Numerical rating scale
Degree of symptom severity, within a time frame of ‘the last week’, were self-reported by participants using 11-point numerical rating scales.34
6MWT
Functional capacity was additionally measured in 2013 as the completed walking distance in the 6MWT.29
Clinical measures of signs
Heart rate was measured in sitting position after rest (>5 min) using an automated non-invasive monitor (Omron Memory Intelli Sense, model 705 IT; Omron Healthcare, Hoofddorp, The Netherlands). Respiratory rate was assessed manually using the pulse clock, at rest within a set of 60 s. Weight was measured with light clothing on a digital scale. Height was measured on-site. Body mass index (BMI) was calculated by weight in kilograms divided by height in metres squared.
In order to limit the numbers of outcome variables in this small sample study, we analysed the effects of MBI on the three most frequent symptoms reported. Outcomes were measured in all patients at baseline and at follow-up after eight weeks of intervention or usual care. Detailed methodology for outcome measures is available as supporting information (Appendices in Supplementary Material online).
Ethical considerations
The study was performed according to the principles of the Declaration of Helsinki35 and was approved by the Regional Ethical Review Board in Gothenburg, Sweden (No. 265:10). The participants obtained both oral and written information about the study and the right of free withdrawal. Written informed consent was obtained from all participants. Patients randomized to the control group were offered participation in the educational MBI programme later, in a cross-over substudy when more knowledge about the results was available.
The mindfulness meditation practice focused attention on thoughts, feelings and bodily sensations which could make the person more aware of illness and limitations in life. This was disclosed in the research personal information. Participants were free to remain in contact with the MBI instructor/RN heart failure specialist during the day by telephone, and if needed arrange an appointment with a counsellor. In addition they were encouraged to stay in contact with their usual care provider or seek emergency medical care if needed. During the study period, a psychologist with expertise in cognitive behavioural therapy and experience of treating patients with heart failure was available for consultation.
Statistical analysis
Descriptive statistics, median, means (SD) and percentages were used to describe baseline characteristics. Statistical analyses were conducted using both IBM SPSS Statistics version 22 (IBM, Riverton, NJ, USA) on baseline characteristics and SAS version 9.3 in the statistical consultation by Healthmetrics, University of Gothenburg, Sweden. The level of statistical significance was set to p<0.05.
The analysis of change from baseline to follow-up in the MBI group versus control group was conducted using the non-parametric Mann–Whitney U test. The rationale for using non-parametric testing and medians was the small number of participants (n<30) in each group, variables not expected to be distributed symmetrically and the ordinal level of our data. Owing to the small sample size, we did not control for age or any other variables. Per-protocol analysis was performed, which meant that patients who attended and completed both the baseline and follow-up visits were considered as adherent. Missing data were imputed, if only one item in the questionnaire/subscale was missing, with the participants’ self-reported response at baseline or follow-up, indicating ‘no change’.
Results
Patient recruitment, dropouts and flow through the study
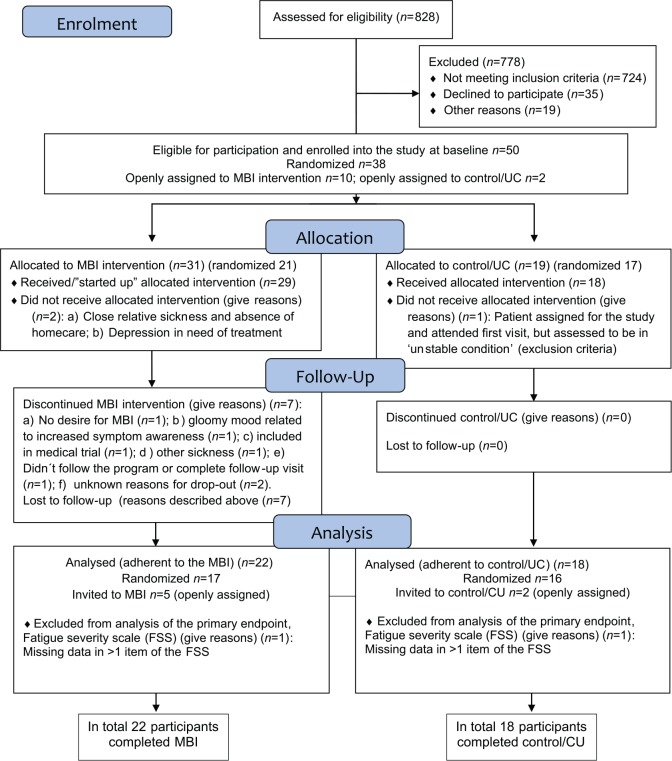
In total 50 patients were included in the study, signed up for it, and attended the baseline visit. Results are based on 40 participants (women, n=17) adhering to and completing the study (MBI, n=22; control, n=18). Of these, 33 were randomized (MBI, n=17; control, n=16) into the study. Results include data on seven openly assigned participants (MBI, n=5; control, n=2), all carefully assessed with no ‘outliers’ among them. The flow of participants is presented in a modified flow diagram (Figure 1). Many patients did not meet inclusion criteria and 35 declined to participate, among whom some felt ‘too tired and sick’, others did not perceive themselves to be needful of intervention, while others hesitated because of the effort and work required during the study. In the first phase from 2010 to 2011 (n=19), 14 completed the study and five dropped out. In the second phase conducted in 2013 (n=31), planned and initiated as a RCT, 26 participants completed and five dropped out. Two ‘dropouts’ were known to be associated with the MBI. One patient did not manage to follow the programme, while the other reported a ‘gloomy mood’ related to increased bodily symptom awareness as a consequence of MBI learning and training. Two other participants did not offer reasons for dropping out, although one of them reported high scores on the Hospital Anxiety and Depression Scales36 at baseline (noticed after the study was closed) and the other disliked group sessions. In the process handling the ‘suspected MBI-related dropouts’, psychological consultation was used. Because of the slow recruitment rate, in 2013 the study was extended to also include patients from another study on follow-up two years after MI with CHF as secondary diagnosis. Five patients (enrolled from the ‘post MI’ study) did not know of their CHF diagnosis and, based on safety and ethics, these five patients, reporting ‘not knowing about their CHF diagnosis’ and with no CHF follow-up, were offered a consultation with the cardiologist and then openly assigned to MBI. In total, 10 participants were openly assigned to MBI (2010−2011, n=5; 2013, n=5) and towards the end two patients were also openly assigned to the control group. Most participants (n=38) were randomized to either the MBI group (n=21) or the control group (n=16). In total 10 patients failed to complete the study, seven of whom dropped out from the MBI because of ‘reasons given’ (Figure 1). However, five of the 10 openly MBI assigned patients are among these ‘dropouts’ (2010−2011, n=2; 2013, n=3). No one in the control group dropped out from this study. One participant in NYHA class IIIb, living on the third floor, completed the MBI at distance via telephone because of a broken elevator. Some participants (n=3) had a ‘slow start’, could not attend group sessions and needed individual meetings with the instructor or additional weeks to complete their training. Because of comorbidities, one patient attended the course together with his wife. In total, five participants experienced adverse events (e.g. falls): two of 18 in the control group and three of 22 in the MBI group. All falls happened outdoors in the community, and no fall was related to mindfulness training at home. One participant in the control group required readmission to hospital because of decompensated heart failure.
Figure 1.
Flow diagram showing patient recruitment, dropouts and flow through the study.
MBI: mindfulness-based intervention; UC: usual care.
Clinical characteristics
The baseline characteristics of the 40 participants adhering to and completing the study in the control and MBI groups, respectively, are presented in Table 2. No significant differences were found when comparing characteristics variables between groups. The median age was 76.0 and HFrEF was equally distributed among both groups. The most frequently reported symptoms were breathlessness and tiredness related to physical exertion, followed by unsteadiness/dizziness and pain. CHF treatment was equivalent in the groups at baseline (Table 2). Self-reported median of fatigue in the FSS sum were comparable at baseline, as well as NYHA classification and walking distance in 6MWT (Table 3). More than half of the participants in both groups (control 56%; MBI 60%) rated their sleep quality on the KSQ-sqi as ‘Good sleep’ (score 18−24). Only one person in the MBI group and none of the controls considered their sleep to be ‘poor’ as rated on the KSQ-sqi (score 4−10). Medication was changed in four of 18 patients in the control group and two of 22 patients in the MBI group during the intervention.
Table 2.
Baseline demographics and other characteristics of participants in the control group and MBI groupa.
| Demographics | Control (n=18) |
MBI (n=22) |
|---|---|---|
| Age median, years (range) | 75.0 (53−84) | 76.5 (45−90) |
| Women, n (%) | 6 (33.3) | 11 (50.0) |
| Comorbidities | ||
| Cardiac artery disease, n (%) | 11 (61.1) | 12 (54.5) |
| Hypertension, n (%) | 8 (44.4) | 12 (54.5) |
| Atrial fibrillation, n (%) | 8 (44.4) | 8 (36.4) |
| Valvular disease, n (%) | 5 (27.8) | 11 (50.0) |
| Cardiomyopathy, n (%) | 5 (27.8) | 4 (18.2) |
| Diabetes, n (%) | 4 (22.2) | 6 (27.3) |
| Renal failure, n (%) | 2 (11.1) | 2 (9.1) |
| Obstructive sleep apnoea, n (%) | 1 (5.6) | 3 (13.6) |
| Chronic obstructive pulmonary disease, n (%) | 1 (5.6) | 2 (9.1) |
| CHF diagnosis | ||
| HFrEF, n (%) | 15 (83.3) | 15 (68.2) |
| HFpEF, n (%) | 3 (16.7) | 6 (27.3) |
| Heart failure within the ‘grey area’b, n (%) | 4 (22.2) | 4 (20.0) |
| Diastolic dysfunction, n (%) | 14 (77.8) | 15 (68.2) |
| Current treatmentc, n (%) | 18 (100) | 22 (100) |
| ACE/ARB inhibitors, n (%) | 18 (100) | 19 (86.3) |
| Beta-blockers, n (%) | 18 (100) | 21 (95.4) |
| MRA, n (%) | 9 (50) | 10 (45) |
| Diuretics, n (%) | 10 (55) | 10 (45) |
| Total numbers of drugs, mean (SD) | 8.39 (3.760) | 7.55 (2.773) |
| Devices, n (%) | 3 (17) | 4 (18) |
| Symptoms (occurrence in the past week, self-reported) | ||
| Breathlessness, n (%) | 16 (89) | 22 (100) |
| Tiredness, n (%) | 17 (94.4) | 20 (90.9) |
| Unsteadiness/dizziness, n (%) | 14 (77.8) | 19 (86.3) |
| Pain at rest/moving, n (%) | 11 (61.1) | 9 (40.9) |
| Thirst/dryness in the throat, n (%) | 3 (16.7) | 5 (22.7) |
| Impaired/reduced appetite, n (%) | 4 (22.2) | 5 (22.7) |
| Ankle swelling, n (%) | 3 (17) | 3 (14) |
| Nausea, n (%) | 0 (0) | 3 (13.6) |
| Stress in the past weekd, n (%) | 1 (16.7) | 3 (13.6) |
| Clinical measures | ||
| Systolic blood pressure, mmHg, mean (±SD) | 123.9 (15.4) | 131.6 (17.3) |
| Diastolic blood pressure, mmHg, mean (±SD) | 78.3 (11.2) | 75.0 (12.4) |
| Respiratory rate, breaths/min, mean (±SD) | 16.1 (3.2) | 15.3 (4.4) |
| BMI, mean (±SD) | 28.2 (6.36) | 27.7 (6.34) |
| LVEF, mean (±SD), % | 34.5 (10.1) | 40.8 (14.4) |
n=40 adherent participants who completed the study from baseline to follow-up in the period 2010 to 2013.
Heart failure within the ‘grey area’: LVEF between 35% and 50% and at least two signs of diastolic dysfunction.
n=40 participants, assessed to be on optimal treatment according to guidelines by a heart failure specialist.
n=14 missing, due to participants who completed the study in 2010 to 2011 (control, n=6; MBI, n=8) and were not asked for/tested on this variable (additional in 2013).
MBI: mindfulness-based intervention; CHF: chronic heart failure; LVEF: left ventricular ejection fraction; HFrEF: heart failure with reduced LVEF; HFpEF: heart failure with preserved LVEF; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; Beta-blockers: beta-receptor-blockers; MRA: mineralocorticoid receptor antagonist; SD: standard deviation; BMI: body mass index.
Table 3.
Self-reported symptom severity and measured functional capacity at baseline in participants in the control group and MBI group.
| Symptom severitya | Control (n=18) |
MBI (n=22) |
|---|---|---|
| FSS sum, median (range) | 39 (21−63) | 42 (12−56) |
| KSQ-sqi sum, median (range) | 18 (13−23) | 18 (7−24) |
| Symptoms (in the past week) | ||
| Breathlessness, five-point scale of 1−5, mean (SD) | 2.56 (1.042) | 2.55 (0.596) |
| Tiredness, five-point scale of 1−5, mean (SD) | 2.78 (1.060) | 2.50 (0.913) |
| Unsteadiness/dizziness, 11-point scale of 0−10, mean (SD) | 3.28 (3.196) | 3.14 (2.376) |
| NYHA classification, mean (SD) | 2.72 (0.826) | 2.82 (0.733) |
| NYHA class I, n (%) | 0 | 0 |
| NYHA class II, n (%) | 9 (50) | 8 (36.4) |
| NYHA class IIIa, n (%) | 5 (27.8) | 10 (45.4) |
| NYHA class IIIb, n (%) | 4 (22.2) | 4 (18.2) |
| NYHA class IV, n (%) | 0 | 0 |
| Functional capacity b | ||
| 6MWT, completed walking distance, m, mean (±SD) | 405.6 (85.1) | 400 (154.5) |
| Perceived physical effort Borg RPE scale (6−20) post 6MWT, median (range) | 12.5 (9−15) | 13 (6−19) |
n=40 adherent participants who completed the study from baseline to follow-up in the period 2010 to 2013.
n=14 missing, due to participants who completed the study in 2010 to 2011 (control, n=6; MBI, n=8) not tested.
MBI: mindfulness-based intervention; FSS: Fatigue severity scale; KSQ-sqi: Karolinska Sleep Questionnaire sleep quality index; NYHA class: New York Heart Association functional classification; 6MWT: six-minute walk test; Borg RPE scale: Borg rating of perceived exertion scale.
Primary outcome
At follow-up participants in the MBI group reported significantly reduced impact of fatigue (effect-size −8.0; p=0.0165) compared with those in the control group (Table 4). By using the median FSS sum-change on the group level as effect-size measure, on average participants in the MBI group decreased about one step (0.89) on each item of the nine-item FSS.
Table 4.
MBI effects on median sum-changes in outcome variables at 10 weeks compared with baseline, in control group versus MBI group participants who completed the study.
| Outcome variables sum-change median (range) | Control group n=18 |
MBI group n=22 |
Z-score (p value) |
|---|---|---|---|
| FSSa | 0.0 (−16.0, 11.0) | −8.0 (−20.0, 12.0) | −2.513 (0.0165) |
| KSQ-sqi | 0.5 (−4.0, 4.0) | 0.0 (−7.0, 9.0) | 0.428 (0.672) |
| Unsteadiness/dizzinessb,c | 0.0 (−10.0, 9.0) | 0.0 (−6.0, 0.0) | −2.138 (0.039) |
| Breathlessnessd | 0.0 (−1.0,1.0) | 0.0 (−1.0, 1.0) | −1.578 (0.123) |
| Tirednessd | 0.0 (−1.0, 2.0) | 0.0 (−2.0, 1.0) | −0.787 (0.436) |
| NYHA class | 0.0 (−1.0, 2.0) | 0.0 (−2.0, 0.0) | −2.762 (0.0087) |
| 6MWT, completed walking distance, me | −4.0 (−102.0, 70.0) | 25.8 (−49.0, 91.5) | 1.647 (0.112) |
| Heart rate, beats/min | 0.0 (−20.0, 35.0) | −2.0 (−21.0, 16.0) | −1.076 (0.289) |
| Respiratory rate, breaths/min | 0.0 (−4.0, 6.0) | 0.0 (−9.0, 6.0) | −0.589 (0.559) |
n=2 missing, due to missing data in >1 item in the FSS (control, n=1; MBI, n=1).
n=1 missing, due to missing data in >1 item (control, n=0; MBI, n=1).
Symptom degree patient self-rated on 11-point scale (0–10).
Symptom degree patient self-rated on five-point scale (1–5).
n=14 missing, due to participants completed the study in 2010 to 2011 (control, n=6; MBI, n=8) not tested.
MBI: mindfulness-based intervention; FSS: Fatigue severity scale; KSQ-sqi: Karolinska Sleep Questionnaire sleep quality index; NYHA class: New York Heart Association functional classification; 6MWT: six-minute walk test.
Secondary outcomes
A significant change in benefit of the MBI was also found in reduced self-reported symptoms of unsteadiness/dizziness (p=0.039) and improved physical functioning as measured by NYHA classification assessed on the basis of patients’ self-reported symptoms of breathlessness and/or tiredness (p=0.0087). No significant median sum changes were found in sleep quality as measured on the KSQ-sqi, walking distance in 6MWT, heart rate or respiratory rate between the groups (Table 4). However, on the individual level patients in the MBI group did improve with respect to secondary endpoints. Regarding sleep quality, several participants reported better sleep in the group session, and the person in the MBI group reporting ‘poor sleep’ at baseline did improve up to ‘average sleep’. Several major changes up to ‘good sleep’ (score 18−24) were also reported in favour of the MBI. Meanwhile others reported better sleep in weeks 3−4 but, because of other circumstances such as spouse or relative’s sickness, reported worse sleep at the endpoint. There were also ceiling effects in the FSS, such as one participant in the control group scoring maximum fatigue at baseline and ‘the same at endpoint’ (maximum score) in tandem reported even worse fatigue experience and ‘improved’ KSQ-sqi due to sleeping ‘all day long’ at the endpoint.
Discussion
Our results showed that a structured eight-week mindfulness-based educational and training programme in addition to usual care in the MBI group might be effective in reducing the self-reported impact of fatigue on daily living, unsteadiness/dizziness and breathlessness/tiredness related to physical function (NYHA class). To our knowledge this is the first study to explore the effects of MBI on fatigue in patients with CHF. Since fatigue is a prevalent, distressing and multidimensional symptom1–6 with negative implications for quality of daily life and possibly even prognosis in patients with CHF,3–6,9,10 our findings are interesting. Unsteadiness/dizziness was found to be a frequent and bothersome symptom and possibly also to be affected by MBI. Reduced unsteadiness/dizziness could be important in preventing fall-related injuries and might also contribute towards improved treatment adherence in patients experiencing the symptom as a side effect of cardiovascular drugs, and thus also be of prognostic significance.11,37 NYHA functional classification based on self-reported breathlessness/tiredness also seemed to improve in the MBI group, indicating possible beneficial physical effects. Physically limiting symptoms are known to have a negative impact in all dimensions of life: psychological, social and also existential.7,38,39 In this study the sum-changes in other secondary variables also showed a favourable tendency towards intervention, as shown in Table 4. Notably, a positive median sum-change in walking distance (25.8 m) was obtained in the 6MWT, indicating improved physical functioning by several participants who were learning and practising MBI, albeit not significantly powered on the group level. On the individual level, MBI was effective regarding sleep quality in MBI group participants. Each positive outcome change potentially contributes to reducing patients’ suffering with CHF.
Major feasibility problems arose from the slow recruitment rate, which might have been for several reasons: one is that our study patients had multiple comorbidities that made them too sick to participate, and another is that there were extensive inclusion criteria. That is why non-randomized participants were included and the interventional study was transformed into a feasibility study. No patient diagnosed with CHF can assuredly be considered to be ‘stable’ or ‘optimally treated’ because of the unpredictable trajectory of CHF. However, these requirements also strengthen the evidence of the study exploring the effects of MBI as an additional treatment. Several patients declined participation because of ‘feeling too sick and tired’ and the amount of work required by the eight-week MBI programme. Feasible problems related to the MBI were few among included participants, but still needed to be considered. Psychologist expertise consultations are also valuable in future trials. However, the programme could perhaps be modified to include fewer group sessions, with the possibility also for only individual-based sessions, together with relatives and/or web-based study with telephone support at a distance. The feasibility in this study turned out to be high because of the efficacy of ‘utilization’, which involves active participants learning and training both in instructor-led group sessions at the clinic and in their daily life at home while using the integrated course material in their own personal way. Giving the opportunity to participants in the control group to attend the MBI programme later on might have contributed to their adherence.
The underlying mechanisms for effects of mindfulness on the cardiovascular system remain unsettled. It appears that mindfulness has a series of favourable effects on cardiac working efficiency and neuro hormonal regulation.23 May and colleagues found that, in a sample of healthy females practising mindfulness meditation for 15 minutes, a strong negative relationship was observed between the myocardial oxygen consumption and LV work. Moreover mindfulness seems to have a strong positive effect on cardiovascular modulation by decreasing cardiac sympathovagal tone, vascular resistance and ventricular workload.23 The respiratory component was probably linked to the focused breathing and low-frequency component of systolic blood pressure, and variation of inhalation and exhalation rates.23 Their results suggest that mindfulness may be clinically pertinent for patients with increased cardiovascular sympathetic activity,23 which is an important characteristic of CHF and the main focus.11,16,17
Kessing and colleagues40 measured general and exertion fatigue in outpatients with CHF using methods similar to ours, and demonstrated that both were significantly associated with poor CHF self-care, which could not be explained by sleep problems or mood symptoms. Neither did most participants in our study suffer from severe sleep disturbances or mood symptoms that contributed to their fatigue experiences. These findings indicate that treatment of fatigue by practising MBI may improve heart failure self-care skills 40 and, ultimately, other outcomes in CHF.9,10
Regarding our secondary outcomes, symptoms of unsteadiness/dizziness have received limited attention in previous research11,12 although it is well known and reported frequently by patients. Therefore it is an interesting finding in the present study that unsteadiness/dizziness could be relieved by MBI. Sullivan and colleagues41 demonstrated clinical symptom improvements over time (one year), after a mindfulness-based stress reduction programme and a psychoeducational intervention (also including coping skill training and an expressive support group) in patients with HFrEF. Our study has extended these findings, including HFpEF in addition to HFrEF. Surprisingly MBI did not affect sleep quality as measured by the KSQ-sqi in this study. A recent secondary analysis of mindfulness-based stress reduction interventions42 indicated that to improve sleep quality, high levels of sleep disturbances must represent the point of departure. Our results may be explained by low degrees of sleep disturbances among most of our participants, therefore allowing less room for improvement in sleep quality. A recent study on younger patients with heart disease by Younge et al.24 showed beneficial physiological effects on exercise capacity as measured by the 6MWT and significantly lower heart rate in favour of the intervention group practising a 12-week online web-based mindfulness programme. Participants had diagnosed heart disease (ischaemic, valvular, congenital heart disease, or cardiomyopathy) and a mean BMI of 25 kg/m2, but were not assessed with regard to symptoms of fatigue, breathlessness or NYHA classification.24 Our small sample study was probably underpowered to be able to reach a significant effect on 6MWT and heart rate. However, changes showed a favourable trend with decreased median change in heart rate of 2 beats/min, comparable to findings from medical trials.43 The strength in our study compared with that of Younge et al.24 lies in the high median age of 76 years, which is more representative of real-life CHF patients.
Limitations and strengths of this study
This study has limitations, the main one being that it was a single-centre study with a small sample and a relatively short-term follow-up. The character of the study, whereby it was not possible for researcher to be blinded to MBI treatment, is a potential limitation. On the other hand we have thoroughly described a representative heart failure population with verified diagnosis, well-defined comorbidities, symptom assessment and median age of 76 years. Other strengths are the systematic application of MBI in a mostly randomized and controlled manner. Although not all participants were randomized into the study, the two groups appear to be comparable regarding characteristics at baseline. Both the FSS and KSQ-sqi were answered at home after visits, sent in to a university address and not opened until the study was closed, in an attempt to reduce experimenter demands on patient self-reported measures. All outcome variables are of clinical relevance and the research methods also feasible and reproducible by others. An additional major strength is that very few internal data are missing with regard to those participants who completed the study. The training programme to become a certified MBI instructor as used in this study is practically oriented, approachable by heart failure RN specialists but also by other clinicians, and, although accessible today only in Swedish, is internet-based and available for translation into other languages.
Clinical implications
Our results support the clinical relevance of MBI as an additional complementary treatment to conventional therapy in outpatients with CHF. Mindfulness seems to hold the potential to succeed by altering aspects of pathological sympathetic nerve activation,16,23 thus contributing to symptom relief, an important factor in the prognosis of patients diagnosed with CHF.9,10,17 The MBI used in the present study comprises eight weekly (2 h) group meetings at a clinic and home-based patient training (20 min for 6/7 days) guided by an audio CD, and may be adapted for implementation in other settings (e.g. primary and home-based care).
Research implications
Further research is needed, namely multicentre RCTs that also include active controls with an MBI ‘placebo’ (i.e. a control that involves equivalent social interaction, learning and reflection, and support, but lacks critical MBI components thought to be responsible for effects). To make the intervention more accessible, RCTs including web-based MBI learning might also be possible with professional telephone support at a distance. Recruiting patients earlier and in parallel with drug titrations might be more feasible, as would less frequent group sessions. Future research should also focus on ‘dose’ response issues (i.e. minimum effective formal training time) and the long-lasting benefits of MBI.
Conclusions
This study confirms that MBI, in addition to conventional treatment, has the potential to reduce the impact of self-reported fatigue, as well as perhaps unsteadiness/dizziness and breathlessness/tiredness related to physical functioning (NYHA class) in stable but symptomatic outpatients with CHF. Our findings also support the role of MBI as a feasible complementary, both clinically and home-based treatment, for further optimized heart failure care and possible reduction of the burden of symptom for patients in daily life with CHF. However, further studies, designed as multicentre RCTs, that include active control groups and instructor-led sessions, are needed.
Acknowledgments
We wish to acknowledge all the participants in this study. We would also like to thank the outpatient heart failure clinicians involved in this study, and also acknowledge the research nurses who contributed to this work at the research unit of the hospital. We specially acknowledge the psychological consultation by Mahnaz Gholipor at the outpatient clinic of the hospital. Finally we would like to acknowledge the statistical consultation by Oscar Hammar, Healthmetrics, at the University of Gothenburg, Sweden.
Footnotes
Author contribution: All authors contributed to the study design and writing of the article. The manuscript was drafted by JN with critical input from KF, MF, IE and LB. All authors contributed to approval of the version submitted. All authors have given final approval of the submitted manuscript.
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This work was supported by the Centre for Person-Centred Care at the University of Gothenburg (GPCC), Sweden. GPCC is funded by the Swedish Government’s grant for Strategic Research Areas, Care Sciences (Application to Swedish Council no. 2009-1088).
Implications for practice
- Mindfulness-based training might reduce the impact of fatigue in patients with CHF.
References
- 1. Zambroski CH, Moser DK, Bhat G, et al. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs 2005; 4: 198–206. [DOI] [PubMed] [Google Scholar]
- 2. Barnes S, Gott M, Payne S, et al. Prevalence of symptoms in a community-based sample of heart failure patients. J Pain Symptom Manage 2006; 32: 208–216. [DOI] [PubMed] [Google Scholar]
- 3. Falk K, Swedberg K, Gaston-Johansson F, et al. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. Eur J Cardiovasc Nurs 2007; 6: 99–104. [DOI] [PubMed] [Google Scholar]
- 4. Falk K, Granger BB, Swedberg K, et al. Breaking the vicious circle of fatigue in patients with chronic heart failure. Qual Health Res 2007; 17: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 5. Falk H, Ekman I, Anderson R, et al. Older patients’ experiences of heart failure – an integrative literature review. J Nurs Scholarsh 2013; 45: 247–255. [DOI] [PubMed] [Google Scholar]
- 6. Janssen DJ, Spruit MA, Uszko-Lencer NH, et al. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med 2011; 14: 735–743. [DOI] [PubMed] [Google Scholar]
- 7. Wu JR, Lennie TA, Frazier SK, et al. Health-related quality of life, functional status, and cardiac event-free survival in patients with heart failure. J Cardiovasc Nurs 2016; 31: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stanek EJ, Oates MB, McGhan WF, et al. Preferences for treatment outcomes in patients with heart failure: Symptoms versus survival. J Card Fail 2000; 6: 225–232. [DOI] [PubMed] [Google Scholar]
- 9. Ekman I, Cleland JG, Swedberg K, et al. Symptoms in patients with heart failure are prognostic predictors: Insights from COMET. J Card Fail 2005; 11: 288–292. [DOI] [PubMed] [Google Scholar]
- 10. Perez-Moreno AC, Jhund PS, Macdonald MR, et al. Fatigue as a predictor of outcome in patients with heart failure: Analysis of CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail 2014; 2: 187–197. [DOI] [PubMed] [Google Scholar]
- 11. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 12. Jonkman NH, Westland H, Groenwold RH, et al. Do self-management interventions work in patients with heart failure? an individual patient data meta-analysis. Circulation 2016; 133: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang TC, Huang JL, Ho WC, et al. Effects of a supportive educational nursing care programme on fatigue and quality of life in patients with heart failure: A randomised controlled trial. Eur J Cardiovasc Nurs 2016; 15: 157–167. [DOI] [PubMed] [Google Scholar]
- 14. Witte KK, Clark AL. Why does chronic heart failure cause breathlessness and fatigue? Prog Cardiovasc Dis 2007; 49: 366–384. [DOI] [PubMed] [Google Scholar]
- 15. McIlvennan CK, Allen LA. Palliative care in patients with heart failure. BMJ 2016; 353: i1010. [DOI] [PubMed] [Google Scholar]
- 16. Grassi G, Seravalle G, Mancia G. Sympathetic activation in cardiovascular disease: Evidence, clinical impact and therapeutic implications. Eur J Clin Invest 2015; 45: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 17. Tawakol A, Ishai A, Takx RAP, et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017; 389: 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: Results from the SHIFT study. Eur Heart J 2011; 32: 2395–2404. [DOI] [PubMed] [Google Scholar]
- 19. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med 1995; 333: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 20. Ekman I, Kjellstrom B, Falk K, et al. Impact of device-guided slow breathing on symptoms of chronic heart failure: A randomized, controlled feasibility study. Eur J Heart Fail 2011; 13: 1000–1005. [DOI] [PubMed] [Google Scholar]
- 21. Gotink RA, Chu P, Busschbach JJV, et al. Standardised mindfulness-based interventions in healthcare: An overview of systematic reviews and meta-analyses of RCTs. PLoS One 2015; 10: e0124344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Sundquist J, Lilja A, Palmer K, et al. Mindfulness group therapy in primary care patients with depression, anxiety and stress and adjustment disorders: Randomised controlled trial. Br J Psychiatry 2015; 206: 128–135. [DOI] [PubMed] [Google Scholar]
- 23. May RW, Bamber M, Seibert GS, et al. Understanding the physiology of mindfulness: Aortic hemodynamics and heart rate variability. Stress 2016; 19: 168–174. [DOI] [PubMed] [Google Scholar]
- 24. Younge JO, Wery MF, Gotink RA, et al. Web-based mindfulness intervention in heart disease: A randomized controlled trial. PLoS One 2015; 10: e0143843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med 1985; 8: 163–190. [DOI] [PubMed] [Google Scholar]
- 26. Teasdale JD, Segal ZV, Williams JM, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000; 68: 615–623. [DOI] [PubMed] [Google Scholar]
- 27. Johansson I, Karlson BW, Grankvist G, et al. Disturbed sleep, fatigue, anxiety and depression in myocardial infarction patients. Eur J Cardiovasc Nurs 2010; 9: 175–180. [DOI] [PubMed] [Google Scholar]
- 28. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 29. Vuckovic KM, Fink AM. The 6-min walk test: Is it an effective method for evaluating heart failure therapies? Biol Res Nurs 2012; 14: 147–159. [DOI] [PubMed] [Google Scholar]
- 30. Schenstrom O. [in Swedish] Här & Nu - Ett program för medveten närvaro. Retrieved from: http://www.mindfulnesscenter.se (2013, accessed 21 March 2013).
- 31. Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 32. Whitehead L. The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage 2009; 37: 107–128. [DOI] [PubMed] [Google Scholar]
- 33. Kecklund G, Akerstedt T. The psychometric properties of the Karolinska Sleep Questionnaire. J Sleep Res 1992; 6 (Suppl. 1: 113): 221–229. [Google Scholar]
- 34. Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991; 7: 6–9. ] [PubMed] [Google Scholar]
- 35. Rickham PP. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 37. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008: Cd000011. [DOI] [PubMed] [Google Scholar]
- 38. Martensson J, Karlsson JE, Fridlund B. Male patients with congestive heart failure and their conception of the life situation. J Adv Nurs 1997; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 39. Martensson J, Karlsson JE, Fridlund B. Female patients with congestive heart failure: How they conceive their life situation. J Adv Nurs 1998; 28: 1216–1224. [DOI] [PubMed] [Google Scholar]
- 40. Kessing D, Denollet J, Widdershoven J, et al. Fatigue and self-care in patients with chronic heart failure. Eur J Cardiovasc Nurs 2016; 15: 337-344 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan MJ, Wood L, Terry J, et al. The Support, Education, and Research in Chronic Heart Failure Study (SEARCH): A mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157: 84–90. [DOI] [PubMed] [Google Scholar]
- 42. Gallegos AM, Moynihan J, Pigeon WR. A secondary analysis of sleep quality changes in older adults from a randomized trial of an MBSR program. J Appl Gerontol. Epub ahead of print 1 August 2016. DOI: 10.1177/0733464816663553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swedberg K, Komajda M, Bohm M, et al. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: Is there an influence of beta-blocker dose?: Findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol 2012; 59: 1938–1945. [DOI] [PubMed] [Google Scholar]