Abstract
Insects and other arthropods transmit devastating human diseases, and these vectors use chemical senses to target humans. Understanding how these animals detect, respond, and adapt to volatile odorants may lead to novel ways to disrupt host localization or mate recognition in these pests. The past decade has led to remarkable progress in understanding odorant detection in arthropods. Insects use odorant-gated ion channels, first discovered in Drosophila melanogaster, to detect volatile chemicals. In flies, 60 “tuning” receptor subunits combine with a common subunit, Orco (odorant receptor coreceptor) to form ligand-gated ion channels. The mechanisms underlying odorant receptor desensitization in insects are largely unknown. Recent work reveals that dephosphorylation of serine 289 on the shared Orco subunit is responsible for slow, odor-induced receptor desensitization. Dephosphorylation has no effect on the localization of the receptor protein, and activation of the olfactory neurons in the absence of odor is sufficient to induce dephosphorylation and desensitization. These findings reveal a major component of receptor modulation in this important group of disease vectors, and implicate a second messenger feedback mechanism in this process.
Keywords: Olfaction, olfactory, Drosophila, coreceptor, desensitization, phosphorylation, adaptation
Introduction
Desensitization to background odorants is essential to maintain responsiveness in a fluctuating odorant environment. This ability is important for the localization of food and mates for most species. The human olfactory system is well known to desensitize to odorants. Everyone has experienced entering a room with a foul odor, but within a few minutes, the perception of the odorant vanishes. Both peripheral and central mechanisms are thought to be responsible for this phenomenon.1 Insects, such as mosquitoes and fruit flies, have odorant receptors that desensitize to the presence of background odorants, but the mechanisms underlying this phenomenon are a mystery. Now, we learn that changes in phosphorylation are involved in insect odorant receptor desensitization, stemming from the depolarization of these neurons.2
Peripheral Olfactory Systems in Vertebrates and Insects
Mammalian odorant receptors are encoded by a large number of Or genes that belong to canonical G protein–coupled receptor (GPCR) family.3 The interaction between odorant ligands and the receptor protein leads to activation of a G protein, Golf,4 that subsequently activates adenylyl cyclase type III (ACIII), an enzyme that catalyzes the production of cyclic adenosine monophosphate (cAMP).5,6 The rise in intracellular cAMP triggers the opening of cyclic nucleotide–gated (CNG) ion channels and membrane depolarization.7 Calcium entering the depolarized neuron triggers activation of calcium-activated chloride channels that augments depolarization.8
Insects have far fewer odorant receptor genes compared with vertebrates. For example, the Drosophila genome only encodes 60 “tuning” Or genes. These genes are predicted to encode 7 transmembrane receptors, but they lack sequence similarity with GPCRs and, compared with GPCRs, are reversed in the membrane with their C termini outside of the cell.9 Each tuning subunit is expressed in a small number of olfactory neurons together with the common subunit Orco (odorant receptor coreceptor) to form ligand-activated odorant receptors. These receptors function on the cilia of the olfactory neurons located in the antenna and maxillary palps. Consistent with species-specific niches, tuning receptors are divergent between species, whereas Orco is highly conserved.10
Odorant Desensitization in Vertebrates and Insects
Desensitization (also called adaptation) can occur through multiple mechanisms and timescales. Some are intrinsic to the primary olfactory neurons, whereas others involve feedback from neurons downstream in the circuit. Desensitization can occur in milliseconds, modulating neuronal output during the stimulus, or can be slow, requiring prolonged odorant exposure over minutes to hours. Here, we focus on the slow adaptation mechanisms that occur within the olfactory neurons on a scale of minutes.
In mammals, slow desensitization of olfactory neurons has been attributed to several feedback mechanisms (Figure 1A). Odorant receptors are phosphorylated by the G protein–coupled receptor kinase 3 gene11 and protein kinase A12 leading to binding and deactivation by β-arrestin 2.13 In addition, multiple feedback mechanisms are triggered by calcium entry into the activated olfactory neurons. These mechanisms include calmodulin activation of CaMKII that phosphorylates and activates phosphodiesterase that degrades cAMP14 and phosphorylation and inhibition of ACIII.15 Additional feedback is provided by calcium-activated potassium channels16 and calmodulin-mediated reduction in CNG channel sensitivity to cAMP.17,18 Work from Anne Menini’s group using caged cAMP and nonhydrolyzable 8-Bromo-cAMP suggests that the strongest adaptation mechanisms act downstream of cAMP, most likely at the CNG channel.19
Figure 1.

Schematic diagram of the signal transduction mechanisms in mammals and insects. (A) Mammalian signaling and sites for regulation. Odorant ligand activates the odorant receptor (Or) that activates the G protein, Golf. Golf activates adenylyl cyclase type III (ACIII) that converts ATP to cAMP. cAMP opens cyclic nucleotide–gated (CNG) channels that allow sodium and calcium to enter the neuron. Calcium has a feed-forward effect by opening chloride channels that augment depolarization. Feedback mechanisms include PKA and GRK3 that phosphorylate activated receptors and facilitate the binding of β-arrestin 2 to block receptor/Golf interactions. The calcium entering through the CNG channels binds calmodulin that activates the Ca2+-calmodulin dependent kinase II (CaMKII). CaMKII phosphorylates and inhibits the activity of ACIII and phosphorylates and activates the phosphodiesterase responsible for the hydrolysis of cAMP. (B) In insects, such as Drosophila, odorants activate the Or/Orco heterodimers leading to calcium influx. The elevated intracellular Ca2+ activates a phosphatase (or inhibits a kinase) resulting in gradual dephosphorylation of OrcoS289, resulting in desensitization of the olfactory receptors. ATP indicates adenosine triphosphate; cAMP, cyclic adenosine monophosphate; Orco, odorant receptor coreceptor.
In insects, slow desensitization also occurs to odorants, pheromones, and repellants,20–22 but the mechanisms mediating this process are poorly understood (Figure 1B). One mechanism important for insect olfactory neuron desensitization occurs from feedback through downstream interneurons called local neurons (LNs) that release γ-aminobutyric acid (GABA) and inhibit olfactory neuron firing23 (Figure 2). There is a high degree of overlap between glomerular odorant activation and inhibitory neuron activation in glomeruli.24 Recurrent coupling through excitatory and inhibitory synapses within a glomerulus can synchronize action potentials from the output projection neurons that may be important for odorant discrimination by activating neurons sensitive to temporal coincidence in the brain in both vertebrates and invertebrates.25–28 Furthermore, the LNs synapse with multiple glomeruli and thus have the potential to process odorant information across glomeruli.24 Additional complexity is introduced by the fact that GABA receptor expression differs among different classes of Drosophila olfactory neurons.29 Ionotropic GABAA receptors are important for rapid inhibition after odorant onset, whereas metabotropic GABAB receptors are important for long-term adaptation23 (Figure 3). Finally, olfactory neuron sensitivity can be inhibited by the Drosophila neuropeptide transmitter tachykinin (DTK) that is released by a subset of LNs in response to odors. The DTK receptors are expressed on olfactory neurons and mediate inhibitory feedback by DTK and may operate independently of GABA.30 One intriguing possibility is that internal state modulates DTK release, allowing coordination between internal state and chemosensory behavior, but more work remains to be done on the role of neuropeptides on olfactory sensitivity.
Figure 2.

Extrinsic desensitization requiring synaptic transmission. (A) Illustration of insect olfactory neuron anatomy. Olfactory neurons project axons into the antenna lobe (equivalent of the olfactory bulb in vertebrates) where they activate second-order PNs that project to higher brain centers. Olfactory neurons also activate LNs that are local feedback neurons. (B) Normal signal transmission between ORNs and PNs in the glomeruli. ORN action potentials result in an influx of Ca2+ that triggers release of acetylcholine (ACh) that activates the downstream PNs. Activity in the ORNs and PNs is highly correlated.24 The ORN axon also synapses with GABAergic and peptidergic Drosophila tachykinin (DTK) LNs. ORNs express both GABAA and GABAB receptors as well as tachykinin receptors (DTKRs) that provide negative feedback to the ORN. (C) Sustained activity in the olfactory receptor neurons suppresses ORN activity through GABA and tachykinin-mediated presynaptic inhibition. Ionotropic GABAA receptors are chloride channels that hyperpolarize the neuron and mediate rapid adaptation, whereas metabotropic GABAB receptors mediate long-term adaptation.23 TKRs also modulate the sensitivity of ORNs.30 GABA, γ-aminobutyric acid; LNs, local neurons; ORN, olfactory receptor neuron; PNs, projection neurons; TKRs, tachykinin receptors.
Figure 3.
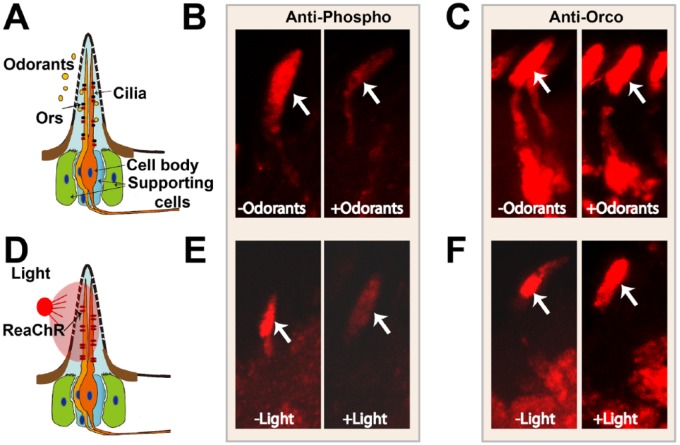
Intrinsic desensitization within insect olfactory neurons. (A-C) Prolonged odorant exposure or (D-F) light activation reduces phosphorylation of OrcoS289 without altering Orco localization in the cilia. (A) ORNs activated by odorants. (B) Phospho-OrcoS289 immunoreactivity in the absence or presence of activating odorant. Odorants trigger the dephosphorylation of OrcoS289. (C) Anti-Orco serum reveals no change in total Orco localization with or without odorant exposure. (D) Red-shifted channelrhodopsin (ReaChR)31 expressed in the ORN allows activation of the ORNs without odorant. (E) Phospho-OrcoS289 immunoreactivity in flies expressing ReaChR in the olfactory neurons without and with red light exposure. Neuronal activation reduces the phospho-OrcoS289 signal. (F) Total Orco levels and localization do not change on light activation in the ORNs expressing ReaChR before or after red light exposure. Orco indicates odorant receptor coreceptor; ORN, olfactory receptor neuron.
Adaptation mechanisms that are intrinsic to the olfactory neurons and those triggered by feedback from inhibitory neurons downstream in the circuit are both important to match gain to stimulus intensity.32 However, it is important to recognize that adaptation mechanisms that occur within the primary olfactory neurons act upstream of these trans-synaptic mechanisms.
Desensitization at the level of the insect olfactory receptor neuron is not well understood. The insect receptors are odor-gated ion channels; therefore, adaptation mechanisms are likely to operate directly on the receptors (see Figure 1B). Furthermore, Orco, being a common subunit of all receptors, is an appealing target for modulation of receptor sensitivity that is independent of the tuning receptor component. The intracellular domains of Orco contain a number of potential phosphorylation sites that are conserved across species. These sites were systematically mutated to alanine and expressed in the olfactory neurons of live flies lacking endogenous Orco.2 Most of these mutants functioned indistinguishably from wild-type Orco. However, when S289 was mutated to alanine, there was a striking reduction in odorant sensitivity compared with wild-type controls.2
Charges at Orco S289 Regulate Sensitivity
If S289 is an important site for regulating sensitivity, and mutating this serine residue to alanine reduces sensitivity, what does replacing serine with a charged (phosphomimetic) residue do? When the S289D mutant Orco was expressed in the Drosophila olfactory system, it resulted in a small but significant increase in odorant sensitivity compared with wild-type Orco controls.2 Thus, negative charges at Orco amino acid 289 are a potential toggle to regulate odorant sensitivity through changes in phosphorylation. Mutants at S289 affect Drosophila desensitization-based behavior as well. Wild-type flies preexposed (desensitized) to apple cider vinegar are not attracted to vinegar traps, whereas flies expressing either OrcoS289A or OrcoS289D are unable to modulate receptor sensitivity normally and are still attracted to vinegar traps following vinegar preexposure.2 These results are consistent with phosphorylation at this position regulating sensitivity of the olfactory receptors in response to background odorants. What is the mechanism of this sensitivity change?
S289 Phosphorylation Does Not Affect Receptor Trafficking
The simplest explanation for the findings described above is that a charge at S289 affects the trafficking of the receptors in the chemosensory cilia, a process known to be dependent on Orco.33 Trafficking of receptors out of the cilia would reduce the receptor density and reduce sensitivity to odorants. Phosphorylation of vertebrate odorant receptors and subsequent trafficking out of the cilia have been proposed as a mechanism of adaptation.12 However, when antiserum to Orco is used to quantify levels in the Orco S289 mutants, Orco protein levels in the chemosensory cilia are not different from wild type.2 Therefore, receptor trafficking is unlikely to account for the changes in odorant sensitivity in insects and suggests that phosphorylation at this position affects the function of the receptor channel.
Is Orco S289 Phosphorylated In Vivo?
Phospho-specific antiserum was raised to a phosphorylated peptide corresponding to OrcoS289 and used to assess the phosphorylation status of this site in living flies. In animals isolated in an odorant-free environment for 1 hour, there is a strong phospho-S289 signal in the chemosensory cilia that is absent in Orco mutants.2 When flies are exposed to a mixture of odorants predicted to activate most olfactory neurons, the phospho-specific signal is strikingly reduced (Figure 3A to C). Therefore, there is an odorant-induced reduction in phosphorylation of OrcoS289. Anti-Orco antiserum (that detects whether Orco is phosphorylated or not) showed Orco that was still present, but no longer phosphorylated, confirming that there is no link between S289 phosphorylation and trafficking. Time course experiments revealed a detectable drop in phosphorylation within 5 minutes that is close to maximal after 30 minutes of odorant exposure. These changes in phosphorylation are mirrored by desensitization of the receptor neurons.2
Neuronal Activation, Not Activation of the Odorant Receptor, Triggers Adaptation
Mammalian GPCRs, including odorant receptors, are phosphorylated by receptor kinases when the receptors are in the activated (ligand bound) conformation.11,34 Phosphorylation by receptor kinase reduces interactions with the downstream signaling machinery by increasing the affinity for arrestins that compete with G proteins for activated receptors.11,13,34 Is odorant-activated receptor conformation important for dephosphorylation of the insect receptors? To explore this possibility, Drosophila olfactory receptor neurons were activated in the absence of odorants using the red-shifted channelrhodopsin (ReaChR).31 The ReaChR-mediated activation of the olfactory neurons was as effective as odorant exposure for inducing OrcoS289 dephosphorylation.2 Red light also desensitizes the olfactory neurons to subsequent odorant exposures. Together, these data demonstrate that neuronal activation, and not activation of the odorant receptors per se, is important for dephosphorylation of Orco at S289 (Figure 3D to F), and dephosphorylation of S289 reduces odorant transduction efficiency.
Blocking Synaptic Transmission Has No Effect on Orco S289 Dephosphorylation
The GABA feedback from LNs activated downstream of olfactory receptor neurons is an important aspect of desensitization.22,29,32,35 To establish that S289 dephosphorylation and desensitization are not a result of influence from downstream circuit components, these processes were measured in flies with olfactory neurons defective for synaptic transmission. Flies expressing tetanus toxin in the olfactory neurons fire action potentials normally, but synaptic transmission is blocked by tetanus toxin–mediated SNARE cleavage.36 In the absence of synaptic transmission, OrcoS289 dephosphorylation and subsequent desensitization are unaffected.2 Therefore, this mechanism is intrinsic to the olfactory neurons and is not a result of LN feedback.
Additional Intrinsic Desensitization Components Remain Unidentified
OrcoS289A and OrcoS289D mutants have striking impairments in adaptation but still show residual desensitization.2 Therefore, additional intrinsic desensitization mechanisms must be present in addition to OrcoS289 dephosphorylation. One possibility is that conserved Orco phosphorylation sites that have little effect on sensitivity when mutated alone could combine to produce stronger effects when multiple residues are modified. This could be important to fine-tune receptor sensitivity under different conditions. Identification of the OrcoS289 dephosphorylation mechanism is an important step but there is more to learn about sensitivity regulation of olfactory neurons.
Future Directions
The kinases and phosphatases responsible for phosphorylation changes at OrcoS289 have not been identified. A major outstanding question is whether the odorant-induced dephosphorylation of OrcoS289 described here results from activation of a phosphatase or inhibition of a kinase in the face of a constitutively active phosphatase. Because calcium influx occurs on olfactory neuron activation, it is tempting to speculate that activation triggers a calcineurin phosphatase that removes the phosphate from OrcoS289, but this prediction awaits further study.
OrcoS289 is a consensus protein kinase C (PKC) phosphorylation site. There are 5 PKC genes in Drosophila, and previous work suggested that Drosophila PKC53E and PKC delta phosphorylate Orco to enhance sensitivity,37 but it is not clear whether these kinases are localized to olfactory neurons or their cilia. A systematic knockdown of these proteins in olfactory neurons should reveal the relevant kinases for OrcoS289. Finally, there is a second family of odorant receptors in insects related to glutamate ionotropic receptors called Ir receptors.38 These receptors do not show the rapid desensitization observed in Or/Orco responses,39 but whether they undergo long-term adaptation is unknown.
Concluding Remarks
Insects are the largest class of animals and colonize every corner of the planet due in part to fine-tuned chemosensory systems. However, molecular dissection of the mechanisms underlying the regulation of odorant receptor sensitivity has lagged behind our understanding of this process in mammals. The identification of OrcoS289 dephosphorylation in adaptation provides an entry point for understanding how odorant sensitivity is regulated in these animals to accommodate an ever-changing environment.
Acknowledgments
The authors apologize to their colleagues whose work is not cited due to space limitations.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH R01DC015230 to D.P.S.
Declaration Of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HG made the figures. HG and DPS wrote the manuscript.
References
- 1. Thomas-Danguin T, Sinding C, Romagny S, et al. The perception of odor objects in everyday life: a review on the processing of odor mixtures. Front Psychol. 2014;5:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo H, Kunwar K, Smith D. Odorant receptor sensitivity modulation in Drosophila. J Neurosci. 2017;37:9465–9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. [DOI] [PubMed] [Google Scholar]
- 4. Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. [DOI] [PubMed] [Google Scholar]
- 5. Pace U, Hanski E, Salomon Y, Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985;316:255–258. [DOI] [PubMed] [Google Scholar]
- 6. Wong ST, Trinh K, Hacker B, et al. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. [DOI] [PubMed] [Google Scholar]
- 8. Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. [DOI] [PubMed] [Google Scholar]
- 9. Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones WD, Nguyen TA, Kloss B, Lee KJ, Vosshall LB. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 2005;15:R119–R121. [DOI] [PubMed] [Google Scholar]
- 11. Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem. 1997;272:25425–25428. [DOI] [PubMed] [Google Scholar]
- 12. Mashukova A, Spehr M, Hatt H, Neuhaus EM. Beta-arrestin2-mediated internalization of mammalian odorant receptors. J Neurosci. 2006;26:9902–9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawson TM, Arriza JL, Jaworsky DE, et al. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–829. [DOI] [PubMed] [Google Scholar]
- 14. Borisy FF, Ronnett GV, Cunningham AM, Juilfs D, Beavo J, Snyder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992;12:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei J, Zhao AZ, Chan GC, et al. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neurons: a mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. [DOI] [PubMed] [Google Scholar]
- 16. Maue RA, Dionne VE. Patch-clamp studies of isolated mouse olfactory receptor neurons. J Gen Physiol. 1987;90:95–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bradley J, Reuter D, Frings S. Facilitation of calmodulin-mediated odor adaptation by cAMP-gated channel subunits. Science. 2001;294:2176–2178. [DOI] [PubMed] [Google Scholar]
- 18. Chen TY, Yau KW. Direct modulation by Ca(2+)-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. [DOI] [PubMed] [Google Scholar]
- 19. Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. [DOI] [PubMed] [Google Scholar]
- 20. Gorur-Shandilya S, Demir M, Long J, Clark DA, Emonet T. Olfactory receptor neurons use gain control and complementary kinetics to encode intermittent odorant stimuli. eLife. 2017;6:e27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stanczyk NM, Brookfield JF, Field LM, Logan JG. Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PLoS ONE. 2013;8:e54438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tachibana S, Touhara K, Ejima A. Modification of male courtship motivation by olfactory habituation via the GABAA receptor in Drosophila melanogaster. PLoS ONE. 2015;10:e0135186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ng M, Roorda RD, Lima SQ, Zeleman BV, Morcillo P, Meisenbock G. Transmission of olfactory information between three populations of neurons in the antenna lobe of the fly. Neuron. 2002;36:463–474. [DOI] [PubMed] [Google Scholar]
- 25. Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci. 2001;24:263–297. [DOI] [PubMed] [Google Scholar]
- 26. Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci. 2002;5:557–565. [DOI] [PubMed] [Google Scholar]
- 27. Raccuglia D, McCurdy L-Y, Demir M, et al. Presynaptic GABA receptors mediate temporal contrast enhancement in Drosophila olfactory sensory neurons and modulate odor-driven behavioral kinetics. eNeuro. 2016;3:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schoppa NE, Westbrook GL. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron. 2001;31:639–651. [DOI] [PubMed] [Google Scholar]
- 29. Root CM, Masuyama K, Green DS, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ignell R, Root CM, Birse RT, Wang JW, Nassel DR, Winther AM. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci U S A. 2009;106:13070–13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–1508.23995068 [Google Scholar]
- 32. Cafaro J. Multiple sites of adaptation lead to contrast encoding in the Drosophila olfactory system. Physiol Rep. 2016;4:e12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. [DOI] [PubMed] [Google Scholar]
- 34. Benovic JL, Bouvier M, Caron MG, Lefkowitz RJ. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol. 1988;4:405–428. [DOI] [PubMed] [Google Scholar]
- 35. Das S, Sadanandappa MK, Dervan A, et al. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci U S A. 2011;108:E646–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sweeney S, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. [DOI] [PubMed] [Google Scholar]
- 37. Getahun MN, Olsson SB, Lavista-Llanos S, Hansson BS, Wicher D. Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors. PLoS ONE. 2013;8:e58889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cao LH, Jing BY, Yang D, et al. Distinct signaling of Drosophila chemoreceptors in olfactory sensory neurons. Proc Natl Acad Sci U S A. 2016;113:E902–E911. [DOI] [PMC free article] [PubMed] [Google Scholar]