Abstract
Hospitalization can negatively impact mobility among older adults. Early detection of older patients most at-risk for mobility decline can lead to early intervention and prevention of mobility loss. This study’s purpose was to identify factors from the International Classification of Functioning, Disability, and Health associated with mobility decline among hospitalized elders. We conducted a secondary analysis of data from 959 hospitalized adults age 65 and older. We estimated the effects of health conditions, environmental, and personal factors on mobility decline using logistic regression. Almost half of the sample declined in mobility function during hospitalization. Younger age, longer length of hospital stay, having a hearing impairment, and non-emergency admit type were associated with mobility decline, after adjusting for covariates. Findings may be used to develop an evidence-based, risk-determination tool for hospitalized elders. Future research should focus on individual, environmental, and policy-based interventions promoting physical activity in the hospital.
Keywords: older adult, aging, physical function, hospitalization
Introduction
Mobility, an important component of physical functioning (PF), is essential to maintaining independence among chronically ill older adults. Mobility impairment has been associated with several important patient-centered outcomes including higher risk of 30-day hospital readmission (Fisher et al., 2013) and long-term disability (Fried, Bandeen-Roche, Chaves, & Johnson, 2000). Mobility impairment is also a predictor of future institutionalization (Hajek et al., 2015). Among women age 65 and older, poor mobility was not only associated with greater risk for hospitalization, but also greater number of inpatient days compared to women with good mobility (Ensrud et al., 2008). Mobility impairment is associated with a greater risk of falls which may contribute to significant injury and subsequent hospitalization among older adults (Enderlin et al., 2015; Ganz, Bao, Shekelle, & Rubenstein, 2007). Thus, mobility impairment can lead to adverse patient outcomes, as well as increase health care utilization and health care cost.
Hospitalization can negatively impact mobility due to disease processes and aspects of clinical care, such as surgical procedures and activity restriction (Brown, Friedkin, & Inouye, 2004; Brown, Redden, Flood, & Allman, 2009; Gill, Allore, Holford, & Guo, 2004; Lafont et al., 2011). Expedient recovery of baseline functioning after hospitalization can improve morbidity and mortality outcomes among older adults (Boyd et al., 2008; Boyd, Xue, Guralnik, & Fried, 2005; Han et al., 2013); however, only about one-third of patients are able to recover within a year of hospital discharge (Boyd et al., 2008, 2005; Han et al., 2013). Therefore, identifying factors associated with a decline in mobility among hospitalized older adults could lead to early identification of subgroups most at-risk, and guide the development of interventions to prevent progression of mobility impairment to disability.
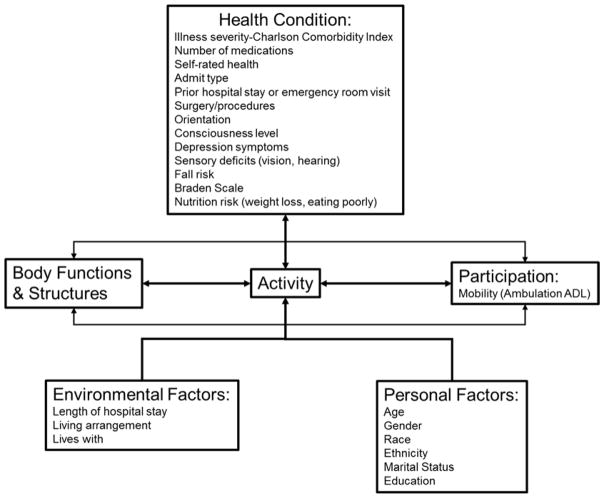
The World Health Organization’s International Classification of Functioning, Disability, and Health (ICF) framework (Figure 1) is a widely-accepted framework used in interdisciplinary research describing factors associated with disability and rehabilitation (Jette, 2006; Stucki, 2016; Stucki, Cieza, & Melvin, 2007; World Health Organization, 2002). Thus, this framework was selected to be consistent with current research on PF among older adults. The ICF suggests that an individual’s PF exists on a continuum where impairment in body function and structures (e.g., knee osteoarthritis, heart failure, respiratory illness) leads to limitations in discreet activities (e.g., ability to walk up and down stairs, ability to rise from a chair, ability to walk a distance) and potential subsequent disability (e.g., inability to do laundry, ambulate in one’s household, perform self-care activities independently). The continuum of PF, from impairment to disability, is affected by health conditions and contextual factors, such as environmental and personal factors (World Health Organization, 2002). Health conditions constitute issues such as chronic or acute illnesses and factors, sensory deficits, comorbidities, and number of medications. Environmental factors may include home environments and exposure to hospital environments. Personal factors include sociodemographic factors, such as age, gender, and race/ethnicity.
Figure 1.
Conceptual Model
Mobility limitations occur later in the ICF model, after impairments in body functions and structures occur, and relates to levels of activity and participation. Impaired mobility at the activity level may be described as impaired gait speed or balance disturbance, which can be measured by assessing performance in an isolated, clinical setting. Impaired mobility at the participation level may be described in the social context, as when an individual’s ambulation is so impaired that they are unable to walk around their house independently. Measures of mobility at this level could include basic activities of daily living (ADLs) and could be operationally defined as using the ambulation component of basic ADLs.
Past research has largely focused on factors that impact composite ADLs as measures of PF outcomes versus specifically examining mobility among hospitalized older patients. For example, among personal factors, older age (Covinsky et al., 2003; Lafont et al., 2011; Mudge, O’Rourke, & Denaro, 2010) and female gender (Buurman et al., 2012; Lafont et al., 2011) appear to be at greater risk for a loss of baseline ADL. Regarding health conditions, multiple studies have demonstrated that cognitive status, especially with the presence of dementia, also could increase risk of mobility loss (Buurman et al., 2012; Lafont et al., 2011; McCusker, Kakuma, & Abrahamowicz, 2002; Mudge et al., 2010; Volpato et al., 2007; Wakefield & Holman, 2007). Additional health conditions, such as hearing and vision impairment (Buurman et al., 2012; Lafont et al., 2011), comorbities (Lafont et al., 2011), nutritional status (Mudge et al., 2010), and fall risk (Buurman et al., 2012; Lafont et al., 2011; Volpato et al., 2007) have also been linked to a decline in PF as measured by ADLs during hospitalization. Past research also suggest that environmental factors, such as living situations (Lafont et al., 2011) and socioeconomic status (Nilsson et al., 2014), could moderate risk. Although the factors listed here have been studied in relation to composite ADLs, it is plausible they also may impact the ambulation component of basic ADLs.
Studies that have specifically examined mobility outcomes among hospitalized older adults have primarily focused on developing mobility measures (Callen, Mahoney, Wells, Enloe, & Hughes, 2004; MacKnight & Rockwood, 1995), exploring mobility as a predictor for other patient outcomes (Fisher et al., 2013; Hubbard et al., 2011; Kozakai, von Bonsdorff, Sipilä, & Rantanen, 2013; Ostir et al., 2013), or testing interventions to increase mobility among hospitalized older adults (Killey & Watt, 2006; Padula, Hughes, & Baumhover, 2009; Şimşek, Yümin, Sertel, Öztürk, & Yümin, 2012; Tucker, Molsberger, & Clark, 2004). Little research has been done to identify environmental and personal factors associated with a decline in mobility or ambulation function (Zisberg et al., 2011). Therefore, the purpose of this study is to investigate a broad range of factors associated with a decline in mobility, defined by the ambulation component of basic ADLs, among hospitalized older adults. Using the ICF (World Health Organization, 2002) as a conceptual framework, we hypothesized that environmental factors, such as living alone and length of hospital stay, and personal factors, such as older age and female gender, would be associated with a decline in mobility among hospitalized older adults. We further hypothesized that health conditions, such as poorer cognitive and nutritional status, illness severity related to comorbidities, and hearing and vision deficits, would also be associated with a decline in mobility among hospitalized older adults.
Materials and Methods
This study was a secondary analysis of data obtained from a parent study designed to develop and test a new clinical decision support tool for post-acute care referrals. For the parent study, researchers obtained assessment data from the electronic health records of a stratified random sample of 1,496 patients from six hospitals located in Pennsylvania, Connecticut, and Illinois (Bowles et al, 2016). Patients were eligible for inclusion for the parent study if they were age 55 and older, were admitted and discharged from one of the four hospitals between October 2011 and July 2012, and whose stay was coded as inpatient versus observational stays. Patient selection for the parent study was stratified by primary diagnosis categories to match percentages of patients in each 16 of the most common primary diagnoses of hospitalized patient nationwide. Patients were then randomly selected within each primary diagnosis category. Data were collected by hospital staff nurses as part of a structured nursing admission assessment conducted on all new patients and from the nursing documentation as the patient was cared for during the hospital stay. The data set included 71 patient characteristics such as sociodemographic and health data as well as clinical assessments (e.g., Braden Pressure Ulcer Risk Assessment and Fall Risk), including an assessment of ADLs collected by registered nurses either upon admission assessment of the patient or in daily documentation during the hospital stay. Patients in the parent study were not followed longitudinally; therefore, no data regarding rehospitalizations were collected.
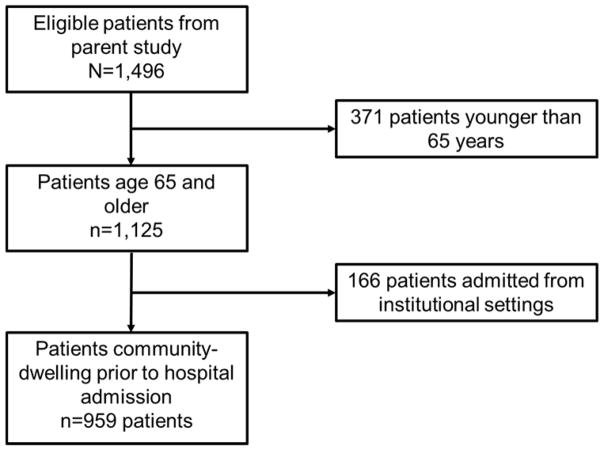
Patients were included in this secondary analysis if they were age 65 and older and were community-dwelling prior to hospital admission. Figure 2 depicts sample selection from the patients included in the parent study. We limited the sample to age 65 and older to be consistent with national and global definitions of “older adult” (Centers for Medicare and Medicaid Services, 2014; World Health Organization, 2016). The ICF framework and relevant past literature were used to guide selection of 25 variables for the present study. Some variables in the original data set were collapsed into categories as described below. Approval for the present study was obtained from the University of Pennsylvania and University of Missouri Institutional Review Boards.
Figure 2.
Sample selection from parent study patients
Independent variables
Independent variables selected for analysis were categorized under the ICF constructs of health conditions and environmental and personal factors (Figure 1).
Health conditions
Comorbidities, number of prescribed medications, and surgeries or procedures for each patient were collected from the patients’ electronic health records. Comorbidities were identified using ICD-9 diagnosis codes on patient admission. Comorbidities were then used to calculate the age-adjusted Charlson Comorbidity Index (CCI) (Charlson, Pompei, Ales, & MacKenzie, 1987; Deyo, Cherkin, & Ciol, 1992). The CCI was developed and validated as a tool to predict mortality and assigns weights to 17 different diagnoses according to 1-year mortality risk. Higher CCI indicates greater illness severity and mortality risk. The age-adjusted CCI incorporates patient age such that an extra point is assigned to patients’ scores for each decade of age above 50 (Charlson, Szatrowski, Peterson, & Gold, 1994). Primary diagnoses were only used to describe the sample due to the large number of different diagnoses entered in patient charts. Admit type was also obtained from patient charts and categorized as emergency or not emergency (e.g., elective or transfer) admit. Self-rated health was defined using three categories: poor-fair, average, good-excellent. Self-report prior hospitalization and prior emergency room visits were dichotomized as yes/no to stays or visits within the prior six months. Patient orientation was assessed by nurses and operationally defined as oriented or not to person, place, time, and situation. To assess orientation to person, patients were asked their name. To assess orientation to place, patients were asked if they knew where they were (e.g., in the hospital). To assess orientation to time, patients were asked to provide the current day of the week, date, and year. To assess situation, patients were asked why they were in the hospital. Patients were categorized as oriented x4, indicating orientation to all four domains, or not oriented x4 (e.g., only oriented to three or less domains). Similarly nursing assessment of consciousness level was operationalized as alert or not alert. Patient-reported depression symptoms, nutrition risk based on weight loss, nutrition risk based on poor eating, and nurse-assessed vision and hearing impairments were all dichotomized (e.g., presence/absence). Fall risk was assessed by nurses using the Morse Fall Scale (Morse, Black, Oberle, & Donahue, 1989; Morse, Morse, & Tylko, 1989; Morse, 1997). The Morse Fall Scale has moderate predictive validity with a sensitivity of 0.76 (95% CI 0.70 – 0.81) and specificity of 0.68 (95% CI 0.66 – 0.70) among hospitalized patients (Aranda-Gallardo et al., 2013). Data were collapsed into risk or no risk categories. Patients who were scored at no risk on the scale were categorized as “no risk” for falls; whereas patients who scored at low, moderate, or high risk on the scale were categorized as “at risk” for falls.” Braden Pressure Ulcer Risk Assessment risk categories were similarly collapsed into risk or no risk options. A recent meta-analysis reported moderate predictive validity for this measure among hospitalized older adults, with a sensitivity of 0.71 (95% CI 0.67 – 0.74) and specificity of 0.68 (95% CI 0.67 – 0.70) (Park, Lee, & Kwon, 2016). Patients who were scored as not at risk category were categorized as “no risk” for pressure ulcers; whereas patients who scored as mild, moderate, high, or severe risk were categorized as “at risk” for pressure ulcers.
Environmental factors
Environmental factors are defined as the external, physical, and social environment in which people live and interact (World Health Organization, 2002). Living arrangement was collapsed into two categories: whether or not the patient lived in a single family home or apartment environment. Data on whether or not the patient lived alone or with someone were also collected. These data were collected by nursing staff. Different hospital environments may contribute to different patient outcomes; thus, we created a variable for each of the four hospitals from which patients were recruited. Hospital length of stay was included in the environmental category, as this variable describes duration of exposure to a specific environment. This information was collected from the hospital administrative data.
Personal factors
Personal factors include demographic variables, such as age, gender, and race/ethnicity. Age was categorized by decades (e.g., 65–74 years, 75–84 years, greater than 85 years old). Although the original data had multiple race categories, there were too few patients in some categories (e.g., Asian, Native American) to include in the analysis. Thus, race was categorized to three levels: White, African-American, or unknown. Ethnicity was similarly categorized into non-Hispanic/Latino, Hispanic Latino, and unknown. Marital status was dichotomized into married/partnered or single. Those who were divorced or separated were determined to be single. Education was defined as up to high school only or college and beyond. These data were collected by nursing staff.
Dependent variable
Mobility was conceptually defined as ambulation function and operationalized using a single-item, ADL assessment of ambulation function performed by nurses on admission to and near discharge from the hospital. Responses were based on level of required assistance categorized as independent, assistive equipment, assistive person, assistive person and equipment, or completely dependent. To describe change in mobility for each patient, admission and discharge responses were compared and responses were dichotomized into decline or no decline in ambulation function. For example, a patient who was independent on admission, but required assistive equipment on discharge was described as having a decline in ambulation function or mobility. The “no decline” category encompassed both improvement and no change in mobility outcomes. The data set for this study only contained information on the dichotomized ambulation function variable.
Statistical Analysis
To summarize all control variables, categorical variables were described using frequencies and percentages while continuous variables were described with means and standard deviations or medians and interquartile ranges. For categorical variables, a Chi-square test of independence was used to examine differences in characteristics of patients who declined versus those that did not decline in mobility as well as to detect differences in complete and incomplete outcome data. Two sample t-tests were conducted for all continuous variables. Univariate logistic regression analyses were used to assess the individual impact of each covariate on the odds of decline in mobility.
Backwards selection was employed to fit a multivariable logistic regression model to examine the effects of environmental, personal, and health condition factors on decline in mobility. All variables significant at the 0.20 level in univariate analyses were considered in the full model. The final multivariable model using odds ratios (OR) was built using backwards selection with a stopping criteria of 0.05. The moderating effect of age×gender was examined. A significance level of 0.10 was selected for the interaction term because it requires more power and we did not wish to dismiss an important finding. The final model was adjusted for gender, age-adjusted CCI, and surgery/procedures, which were forced into the model on the basis of existing literature. Model fit was examined using the Hosmer Lemeshow goodness of fit test and the c-statistic. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Inc., 2013).
Results
Table 1 shows descriptive statistics of the sample. Of the 959 patients included in this secondary analysis, 49% experienced a decline in mobility. Age range for patients was 65 to 103. Mean age of the sample was 78.14 years (SD 8.29). Patients were predominantly White (88%), and female (56%). Only 1% of participants identified themselves as Hispanic/Latino. Fifty-three percent of patients were married and 67% did not live alone. Only about 32% of patients were educated beyond the high school level. Patients spent from one to 33 days in the hospital, with a median of 5 days (Interquartile Range=4–8). The sample was clinically complex overall, as patients took an average of 8.51 medications (SD 5.43, range 0–29) and had a mean age-adjusted CCI score of 5.58 (SD 2.36, range 2–16). Furthermore, based on Morse Fall Scale scores, 81% of patients were at risk for falls. The five most common primary diagnoses, in order of most frequent, were pneumonia, atrial fibrillation, urinary tract infection, obstructive chronic bronchitis and osteoarthrosis. Over half of the sample (57%) had a surgery or procedure during hospitalization. Most had not had a hospital stay (60%) or emergency room (58%) visit six months prior to this hospitalization. Overall 48% patients rated their health as good to excellent, about 90% were alert, and 91% were oriented to person, place, time, and situation. Most patients were not experiencing recent weight loss (96%), poor eating (93%), or depressive symptoms (93%). Although 51% of patients reported some vision impairment, 78% did not have a hearing impairment.
Table 1.
Participant Characteristics of Decline vs. No Decline in Ambulation Function
| Variable | Overall N (%) Mean±SD |
Decline N (%) Mean±SD |
No Decline N (%) Mean±SD |
P–value* |
|---|---|---|---|---|
|
| ||||
| Sex | .545 | |||
|
| ||||
| Female | 486 (55.61) | 243 (56.64) | 243 (54.61) | |
| Male | 388 (44.39) | 186 (43.36) | 202 (45.39) | |
|
| ||||
| Race | .255 | |||
|
| ||||
| White | 737 (88.16) | 365 (89.46) | 372 (86.92) | |
| Black/African-American | 99 (11.84) | 43 (10.54) | 56 (13.08) | |
|
| ||||
| Ethnicity | .008 | |||
|
| ||||
| Non-Hispanic/Latino | 523 (59.84) | 236 (55.01) | 287 (64.49) | |
| Hispanic/Latino | 13 (1.49) | 5 (1.17) | 8 (1.80) | |
| Unknown | 338 (38.67) | 188 (43.82) | 150 (33.71) | |
|
| ||||
| Education | .274 | |||
|
| ||||
| Up to high school only | 550 (68.24) | 263 (66.41) | 287 (70.00) | |
| College and Beyond | 256 (31.76) | 133 (33.59) | 123 (30.00) | |
|
| ||||
| Age | <.001 | |||
|
| ||||
| 65–74 years old | 375 (39.10) | 183 (42.66) | 172 (38.65) | |
|
| ||||
| 75–84 years old | 340 (35.45) | 160 (37.30) | 127 (28.54) | |
|
| ||||
| 85 years and older | 244 (25.44) | 86 (20.05) | 146 (32.81) | |
|
| ||||
| Living Arrangement | .500 | |||
|
| ||||
| House/Mobile home | 712 (84.56) | 357 (85.41) | 355 (83.73) | |
| Other community dwelling (e.g., apartment) | 130 (15.44) | 61 (14.59) | 69 (16.27) | |
|
| ||||
| Lives With | .323 | |||
|
| ||||
| Alone | 273 (32.54) | 128 (30.92) | 145 (34.12) | |
| Not alone | 566 (67.46) | 286 (69.08) | 280 (65.88) | |
|
| ||||
| Marital Status | .294 | |||
|
| ||||
| Married or life partner | 408 (46.90) | 235 (54.91) | 227 (51.36) | |
| Not married | 462 (53.10) | 193 (45.09) | 215 (48.64) | |
|
| ||||
| Hospital Stay Past 6 Months | .287 | |||
|
| ||||
| None | 484 (59.98) | 226 (57.22) | 258 (62.62) | |
| Once | 199 (24.66) | 105 (26.58) | 94 (11.65) | |
| Two or More | 124 (15.37) | 64 (16.20) | 60 (14.56) | |
|
| ||||
| Emergency Room Visit Past 6 Months | .215 | |||
|
| ||||
| None | 465 (57.98) | 219 (27.31) | 246 (59.85) | |
| Once | 204 (25.44) | 98 (25.06) | 106 (25.79) | |
| Two or More | 124 (15.37) | 74 (18.93) | 59 (14.36) | |
|
| ||||
| Surgery or Procedure | .010 | |||
|
| ||||
| No | 373 (42.73) | 164 (38.32) | 209 (46.97) | |
| Yes | 500 (57.27) | 264 (61.68) | 236 (53.03) | |
|
| ||||
| Hospital | .731 | |||
|
| ||||
| 1 | 238 (24.82) | 104 (24.24) | 108 (24.27) | |
|
| ||||
| 2 | 243 (25.34) | 110 (25.64) | 117 (26.29) | |
|
| ||||
| 3 | 231 (24.09) | 98 (22.84) | 112 (25.17) | |
|
| ||||
| 4 | 247 (25.76) | 117 (27.27) | 108 (24.27) | |
|
| ||||
| Length of Hospital Stay Median (IQR=Q1-Q3) | 5 (4) | 6 (4) | 5 (3) | .005 |
|
| ||||
| Age-adjusted Charlson Comorbidity Index | 5.58 ± 2.36 | 5.53 ± 2.37 | 5.61 ± 2.35 | .607 |
|
| ||||
| Number of Medications | 8.51 ± 5.43 | 8.63 ± 5.51 | 8.51 ± 5.42 | .738 |
|
| ||||
| Admit Type | <.001 | |||
|
| ||||
| Emergency | 730 (83.52) | 337 (78.55) | 393 (88.31) | |
| Not emergency | 144 (16.48) | 92 (21.45) | 52 (11.69) | |
|
| ||||
| Self-Rated Health | .974 | |||
|
| ||||
| Poor to fair | 171 (20.28) | 82 (20.00) | 89 (20.55) | |
| Average | 269 (31.91) | 137 (31.64) | 132 (32.20) | |
| Good to excellence | 403 (47.81) | 207 (47.81) | 196 (47.80) | |
|
| ||||
| Orientation | .020 | |||
|
| ||||
| Oriented x4** | 765 (91.40) | 386 (93.69) | 379 (89.18) | |
| Not oriented x4 | 72 (8.60) | 26 (6.31) | 46 (10.82) | |
|
| ||||
| Consciousness Level | .323 | |||
|
| ||||
| Alert | 761 (89.85) | 379 (90.89) | 382 (88.84) | |
| Confused or not alert | 86 (10.15) | 38 (9.11) | 48 (11.16) | |
|
| ||||
| Fall Risk Score | .715 | |||
|
| ||||
| Not at fall risk | 165 (18.94) | 83 (19.44) | 82 (18.47) | |
| At fall risk | 706 (81.06) | 344 (80.56) | 362 (81.53) | |
|
| ||||
| Braden Scale Score | .473 | |||
|
| ||||
| Not at risk | 595 (68.08) | 297 (69.23) | 298 (66.97) | |
| At risk | 279 (31.92) | 108 (25.17) | 127 (28.54) | |
|
| ||||
| Weight Loss | .657 | |||
|
| ||||
| No | 822 (96.14) | 406 (96.44) | 416 (95.85) | |
| Yes | 33 (3.86) | 15 (3.56) | 18 (4.15) | |
|
| ||||
| Eating Poorly | .879 | |||
|
| ||||
| No | 797 (93.22) | 393 (93.35) | 404 (93.09) | |
| Yes | 58 (6.78) | 28 (6.65) | 30 (6.91) | |
|
| ||||
| Depression Symptoms | .473 | |||
|
| ||||
| No | 713 (91.29) | 345 (90.55) | 368 (92.00) | |
| Yes | 68 (8.71) | 36 (9.45) | 32 (8.00) | |
|
| ||||
| Vision Impairment | .669 | |||
|
| ||||
| No | 387 (48.93) | 179 (48.12) | 208 (49.64) | |
| Yes | 404 (51.07) | 193 (51.88) | 211 (50.36) | |
|
| ||||
| Hearing Impairment | .018 | |||
|
| ||||
| No | 636 (78.23) | 288 (74.61) | 348 (81.50) | |
| Yes | 177 (21.11) | 98 (25.39) | 79 (18.50) | |
Note.
p-values from chi-square test for categorical variables or two-sample t-test for continuous variables.
Orientedx4 indicates orientation to person, place, time, and situation.
Table 1 provides descriptive statistics for ICF variables by decline status, along with statistical comparisons. Table 2 lists the results for the univariate analyses. The variables admit type, having a surgery/procedure, orientation, having a hospital stay in the past 6 months, presence of a hearing impairment, length of hospital stay, and age were included in a multivariable logistic regression and backward selection was used to construct the final adjusted model. For the final adjusted model (Table 3), age×gender (p=.280), was not statistically significant; therefore, it was not included in the final model. Good model fit was determined based on the Hosmer-Lemeshow Test (p = .267) and c-statistic (0.624).
Table 2.
Odds of Decline in Ambulation Function Based on Univariate Analysis
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Sex | |||
|
| |||
| Female | 1.09 | 0.83 – 1.42 | .545 |
| Male | REF | REF | |
|
| |||
| Race | |||
|
| |||
| White | REF | REF | |
| Black/African-American | 0.78 | 0.51 – 1.19 | .256 |
|
| |||
| Education | |||
|
| |||
| Up to high school only | 0.85 | 0.63 – 1.14 | .275 |
| College and Beyond | REF | REF | |
|
| |||
| Age | |||
|
| |||
| 65–74 years old | 1.81 | 1.29 – 2.53 | .001 |
|
| |||
| 75–84 years old | 2.14 | 1.50 – 3.05 | <.001 |
|
| |||
| 85 years and older | REF | REF | |
|
| |||
| Living Arrangement | |||
|
| |||
| House/Mobile home | REF | REF | |
| Other community dwelling (e.g., apartment) | 0.88 | 0.61 – 1.28 | .501 |
|
| |||
| Lives With | |||
|
| |||
| Alone | REF | REF | |
| Not alone | 1.16 | 0.87 – 1.55 | .323 |
|
| |||
| Marital Status | |||
|
| |||
| Married or life partner | REF | REF | |
| Not married | 1.15 | 0.88 – 1.51 | .295 |
|
| |||
| Hospital Stay Past 6 Months | |||
|
| |||
| No | REF | REF | |
| Once | 1.28 | 0.92 – 1.78 | .150 |
| Two or More | 1.22 | 0.82 – 1.81 | .329 |
|
| |||
| Emergency Room Visit Past 6 Months | |||
|
| |||
| No | REF | REF | |
| Once | 1.04 | 0.75 – 1.44 | .822 |
| Two or More | 1.41 | 0.96 – 2.08 | .083 |
|
| |||
| Surgery or Procedure | |||
|
| |||
| No | REF | REF | |
| Yes | 1.43 | 1.09 – 1.87 | .010 |
|
| |||
| Hospital | |||
|
| |||
| 1 | 0.89 | 0.61 – 1.29 | .539 |
|
| |||
| 2 | 0.87 | 0.60 – 1.26 | .452 |
|
| |||
| 3 | 0.81 | 0.55 – 1.18 | .267 |
|
| |||
| 4 | REF | REF | REF |
|
| |||
| Length of Hospital Stay | 1.05 | 1.02 – 1.09 | .006 |
|
| |||
| Age-adjusted Charlson Comorbidity Index | 0.99 | 0.93 – 1.04 | .607 |
|
| |||
| Number of Medications | 1.00 | 0.98 – 1.03 | .737 |
|
| |||
| Admit Type | |||
|
| |||
| Emergency | REF | REF | |
| Not emergency | 2.06 | 1.43 – 2.99 | <.001 |
|
| |||
| Self-Rated Health | |||
|
| |||
| Poor to fair | 0.96 | 0.65 – 1.40 | .819 |
| Average | REF | REF | |
| Good to excellence | 0.98 | 0.72 – 1.34 | .912 |
|
| |||
| Orientation | |||
|
| |||
| Oriented x4* | REF | REF | |
| Not oriented x4 | 0.56 | 0.34 – 0.92 | .021 |
|
| |||
| Consciousness Level | |||
|
| |||
| Alert | REF | REF | |
| Confused or not alert | 0.80 | 0.51 – 1.25 | .324 |
|
| |||
| Fall Risk Score | |||
|
| |||
| Not at fall risk | REF | REF | |
| At fall risk | 0.94 | 0.67 – 1.32 | .715 |
|
| |||
| Braden Scale Score | |||
|
| |||
| Not at risk | REF | REF | |
| At risk | 0.90 | 0.68 – 1.20 | .473 |
|
| |||
| Weight Loss | |||
|
| |||
| No | REF | REF | |
| Yes | 0.85 | 0.43 – 1.72 | .659 |
|
| |||
| Eating Poorly | |||
|
| |||
| No | REF | REF | |
| Yes | 0.96 | 0.56 – 1.64 | .879 |
|
| |||
| Depression Symptoms | |||
|
| |||
| No | REF | REF | |
| Yes | 1.20 | 0.73 – 1.98 | .473 |
|
| |||
| Vision Impairment | |||
|
| |||
| No | REF | REF | |
| Yes | 1.06 | 0.80 – 1.41 | .669 |
|
| |||
| Hearing Impairment | |||
|
| |||
| No | REF | REF | |
| Yes | 1.50 | 1.07 – 2.10 | .018 |
Note. OR=odds ration; 95% CI=95% confidence interval; REF=reference category.
Orientedx4 indicates orientation to person, place, time, and situation.
Table 3.
Odds ratios for Multivariable Logistic Regression Analysis of Decline in Ambulation Function on Various Characteristics
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Age | |||
|
| |||
| 65–74 years old | 1.68 | 1.15 – 2.47 | .008 |
|
| |||
| 75–84 years old | 2.19 | 1.50– 3.20 | <.001 |
|
| |||
| 85 years and older | REF | REF | REF |
|
| |||
| Length of Hospital Stay | 1.04 | 1.00 – 1.08 | .035 |
|
| |||
| Admit Type | |||
|
| |||
| Emergency | REF | REF | |
| Not emergency | 1.62 | 1.06 – 2.49 | .026 |
|
| |||
| Surgery or Procedure | |||
|
| |||
| No | REF | REF | |
|
| |||
| Yes | 1.26 | 0.93 – 1.70 | .136 |
|
| |||
| Hearing Impairment | |||
|
| |||
| No | REF | REF | |
| Yes | 1.58 | 1.12 – 2.23 | .009 |
|
| |||
| Age-adjusted Charlson Comorbidity Index | 1.03 | 0.97 – 1.10 | .376 |
|
| |||
| Gender | |||
|
| |||
| Male | REF | REF | |
| Female | 1.15 | 0.86 – 1.54 | .338 |
Note. OR=odds ration; 95% CI=95% confidence interval; REF=reference category
Health conditions
In the univariate analyses, health condition factors of having surgery/procedure (p=.010), non-emergency admit type (p<.001), orientation times four (p =.020), and having a hearing impairment (p =.018) were statistically significantly associated with a decline in mobility. Table 3 displays the results of the final, adjusted, multivariable logistic regression model. Non-emergency admit type and having a hearing impairment remained significant health condition factors. The odds of decline for patients who had a hearing impairment were 1.58 times as large in those who did not decline in mobility (95% CI: 1.12–2.23). Patients that were non-emergency admits were 1.62 times as likely to decline in mobility versus not decline compared to patients that were emergency admits (95% CI: 1.06–2.49).
Environmental factors
Having one hospital stay in the past 6 months (p=.010) and length of hospital stay (p=.006) were significantly associated with mobility decline in the univariate analysis. In the final adjusted model, however, only longer length of hospital stay (OR 1.04; 95% CI: 1.00–1.08) remained statistically significant (Table 3).
Personal factors
Personal factors significantly associated with a decline in mobility were unknown ethnicity (p =.008) and age (p<.001) in the univariate analysis. In the final adjusted model, patients who were age 65–74 (OR: 1.68; 95% CI 1.15–2.47) or age 75–84 (OR: 2.19; 95% CI: 1.50–3.20) were more likely to decline in mobility versus not decline compared to those patients 85 years or older.
Discussion
Mobility is essential to maintaining independence and quality of life among older adults. Our study addresses an important research gap concerning the identification of factors associated with a decline in mobility among hospitalized older adults. Identifying risk factors for mobility decline in this population could lead to early identification of at-risk subgroups and guide the development of targeted interventions to prevent impairment and subsequent disability in mobility.
We hypothesized that health conditions, environmental factors, and personal factors would be associated with a decline in mobility among hospitalized patients. Although our hypotheses were supported, only a few variables from each ICF construct reached statistical significance. Two health condition-related factors were statistically significant. In our study, older patients who were admitted non-emergently were more likely to decline in mobility versus not decline. It is possible that, in our sample, patients admitted non-emergently had greater room for decline versus patients who were admitted emergently. Patients admitted non-emergently included elective admissions who may have been more ambulatory prior to admission and were being admitted for surgeries or procedures. Additional research may be needed to examine and compare admission sources as a predictor of mobility outcome in a larger, more diverse sample.
Previous research has linked sensory deficits with poor PF (Lafont et al., 2011). In our study, hearing impairment, but not vision impairment, was associated with a decline in mobility. This association could have multiple explanations. Some older patients may not have hearing aids or amplifiers given their cost, or may have chosen not to bring their hearing devices fearing they may get lost in the hospital. As a result, communication challenges with clinical staff may impact hearing impaired older patients’ ability or willingness to engage in physical activity to prevent a decline in mobility (Gispen, Chen, Genther, & Lin, 2014). Research evidence has also suggested a link between hearing and balance function given the close proximity of physiologic structures attributed to both functions (Chen et al., 2015; Mikkola et al., 2015). Balance disturbances could limit activity, especially in an unfamiliar setting such as the hospital, and further impact mobility (Chen et al., 2015; Gispen et al., 2014; Mikkola et al., 2015). Limited environmental awareness and perception has also been posited as a potential factor linking hearing impairment to poor PF (Chen et al., 2015; Mikkola et al., 2015). Clinicians caring for hospitalized older patients should assess them for hearing impairments given the potential connections between sensory deficit and mobility. Efforts should be made to ensure hearing impaired older patients have access to their hearing aids or amplifiers. Furthermore, research is needed to explore the impact of interventions at the individual level, such as hearing rehabilitative interventions and the policy level, such as financial support for hearing aids, on mobility outcomes.
We also found that hospital length of stay, an environmental factor, was associated with increased odds of decline in mobility. Older adults who spend more time in the hospital may have greater illness severity, affecting their mobility. Additionally, more time in the hospital could expose older adults to more procedures, such as surgeries or invasive testing, that require limited mobility afterwards. It is well known that limited mobility and immobility during hospitalization greatly increase the risk of functional decline in this population (Brown et al., 2004, 2009; Zisberg et al., 2011). Clinicians should be cognizant of older patients’ mobility in the hospital setting and evaluate rationales for orders to limit mobility. Reviewing the appropriateness of bed rest and limited mobility orders could reveal circumstances in which activity limitations could be modified to encourage greater activity among older adult patients to prevent mobility decline (Brown et al., 2004; Gill et al., 2004; Kleinpell, 2007; Yoon et al., 2015). Interventions targeting hospital environments are also needed to ensure ample opportunities to mobilize older patients. Researchers have tested physical activity interventions to increase mobility among hospitalized older adults (Drolet et al., 2013; Killey & Watt, 2006; Padula et al., 2009), as well as ways to accurately and consistently quantify mobility in this population (Brown, Roth, & Allman, 2008; Fisher et al., 2011). However, additional research is needed to test feasibility of different technologies, such as sensors or accelerometers, to evaluate continuous, real-time mobility among older adults for the duration of their hospital stay. These types of measures could be useful in early detection and prevention of mobility impairment and disability related to hospitalization.
Age was the only personal factor associated with a decline in mobility in our study. Physical and cognitive changes associated with older age may increase sedentary behavior, which could impact mobility and overall PF (Manns, Ezeugwu, Armijo-Olivo, Vallance, & Healy, 2015). Although younger age was associated with a decline in mobility in our sample, past research has demonstrated a relationship between older age and worsening PF (Buurman et al., 2012; Covinsky et al., 2003; Lafont et al., 2011; Mudge et al., 2010). Prior to hospitalization, young-older patients may be more ambulatory and have better mobility function prior to hospitalization; whereas old-older patients may already have some mobility limitations. It is possible that, in our sample, the young-older patients had more room to decline in mobility compared to the old-old. In contrast, pre-hospitalization mobility limitations among old-older patients may prompt providers to order rehabilitative therapies possibly resulting in an improvement in mobility function. When appropriate, promoting independence in activities and limiting sedentary time among all hospitalized older patients could optimize mobility function and prevent mobility decline (Boltz, Chippendale, Resnick, & Galvin, 2015; Boltz, Resnick, Capezuti, Shabbat, & Secic, 2011; Boltz, Resnick, Capezuti, Shuluk, & Secic, 2012; Resnick & Galik, 2013). For example, Boltz and colleagues (2015) demonstrated better walking performance and less cognitive symptoms among older adults with dementia who received nursing care designed to engage older patients in ADLs and physical activity. Additional research is needed to scale this type of nursing intervention to larger, more diverse older adult samples.
Study Limitations
This study has some limitations which may impact interpretation and generalizability of findings. This study is a secondary analysis of data used to develop a clinical decision support tool (Bowles et al, 2016). Thus, limitations regarding the operationalization of mobility are present. The data set used for this study included the final categorization of decline/no decline for ambulation function. Therefore, patients who may have been completely dependent in mobility function at baseline were included in this sample, creating a floor effect (e.g., patients who are completely dependent have no further room to decline based on the specific ADL ambulation categories). We excluded older adults who were admitted to the hospital from institutional settings, such as nursing homes or assisted living facilities, to mitigate the potential inclusion of patients who may be completely dependent in mobility. Regardless, such ceiling and floor effects may limit using ADLs as research measures (Hartigan, 2007). Performance-based measures of mobility, such as gait speed or 6-minute walk test, may be more accurate assessments to determine objective changes in mobility. Replication of this study or future primary research examining mobility among hospitalized older adults should involve performance-based measures of mobility.
Although the sample for the parent study was taken from multiple hospital locations across the country, racially and ethnically diverse older adults were not well represented in this study; therefore, generalizing this study’s findings to more diverse populations is limited. Past research has demonstrated socioecomonic status as an important factor in mobility status (Nilsson et al., 2014). Unfortunately, we had little information on socioeconomic status of the sample, limiting our ability to fully evaluate this variable. Future research examining PF among older adults should explore important social determinants of health by including more diverse samples and examining socioeconomic variables, to ensure generalizability to a larger population, and to identify possible disparities in patient outcomes.
The analytic sample size was limited due to our age inclusion criteria. Thus it is possible that some associations that have been recognized in prior research as factors linked to PF decline, such as cognition, depression, or social factors, might also be statistically significant with a larger, more diverse sample. We used common nursing assessments to evaluate patient orientation; however, we do not have information regarding reliability and validity of these nursing assessments. Researchers may consider using more validated, objective measures of orientation in future studies.
Conclusion
Hospitalized older adults are at risk for a decline in mobility, which could ultimately impact their quality of life. Determining which older adults are at most risk for mobility decline during hospitalization is an initial step to developing interventions to prevention mobility loss. Findings from this study demonstrated non-emergent admission, the presence of a hearing impairment, longer length of hospital stay, younger age, and fewer comorbidities were significantly associated with a decline in mobility. Further research is needed to implement early risk determination among older adults admitted to the hospital, to develop feasible, real-time mobility measures, and to examine individual, environmental, and policy-based interventions to prevent mobility decline among hospitalized older adults. Moreover, additional research is needed to identify factors associated with mobility decline among more racially and ethnically diverse older patients.
Acknowledgments
Research reported in this publication was supported by the National Institute Of Nursing Research of the National Institutes of Health under Award Number R01NR007674 (Bowles PI) T32NR009356 (Chase, postdoctoral fellow). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography
Dr. Chase is a Ruth L. Kirschtein Postdoctoral Fellow, Ms. Lozano is a Master’s Level Data Analyst, Dr. Hanlon is a Research Professor of Biostatistics, Dr. Bowles is a van Ameringen Professor in Nursing Excellence at the University of Pennsylvania School of Nursing, Philadelphia, Pennsylvania. Dr. Chase is also Assistant Professor at the University of Missouri Sinclair School of Nursing, Columbia, Missouri. Dr. Bowles is also Director of the Center for Home Care Policy and Research at the Visiting Nurse Service of New York, New York, New York.
Contributor Information
Jo-Ana D. Chase, Post-doctoral Fellow, University of Pennsylvania, 338G School of Nursing, 418 Curie Blvd., Philadelphia, PA 19104, Assistant Professor, University of Missouri – Columbia.
Alicia Lozano, Master’s Level Data Analyst, 418 Curie Blvd, Suite 479L, Claire M. Fagin Hall, School of Nursing, University of Pennsylvania.
Alexandra Hanlon, Research Professor of Biostatistics, 418 Curie Blvd, Suite 479L, Claire M. Fagin Hall, School of Nursing, University of Pennsylvania.
Kathryn H. Bowles, van Ameringen Professor in Nursing Excellence, University of Pennsylvania School of Nursing, 418 Curie Boulevard Room 340, Philadelphia, PA 19104; Director of the Center for Home Care Policy and Research, Visiting Nurse Service of New York.
References
- Boltz M, Chippendale T, Resnick B, Galvin JE. Testing family-centered, function-focused care in hospitalized persons with dementia. Neurodegenerative Disease Management. 2015;5(3):203–215. doi: 10.2217/nmt.15.10. http://doi.org/10.2217/nmt.15.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltz M, Resnick B, Capezuti E, Shabbat N, Secic M. Function-focused care and changes in physical function in Chinese American and non-Chinese American hospitalized older adults. Rehabilitation Nursing: The Official Journal of the Association of Rehabilitation Nurses. 2011;36(6):233–240. doi: 10.1002/j.2048-7940.2011.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Boltz M, Resnick B, Capezuti E, Shuluk J, Secic M. Functional decline in hospitalized older adults: can nursing make a difference? Geriatric Nursing (New York, NY) 2012;33(4):272–279. doi: 10.1016/j.gerinurse.2012.01.008. http://doi.org/10.1016/j.gerinurse.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Bowles KH, Ratcliffe S, Potashnik S, Topaz M, Holmes J, Shih NW, Naylor MD. Using electronic case summaries to elicit multi-disciplinary expert knowledge about referrals to post-acute care. Applied Clinical Informatics. 2016;7(2):368–379. doi: 10.4338/ACI-2015-11-RA-0161. org/10.4338/ACI-2015-11-RA-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, … Covinsky KE. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. Journal of the American Geriatrics Society. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. http://doi.org/10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CM, Xue QL, Guralnik JM, Fried LP. Hospitalization and development of dependence in activities of daily living in a cohort of disabled older women: the Women’s Health and Aging Study I. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2005;60(7):888–893. doi: 10.1093/gerona/60.7.888. [DOI] [PubMed] [Google Scholar]
- Brown C, Roth D, Allman R. Validation of use of wireless monitors to measure levels of mobility during hospitalization. Journal of Rehabilitation Research. 2008;45(4):551–8. doi: 10.1682/jrrd.2007.06.0086. [DOI] [PubMed] [Google Scholar]
- Buurman BM, Hoogerduijn JG, van Gemert EA, de Haan RJ, Schuurmans MJ, de Rooij SE. Clinical characteristics and outcomes of hospitalized older patients with distinct risk profiles for functional decline: a prospective cohort study. PloS One. 2012;7(1):e29621. doi: 10.1371/journal.pone.0029621. http://doi.org/10.1371/journal.pone.0029621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen B, Mahoney J, Wells T, Enloe M, Hughes S. Admission and discharge mobility of frail hospitalized older adults. MEDSURG Nursing. 2004;13(3):156–63. [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Overview. 2014 Jul 25; Retrieved June 15, 2016, from https://www.cms.gov/Medicare/Medicare-General-Information/MedicareGenInfo/index.html. [PubMed]
- Charlson M, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, Martin KR … Health ABC study. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2015;70(5):654–661. doi: 10.1093/gerona/glu207. http://doi.org/10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, … Landefeld CS. Loss of Independence in Activities of Daily Living in Older Adults Hospitalized with Medical Illnesses: Increased Vulnerability with Age. Journal of the American Geriatrics Society. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. http://doi.org/10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Drolet A, DeJuilio P, Harkless S, Henricks S, Kamin E, Leddy EA, … Williams S. Move to improve: the feasibility of using an early mobility protocol to increase ambulation in the intensive and intermediate care settings. Physical Therapy. 2013;93(2):197–207. doi: 10.2522/ptj.20110400. http://doi.org/10.2522/ptj.20110400. [DOI] [PubMed] [Google Scholar]
- Enderlin C, Rooker J, Ball S, Hippensteel D, Alderman J, Fisher SJ, … Jordan K. Summary of factors contributing to falls in older adults and nursing implications. Geriatric Nursing. 2015;36(5):397–406. doi: 10.1016/j.gerinurse.2015.08.006. http://doi.org/10.1016/j.gerinurse.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Ensrud K, Ewing S, Taylor B, Fink H, Cawthon P, Stone K, … Cummings S. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Archives of Internal Medicine. 2008;168(4):382–9. doi: 10.1001/archinternmed.2007.113. http://doi.org/http://dx.doi.org/10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Ambulatory activity of older adults hospitalized with acute medical illness. Journal of the American Geriatrics Society. 2011;59(1):91–95. doi: 10.1111/j.1532-5415.2010.03202.x. http://doi.org/10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SR, Kuo YF, Sharma G, Raji MA, Kumar A, Goodwin JS, … Ottenbacher KJ. Mobility after hospital discharge as a marker for 30-day readmission. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2013;68(7):805–810. doi: 10.1093/gerona/gls252. http://doi.org/10.1093/gerona/gls252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2000;55(1):M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77–86. doi: 10.1001/jama.297.1.77. http://doi.org/10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- Gispen FE, Chen DS, Genther DJ, Lin FR. Association between hearing impairment and lower levels of physical activity in older adults. Journal of the American Geriatrics Society. 2014;62(8):1427–1433. doi: 10.1111/jgs.12938. http://doi.org/10.1111/jgs.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek A, Brettschneider C, Lange C, Posselt T, Wiese B, Steinmann S, … König H-H. Longitudinal Predictors of Institutionalization in Old Age. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0144203. http://doi.org/10.1371/journal.pone.0144203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Allore H, Murphy T, Gill T, Peduzzi P, Lin H. Dynamics of functional aging based on latent-class trajectories of activities of daily living. Annals of Epidemiology. 2013;23(2) doi: 10.1016/j.annepidem.2012.11.010. http://doi.org/10.1016/j.annepidem.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan I. A comparative review of the Katz ADL and the Barthel Index in assessing the activities of daily living of older people. International Journal of Older People Nursing. 2007;2(3):204–212. doi: 10.1111/j.1748-3743.2007.00074.x. http://doi.org/10.1111/j.1748-3743.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, Eeles EMP, Rockwood MRH, Fallah N, Ross E, Mitnitski A, Rockwood K. Assessing balance and mobility to track illness and recovery in older inpatients. Journal of General Internal Medicine. 2011;26(12):1471–1478. doi: 10.1007/s11606-011-1821-7. http://doi.org/10.1007/s11606-011-1821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette AM. Toward a Common Language for Function, Disability, and Health. Physical Therapy. 2006;86(5):726–734. [PubMed] [Google Scholar]
- Killey B, Watt E. The effect of extra walking on the mobility, independence and exercise self-efficacy of elderly hospital in-patients: a pilot study. Contemporary Nurse. 2006;22(1):120–133. doi: 10.5172/conu.2006.22.1.120. http://doi.org/10.5555/conu.2006.22.1.120. [DOI] [PubMed] [Google Scholar]
- Kozakai R, von Bonsdorff M, Sipilä S, Rantanen T. Mobility limitation as a predictor of inpatient care in the last year of life among community-living older people. Aging Clinical and Experimental Research. 2013;25(1):81–87. doi: 10.1007/s40520-013-0013-1. http://doi.org/10.1007/s40520-013-0013-1. [DOI] [PubMed] [Google Scholar]
- Lafont C, Gérard S, Voisin T, Pahor M, Vellas B Members of I.A.G.G./A.M.P.A Task Force. Reducing “iatrogenic disability” in the hospitalized frail elderly. The Journal of Nutrition, Health & Aging. 2011;15(8):645–660. doi: 10.1007/s12603-011-0335-7. [DOI] [PubMed] [Google Scholar]
- MacKnight C, Rockwood K. A Hierarchical Assessment of Balance and Mobility. Age and Ageing. 1995;24(2):126–130. doi: 10.1093/ageing/24.2.126. [DOI] [PubMed] [Google Scholar]
- Manns P, Ezeugwu V, Armijo-Olivo S, Vallance J, Healy GN. Accelerometer-Derived Pattern of Sedentary and Physical Activity Time in Persons with Mobility Disability: National Health and Nutrition Examination Survey 2003 to 2006. Journal of the American Geriatrics Society. 2015;63(7):1314–1323. doi: 10.1111/jgs.13490. http://doi.org/10.1111/jgs.13490. [DOI] [PubMed] [Google Scholar]
- McCusker J, Kakuma R, Abrahamowicz M. Predictors of functional decline in hospitalized elderly patients: a systematic review. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2002;57(9):M569–577. doi: 10.1093/gerona/57.9.m569. [DOI] [PubMed] [Google Scholar]
- Mikkola TM, Polku H, Portegijs E, Rantakokko M, Rantanen T, Viljanen A. Self-Reported Hearing Status Is Associated with Lower Limb Physical Performance, Perceived Mobility, and Activities of Daily Living in Older Community-Dwelling Men and Women. Journal of the American Geriatrics Society. 2015;63(6):1164–1169. doi: 10.1111/jgs.13381. http://doi.org/10.1111/jgs.13381. [DOI] [PubMed] [Google Scholar]
- Mudge AM, O’Rourke P, Denaro CP. Timing and risk factors for functional changes associated with medical hospitalization in older patients. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2010;65(8):866–872. doi: 10.1093/gerona/glq069. http://doi.org/10.1093/gerona/glq069. [DOI] [PubMed] [Google Scholar]
- Nilsson CJ, Siersma V, Mänty M, Avlund K, Vass M, Lund R. Mobility decline in old age: the combined effect of mobility-related fatigue and socioeconomic position. Journal of Epidemiology and Community Health. 2014;68(6):510–515. doi: 10.1136/jech-2013-203060. http://doi.org/10.1136/jech-2013-203060. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Berges IM, Kuo YF, Goodwin JS, Fisher SR, Guralnik JM. Mobility activity and its value as a prognostic indicator of survival in hospitalized older adults. Journal of the American Geriatrics Society. 2013;61(4):551–557. doi: 10.1111/jgs.12170. http://doi.org/10.1111/jgs.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CA, Hughes C, Baumhover L. Impact of a nurse-driven mobility protocol on functional decline in hospitalized older adults. Journal of Nursing Care Quality. 2009;24(4):325–331. doi: 10.1097/NCQ.0b013e3181a4f79b. http://doi.org/10.1097/NCQ.0b013e3181a4f79b. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E. Using function-focused care to increase physical activity among older adults. Annual Review of Nursing Research. 2013;31:175–208. doi: 10.1891/0739-6686.31.175. http://doi.org/10.1891/0739-6686.31.175. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. SAS 9.4 (Version 9.4) SAS Institute, Inc; 2013. [Google Scholar]
- Şimşek T, Tarsuslu, Yümin E, Tütün, Sertel M, Öztürk A, Yümin M. Assistive Device Usage in Elderly People and Evaluation of Mobility Level. Topics in Geriatric Rehabilitation. 2012;28(3):190–194. http://doi.org/10.1097/TGR.0b013e3182581d72. [Google Scholar]
- Stucki G. Olle Höök Lectureship 2015: The World Health Organization’s paradigm shift and implementation of the International Classification of Functioning, Disability and Health in rehabilitation. Journal of Rehabilitation Medicine. 2016;48(6):486–493. doi: 10.2340/16501977-2109. http://doi.org/10.2340/16501977-2109. [DOI] [PubMed] [Google Scholar]
- Stucki G, Cieza A, Melvin J. The International Classification of Functioning, Disability and Health: A unifying model for the conceptual description of the rehabilitation strategy. Journal of Rehabilitation Medicine. 2007;39(4):279–285. doi: 10.2340/16501977-0041. http://doi.org/10.2340/16501977-0041. [DOI] [PubMed] [Google Scholar]
- Tucker D, Molsberger SC, Clark A. Walking for wellness: a collaborative program to maintain mobility in hospitalized older adults. Geriatric Nursing (New York, NY) 2004;25(4):242–245. doi: 10.1016/j.gerinurse.2004.06.009. http://doi.org/10.1016/j.gerinurse.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Volpato S, Onder G, Cavalieri M, Guerra G, Sioulis F, Maraldi C … Italian Group of Pharmacoepidemiology in the Elderly Study (GIFA) Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. Journal of General Internal Medicine. 2007;22(5):668–674. doi: 10.1007/s11606-007-0152-1. http://doi.org/10.1007/s11606-007-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield BJ, Holman JE. Functional trajectories associated with hospitalization in older adults. Western Journal of Nursing Research. 2007;29(2):161–177. doi: 10.1177/0193945906293809. discussion 178–182. http://doi.org/10.1177/0193945906293809. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Towards a common language for functioning, disability, and health; ICF, the International Classification of Functioning, Disability and Health. Geneva, Switzerland: 2002. Retrieved from http://www.who.int/classifications/icf/en/ [Google Scholar]
- World Health Organization. WHO: Definition of an older or elderly person. 2016 Retrieved June 15, 2016, from http://www.who.int/healthinfo/survey/ageingdefnolder/en/
- Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. Journal of the American Geriatrics Society. 2011;59(2):266–273. doi: 10.1111/j.1532-5415.2010.03276.x. http://doi.org/10.1111/j.1532-5415.2010.03276.x. [DOI] [PubMed] [Google Scholar]