Abstract
In this issue of Neuron, Kuo et al. (2016) show that coordinated interaction between electrical and chemical synapses in a defined retinal circuit enhances sensitivity to moving objects. Their work demonstrates how electrical and chemical synapses combine to improve information processing in a specific area of the CNS.
The vertebrate retina encodes the visual environment in several stages of processing. Photoreceptor responses to light are processed by parallel networks of interneurons that provide synaptic input to ganglion cells (GCs), the output neurons that convey information to the brain. There are multiple types of GCs, each of which receives input from a unique presynaptic circuit and is tuned to particular features of the visual world, including motion, contrast, orientation, and color (Figure 1A) (Demb and Singer, 2015; Baden et al., 2016). Each GC encodes visual information within a region of space known as its receptive field. Kuo et al. (2016) describe how interactions between the electrical and chemical synapses in a specific interneuron circuit of the mouse retina increase the sensitivity of some GC types to stimuli that are correlated across space and time, thereby improving the visibility of moving objects.
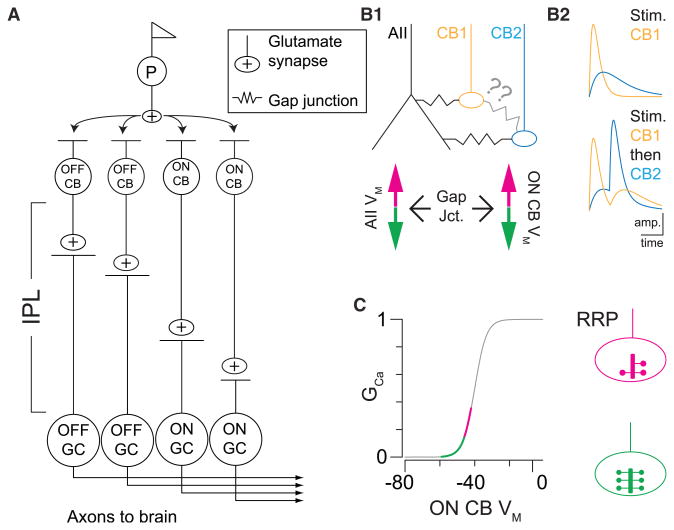
Figure 1. Signal Propagation through the Retinal Network.
(A) Parallel, excitatory retinal pathways: a uniform photoreceptor output (p) is distributed to various cone bipolar (CB) cell types, each of which makes synapses onto a unique population of GCs. CBs and postsynaptic GCs are characterized broadly as either ON or OFF, excited by increments or decrements in luminance, respectively, and then more specifically by response properties. The dendrites of different GC types, and the axon terminals of their specific presynaptic CB partners, costratify in narrow laminae within the inner plexiform layer (IPL).
(B1) Electrical coupling within the AII-ON CB network (top) allows AII VM to alter ON CB VM (bottom). Coupling between AIIs and ON CBs in the mouse retina is well established; direct coupling between CBs has not been demonstrated (question marks).
(B2) Electrical coupling permits synaptic input to one ON CB to influence VM in a different ON CB. As illustrated schematically, a postsynaptic potential (PSP) evoked by a stimulus in the receptive field of CB1 (e.g., an ∼18 μm bar, as in Figures 1C and 1D; Kuo et al., 2016) changes VM in CB2. If CB2 receives a synaptic input soon after a PSP is generated in CB1, the resulting PSP in CB2 is amplified. And the PSP in CB2 then serves to lengthen the duration of depolarization in CB1.
(C) Depolarization or hyperpolarization of the AII-ON CB network alters the gain of signaling at ON CB→ON GC synapses by shifting mean VM relative to the conductance (G)-voltage relationship of the presynaptic Ca channels (left). Changes in tonic Ca conductance alter the tonic release rate and thereby affect the availability of vesicles in the RRP, as illustrated schematically at right.
Kuo et al. studied the ON α GC, which responds to increased light intensity relative to the background and to motion in any direction within its ∼400 μm wide receptive field. This GC receives excitatory, glutamatergic input from ON cone bipolar cells that are depolarized by increments in light intensity. Hundreds of cone bipolar cells converge onto a single ON α GC, and the receptive field of each cone bipolar cell forms a small subunit of the larger GC receptive field (Demb and Singer, 2015). Each ON cone bipolar cell presynaptic to an ON α GC has a relatively small dendritic tree ∼18 μm wide, but its receptive field is ∼45 μm wide (Schwartz et al., 2012). The relatively large receptive fields of ON cone bipolar cells were posited to depend on electrical coupling between their terminals and an interneuron called the AII amacrine cell (Figure 1A) (Schwartz et al., 2012).
The ON α GC does not sum the output of its presynaptic subunits in a linear manner; responses to increments in light intensity in one part of the ON α GC receptive field do not cancel responses to decrements in light intensity in another part (Demb et al., 2001). Kuo et al. further examined this nonlinear interaction between bipolar cell subunits. They designed a linearity test using two bars of light, each the width of a bipolar cell receptive field. Alone, each bar evoked a similar response in an ON α GC. Presented together, however, the bars evoked a response that exhibited a nonlinear amplification in that it exceeded the sum of the responses to each presented alone. This nonlinear amplification occurred when two bars were presented over a confined region of space (<60 μm) and within a brief time window (<60 ms; Figure 6), and it was independent from presynaptic inhibitory circuits (Figure S3). The dependence of nonlinear amplification on spatial and temporal correlations in the stimulus generated sensitivity to objects moving at up to 40 deg/s, and amplification was further enhanced by action potential generation in the GC (Figures 7 and S6).
Several lines of evidence support a model in which nonlinear amplification depends on linear integration within the electrically coupled ON cone bipolar-AII amacrine cell network, followed by a synaptic nonlinearity at the ON cone bipolar cell synapse. First, by suppressing the responses of ON cone bipolar cells to direct cone input, Kuo et al. found significant light-evoked electrical input to ON cone bipolar cells from the coupled network (Figure 3) (Demb and Singer, 2012). Indeed, nonlinear amplification was absent in a mouse lacking connexin (Cx) 36, the Cx found in AIIs (Figure 3) (Deans et al., 2002), and was suppressed in the presence of a blocker of gap junctions (Figure S3A). Cx36 is not found in all ON cone bipolar cells (Han and Massey, 2005; Maxeiner et al., 2005), and hence, electrical coupling responsible for lateral interactions between ON cone bipolar cells likely is indirect, via the AIIs. Finally, Kuo et al. demonstrated that responses to paired bar stimuli were summed linearly by AIIs, indicating that summation within the electrically coupled network itself is linear (Figure 4). This finding also implies that cone to ON cone bipolar cell synapses behave linearly (Borghuis et al., 2013).
Because nonlinear amplification was observed in ON α GCs, but not in AIIs, the key nonlinearity in the circuit is found at the glutamatergic synapses of ON cone bipolar cells. This finding is consistent with descriptions of ON bipolar cell synapses as accelerating nonlinearities (Grimes et al., 2014; Jarsky et al., 2011). Kuo et al., however, show that the nonlinear behavior of individual ON cone bipolar cell synapses depends on lateral interactions between ON cone bipolar cells mediated by electrical coupling in the AII-ON cone bipolar cell network (Figure 1B1) (Demb and Singer, 2012). Light-evoked depolarization of one ON cone bipolar cell can depolarize a neighboring cell, thereby potentiating subsequent light-evoked glutamate release from the second cell (Figure 1B2).
The synaptic nonlinearity of an ON cone bipolar cell depends on its activity, including its presynaptic membrane potential (VM) and the availability of vesicles in a readily releasable pool (RRP). Ca2+-dependent transmission at bipolar cell synapses is steeply dependent on presynaptic VM in the activation range of presynaptic voltage-gated Ca channels (Jarsky et al., 2011). When bipolar cell synapses are tonically depolarized, not only is the relationship between VM and Ca current linear, but tonic Ca2+ influx drives sustained exocytosis that depletes the RRP (Figure 1C) (Jarsky et al., 2011).
ON cone bipolar cell VM can be controlled at the circuit level by a number of mechanisms, including light-intensity-dependent changes in AII VM (Grimes et al., 2014; Ke et al., 2014). Also, AIIs possess a number of intrinsic membrane conductances that can promote bursting and bistability (Cembrowski et al., 2012), and it is possible that particular modulatory states could amplify or suppress nonlinear subunit summation. Furthermore, the conductance of gap junctions in AIIs may be modulated by a cAMP-dependent mechanism (Demb and Singer, 2012), providing a pathway for neuromodulatory control of the nonlinearity at ON cone bipolar cell synapses. Understanding network-level modulation could yield valuable insights into how retinal processing is specialized within specific neural circuits to permit function across the wide range of lighting conditions in various visual environments.
Indeed, Kuo et al. found that nonlinear summation of cone bipolar cell outputs differed between retinal circuits and among lighting conditions (Figures S4 and S5). Integration in one type of OFF GC, which responds to decrements in luminance, did not exhibit nonlinear amplification, suggesting that presynaptic OFF cone bipolar cells differ from the ON bipolar cells described above. An ON transient GC exhibited more significant nonlinear amplification than the ON α GC, and the ON α and ON transient GC types apparently receive input from different presynaptic ON cone bipolar cell types. More generally, the approximately five ON cone bipolar cell types identified likely differ in their degree of nonlinear output; this might be explained by differences in VM, in coupling to the AII network, and in the intrinsic dynamics of transmission at their synapses.
Nonlinear amplification likely serves specific roles to permit efficient coding of natural scenes. For example, nonlinear amplification of ON GC responses was strongest at low contrasts (Figures 2D, 5, S2C, and S4G), which are most abundant in natural scenes (Simoncelli, 2003). Furthermore, natural scenes contain relatively large areas of light increments—encoded by ON cone bipolar cells—interspersed with relatively small areas of light decrements—encoded by OFF cone bipolar cells (Ratliff et al., 2010). This might explain why electrical coupling is more prominent in ON bipolar cells; it would tune their sensitivity to relatively large objects moving within the visual scene.
References
- Baden T, Berens P, Franke K, Román Rosó n M, Bethge M, Euler T. Nature. 2016;529:345–350. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghuis BG, Marvin JS, Looger LL, Demb JB. J Neurosci. 2013;33:10972–10985. doi: 10.1523/JNEUROSCI.1241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, Logan SM, Tian M, Jia L, Li W, Kath WL, Riecke H, Singer JH. Cell Rep. 2012;1:155–166. doi: 10.1016/j.celrep.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Vis Neurosci. 2012;29:51–60. doi: 10.1017/S0952523811000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Singer JH. Annu Rev Vis Sci. 2015;1:263–289. doi: 10.1146/annurev-vision-082114-035334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. J Neurosci. 2001;21:7447–7454. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Schwartz GW, Rieke F. Neuron. 2014;82:460–473. doi: 10.1016/j.neuron.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Massey SC. Proc Natl Acad Sci USA. 2005;102:13313–13318. doi: 10.1073/pnas.0505067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, Demb JB, Singer JH. J Neurosci. 2011;31:11003–11015. doi: 10.1523/JNEUROSCI.2631-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke JB, Wang YV, Borghuis BG, Cembrowski MS, Riecke H, Kath WL, Demb JB, Singer JH. Neuron. 2014;81:388–401. doi: 10.1016/j.neuron.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Schwartz GW, Rieke F. Neuron. 2016;90:320–332. doi: 10.1016/j.neuron.2016.03.012. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermüller J, Brune H, Kirsch T, Pieper M, Degen J, Krüger O, Willecke K, Weiler R. J Neurosci. 2005;25:566–576. doi: 10.1523/JNEUROSCI.3232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff CP, Borghuis BG, Kao YH, Sterling P, Balasubramanian V. Proc Natl Acad Sci USA. 2010;107:17368–17373. doi: 10.1073/pnas.1005846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, Wong RO, Rieke F. Nat Neurosci. 2012;15:1572–1580. doi: 10.1038/nn.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncelli EP. Curr Opin Neurobiol. 2003;13:144–149. doi: 10.1016/s0959-4388(03)00047-3. [DOI] [PubMed] [Google Scholar]