Abstract
Background
The advent of cancer immunotherapy has made autoimmune disease in oncology populations clinically important. We analyzed the association of autoimmune disease with treatment and outcomes among lung cancer patients.
Methods
Using linked Surveillance Epidemiology and End Results (SEER)-Medicare data, we identified lung cancer patients diagnosed between 1992–2009 with autoimmune diseases. We recorded number and timing of autoimmune disease diagnoses, lung cancer treatment, and markers of healthcare utilization including emergency department visits, hospitalizations, and outpatient visits. To account for potential lead-time bias, we used a matched case-control analysis wherein living and deceased patients were matched on survival time. We performed unadjusted and multivariable adjusted logistic regressions separately by cancer stage for all-cause and lung cancer-specific mortality.
Results
Among 172,285 lung cancer patients, 23,084 (13.4%) had ≥1 autoimmune disease at any time. Overall, 10,927 patients (6.3%) had one autoimmune disease before cancer diagnosis; 9,338 (5.4%) had two or more before cancer diagnosis; and 2,819 (1.6%) had one or more after cancer diagnosis. Healthcare utilization was higher in the autoimmune disease population. Treatment patterns were similar among patients with and without autoimmune disease and there was no significant association with mortality.
Conclusions
Among patients with lung cancer, autoimmune disease does not influence treatment patterns and is not associated with mortality.
Keywords: lung cancer, autoimmune disease, immune therapy, SEER-Medicare
Introduction
The intersection of autoimmune disease and cancer has long been the subject of laboratory research, clinical observations, and epidemiologic studies. While chronic inflammatory conditions predispose to cancer, an abundance of intra-tumoral infiltrating lymphocytes is associated with improved prognosis.[1] Hypothetically, a concurrent autoimmune disease could convey anti-cancer effects, as has been shown in patients with small cell lung cancer and certain autoimmune paraneoplastic syndromes, resulting in better clinical outcomes.[2] Conversely, the administration of chronic immunosuppression—the mainstay of treatment of autoimmune disease—increases risk and aggressiveness of cancer.[3, 4] Some treatments for autoimmune diseases, such as tumor necrosis factor inhibitors and methotrexate, have been linked to heightened cancer risk.[5–7] In some instances, underlying autoimmune disease increases treatment-related toxicities.[8, 9] Despite these observations, it was not until the recent emergence of immune checkpoint inhibitor therapy—for which pre-existing autoimmune conditions are often considered a contraindication—that the occurrence of autoimmune diseases among patients with cancer has received general interest.
Indeed, in the current era of cancer immunotherapy, autoimmune diseases have assumed a critical role in patient evaluation. Due to concerns over increased risk of immune-mediated adverse events—which may be unpredictable, possibly severe, and potentially permanent—patients with active autoimmune disease have been universally excluded from cancer immunotherapy clinical trials. Although small series have reported the successful administration of immune checkpoint inhibitor therapy to this population,[10–12] and some checkpoint inhibitor clinical trials are tentatively expanding to include patients with pre-existing autoimmune disease,[13] it remains unclear how best to approach these patients.
It is estimated that at least 13% of patients with lung cancer also have autoimmune disease, with rheumatoid arthritis, psoriasis, polymyalgia rheumatica, Addison’s disease, and systemic lupus erythematosis the most common.[14] Overall, the number of patients with autoimmune disease is estimated at 20–50 million individuals in the U.S.[15] Despite the heightened attention paid to these conditions in the age of cancer immunotherapy, little is known about their general impact on cancer treatment and outcomes. To address these knowledge gaps, we analyzed these effects among patients with lung cancer using a large, representative, population-based dataset.
Materials and Methods
Data and Study Population
Data were obtained from linked 1992–2009 National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) program data and 1991–2011 Medicare claims data. Data were available from 17 registries broadly representing approximately 26% of the U.S. population.[16] This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center (IRB# STU 082012-040).
We included Medicare patients age ≥66 years diagnosed with a primary lung cancer diagnosed between 1992–2009. These years were selected because Medicare claims were first available in 1991, allowing a one-year lead-in time to measure autoimmune disease and covariates prior to lung cancer diagnosis. Likewise, 2009 was the most recent year of available data for the analysis. We included patients age ≥66 years to allow for one-year of complete Medicare claims data pre-diagnosis. All patients had full coverage of Medicare Parts A and B from one year before and one year after the lung cancer diagnosis. We excluded HMO members and patients with only autopsy or death certificate records to ensure complete claims data. We included only patients with non-small cell lung cancer or small cell lung cancer histology. We excluded patients with incomplete diagnosis or death dates or discrepancies in SEER and Medicare birth dates of one year or more.
Autoimmune Disease
We identified patients with autoimmune disease using ICD-9 codes for 7 systemic and 36 organ-specific autoimmune diseases. As described in our prior study, we did not include autoimmune diseases that are not routinely excluded from cancer immunotherapy (e.g., type 1 diabetes).[14] For this study, we counted only those diseases documented in 1 or more inpatient claims or 2 or more outpatient claims at least 30 days apart (claim “rule-out” approach, which provides a more conservative estimate than using a single outpatient claim).
We classified presence of autoimmune disease using four categories depending on the number and timing of the first diagnosis of autoimmune disease relative to the lung cancer diagnosis: none; one disease before; two or more diseases before; and one or more diseases after. Timing of the first documented diagnosis was used to determine whether patients were diagnosed before vs. after cancer diagnosis (e.g., patients with the same condition documented in claims both before and after lung cancer were considered to have been diagnosed before cancer). We separated those with one vs. more than one because the number of conditions may be differentially associated with mortality and may reflect ascertainment bias. For example, those with more than one disease may be more likely to be alive at follow-up as patients living longer have more opportunity for both development, documentation, and treatment of conditions. This could occur as a result of having more available claims data among those with greater healthcare utilization, a longer-time period before the lung cancer (e.g., claims are only available starting at age 65), or a longer survival time. We examined timing of diagnosis relative to lung cancer because autoimmune diseases diagnosed before vs. after lung cancer may have differential effects on treatment selection, toxicity, and mortality. For example, compared to those diagnosed after lung cancer, patients diagnosed with autoimmune disease before lung cancer may experience an earlier lung cancer diagnosis due to greater healthcare utilization; for these patients, lead time bias may result in an apparent survival advantage. Similarly, patients with shorter survival time after lung cancer diagnosis would have fewer available claims and fewer opportunities for the diagnosis of autoimmune disease. Given small sample sizes, we could not distinguish between those with only one vs. more than one autoimmune condition diagnosed after their lung cancer.
Outcomes
The primary outcomes of interest were all-cause and lung cancer-specific mortality defined using the cause of death to site recode of “039” for lung cancer-specific death and the non-missing date of death in SEER for all-cause death.
Stratifying variables and covariates
We defined lung cancer stage as American Joint Committee on Cancer (AJCC) Stage I/II, III, or IV per the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Third and Sixth Editions. We included multiple covariates associated with mortality among lung cancer patients. These included sociodemographic factors such as age at diagnosis (<75, 75–84, or ≥85 years), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic or other), marital status (married/common law, separated/widowed/divorced, single/never married, or unknown). We categorized tumor histology as small cell, adenocarcinoma, squamous, or other non-small cell histology. We measured lung cancer treatment (surgery only, chemotherapy only, radiation only, more than one treatment, or no treatment) using previously described methods. [17, 18] We measured comorbidity in the 12 months before lung cancer using the Charlson comorbidity index-Klabunde adaptation [19, 20]; for this analysis, we removed rheumatologic (ie, autoimmune) diseases from the Charlson score. Patients with Medicaid were identified using the state buy-in variable.[21] We measured healthcare utilization in the one year prior to lung cancer diagnosis, including the number of inpatient hospitalizations and outpatient visits and whether or not the patient had emergency department visit (yes/no). We focused on measuring overall utilization and not lung cancer-specific diagnostic procedures, thus did not measure utilization in the week prior to lung cancer diagnosis. To improve model fit, we included outpatient visits as a continuous variable and inpatient hospitalizations as 0, 1, or ≥2.
Statistical analysis
First, we compared all measured variables across the four autoimmune groups (none, 1 before, ≥2 before, ≥1 after) using descriptive statistics (number, percent). Next, we conducted our primary outcome analysis, which was designed to account for lead-time bias. Given the potential for closer monitoring, more imaging, and faster work-up of symptoms, patients with existing autoimmune disease may be diagnosed with earlier stage cancer compared to those without autoimmune disease. A diagnosis earlier in the natural history of disease could result in lead-time bias (i.e., wherein survival time appears longer for those with vs. without autoimmune disease). To account for this, we used Coarsened Exact Matching (CEM) to match case-control patients on survival time. In our analysis cases (patients who died during follow-up) were matched to controls (alive at the end of follow-up) on survival time in one-month increments. After matching, the association of autoimmune disease with mortality (yes/no) was examined using logistic regression. We chose Coarsened Exact Matching (CEM) because it offers several advantages over traditional matching methods, and is described in detail elsewhere.[22] We have previously used this approach to adjust for lead-time bias.[23] Survival was measured as the interval in months between lung cancer diagnosis date (defined as the 15th of the month because SEER provides only month and year of diagnosis) and death date per SEER. Patients were followed until date of death or the end of 2009 (last date of death in 2011 SEER submission).
We fitted unadjusted and multivariable covariate-adjusted logistic regression models separately by cancer stage (I/II, III, IV) and separately for both outcomes (all-cause and lung-cancer specific mortality). Lung cancer stages I and II were combined due to small numbers diagnosed with stage II disease. Coarsened exact matching was performed using Stata 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.). Specifically, we used the -cem- command followed by weighted logistic regression with iweight=cem_weights under the condition of cem_matched=1, in which weights and matching analyses reflect the matching ratio of the number of controls and to the number of cases(e.g., 1:2 or 1:40).
Results
We identified 172,285 patients with lung cancer, of whom 23,084 (13.4%) had at least one autoimmune disease either before or after their lung cancer diagnosis. Across all stages, 10,927 patients (6.3%) had one autoimmune disease before lung cancer diagnosis, 9,338 (5.4%) had two or more autoimmune diseases before lung cancer diagnosis, and 2,819 (1.6%) had one or more autoimmune diseases diagnosed after lung cancer diagnosis.
Table 1 demonstrates that all characteristics were significantly different across the four patient groups. In brief, men were less likely to be represented in any of the autoimmune disease groups and the percent of women was highest among patients with ≥2 diseases diagnosed before lung cancer. Lung cancer stage distribution varied across groups; stage I was most common and stage IV was least common among patients with ≥1 disease diagnosed after cancer. Patients with ≥2 diseases diagnosed before cancer had the most healthcare utilization in the year prior to cancer diagnosis, followed by those with ≥1 disease before cancer, then patients with no autoimmune disease; patients with ≥1 disease diagnosed after cancer had the least healthcare utilization.
Table 1.
Characteristics of lung cancer patients by the number and timing of diagnosed autoimmune disease (AI) (N=172,285)
| Patient Characteristics | No AI Disease N (%) |
1 AI before Lung cancer N (%) |
≥2 AIs before lung cancer N (%) |
≥1 AIs after lung cancer N (%) |
Chisq p-value |
|---|---|---|---|---|---|
| Age, yr | <.0001 | ||||
| ≥66–74 | 71254(47.8) | 4461(40.8) | 3626(38.8) | 1672(59.3) | |
| ≥75–84 | 63739(42.7) | 5269(48.2) | 4712(50.5) | 1014(36.0) | |
| ≥85 | 14208(9.5) | 1197(11.0) | 1000(10.7) | 133(4.7) | |
| Sex | <.0001 | ||||
| Female | 65923(44.2) | 6003(54.9) | 5756(61.6) | 1450(51.4) | |
| Male | 83278(55.8) | 4924(45.1) | 3582(38.4) | 1369(48.6) | |
| Race | <.0001 | ||||
| White | 128350(86.0) | 9505(87.0) | 8312(89.0) | 2440(86.6) | |
| Black | 12406(8.3) | 878(8.0) | 601(6.4) | 216(7.7) | |
| Hispanic | 1589(4.6) | 419(3.8) | 330(3.5) | 130(4.6) | |
| Other | 6856(1.1) | 125(1.1) | 95(1.0) | 33(1.2) | |
| Marriage status | <.0001 | ||||
| Married | 77146(51.7) | 5344(48.9) | 4548(48.7) | 1606(57.0) | |
| Sep/Div/Wida | 56288(37.7) | 4541(41.6) | 3966(42.5) | 958(34.0) | |
| Single | 10947(7.3) | 682(6.2) | 537(5.8) | 169(6.0) | |
| Unknown | 4820(3.2) | 360(3.3) | 287(3.1) | 86(3.1) | |
| Histology | <.0001 | ||||
| Non-small cell | |||||
| Adenocarcinoma | 51823(34.7) | 3784(34.6) | 3583(38.4) | 1268(45.0) | |
| Squamous | 31991(21.4) | 2344(21.5) | 2035(21.8) | 668(23.7) | |
| Other | 44759(30.0) | 3306(30.3) | 2573(27.6) | 561(19.9) | |
| Small cell | 20628(13.8) | 1493(13.7) | 1147(12.3) | 322(11.4) | |
| Charlson Comorbidityc | <.0001 | ||||
| 0 | 63093(42.3) | 4179(38.2) | 3503(37.5) | 1342(47.6) | |
| 1 | 42715(28.6) | 3364(30.8) | 2847(30.5) | 833(29.5) | |
| ≥2 | 33799(22.7) | 3239(29.6) | 2950(31.6) | 532(18.9) | |
| Not calculatedd | 9594(6.4) | 145(1.3) | 38 (0.4) | 112(4.0) | |
| Medicaid | <.0001 | ||||
| Yes | 25150(16.9) | 2109(19.3) | 1502(16.1) | 380(13.5) | |
| No | 124051(83.1) | 8818(80.7) | 7836(83.9) | 2439(86.5) | |
| Treatment | <.0001 | ||||
| Surgery only | 19410(13.0) | 1574(14.4) | 187(20.1) | 1007 (35.7) | |
| Chemotherapy only | 14664(9.8) | 1113(10.2) | 908(9.7) | 262(9.3) | |
| Radiation only | 28167(18.9) | 2053(18.8) | 1684(18.0) | 308(10.9) | |
| ≥2 treatments | 38008(25.5) | 2604(23.8) | 2193(23.5) | 894(31.7) | |
| No Surg/Chemo/Rade | 48952(32.8) | 3583(32.8) | 2679(28.7) | 348(12.3) | |
| Stage (AJCC) | <.0001 | ||||
| I | 29987(20.1) | 2395(21.9) | 2610(28.0) | 1160(41.1) | |
| II | 5751(3.9) | 445(4.1) | 382(4.1) | 180(6.4) | |
| III | 44903(30.1) | 3235(29.6) | 2667(28.6) | 737(26.1) | |
| IV | 68560(46.0) | 4852(44.4) | 3679(39.4) | 742(26.3) | |
| Healthcare Utilization (one year before lung cancer) | |||||
| ED visits | <.0001 | ||||
| 0 | 70222(47.1) | 4436(40.6) | 3724(39.9) | 1543(54.74) | |
| ≥1 | 78979(52.9) | 6491(59.4) | 5614(60.1) | 1276(45.3) | |
| Number of Hospitalizations | <.0001 | ||||
| 0 | 83820(56.2) | 5288(48.4) | 4478(48.0) | 1725(61.2) | |
| 1 | 40072(26.9) | 3090(28.3) | 2493(26.7) | 724(25.7) | |
| ≥2 | 25309(17.0) | 2549(23.3) | 2367(25.3) | 370(13.1) | |
| Number of Outpatient visits (continuous) | |||||
| M (SD) | M (SD) | M (SD) | M (SD) | ||
| 30.4 (41.5) | 38.4(50.8) | 44.5(52.9) | 32.7(34.32) | <.0001 | |
| Cause of deathf | <.0001 | ||||
| Alive | 22360(15.0) | 1722(15.8) | 1793(19.2) | 814(28.9) | |
| Lung cancer specific | 101729(68.2) | 7257(66.4) | 5744(61.5) | 1394(49.5) | |
| All other causes | 25112(16.8) | 1948(17.8) | 1801(19.3) | 611(21.7) | |
| Total | 149201 (86.6) | 10927(6.3) | 9338(5.4) | 2819(1.6) | |
Separated/Divorced/Widowed.
Non-small cell lung cancer.
The category of Rheumatologic diseases (710.0, 710.1, 710.4, 714.0 – 714.2, 714.81, 725) was removed from the comorbidity index
Because of rule-out rule.
No surgery/chemotherapy/radiation.
Dependent variable.
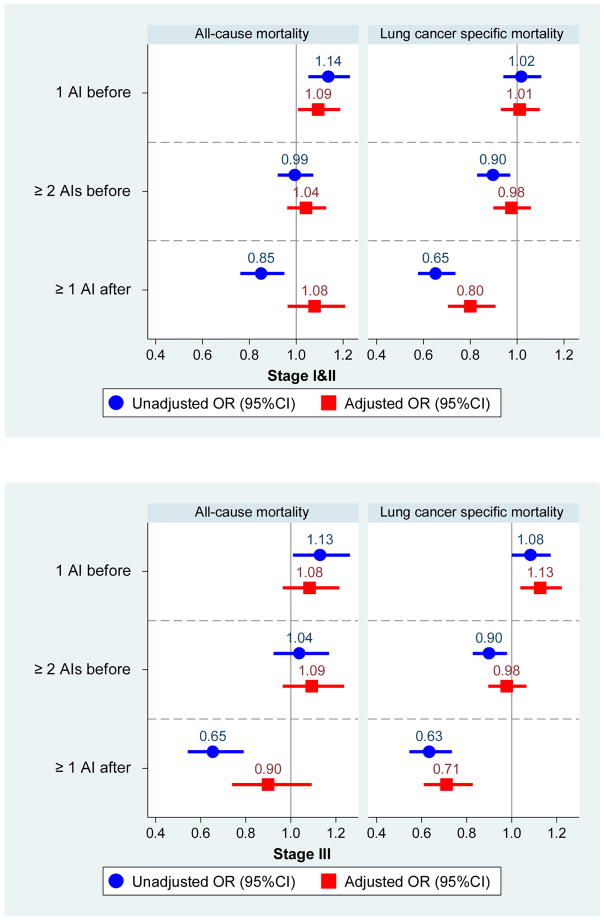
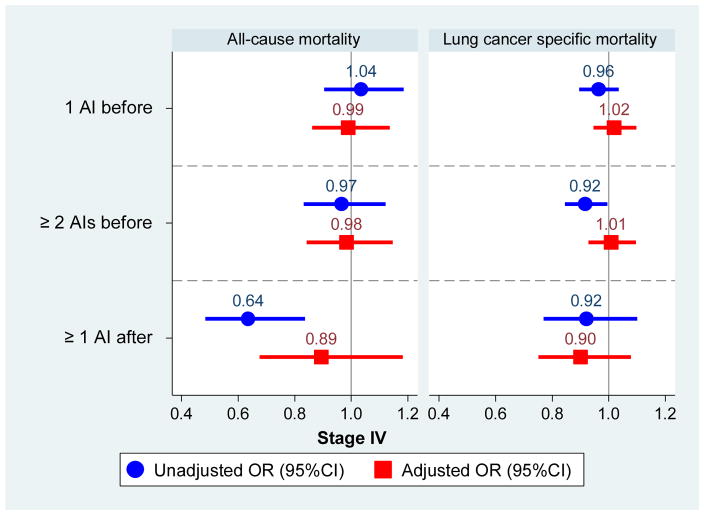
Results of our lead-time-corrected analysis are displayed in Figure 1 and described briefly below. Figure 1 shows odds ratios and 95% confidence intervals for all autoimmune disease groups compared to those with no autoimmune disease across unadjusted and adjusted models, presented by lung cancer stage and cause of death. While several of the unadjusted models demonstrated both positive and negative associations with mortality, they were all attenuated in the adjusted models. Separately by stage, we describe the statistically significant results emerging from the adjusted models below.
Figure 1.
Odds of death among lung cancer patients by the number and timing of diagnosed autoimmune diseases, compared to those with no diagnosed autoimmune conditions, separately by lung cancer stage at diagnosis.
Among patients with Stage I/II disease, the association with mortality varied by the number and timing of autoimmune diseases and cause of death. For all-cause mortality, having one autoimmune disease before lung cancer was associated with increased odds of all-cause death compared to those with no autoimmune conditions (adjusted OR [aOR]=1.09, 95% CI: 1.01–1.19). In contrast, for lung-cancer specific mortality, having one or more autoimmune disease after the diagnosis of lung cancer was associated with lower mortality in adjusted models (aOR=0.80, 95% CI: 0.70–0.91).
Among patients with Stage III disease, no combination of number and timing of autoimmune diseases was significantly associated with all-cause death. For lung-cancer specific mortality, associations differed in direction as follows. Compared to patients with no autoimmune diseases, those with one disease diagnosed before cancer had higher odds of death (adjusted OR=1.13, 1.04–1.22) whereas those with one or more diseases diagnosed after cancer had decreased odds of death (adjusted OR=0.71, 0.61–0.83).
Among patients with Stage IV disease, no combination of number or timing of autoimmune diseases was significantly associated with either cause of death.
Discussion
We present the first comprehensive analysis of the prognostic impact of autoimmune disease among lung cancer patients using a large, robust, population-based SEER-Medicare data. Our analysis reveals that autoimmune disease in general is not associated with increased mortality. However, we found two exceptions in adjusted models where a very modest increased risk of death was observed for patients with one autoimmune disease diagnosed before lung cancer: among patients with stage I/II cancer who had increased all-cause mortality and patients with stage III cancer who had increased lung cancer-specific mortality. This was not the case for individuals with more than one autoimmune disease. It is possible that this pattern is related to intolerance of therapy. While we controlled for use of treatment, SEER-Medicare data cannot be used to test the impact of treatment intolerance. Notably, in both cases where statistically significant increased risk of death was observed, the lower bound of the confidence intervals was very close to 1.
We also observed two instances in which patients with one or more autoimmune diseases diagnosed after lung cancer had decreased risk of lung-cancer specific mortality: among patients with stage I/II and stage III cancer. That some patients with an autoimmune disease diagnosed after their lung cancer had improved outcomes presumably suggests resilience or reflects the favorable clinical state required for patients to live long enough and be healthy enough after their lung cancer diagnosis to develop and be evaluated for a new medical condition. While our case-control analysis in which we matched living and deceased patients on survival time likely lessened the likelihood of ascertainment bias, these results could nevertheless reflect such a bias. Theoretically, the emergence of post-cancer autoimmune disease diagnosis could also imply anti-tumor immune effects. However, in thoracic oncology, clinically evident immune cross-reactivity between host and tumor tissues has generally been limited to a subset of small cell lung cancer, and these conditions typically present before cancer diagnosis.[24]
In general, lung cancer treatment patterns were similar between patients with and without pre-existing autoimmune disease. In particular, rates of radiation therapy alone (approximately 20% of cases) and combined modality therapy (approximately 25% of cases) were comparable. This observation contrasts practice recommendations and patterns in breast cancer, where a history of autoimmune/connective tissue disease is considered a contraindication to radiation therapy due to heightened toxicities.[8, 9] That pre-existing autoimmune disease did not seem to impact lung cancer treatment selection is particularly notable because it did clearly impact healthcare utilization leading up to the lung cancer diagnosis, including outpatient visits (median 30 for patients without autoimmune disease, median 38–45 for patients with autoimmune disease) and emergency department visits (59% of patients with autoimmune disease, 53% of patients with autoimmune disease).
Our results also raise important questions for further study using SEER-Medicare or other large databases. For example, future studies could explore how specific autoimmune diseases are associated with mortality in cancer patients. Additionally, effective treatments for autoimmune diseases such as disease modifying anti-rheumatic drugs (DMARDs) and anti-tumor necrosis factor (TNF) agents may affect tolerability and response to subsequent anticancer therapy compared to those who did not receive therapy. Given that TNF inhibitors became widely used in the 2000s, this dataset (which spans the pre and post TNF inhibitor era) covers a unique period of time in lung cancer therapy. Importantly, given the time-frame of our data set, presumably none of the patients in the present analysis received immune checkpoint therapy, which is now a mainstay of treatment for advanced disease. Further research is warranted to understand the impact of immune checkpoint inhibitors on existing and incident autoimmune disease. While our analysis shows that lung cancer patients with autoimmune disease had similar mortality as those without autoimmune disease, it is unclear how this may change with the increasing use of immune checkpoint inhibitors.
A potential limitation of this study is our use of administrative claims to define autoimmune disease, which may lead to misclassification. In contrast to a cancer diagnosis, which is typically a binary determination based on pathologic findings, the clinical diagnosis of autoimmune disease incorporates a combination of clinical, serologic, radiographic, and histologic data. For instance, establishing a diagnosis of lupus is challenging, as more than 25% of healthy adults have low-level antinuclear antibody (ANA) positivity, but only 1% of the general population is estimated to have the disease.[25, 26] Although our use of a conservative method to identify autoimmune disease (≥2 outpatient claims separated by 30 days; or ≥1 inpatient claim) is intended to limit misclassification, we recognize that it may also exclude some patients with bona fide autoimmune diagnoses. Importantly, it is possible that autoimmune diseases noted in a patient’s medical history, even if inaccurate, could impact immunotherapy utilization, as oncologists may not have the time or resources to verify these diagnoses in their busy clinical practices. We cannot determine with these data what proportion of autoimmune diseases were active requiring systemic immune suppression—the threshold generally employed for patient exclusion from cancer immunotherapy clinical trials. However, by excluding autoimmune conditions that do not require chronic immune suppression (eg, type 1 diabetes, autoimmune thyroid disease) from our analysis, we increase the likelihood that patients in our cohort may have ongoing immune modification leading up to or following their lung cancer diagnosis. Ultimately, whether potential diagnostic misclassification could mask a potential impact of autoimmune disease on treatment selection or clinical outcomes (and what direction the impact would be) remains unknown. Further, using these data, we are not able to determine how treatment with disease modifying anti-rheumatic drugs or TNF inhibitors influences or primes the immune system to respond to cancer therapy. Although immune checkpoint inhibitors are currently approved only for patients with stage IV NSCLC, we believe our inclusion of patients with earlier stage NSCLC and with small cell lung cancer is relevant because (1) a substantial proportion of patients with earlier-stage NSCLC subsequently develop recurrent, advanced disease, and (2) immune checkpoint inhibitors are currently under investigation in stage I–III NSCLC and in small cell lung cancer, with encouraging preliminary results.[27–29] Importantly, our findings cannot be generalized to other populations currently treated with immune checkpoint inhibitors, which have varying clinical characteristics and outcomes. Use of linked SEER-Medicare data in this study limits our observations to those patients over age 65 years. However, given the advanced age at diagnosis, this age group still accounts for more than two-thirds of lung cancer patients.[30] Finally, because our study period predates the approval of immunotherapy for lung cancer by more than 5 years, we cannot determine the impact of autoimmune disease on selection, toxicity, or efficacy of immune checkpoint inhibitors for lung cancer.
Our study has several methodologic strengths. We carefully controlled for ascertainment bias, common in administrative claims data, by modeling both the number and timing of autoimmune disease relative to the lung cancer diagnosis. We controlled for the potential of lead-time bias using an innovative case-control approach. These advancements improve upon standard approaches used in observational, administrative claims research on cancer outcomes and greatly improve the validity of our results.
In conclusion, cancer immunotherapy represents a rapidly changing and expanding field, with recent drug approvals in melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer, Hodgkin lymphoma, Merkel cell cancer, head and neck cancer, and any cancer that is microsatellite instability (MSI) high—and others likely forthcoming. The emergence of these promising therapies has, for the first time, generated widespread interest in the intersection of autoimmunity and cancer. However, clinicians have had little or no guidance on the evaluation and management of patients with both oncologic and autoimmune diagnoses. The present study demonstrates that, despite having higher healthcare utilization rates leading up to their lung cancer diagnosis, patients with autoimmune disease (1) receive similar treatments, and (2) appear to have similar lung cancer-specific and all-cause mortality, as patients without autoimmune disease. Future research into how these patients are best approached in the present era of cancer immunotherapy will be critical to optimizing treatment and outcomes.
Highlights.
Autoimmune disease’s impact on cancer treatment and outcomes is examined.
Healthcare utilization is increased in cancer patients with autoimmune disease.
The presence of autoimmune disease doesn’t impact lung cancer treatment selection.
There is no significant association between autoimmune disease and mortality.
Acknowledgments
Funding:
This work was supported by the National Cancer Institute (NCI) (1R03CA191875-01A1; to DEG, SLP), a National Institutes of Health (NIH) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01) (to DEG), Cancer Prevention Research Institute of Texas (CPRIT) R1208 (to SLP), the UT Southwestern Center for Patient-Centered Outcomes Research (PCOR), Agency for Healthcare Research and Quality (1R24HS022418-01) (to SLP, LX) and the National Center for Advancing Translational Sciences UT Southwestern Center for Translational Medicine (U54 RFA-TR-12-006) (to SLP).
The authors thank Helen Mayo, MLS, from the UT Southwestern Medical Library, for assistance with literature searches and Dru Gray for assistance with manuscript preparation. The authors also acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Prior presentations:
Presented in abstract form at the 52nd annual meeting of the American Society of Clinical Oncology, Chicago IL, June 3–7, 2016
Disclosure: all authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 2.Graus F, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15(8):2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 3.Dantal J, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351(9103):623–8. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 4.Miao Y, et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87(9):1347–59. doi: 10.1097/TP.0b013e3181a238f6. [DOI] [PubMed] [Google Scholar]
- 5.Bongartz T, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Michaud K. Lymphoma in rheumatoid arthritis: the effect of methotrexate and anti-tumor necrosis factor therapy in 18,572 patients. Arthritis Rheum. 2004;50(6):1740–51. doi: 10.1002/art.20311. [DOI] [PubMed] [Google Scholar]
- 7.Solomon DH, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2014;43(4):489–97. doi: 10.1016/j.semarthrit.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JM, et al. Breast conservation therapy. Severe breast fibrosis after radiation therapy in patients with collagen vascular disease. Cancer. 1991;68(3):502–8. doi: 10.1002/1097-0142(19910801)68:3<502::aid-cncr2820680310>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Fleck R, et al. Consequences of breast irradiation in patients with pre-existing collagen vascular diseases. Int J Radiat Oncol Biol Phys. 1989;17(4):829–33. doi: 10.1016/0360-3016(89)90074-6. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen M, et al. Successful treatment with Ipilimumab and Interleukin-2 in two patients with metastatic melanoma and systemic autoimmune disease. Cancer Immunol Immunother. 2014;63(12):1341–6. doi: 10.1007/s00262-014-1607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyi C, et al. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer. 2014;2(1):35. doi: 10.1186/s40425-014-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DB, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016;2(2):234–40. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DB, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2015:1–7. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed]
- 14.Khan SA, et al. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.February 11th, 2016; Available from: http://www.aarda.org/autoimmune-information/autoimmune-statistics.
- 16.Chaturvedi AK, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laccetti AL, et al. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/djv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JL, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–97. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Mazumder A, Vesole DH, Jagannath S. Vorinostat plus bortezomib for the treatment of relapsed/refractory multiple myeloma: a case series illustrating utility in clinical practice. Clin Lymphoma Myeloma Leuk. 2010;10(2):149–51. doi: 10.3816/CLML.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 21.Koroukian SM, et al. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health Serv Res. 2010;45(1):265–82. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacus SM, King G, Porro G. Causal inference without balance checking: Coarsened exact matching. Political analysis. 2012;20(1):1–24. [Google Scholar]
- 23.Pruitt SL, et al. Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br J Cancer. 2017;116(6):717–725. doi: 10.1038/bjc.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelosof LC, Gerber DE. Mayo Clinic Proceedings. Elsevier; 2010. Paraneoplastic syndromes: an approach to diagnosis and treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiden PL, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–33. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 26.Mahler M, et al. Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Res Ther. 2008;10(6):R131. doi: 10.1186/ar2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott PA, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. ASCO Annual Meeting Proceedings; 2015. [Google Scholar]
- 28.Antonia SJ, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-lavel, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 29.Antonia SJ, et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2017 Sep 8; doi: 10.1056/NEJMoa1709937. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Rasco DW, et al. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5(10):1529–35. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]