SUMMARY
Here, I revisit our early experiments published in Cell Host & Microbe (Turnbaugh et al., 2008) showing that a diet rich in fat and simple sugars alters the gut microbiome in a manner that contributes to host adiposity, and reflect upon the remarkable advances and remaining challenges in this field.
Keywords: Gut microbiome, diet-induced obesity, metabolic disease, high-fat, high-sugar diets, metagenomics, metatranscriptomics, multi-omics, nutrition, microbial dynamics, cellular memory
“Well, isn’t that a bit obvious?” asked an esteemed Professor at my graduate program departmental retreat. I had just finished giving an oral presentation on our unpublished experiments demonstrating that the consumption of a high-fat, high-sugar “Western” diet re-shapes the murine gut microbiome, potentially contributing to host adiposity (Turnbaugh et al., 2008). My thesis advisor at Washington University in Saint Louis, Jeffrey Gordon, the eternal optimist, might have replied that the translational implications for addressing one of the most important health issues of modern civilization (obesity and metabolic disease) were profound. Yet, part of me couldn’t help but agree with the faculty member asking the question – the main source of nutrients for the gut microbiome is the diet, so wasn’t this result expected? No microbiologist would be surprised that changing the in vitro cell culture medium affects bacterial growth, so why is the fact that this also happens inside the body interesting? Hadn’t nearly a century passed since Arthur Kendall concluded, based on his microscopic and metabolic observations of stool bacteria following a dietary intervention on monkeys, that “the nature of the diet practically determines the dominant types of intestinal bacteria” (Kendall, 1909)?
I don’t remember my exact answer, probably a lot of scientific jargon about the benefits of metagenomic sequencing, but perhaps a better response would have been to say that “yes, it is not at all surprising that diet impacts the gut microbiome, but no one could have predicted which particular bacteria would be responsive to diet or what consequence (if any) those changes would have for the host.” Our findings raised more questions than answers. Why did the Western diet increase the relative abundance of the Firmicutes phylum at the expense of the Bacteroidetes? Why were the Mollicutes (sometimes assigned to a distinct phylum called the Tenericutes) enriched? Were the observed changes in the gut microbiome solely driven by the direct impact of altering the luminal concentration of dietary substrates required for bacterial growth, or due to a more complex series of diet-induced changes to host tissues, inflammation, and/or microbial interactions?
Even now, nearly a decade since those results were published, the microbiome field struggles to provide definitive answers to these questions. It is equally unclear how many distinct molecular mechanisms link changes in the gut microbiome to host adiposity and whether or not they can be co-opted to improve the treatment of human obesity or other metabolic diseases. Here, I invite you to join me on a voyage back in time to revisit the major findings from our early studies on diet-induced obesity published in Cell Host & Microbe (Turnbaugh et al., 2008) (Figure 1a). In addition to discussing the rationale and highlights from our original experiments, I will attempt to briefly touch upon the degree to which these results have been confirmed and extended by our research group and the field at large. I will also discuss some of the major unanswered questions and broader implications of this line of research.
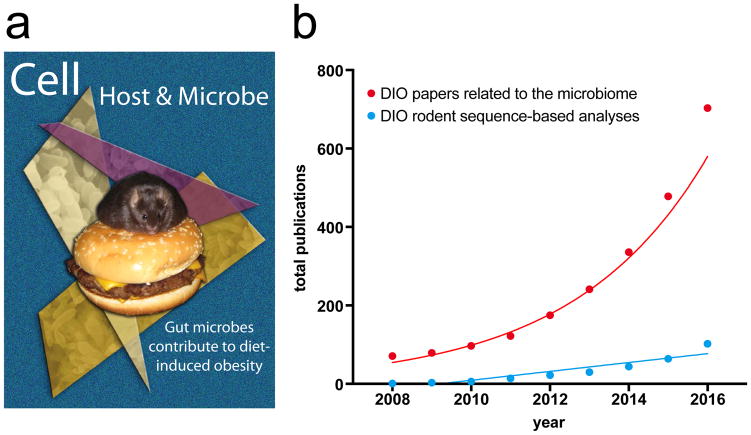
Figure 1. Publications related to diet-induced obesity (DIO) and the gut microbiome.
(a) The cover art that we submitted to Cell Host & Microbe for consideration in 2008 but was not selected. The depicted mouse was actually leptin-deficient (ob/ob) providing an exaggerated obese phenotype and an animal that would sit still on a burger long enough to get a high-resolution photo. The bacterial images are Bacteroides thetaiotaomicron, not complex gut microbial communities. I can’t justify the stained glass effect, but it seemed like a good idea at the time. (b) The number of publications related to diet-induced obesity and the gut microbiome is rising at an exponential rate. Red dots represent the PubMed query “(high-fat diet OR western diet OR diet-induced obesity) AND (microflora OR microbiota OR microbiome) NOT Review[pt]” fit with an exponential growth function (red line) in GraphPad Prism version 7.0 (R2=0.95). 62 additional publications were found dating back to 1965 (≤4 publications/year). Restricting the search term to manuscripts that include rodent models and sequencing-based methods [(high-fat diet OR western diet OR diet-induced obesity) AND (microflora OR microbiota OR microbiome) AND (sequencing OR pyrosequencing OR metagenomic) AND (murine OR rodent OR mice OR rats) NOT Review[pt]] reduces the numbers substantially (110 papers starting with our 2008 study) resulting a linear trend (R2=0.86) projected to reach 710 publications by 2072.
Do mouse models of obesity have an altered gut microbiome?
As a graduate student trained by Ruth Ley (a microbial ecologist in the Gordon lab) we turned to a well-studied model of obesity, mice deficient for leptin (a hormone produced by adipose tissue that regulates appetite). These animals exhibit a remarkable increase in adiposity, but it was unknown how this change in energy balance would impact their associated microbial communities. We found that leptin-deficient animals have a shift in their gut microbiome favoring the Firmicutes phylum over the Bacteroidetes (Ley et al., 2005). Remarkably, the colonization of germ-free mice with microbes from an obese donor resulted in significantly greater adiposity relative to a lean donor (Turnbaugh et al., 2006), providing the first evidence that differences in the murine gut microbiome are sufficient to alter host body composition.
The obvious caveat was that leptin deficiency is rare in humans, resulting in a severe form of obesity driven by a profound increase in appetite and decrease in energy expenditure. To better address the translational relevance of our findings, I turned to diet-induced obesity, a simple and widely used model in mice and other rodents wherein increased adiposity is driven by the consumption of a “Western” diet rich in simple sugars and fat.
The Western diet alters the gut microbiome
We collected distal gut contents (cecum) from 10 formerly germ-free C57BL/6J mice that had been colonized with the same conventional mouse donor sample prior to consuming a high-fat, high-sugar “Western” diet or a low-fat, plant polysaccharide-rich (LFPP) control diet for three months. The advantage of this experimental design was that all the mice started with a similar gut microbial community structure, unlike in conventional animals that can have long-term legacy effects of kinship (Ley et al., 2005) and co-housing (Carmody et al., 2015). Remarkably, we found that the gut microbiomes of LFPP controls clustered with the donor animal [also fed a LFPP (chow) diet] whereas all 5 of the Western diet-associated gut microbiomes clustered in a distinct group. Similar to our prior leptin-deficient mouse studies (Ley et al., 2005; Turnbaugh et al., 2006), mice fed the Western diet were enriched for Firmicutes at the expense of the Bacteroidetes. However, we were surprised to find a dramatic enrichment within the Firmicutes phylum for the Mollicutes lineage, reaching on average 70% of the gut microbiome accompanied by an overall decrease in diversity. A similar shift was observed in conventional animals colonized at birth (Turnbaugh et al., 2008).
One of the most surprising findings from this study was that the shift in bacterial abundance was independent of an intact innate or adaptive immune system. We fed the same Western diet to MyD88−/− and Rag1−/− animals deficient for innate and adaptive immunity, respectively. Although we observed a difference between wild-type and MyD88−/− mice on the LFPP diet, the microbial response to the Western diet was consistent across genotypes. Since then, we have confirmed and extended these findings (Carmody et al., 2015), showing that the gut bacterial response to a high-fat, high-sugar diet is remarkably reproducible across inbred and outbred mice of diverse genotypes and transgenic mice with defects in immunity and metabolism. These results suggest that the effects of dietary intake on the gut microbiome can outweigh host genetics and immune response, underscoring the critical role external factors (e.g., diet, antibiotics, other drugs, pathogens, and environmental toxins) play in shaping host-associated microbial communities.
A related prediction is that the observed changes in gut microbial community structure (at least in the lumen) may not simply reflect the inflammation associated with diet-induced obesity and perhaps could even be de-coupled from weight gain and adiposity. Consistent with this, transgenic mice that are resistant to diet-induced obesity exhibit similar shifts in gut microbial community structure (Hildebrandt et al., 2009) and weight loss induced by caloric restriction is insufficient to counter-act the impact of a Western diet on the gut microbiome (Liou et al., 2013). Massive changes to gut microbial community structure are detectable within 1 day of consuming the Western diet, well before a clear host phenotype emerges (Turnbaugh et al., 2009). The speed at which bacterial groups reach their new steady state is remarkable, taking on average just 3.5 days (Carmody et al., 2015). The rapid response of the gut microbiome to the Western diet raises the intriguing possibility that an overall shift in microbial ecology could be a contributing factor and/or biomarker for the risk of diet-induced obesity and its associated metabolic diseases.
The Western diet-associated gut microbiome promotes adiposity
A major challenge in microbiome research is that it is difficult, if not impossible, to use data about gut microbial community structure to infer function, especially when the function we care about is far removed from bacterial genetics, such as increased host adiposity. Our prior study had implicated a similarly Firmicutes-dominated gut microbiome from leptin-deficient mice in contributing to host body fat (Turnbaugh et al., 2006), so we hypothesized that transplantation of the Western diet-associated gut microbiome into germ-free mice fed a LFPP diet would increase host body fat relative to controls. This turned out to be the case, resulting in a 43% increase in total body fat two weeks after colonization, nearly twice that of the control group, despite no difference in food consumption. However, the Western diet had a distinctive impact on gut microbial community structure relative to leptin-deficient animals, most notably the bloom within the Mollicutes class that led to a marked decrease in overall diversity.
To this day, little is known about these mysterious bacteria and their impact on human health and disease. We sequenced and annotated Eubacterium dolichum DSM3991 (a human isolate), revealing substantial gene loss and genome size reduction common in the Mollicutes lineage (Turnbaugh et al., 2008), but rarely seen in other members of the Firmicutes phylum. The E. dolichum genome lacks genes for motility, and exhibits potential autotrophies for multiple vitamins and amino acids. In contrast with their evolutionary relatives the Mycoplasma, we detected genes for cell wall biosynthesis in the E. dolichum genome, consistent with their ability to grow in pure culture using standard anaerobic media. We were able to annotate genes for the degradation, import, and metabolism of sucrose (prominent in the Western diet), although the role of these, or other, pathways in bacterial fitness and their downstream consequences for host physiology remain to be determined.
The microbial response to diet can be reversed, or can it?
Clinical studies had previously suggested that diet can alter the gut microbiomes of obese individuals over timescales of weeks (Duncan et al., 2007) to years (Ley et al., 2006), but these changes cannot be conclusively attributed to diet due to other confounding factors like weight loss, exercise, and other lifestyle changes. Furthermore, the diets used to induce obesity in rodents are “semi-purified”, in contrast to the relatively unprocessed standard chow, making it impossible to determine if the observed changes in response to the Western diet were due to macronutrient intake, or additional factors like the extent of food processing, the specific dietary ingredients used, or other dietary bioactive compounds.
To circumvent this caveat and to model actual human weight loss diets, we designed two additional semi-purified diets with the same ingredients as our Western diet, but with lower carbohydrate or fat content. Both diets resulted in weight stabilization and reduction in adiposity when fed to diet-induced obese animals. They also partially rescued the effect of the Western diet on gut microbial community structure and the ability of the gut microbiome to promote host adiposity in germ-free recipients (Turnbaugh et al., 2008). I found this result to be incredibly exciting, as it suggested that the gut microbiome would not be permanently affected by a dietary perturbation, providing a clear potential for the rational dietary manipulation of the gut microbiome to treat disease. However, it also raised a critical caveat about our microbiome transplantation experiment that remains unaddressed today—wouldn’t the diet of the recipient mice (LFPP) rapidly impact the gut microbiome? If diet-induced changes to microbial community structure can be reversed in a single day (Carmody et al., 2015) why are there long-term impacts to the phenotype of the recipient animals?
One simple explanation might be that there are subtle differences in either the structure or function of the gut microbiome that cannot be restored after returning the animals to their original diet. Are there mechanisms by which the microbiome can remember past diets and act accordingly or do microbes purely live in the moment? One argument for this type of diet-driven “hysteresis” is that while numerous studies have shown correlations between long-term dietary intake and the gut microbiome, short-term dietary interventions do not markedly disrupt the strong signature of individuality found in the gut microbiome (David et al., 2014). Dynamical modeling of the gut microbiomes of mice subjected to repeated shifts between the LFPP and Western diet (44.6% kcal fat) revealed a subset of gut bacteria that exhibit hysteretic patterns (Carmody et al., 2015), i.e., their abundance depends not just on the current diet but on the past history of dietary intake. Experiments in which mice were fed a higher-fat lard-based diet (60% kcal fat), demonstrated an even more incomplete microbial recovery upon returning mice to standard chow, contributing to accelerated weight regain upon a second exposure to the Western diet (Thaiss et al., 2016).
Experiments in “humanized” mice, formerly germ-free animals colonized with human donor samples, also provide evidence for microbial hysteresis. The dietary history of the human donor used to colonize germ-free mice correlates with the extent to which the gut microbiome responds to a dietary perturbation (Griffin et al., 2017). Over longer time-scales these effects can be amplified; the consumption a high-sugar diet deficient in plant polysaccharides by humanized mice leads to successive decreases in microbial diversity that are transmissible to the next generation (Sonnenburg et al., 2016). In humans and other primates, the infant gut microbiome is even affected by maternal dietary intake, potentially contributing to metabolic disease later in life (Chu et al., 2016). This type of “cellular memory” is not uncommon in the microbial world, enabling microorganisms to anticipate future stressors or changes to the available nutrients. More work is needed to study the mechanistic basis for this phenomenon and its role in shaping host-associated microbial communities.
When one “ome” is not enough
To gain more insight into the Western diet-associated gut microbiome we conducted a primitive form of “multi-omics”. First, we performed metagenomic shotgun sequencing of DNA extracted from the cecal samples of mice on the Western diet, in addition to samples harvested from animals fed the carbohydrate- and fat-restricted weight loss diets. We were able to confirm the observed changes in the abundance of the Bacteroidetes and the Mollicutes detected by 16S rRNA gene sequencing. We were also able to generate functional annotations for the sequencing reads and predicted proteins from metagenomic assemblies, highlighting Western diet-associated pathways involved in starch and sucrose degradation, monosaccharide import, anaerobic fermentation, and cell wall biosynthesis (Turnbaugh et al., 2008). Many of these annotations were consistent with the sequenced genome of E. dolichum, a representative member of the Mollicutes class, providing more insight into these poorly characterized and Western diet-associated gut bacteria.
A major caveat of our results, and [meta]genomics in general, is that they only provided a prediction of metabolic potential, not actual metabolic activity. To get one step closer to function, we performed our first metatranscriptomic analysis wherein we extracted RNA from a single cecal sample collected from a mouse fed the Western diet, converted it to cDNA, and performed Sanger sequencing. Due to a combination of low sequencing depth and insufficient methods for rRNA depletion, we could only make limited conclusions about the expression of protein-coding genes; however, we were able to confirm the high level of 16S rRNA transcripts matching the Mollicutes bloom. Finally, we used targeted mass spectrometry to confirm our sequencing-based predictions of enhanced bacterial fermentation on the Western diet relative to the carbohydrate-restricted weight loss diet (Turnbaugh et al., 2008).
Despite the small number of samples we analyzed, I think this study still provides a useful lesson about the benefits of using many complementary experimental approaches to study the microbiome. The integration of disparate datasets, while challenging, strengthens the conclusions that can be drawn and can provide surprising insights that might not be predicted from a single approach.
Some food for thought
The number of microbiome studies related to diet-induced obesity is rising at an exponential rate (Figure 1b), but with each new discovery comes new questions. What functional roles do the Mollicutes, or the other members of the “dark matter” of the microbiome, play in metabolic disease and host physiology in general? Considerable attention has been given to the two most abundant phyla, the Firmicutes and the Bacteroidetes, but entire phyla that are prevalent in adults (e.g., Actinobacteria and Verrucomicrobia) remain understudied. How predictable are diet-induced changes to the gut microbiome between individuals and between mammalian species, including humans? How important is overall macronutrient intake relative to other nutritional variables like dietary additives, environmental contaminants, bioactive compounds, and micronutrients? The number of distinct mechanisms by which the gut microbiome can be shaped by diet and in turn influence obesity and other diseases continues to multiply, but few comparative studies have addressed the relative strength of each mechanism relative to one another. Which side of the energy balance equation matters more, microbial contributions to caloric intake or microbe-induced changes to host energy expenditure? For that matter, does the microbiome itself expend a significant amount of energy or induce an energetic cost for host tissues to maintain colonization?
A skeptic might argue that host-associated microbial communities are irreducibly complex and experimentally intractable, that we will never have satisfying answers to these questions. Hundreds of preclinical studies have provided evidence for microbiome-related interventions that affect host energy balance, but compelling causal evidence from clinical trials has lagged behind. Personally, I am optimistic given the innovative computational, experimental, and theoretical approaches now being leveraged to study the microbiome—microbiology has spread like an epidemic across nearly every traditional field of study and new breakthroughs are coming at an ever more rapid pace. But at the very least, what is now clear is that the impact of diet on the gut microbiome is not only non-obvious, but its implications are far-reaching and essential for understanding and combating human disease.
Acknowledgments
I sincerely apologize to the many outstanding colleagues whose work was left out due to space and reference limits. This work was supported by the National Institutes of Health (R01HL122593), the UCSF Nutrition Obesity Research Center, the G.W. Hooper Foundation, and the UCSF Department of Microbiology and Immunology. I am a Nadia’s Gift Foundation Innovator supported, in part, by the Damon Runyon Cancer Research Foundation (DRR-42-16), the UCSF Program for Breakthrough Biomedical Research (partially funded by the Sandler Foundation), and the Searle Scholars Program.
References
- Carmody RN, Gerber GK, Luevano JM, Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Meyer KM, Prince AL, Aagaard KM. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016;7:459–470. doi: 10.1080/19490976.2016.1241357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin NW, Ahern PP, Cheng J, Heath AC, Ilkayeva O, Newgard CB, Fontana L, Gordon JI. Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe. 2017;21:84–96. doi: 10.1016/j.chom.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, Knight R, Ahima RS, Bushman F, Wu GD. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. e1711–1712. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AI. Some observations on the study of intestinal bacteria. Journal of Biological Chemistry. 1909;6:499–507. [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Itav S, Rothschild D, Meijer M, Levy M, Moresi C, Dohnalova L, Braverman S, Rozin S, Malitsky S, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016 doi: 10.1038/nature20796. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]