Abstract
Background
Inflammatory bowel disease (IBD) increases the risk of developing colon cancer. This risk is higher in men compared to women, implicating a role for female hormones in the protection against this disease. Studies from our laboratory demonstrated that estradiol (E2) protects against inflammation-associated colon tumor formation when administered following chemical carcinogen and induction of chronic colitis.
Aim
This study seeks to better understand the effect of E2 on acute colitis in the presence and absence of estrogen receptor β (ERβ).
Methods
Inflammation was induced by 2,4,6-trinitrobenzenesulfonic acid in wild-type (WT) and ERβ knockout (ERβKO) mice implanted with a control or E2-containing pellet and killed 5 days later. Inflammation and injury were scored by a pathologist. Apoptosis and proliferation were assessed by immunohistochemistry. Cytokines were measured by multiplex analysis.
Results
E2 treatment reduced inflammation in the middle colon in WT mice and the distal colon in ERβKO mice compared to control mice. WT mice had reduced IL-6, IL-12, IL-17, GM-CSF, IFN-γ, MCP-1, MIP-1α, and TNF-α, and ERβKO had reduced IL-6 and IFN-γ expression in response to E2. Injury scores were lower in E2-treated ERβKO mice compared to control ERβKO mice. ERβKO mice had increased proliferation in the basal third of crypts in the distal colon and decreased apoptosis in the proximal colon.
Conclusions
These data suggest that E2 has differential protective effects against acute colitis in the presence or absence of ERβ and provide insight into how E2 may protect against IBD.
Keywords: Colon cancer, Estradiol, Estrogen receptor beta, Crohn’s disease, Inflammation
Introduction
Inflammatory bowel disease (IBD) affects over one million people in the USA. Typically, IBD occurs in persons in their twenties and thirties at which point symptoms lesson for a number of years followed by flare-ups of the disease later in life. The two primary forms of IBD, Crohn’s disease and ulcerative colitis, both confer a greater lifetime risk of developing colon cancer [1, 2]. This increased risk is most likely due to an increased rate of growth due to the exposure to pro-inflammatory cytokines. Interestingly, women have been observed to be 60% less at risk than men to develop inflammation-associated colon cancer, suggesting that female hormones may play a role in the prevention of this disease [3].
A number of experimental models have demonstrated that estrogens have anti-inflammatory properties in tissues other than the intestine and one way in which estradiol (E2), the most biologically active form of estrogen in the human body, may be protecting against inflammation-associated colon carcinogenesis is through the suppression of intestinal inflammation [4–7]. Epidemiological studies have observed that pre-menopausal women with Crohn’s disease reported a worsening of their IBD-related symptoms during menses, the time during the estrous cycle that E2 concentrations are at their lowest [8]. Not only does this suggest that E2 could be protecting against intestinal inflammation, but the fact that the same worsening of symptoms was not observed in women with ulcerative colitis suggests that the role of E2 in protecting against inflammation could be dependent on the subtype of IBD.
Experimental data to support the protective effects of E2 on the development of inflammation-associated colon tumors, however, are conflicting. Data from our laboratory indicate that treatment with E2 in mice following induction of inflammation-associated colon cancer using cotreatment with the colon-specific carcinogen azoxymethane (AOM) and dextran sulfate sodium (DSS) was effective at reducing both the number and size of colon tumors [9]. Other groups, however, have observed that in mice pre-treated with E2 and then subjected to AOM/DSS, polyp number and size were increased [10]. In a separate study investigating the role of estrogen receptor (ER)β in AOM/DSS-induced polyp formation in the presence of endogenous E2 levels, Saleiro et al. determined that mice lacking ERβ (ERβKO) had more polyps at 9 weeks compared to wild-type (WT) littermates. This study further saw an induction of pro-inflammatory cytokines in the absence of ERβ at 16 weeks; however, at 9 weeks, no significant differences in cytokine expression were detected [11].
The disparity in the observed results is not limited to eventual tumor development. Studies investigating the effects of E2 solely on inflammation have also reached varying data. Here though, the incongruent findings are likely the result of the models of inflammation used. Studies using DSS as the inflammatory agent found that E2 worsened disease severity, whereas colitis induced by dinitrobenzene sulfonic acid was improved in the presence of E2 [10, 12]. When 2,4,6-trinitrobenzenesulfonic acid (TNBS) was used to induce colonic inflammation in rats, estradiol benzoate and the phytoestrogen genistein improved pathological scores in models of acute and chronic inflammation, respectively [13, 14]. DSS and TNBS induce inflammation through distinct mechanisms resulting in colitis that resembles ulcerative colitis and Crohn’s disease, respectively, and the differences in response to E2 observed between these two models may be reflective of distinct effects of E2 on these two separate forms of IBD.
The focus of the present study is to investigate the physiological actions of E2 during acute TNBS-induced colitis in WT and ERβKO mice. While previous studies suggest that estrogen is protective against TNBS colitis, the mechanism behind this protection is still poorly understood. Studies in our laboratory in both sporadic and inflammation-associated colon cancer models suggest that ERβ, the primary ER in the colon, mediates the protective effect of E2 in this tissue [9, 15]. To date, however, the role of ERβ in TNBS-induced colitis is unknown. Understanding the function of E2 and ERβ during acute inflammation is important, particularly when you take into consideration the fact that persons with active Crohn’s disease have lower colonic expression of ERβ compared to when their disease is in remission [16].
Methods and Materials
Animals
C57Bl6/J mice heterozygous for ERβKO (±) were originally obtained from the Jackson Laboratory. The mice were housed at the Laboratory Animal Resources and Research Facility at Texas A&M University. Mice were bred to produce WT and ERβKO offspring, and genotype was confirmed using genomic tail DNA. All procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee at Texas A&M University.
Induction of Colitis
While it does not perfectly recapitulate human Crohn’s disease, TNBS-induced colitis shares many characteristics with human Crohn’s disease including the involvement of NOD2, the macroscopic pattern of inflammation, induction of mucosal edema, crypt distortion, and abscess formation [17].
For this study, female mice were weight and age matched (mean 27.8 g and 5.8 months) and divided into treatment groups (n = 12–16 mice per group). On day 1, mice were ovariectomized (OVX) and implanted with either a 20-mg cholesterol containing pellet or a 0.5-mg E2 + 19.5-mg cholesterol (Sigma-Aldrich, St. Louis, MO) pellet subcutaneously and placed on a phytoestrogen-free diet. Corresponding plasma E2 levels for the groups at the end of the study were 1.2 nM in the mice receiving E2 and undetectable in the cholesterol mice with no significant differences between genotype and TNBS treatment group. Two weeks after surgery and pellet implant, mice were pretreated with 1% TNBS dermal absorption through the skin on their backs. One week following pre-treatment, a 2.5% TNBS solution was administered intrarectally 2–4 cm into the colon using a flexible plastic gavage tube (Instech Solomon) after a 12-h fast. Mice were killed 5 d postintrarectal TNBS. Colons were resected and longitudinally bisected. One half of the colon was rolled into a Swiss roll and fixed in PFA. The other half was snap-frozen in liquid nitrogen for cytokine analysis. Inflammation and injury in the colons were assessed by a board-certified pathologist on H&E-stained colon Swiss rolls on a scale of 0–3. In brief, a score of 0 would indicate no inflammation or injury noted, while a score of 3 would indicate severe inflammation or injury.
Cytokine Multiplex Analysis
Snap-frozen colon tissues representative of the whole colon were homogenized in 1 mL Tissue Protein Extraction Reagent (Thermo Scientific). Total protein content was assessed using the DC Protein Assay (Bio-Rad) as per the manufacturer’s instructions. The Magnetic Mouse Cytokine/Chemokine Milliplex Map Kit was used to measure tissue levels of IL-6, IL-10, IL-12(p40), IL-17, GM-CSF, IFN-γ, MCP-1, MIP-1α, and TNF-α following the manufacturer’s instructions. All samples were diluted to 10 M. In brief, 25 µL diluted sample was added per well of a 96-well plate in addition to 25 µL assay buffer and 25 µL of the magnetic beads provided in the kit. The plate was sealed and agitated on a plate shaker overnight at 4 °C. The following day, the 96-well plate was placed on a handheld magnet, the contents of the wells removed, and then the plate was washed twice. Following washing, 25 µL of detection antibodies was added to each well and the plate was incubated at room temperature for 1 h with agitation. Next, 25 µL of streptavidin–phycoerythrin was added to each well and the plate was incubated at room temperature for half an hour with agitation. The plate was then washed twice, the magnetic beads resuspended in 150 µL Luminex sheath fluid, and the plate was run on a Luminex 200.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling Assay
Paraffin-embedded, paraformaldehyde (PFA)-fixed colon Swiss rolls were rehydrated, and the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore; Billerica, MA) was used for the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assays following the manufacturer’s instructions with slight modifications. PFA-fixed tissues were rehydrated and treated with 10 µg/mL proteinase K for 3 min at 37 °C. Endogenous peroxidase activity was blocked using 0.3% H2O2 in methanol for 30 min. Tissues were incubated at room temperature (RT) for 20 s in equilibration buffer and then incubated for 1 h at 37 °C in reaction buffer plus TDT enzyme. The TDT enzyme was omitted from an individual section as a negative control for the stain. Subsequently, slides were placed in stopwash solution for 10 min followed by 30 min at RT in anti-digoxigenin in a humidified chamber. A 0.5% DAB solution for 20 s was used as the chromogen, and nuclei were counterstained in 0.5% methyl green for 5 s. Lastly, slides were dehydrated and coverslipped.
Immunohistochemistry for BrdU
Four micrometer sections were taken from the PFA-fixed tissues. Sections were deparaffinized and rehydrated. Endogenous peroxidase was quenched using 3% H2O2 in methanol for 30 min, and antigen retrieval was achieved by microwaving in 10 mM citrate buffer for 20 min. Slides were then incubated in the primary antibody, anti-BrdU (Roche, Basel, Switzerland) diluted 1:20 at 4 °C overnight in a humidified chamber. During each stain, the primary antibody was left off of a single slide to serve as a negative control. The following morning, the slides were washed and then incubated in the secondary antibody, goat anti-mouse HRP (Abcam, Cambridge, MA) diluted 1:250. Meyer’s hematoxylin was used as the counterstain. Lastly, slides were dehydrated and coverslipped.
Immunohistochemistry Analysis
The TUNEL and BrdU assays were analyzed in the same manner. For each, 20 well-oriented crypts per animal from both the distal and proximal colon were symmetrically bisected and the right halves of each were analyzed. The total number of positively stained cells was divided by the total number of cells in the crypt column to generate the percentage of apoptotic or proliferative cells for each crypt column.
Statistics
Analysis for all data was determined using one-way ANOVA or Student’s t test using JMP Pro 10. Differences were considered significant if P < 0.05.
Results
E2 Protects Against TNBS-Induced Weight Loss and Increases Colon Length
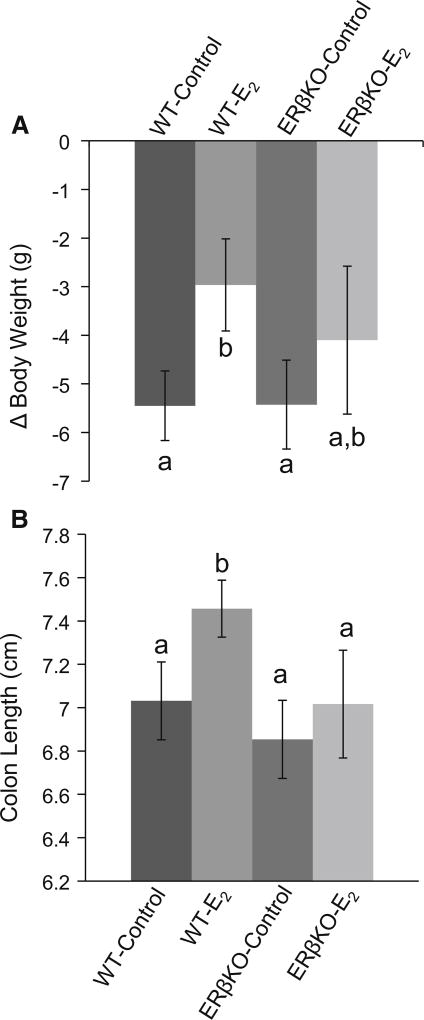
All four groups of mice had a significant weight loss from their pre-study weights. WT control mice lost significantly more weight than WT E2-treated mice. There was no difference in weight loss between ERβKO mice in response to E2 (Fig. 1a). Additionally, as it has been routinely observed in our laboratory, E2 treatment increased the length of the colon in WT but not ERβKO mice (Fig. 1b).
Fig. 1.
a Effect of E2 on TNBS-induced weight loss. Values are mean weight loss per animal in grams ± SEM and b effect of E2 on colon length. Values are mean colon length ± SEM. n = 12–16 mice per group. Bars without a common letter differ, P ≤ 0.05
E2 Protects Against Inflammation and Injury in Regions of the Colon
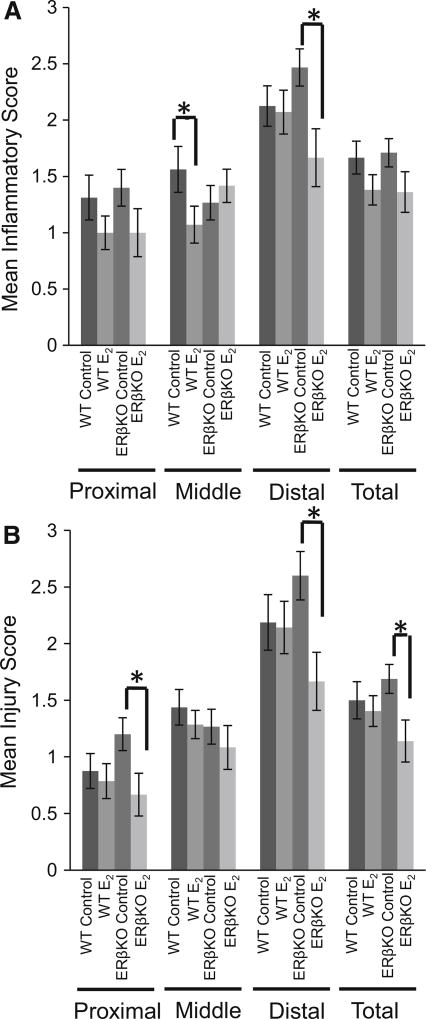
After induction of acute colitis using TNBS, E2-treated WT mice had reduced inflammation in the middle region of the colon. ERβKO mice had reduced inflammation in response to E2 in the distal end of the colon (Fig. 2a). There were no changes in injury scoring in the WT mice for any region of the colon. In ERβKO E2-treated mice, however, a reduction in injury was observed in the proximal, distal, and overall colon compared to ERβKO control-treated mice (Fig. 2b).
Fig. 2.
Inflammation was induced in the colon of ovariectomized mice with TNBS. Sectioned tissues were H&E stained and analyzed by a board-certified pathologist. a Inflammation scores and b injury scores. Values are mean score ± SEM. n = 12–16 mice per group. “*” denotes significance between indicated groups, P < 0.05
E2 Reduces the Expression of Pro-inflammatory Cytokines
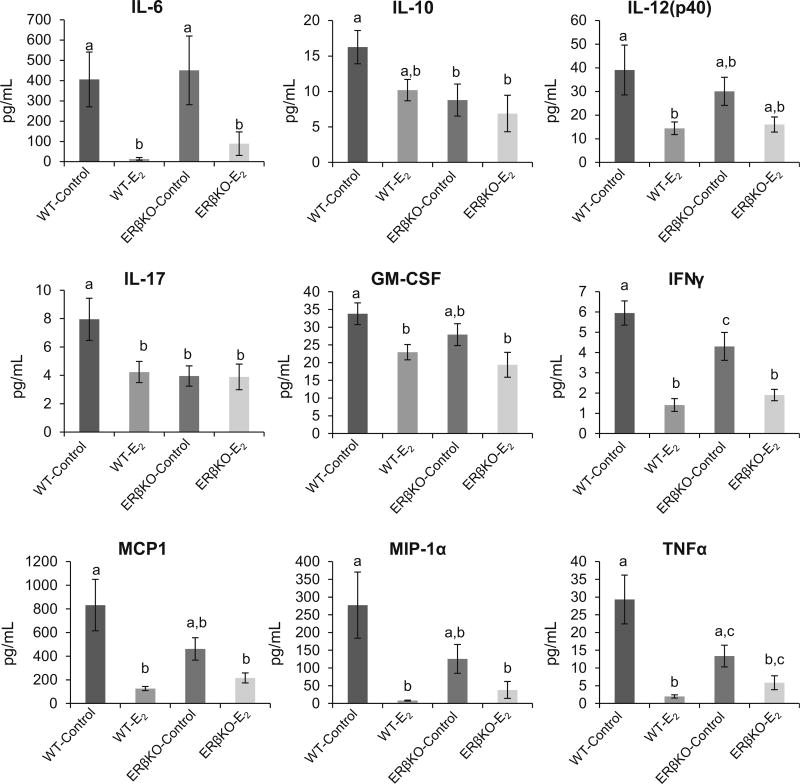
Cytokine expression profiles were determined using a multiplex magnetic bead assay. In WT mice, treatment with E2 resulted in reduced expression of IL-6, IL-12(p40), IL-17, GM-CSF, IFN-γ, MCP-1, MIP-1α, and TNF-α. In ERβKO mice, IL-6 and TNF-α expression was significantly downregulated by E2. Several other cytokines, IL-12(p40), GM-CSF, MCP-1, MIP-1α, and TNF-α, trended toward reduced expression in response to E2 in ERβKO mice; however, the differences did not reach statistical significance (Fig. 3).
Fig. 3.
Cytokine expression in colon tissue was assessed using a multiplex assay. Values are mean expression ± SEM. Bars without a common letter differ, P < 0.05
Proliferation Is Increased in ERβKO Mice
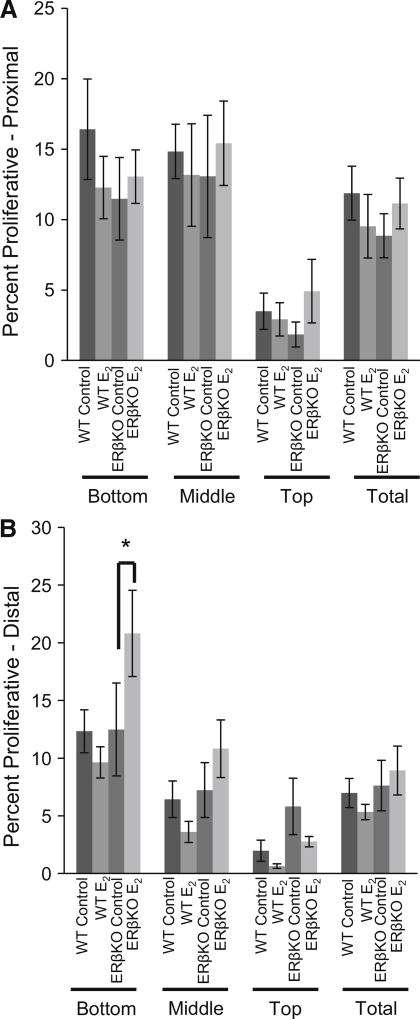
Proliferation was assessed by BrdU incorporation into actively dividing cells. Both the proximal and distal ends of the colon were analyzed. The crypts were further divided into bottom, middle, and top regions. No changes in proliferation were observed in either genotype in response to E2 in the proximal colon for any region in the crypt (Fig. 4a). In the distal colon, E2-treated ERβKO mice had increased proliferation compared to control-treated ERβKO mice in the bottom third of crypts. No other significant differences were observed in the distal colon (Fig. 4b).
Fig. 4.
Effect of E2 on proliferation during acute inflammation. Two hours prior to kill, mice were injected with 5-bromo-2′-deoxyuridine (BrdU). Immunohistochemistry for BrdU was performed on sectioned tissues from the proximal and distal colon. Data are expressed as the percentage of proliferative cells compared to the total number of cells for either the full crypt column or the indicated region of the crypt. Data are representative of 20 well-oriented crypts per animal per proximal or distal colon with n = 12–16 mice per group. a Proliferation in the proximal colon and b proliferation in the distal colon. “*” denotes significance between indicated groups, P < 0.05
Apoptosis Is Decreased in ERβKO Mice
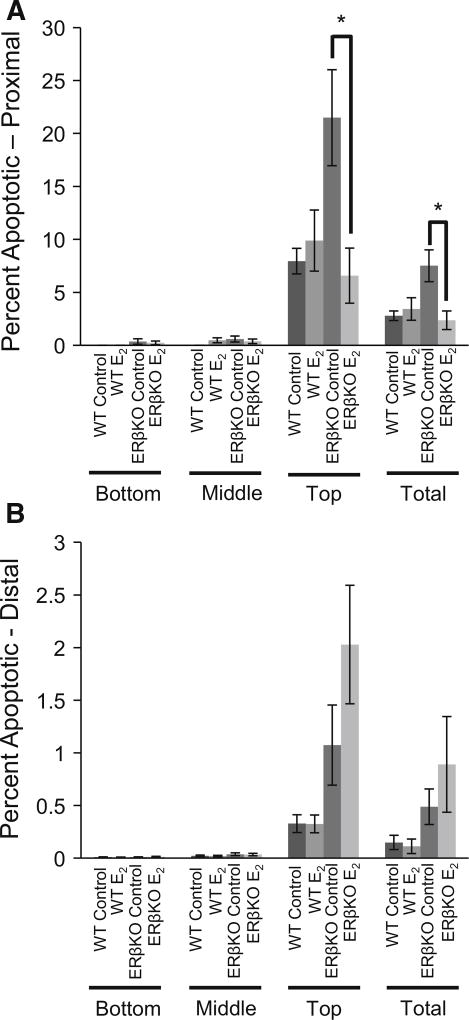
Apoptosis was analyzed in the proximal and distal colon using a TUNEL assay. Again, the crypts were divided into bottom, middle, and top regions to observe changes in the different populations of colonocytes. In the proximal colon, E2-treated ERβKO mice had reduced apoptosis both overall and in the top third of colon crypts compared to control ERβKO mice (Fig. 5a). There was a trend for increased apoptosis in E2-treated ERβKO mice in the distal colon compared to ERβ controls; however, no significant changes occurred in the distal colon in response to E2 in any region of the crypt for either genotype (Fig. 5b).
Fig. 5.
A TUNEL assay was performed on sectioned tissues from the proximal and distal colon. a Apoptosis in the proximal colon and b apoptosis in the distal colon. Data are expressed as the mean percentage of apoptotic cells compared to the total number of cells for either the full crypt column or the indicated region of the crypt ± SEM. Data are representative of 20 well-oriented crypts per animal per proximal or distal colon with n = 12–16 mice per group. “*” denotes significance between indicated groups, P < 0.05
Discussion
Epidemiological studies suggest that E2 is protective against inflammation-associated colon tumor formation [3]. One mechanism by which this protection may occur is through the modulation of acute inflammatory episodes in the colon. Thus far, however, results from in vivo studies investigating the effects of E2 on inflammation and subsequent tumor formation in colon cancer models have been inconclusive. The differences observed in response to the presence of E2 may be due, in part, to the inherent differences in the pathology of each disease as well as the variety of models used to study them. This study utilized TNBS to mimic acute Crohn’s disease inflammation.
In this model, E2 was observed to protect against inflammation in WT and ERβKO mice in the middle and proximal regions of the colon, respectively. The likely mediator of the reduced inflammation in the middle and distal colon in WT and ERβKO E2-treated mice, respectively, is the decreased expression of pro-inflammatory cytokines observed in the presence of E2. Aberrant expression of cytokines is a hallmark for Crohn’s disease, and several of the current therapies are aimed at blocking cytokine activity [18]. Highlighting the fact that E2 has varying effects depending on ERβ expression, cytokine production was reduced in both WT and ERβKO mice; however, only expression of two of the targets analyzed, IFN-γ and IL-6, was significantly downregulated in the ERβKO mice compared to the eight cytokines affected in the WT mice. Studies have shown that macrophages express ERβ and that ERβ-specific agonists can influence cytokine production [19, 20]. The lack of ERβ expression in the ERβKO mice reduces the ability of these cells to respond to E2 and might partially explain the reduced physiological effects observed in these mice in regard to cytokine production.
While the ERβKO mice lack ERβ, they still express ERα and other, non-classical, ERs such as G protein-coupled estrogen receptor 1 (GPER or GPR30). Other studies have observed that ERα-specific agonists influence leukocyte rolling and adhesion to protect against sepsis in the colon, demonstrating that ERα has a protective roll in the tissue [21]. GPER has also been implicated in mitigating inflammation; treatment of endothelial cells with a GPER-specific agonist has been demonstrated to reduce the expression of pro-inflammatory cytokines [22]. It is also important to note that the colonic inflammatory process is complex and does not rely solely on the colonic epithelia but is rather a systemic process with immune cells migrating into the tissue upon initiation of the inflammatory cascade. Unlike the colonic epithelia, ERβ is not necessarily the primary ER in these immune cells. In human monocytes, Pelekanou et al. observed expression of GPER and ERα36, a splice variant of ERα, but they did not detect any expression of ERβ or full-length ERα. Furthermore, they demonstrated that in these cells, E2 treatment inhibited LPS-induced IL-6 inflammatory response and this could be inhibited by the addition of either the nuclear ER antagonist ICI or the GPER-specific antagonist G15 [23]. Another study observed GPER expression in mast cells in human colon biopsies. Contrary to the protective effects of E2 in the colon observed in our study, this group correlated increased GPER expression in mast cells to enhanced abdominal pain severity in diarrhea-predominant irritable bowel syndrome patients [24]. Together, these studies suggest that ERα36 and/or GPER has a role in inflammation and in the colon. Being as the ERβKO mice in our study retain ERα and GPER expression, it is possible that these estrogen receptors are responsible for the anti-inflammatory effects of E2 observed in our model. While it goes beyond the scope of the presented study, ongoing experiments in our laboratory are investigating the contribution of immune cells as well as GPER and ERα and its variants in response to E2 in both acute and chronic colitis.
Injury scores were also reduced by E2 treatment. For all groups, injury scoring was highest in the distal colon, possibly a result of the intrarectal method of TNBS administration: Insertion of the gavage tube into the colon physically damages this portion of the tissue which is a limitation as to how data from this model may be compared to Crohn’s disease in humans. However, due to the physical damage, the results observed in response to E2 in the distal colon in regard to injury are partially reflective of wound healing and not solely inflammation. E2 treatment was associated with a lower injury score in ERβKO mice in the proximal, distal, and overall colon. In the distal colon, in addition to the reduction in injury, an increase in proliferation was observed in ERβKO mice. This increase in proliferation could be indicative of damaged colonocytes being replaced and may be the underlying mechanism behind the increased healing in the distal colons of these mice resulting in reduced injury scores. Future studies will explore the timing of E2 exposure with the goal to clearly define its potential role in wound healing in the colon.
The fact that the protection against inflammation was observed in different regions of the colon for WT and ERβKO animals and that injury was only reduced in the presence of E2 in ERβKO mice suggests that during acute inflammation separate mechanisms of protection are being utilized for different regions of the colon in the presence and absence of ERβ. The proximal and distal colon is distinct in developmental origin, morphology, and gene expression profiles [25–27]. Additionally, the microbial population also varies between regions of the colon, due in part to changes in the viscosity of the mucus allowing for different microbes to reach the mucosa [28]. These physiological variances between regions of the colon support the hypothesis that the mechanism of action of E2 differs between the proximal, middle, and distal colon.
Overall, data from this study suggest that E2 is partially protective against acute TNBS-induced colitis and reduces the expression of pro-inflammatory cytokines in the colon. This protective effect differs between WT and ERβKO mice in regard to location of protection against inflammation, injury, and the cytokine expression profiles. This suggests unique actions of E2 in the presence and absence of ERβ which has clinical relevance due to the reduced ERβ expression in the colon of people with active Crohn’s disease. Furthermore, reduction of acute inflammation could be a contributing mechanism by which E2 reduces the risk of inflammation-associated colon cancer.
Acknowledgments
Funding was provided by American Cancer Society (Grant No. RSG-11-179-01-TVE).
Footnotes
Conflict of interest
None of the authors have any conflicts of interest to declare.
References
- 1.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soderlund S, Granath F, Brostrom O, et al. Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males. Gastroenterology. 2010;138:1697–1703. doi: 10.1053/j.gastro.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Cuzzocrea S, Mazzon E, Sautebin L, et al. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7:478–487. [PMC free article] [PubMed] [Google Scholar]
- 5.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89:823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Lai K, Shaw LJ, Harnish DC, Chadwick CC. Estrogen receptor alpha inhibits IL-1beta induction of gene expression in the mouse liver. Endocrinology. 2002;143:2559–2570. doi: 10.1210/endo.143.7.8919. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto N, Mandai M, Suzuma I, et al. Estrogen protects against cellular infiltration by reducing the expressions of E-selectin and IL-6 in endotoxin-induced uveitis. J Immunol. 1999;163:374–379. [PubMed] [Google Scholar]
- 8.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93:1867–1872. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong CM, Billimek AR, Allred KF, et al. A novel shift in estrogen receptor expression occurs as estradiol suppresses inflammation-associated colon tumor formation. Endocr Relat Cancer. 2013;20:515–525. doi: 10.1530/ERC-12-0308. [DOI] [PubMed] [Google Scholar]
- 10.Heijmans J, Wielenga MC, Rosekrans SL, et al. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut. 2014;63:310–316. doi: 10.1136/gutjnl-2012-304216. [DOI] [PubMed] [Google Scholar]
- 11.Saleiro D, Murillo G, Benya RV, et al. Estrogen receptor-beta protects against colitis-associated neoplasia in mice. Int J Cancer. 2012;131:2553–2561. doi: 10.1002/ijc.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdu EF, Deng Y, Bercik P, Collins SM. Modulatory effects of estrogen in two murine models of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G27–G36. doi: 10.1152/ajpgi.00460.2001. [DOI] [PubMed] [Google Scholar]
- 13.Houdeau E, Moriez R, Leveque M, et al. Sex steroid regulation of macrophage migration inhibitory factor in normal and inflamed colon in the female rat. Gastroenterology. 2007;132:982–993. doi: 10.1053/j.gastro.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48:213–220. doi: 10.1007/s00394-009-0004-3. [DOI] [PubMed] [Google Scholar]
- 15.Weige CC, Allred KF, Allred CD. Estradiol alters cell growth in nonmalignant colonocytes and reduces the formation of preneoplastic lesions in the colon. Cancer Res. 2009;69:9118–9124. doi: 10.1158/0008-5472.CAN-09-2348. [DOI] [PubMed] [Google Scholar]
- 16.Looijer-van Langen M, Hotte N, Dieleman LA, et al. Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621–G626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou E, Margonis GA, Angelou A, et al. The TNBS-induced colitis animal model: an overview. Annal Med Surg. 2016;11:9–15. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macdonald TT. Inside the microbial and immune labyrinth: totally gutted. Nat Med. 2010;16:1194–1195. doi: 10.1038/nm1110-1194. [DOI] [PubMed] [Google Scholar]
- 19.Huang SY, Xin H, Sun J, et al. Estrogen receptor beta agonist diarylpropionitrile inhibits lipopolysaccharide-induced regulated on activation normal T cell expressed and secreted (RANTES) production in macrophages by repressing nuclear factor kappaB activation. Fertil Steril. 2013;100:234–240. doi: 10.1016/j.fertnstert.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian M, Shaha C. Oestrogen modulates human macrophage apoptosis via differential signalling through oestrogen receptor-alpha and beta. J Cell Mol Med. 2009;13:2317–2329. doi: 10.1111/j.1582-4934.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharawy N, Ribback S, Al-Banna N, et al. Estradiol receptors agonists induced effects in rat intestinal microcirculation during sepsis. Microvasc Res. 2013;85:118–127. doi: 10.1016/j.mvr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Davidge ST. G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PLoS ONE. 2012;7:e52357. doi: 10.1371/journal.pone.0052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelekanou V, Kampa M, Kiagiadaki F, et al. Estrogen anti-inflammatory activity on human monocytes is mediated through cross-talk between estrogen receptor ERalpha36 and GPR30/GPER1. J Leukoc Biol. 2016;99:333–347. doi: 10.1189/jlb.3A0914-430RR. [DOI] [PubMed] [Google Scholar]
- 24.Qin B, Dong L, Guo X, et al. Expression of G protein-coupled estrogen receptor in irritable bowel syndrome and its clinical significance. Int J Clin Exp Pathol. 2014;7:2238–2246. [PMC free article] [PubMed] [Google Scholar]
- 25.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755–762. [PubMed] [Google Scholar]
- 26.Distler P, Holt PR. Are right- and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–311. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 27.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 28.Fredricks DN. The human microbiota: how microbial communities affect health and disease. Hoboken: Wiley; 2013. [Google Scholar]