Abstract
Treatment of tumors with Toca 511, a gamma retroviral replicating vector encoding cytosine deaminase, followed by 5-fluorocytosine (5-FC) kills tumors by local production of 5-fluorouracil (5-FU). In brain tumor models, this treatment induces systemic anti-tumor immune responses and long-term immune-mediated survival. Phase 1 Toca 511 and Toca FC (extended-release 5-FC) clinical trials in patients with recurrent high-grade glioma show durable complete responses and promising survival data compared to historic controls. The work described herein served to expand on our earlier findings in two models of metastatic colorectal carcinoma (mCRC). Intravenous (i.v.) delivery of Toca 511 resulted in substantial tumor-selective uptake of vector into metastatic lesions. Subsequent treatment with 5-FC resulted in tumor shrinkage, improved survival, and immune memory against future rechallenge with the same CT26 CRC cell line. Similar results were seen in a brain metastasis model of mCRC. Of note, 5-FC treatment resulted in a significant decrease in myeloid-derived suppressor cells (MDSCs) in mCRC tumors in both the liver and brain. These results support the development of Toca 511 and Toca FC as a novel immunotherapeutic approach for patients with mCRC. A phase 1 study of i.v. Toca 511 and Toca FC in solid tumors, including mCRC, is currently underway (NCT02576665).
Keywords: retroviral vector, immunotherapy, MDSC, mCRC, 5-FU, chemoimmunotherapy, durable response
Introduction
Toca 511 (vocimagene amiretrorepvec), a retroviral replicating vector, selectively replicates and spreads in malignant cells and encodes an optimized yeast cytosine deaminase (CD) protein. Toca 511 is designed to selectively infect cancer cells because retroviral replicating vectors (RRVs) selectively infect cancer cells because viral replication is restricted by innate and adaptive immune responses that are defective in malignant cells but intact in normal tissues.1, 2 As a further restriction to cancer cells, RRVs only infect actively dividing cells. In infected cells, CD enzyme is expressed and converts 5-fluorocytosine (5-FC) (an oral anti-fungal drug) to 5-fluorouracil (5-FU) (an anti-cancer drug). Our previous data demonstrated that Toca 511 administered intratumorally results in tumor cell death and provides a long-term survival benefit against brain cancer in preclinical models.1, 3, 4, 5 Administration of Toca 511 and subsequent treatment with the prodrug 5-FC is designed to generate higher levels of 5-FU in the tumor than can be achieved with systemic 5-FU delivery, allowing 5-FU tumor killing with fewer systemic toxicities. Direct tumor cytotoxicity and extended survival attributed to immunotherapeutic effects have been reported using this approach.1, 4, 5, 6
Approximately 50% of patients with colorectal cancer (CRC) develop metastases (mCRC) during the course of the disease, with liver being the most frequent site. Standard treatment for mCRC is 5-FU-based combination therapy, which extends median survival from 6 to 24 months.7 Brain metastases (BMs) from colorectal cancer are historically quite rare, representing only 4%–6% of all BM cases.8, 9 However, because first-line treatments are improving survival, brain metastasis is now becoming more frequent.10, 11 The current treatment options for BM from colorectal cancer include surgery or stereotactic radiosurgery, with or without whole-brain radiotherapy and, in rare cases, chemotherapy.12 However, the prognosis for patients with BMs from colorectal cancer remains poor, with median survival ranging from 2 to 15 months.13 Further, trials with new immunotherapeutic agents have had a limited therapeutic impact on mCRC.14 Taken together, mCRC represents an area in which novel therapeutic approaches are desperately needed.
Recent studies suggest myeloid-derived suppressor cells (MDSCs) contribute to cancer immune evasion by suppressing anti-tumor immune response.15, 16, 17, 18 MDSCs are immature myeloid cells that are attracted to, and develop in, the tumor microenvironment by tumor-associated signals, and once there, continue to proliferate and actively suppress the immune system through multiple mechanisms. MDSCs are thought to be a key player in setting up and maintaining local immune suppression in numerous cancers, including mCRC.19 There are no currently approved therapies specifically targeting MDSCs; however, it has been shown that 5-FU can deplete MDSCs,20 presumably because of their continued proliferation and low levels of thymidylate synthase.21 Therefore, treatment with Toca 511 and 5-FC to generate high local concentrations of 5-FU at the site of the tumor may have additional benefits outside of its ability to directly kill tumor cells. 5-FU may also confer an immunotherapeutic effect through depletion of highly immunosuppressive cells from the tumor microenvironment. There is a clear unmet medical need for new treatments for liver and brain metastases, and immunotherapeutic strategies that impact immunosuppressive tumor microenvironments appear to be useful candidates for this role. We show here that treatment with Toca 511 and 5-FC incorporates such a strategy.
We assessed the effects of Toca 511 and 5-FC treatment on survival and anti-tumor immune activation in syngeneic models of CRC metastases to the liver and brain. The results reported here support the development of Toca 511 and Toca FC as a novel immunotherapeutic approach for patients with mCRC and potentially other metastatic solid tumors. Toca 511 administered locally or intravenously (i.v.) combined with oral Toca FC (extended release 5-FC) is under investigation in patients with recurrent primary brain tumors (NCT01156584, NCT01470794, NCT02414165, and NCT01985256). Potential benefits have been observed, including durable complete responses, extended overall survival compared to historic controls, and a favorable safety profile.22 A phase 1 study of i.v. Toca 511 followed by cycles of oral Toca FC in patients with solid tumors, including mCRC, is currently underway (NCT02576665).
Results
Toca 511 in Combination with 5-FC Prolonged Survival in a Liver Metastasis Model of mCRC
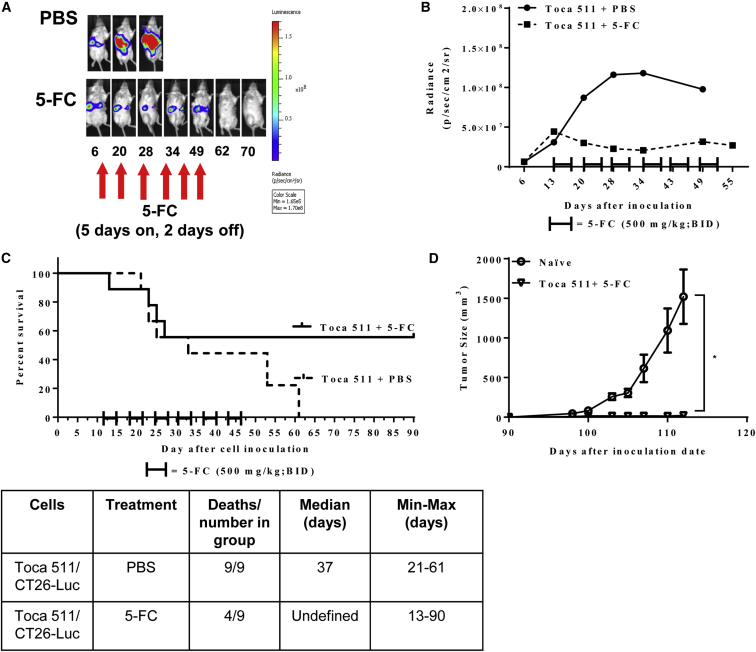
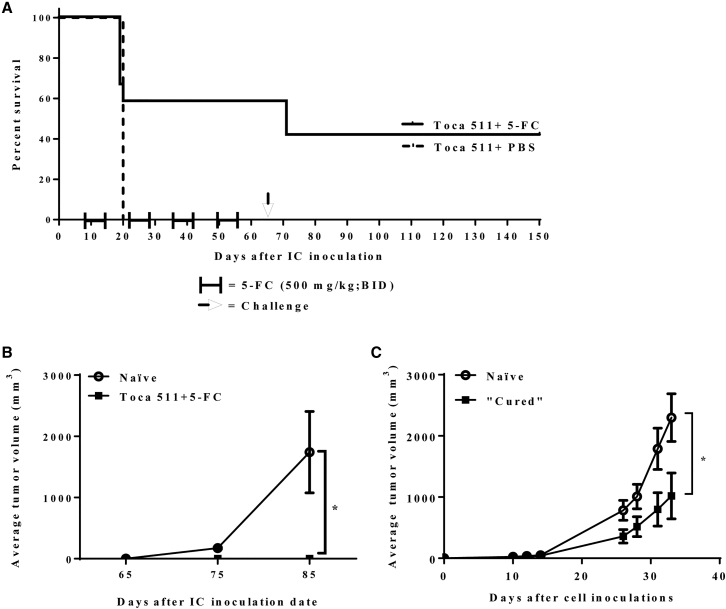
Survival was assessed in a multifocal liver metastasis model of murine mCRC after treatment with Toca 511 and 5-FC. In order to monitor tumor take and progression, CT26 cells were engineered to express luciferase (CT26-Luc) and mice were monitored through noninvasive imaging throughout the study (Figures 1A, S1A, and S1B). Mice that were inoculated intrasplenically with Toca 511 pre-transduced CT26-Luc cells developed liver metastases as early as 6 days after inoculation. 5-FC treatment cycles were initiated on day 13 and continued for a total of 6 cycles. All animals in the PBS control group showed tumor progression, as evidenced by increased bioluminescence signal over time. The Toca-511- and 5-FC-treated animals showed slower tumor progression than the control group after 6 cycles of 5-FC treatment. On average, progression in the Toca 511 and 5-FC treatment group was blunted over time and stabilized through cycles of 5-FC (Figures 1B and S1B). Treatment with 5-FC resulted in prolonged survival compared to PBS control (p = 0.05) (Figure 1C). Five of 9 tumor-bearing mice (55%) remained tumor free after cessation of 5-FC until the end of the study (day 90). Prolonged survival after cessation of treatment suggested that survival was at least partially due to the induction of anti-tumor immune response. To further evaluate the effect of Toca 511 and 5-FC treatment on the induction of anti-tumor immune responses, CT26 cells were implanted into the right flanks of “cured” mice as well as naive, age-matched mice on day 90 after the original intrasplenic tumor implant. Tumors engrafted and grew in all naive animals; however, tumors were rejected in mice that had previously cleared CT26 liver metastases through treatment with Toca 511 and 5-FC (p = 0.028 versus naive) (Figure 1D). Animals that received Toca 511 and 5-FC did not exhibit signs of toxicity (Table S1).
Figure 1.
Toca 511 in Combination with 5-FC Was Efficacious in a Multifocal Liver Metastasis Model of mCRC
(A) Representative radiance intensities (photon/s/mouse) from the PBS and 5-FC treatment group at various time points throughout the study. After liver metastases were established with Toca 511 pre-transduced CT26-Luc cells delivered intrasplenically, each mouse was imaged by IVIS at indicated time points. 5-FC treatment (500 mg/kg, i.p., BID) was initiated on day 13 for 5 days on and 2 days off for a total of six cycles. The control group received PBS (see also Figure S1). (B) CT26-Luc bioluminescence average signal intensity at indicated time points (n = 9/group). (C) Kaplan-Meier survival analysis. After liver metastases were established with Toca 511 pre-transduced CT26 cells delivered intrasplenically, PBS or 5-FC treatment was started at day 13 post cell inoculation (n = 9/group). A total of six cycles of PBS or 5-FC treatment were administered and survival was examined out to 90 days. The table is the summary of the survival analysis out to 90 days post tumor cell implantations. (D) Cured mice from survival studies (Toca 511/CT26+5-FC; n = 5) were subcutaneously challenged with wild-type CT26 cells on day 0 (day 90 post initial tumor inoculation) at a cell dose of 5 × 105. As a control, CT26 cells were implanted into naive BALB/cJ mice (n = 9) (*p = 0.028; naive versus Toca 511/CT26+5-FC). *Statistical significance was defined as p < 0.05. Error bars represent SEM.
Toca 511 Efficiently Infects and Spreads in Multifocal Liver Metastases after Intrasplenic, i.v., or Intraportal Delivery
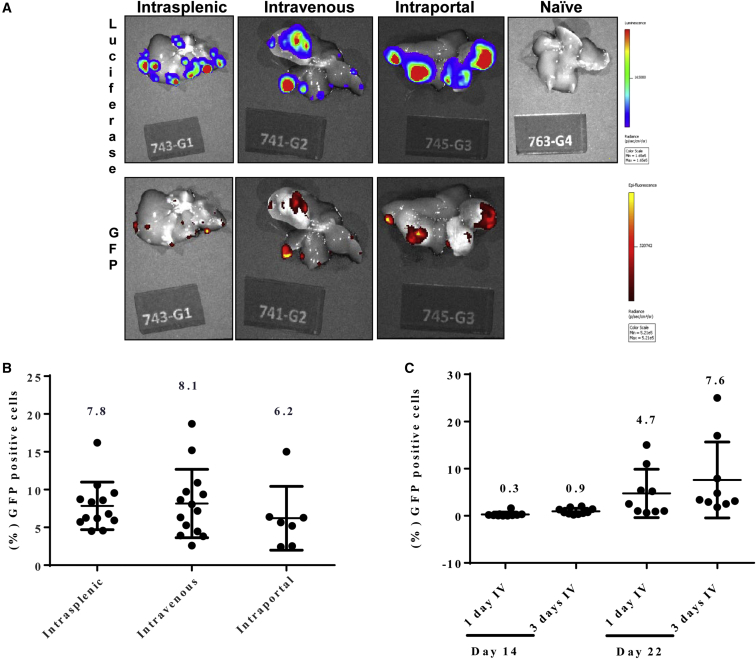
In order to obtain maximal spread of Toca 511, initial distribution of vector through an optimal delivery modality is a key factor. In this study, instrasplenic, i.v., and intraportal vein routes were compared to evaluate vector delivery to multifocal liver metastases. 3 days post cell inoculations, one dose of vector (3.4 × 107 TU) was delivered via three different routes. A GPF-expressing vector (Toca GFP) was utilized in order to facilitate imaging of the vector within metastases in the liver. Metastatic lesions, as mentioned above, expressed luciferase and were therefore visualized by bioluminescence imaging. 18 days post vector administration, all delivery routes resulted in vector expression in tumor foci, but not in normal liver tissue (Figure 2A). Excised multifocal liver metastases were also analyzed by flow cytometry. The average percentage of GFP+ cells for intrasplenic, i.v., and intraportal delivery modalities were similar: 7.8 ± 0.9, 8.2 ± 1.2, and 6.2 ± 1.6, respectively (Figure 2B). i.v. vector administration was selected for all subsequent experiments. A total of three consecutive doses of Toca GFP versus a single dose of i.v. delivery of Toca GFP resulted in higher average GFP+ cells, as seen by vector spread at 14 and 22 days post vector administration (Figure 2C).
Figure 2.
Upon Intrasplenic, Intravenous, or Intraportal Delivery, GFP-Expressing Vector Concentrates in Metastatic Foci in the Liver and Not in Normal Liver Tissue
(A) Representative images showing luciferase (CT26-Luc cells) and GFP (Toca GFP vector) signal in livers from mice receiving intrasplenic, intravenous, or intraportal delivery of Toca GFP vector. (B) Flow cytometric analysis of excised metastatic lesions from livers collected 18 days post vector delivery. (C) CT26-Luc tumor-bearing animals received 1 day or 3 days of intravenous delivery of Toca GFP vector. Flow cytometric analysis of excised metastatic lesions from livers collected 14 or 22 days post vector delivery (n = 9/group). Numbers above the columns indicate the average GFP+ cells per group. Error bars represent SEM.
i.v. Delivery of Toca 511 and Treatment with 5-FC Was Efficacious in a Murine Liver Metastasis Model of mCRC
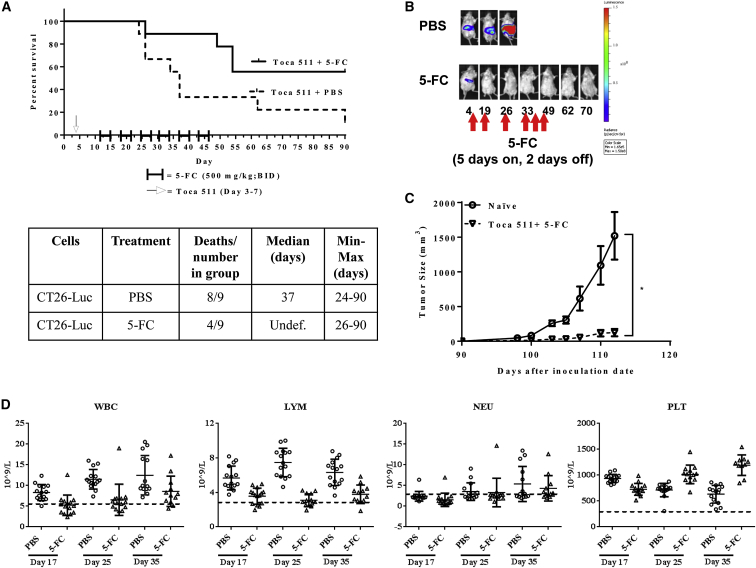
Although pre-transduced CT26 cells provided insight into the feasibility of treating metastatic disease with this therapeutic platform, we wanted to confirm these results in a clinically relevant delivery model. Therefore, in order to assess the therapeutic efficacy of 5-FC treatment after i.v. delivery of Toca 511, an additional survival study was conducted. Mice were administered 5 consecutive (one injection per day for 5 days) i.v. injections of Toca 511 (6.6 × 107 TU/injection/day) starting 4 days post tumor cell inoculation, followed by six cycles of 5-FC. Animals that were treated with 5-FC lived significantly longer compared to PBS control (p = 0.037) (Figures 3A, S1A, and S2B). Six of 9 mice remained tumor free, even after cessation of 5-FC (Figures 3B and S2B). Complete blood counts were collected at 17, 25, and 35 days post cell inoculations. 5-FC treatment caused a transient decrease in white blood cell (WBC), lymphocyte (LYM), neutrophil (NEU), and platelet (PLT) counts after the first and second 5-FC cycles compared to PBS treatment. However, values were above the lower limits of normal (LLN) by the end of the fourth cycle of 5-FC treatment (Figure 3D). Modest lymphoid suppression was observed with the use of Toca 511 and 5-FC relative to systemic 5-FU. As above, CT26 cells were implanted into the right flanks of cured as well as naive, age-matched, mice on day 90 after the original intrasplenic tumor implantation. Tumors engrafted and grew in all naive animals; however, tumors were rejected in mice that had previously cleared CT26 liver metastases through treatment with Toca 511 and 5-FC (p = 0.001) (Figure 3C).
Figure 3.
Intravenous Delivery of Toca 511, Followed by Treatment with 5-FC, Was Efficacious against Colorectal Liver Metastasis
(A) Kaplan-Meier survival analysis. Mice inoculated with CT26-Luc cells intrasplenically were then administered Toca 511 i.v. for 5 consecutive days starting on day 3 post cell inoculation. PBS or 5-FC treatment started at day 12 post cell inoculation. Six cycles of PBS or 5-FC treatment were administered, and survival was examined out to 90 days. The table is the summary of the survival analysis out to 90 days post tumor cell implantations. Toca 511 and 5-FC treated animals lived significantly longer compared to Toca 511 and PBS control animals (p = 0.037). (B) Radiance intensities (photon/s/mouse) from representative animals at indicated time points. 5-FC treatment (500 mg/kg, i.p., BID) started on day 13 for 5 days on and 2 days off for 6 cycles. Control group received PBS (see also Figure S2). (C) Cured mice from survival studies (Toca 511+5-FC; n = 5) were subcutaneously challenged with wild-type CT26 cells on day 0 (day 90 post initial tumor inoculation) at a cell dose of 5 × 105. As a control, CT26 cells were implanted into naive BALB/cJ mice (n = 9) (*p = 0.04; naive versus Toca 511+5-FC). *Statistical significance was defined as p < 0.05. (D) WBCs, LYMs, NEUs, and PLTs were determined in the PBS and 5-FC treatment groups. Dotted line represents LLN for age-matched mice. Age-matched mouse LLN = WBC, 6 × 109 cells/L; LYM, 3.4 × 109 cells/L; PLT, 200 × 109 cells/L; and NEU, 3.4 × 109 cells/L. Error bars represent SEM.
i.v. Delivery of Toca 511, Followed by Treatment with 5-FC, Resulted in Concentrated 5-FU within Liver Metastases in a Murine Model of mCRC
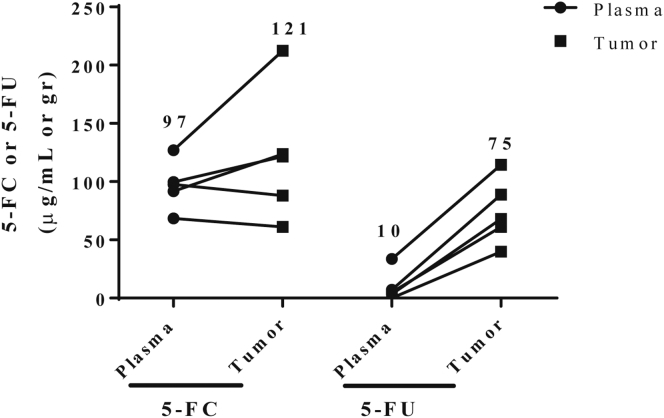
Because Toca 511 gene transfer of CD enzyme is designed to convert the well-tolerated prodrug 5-FC into the potent anti-metabolite 5-FU in infected tumor cells, we expect that prolonged presence of high 5-FU concentrations is likely to enhance the antitumor effect. 23 days post cell inoculation, pharmacokinetics of 5-FC and 5-FU were evaluated after the seventh administration of 5-FC (500 mg/kg BID) in CRC liver metastases and plasma. Mean 5-FU levels in tumor and plasma were 75 μg/g and 10 μg/mL, respectively, 1 hr after the final 5-FC dose (Figure 4). Gastrointestinal toxicity, a common toxicity of 5-FU, was not observed.
Figure 4.
Toca 511 and 5-FC Resulted in Concentrated 5-FU within Liver Metastases in a Murine Model of mCRC
LC/MS measurement of 5-FC and 5-FU in plasma and tumor from mice with Toca 511 pre-transduced CT26-Luc liver metastases treated for 3.5 days with 5-FC (500 mg/kg, i.p., BID). Numbers above columns indicate average values for each group. Error bars represent SEM.
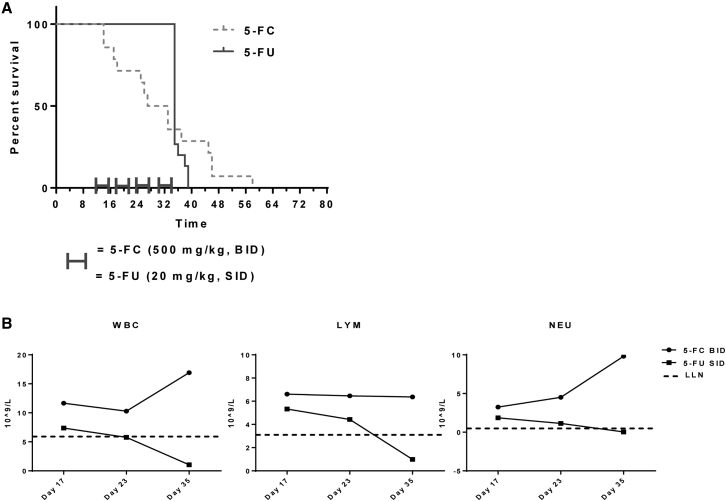
Systemic 5-FU Treatment Was Not Efficacious in a Murine Liver Metastasis Model of mCRC and Resulted in Hematological Toxicity
Efficacy of systemic 5-FU was tested in the CT26 syngeneic liver metastasis model and was used as a positive control in the evaluation of Toca 511 and 5-FC therapy. First, hematological toxicity of systemic 5-FU treatment was determined after delivery at 20 mg/kg/day and 40 mg/kg/day for 5 consecutive days, followed by 2 days without the drug in non-tumor-bearing mice. The dose of 40 mg/kg/day was lethal to mice. The 20 mg/kg/day dose caused leukopenia but was not lethal to mice (Figure S3). For efficacy studies, 5-FU was administered to tumor-bearing animals at 20 mg/kg/day for 5 consecutive days, followed by 2 days without drug for a total of 4 cycles. A control group was treated with 5-FC (500 mg/kg BID). These animals did not receive Toca 511 and represent a negative control. The median survival of animals treated with 5-FU was not significantly different than the 5-FC negative control group (35 days versus 30 days, respectively; p = 0.7). Additionally, there were no long-term survivors with 5-FU treatments after four cycles of treatments (Figure 5A), and therefore it was not possible to perform a tumor rechallenge experiment with this regime. WBC, LYM, and NEU counts were above the LLN during the four cycles of 5-FC treatment. However, systemic 5-FU treatment caused reductions in WBC, LYM, and NEU values, with counts below the LLN (Figure 5B).
Figure 5.
Systemic 5-FU Treatment Was Not Efficacious in a Mouse Model of Colorectal Liver Metastasis
(A) Kaplan-Meier analysis showing the survival of animals with liver metastasis treated with 5-FC (500 mg/kg, i.p., BID) or 5-FU (20 mg/kg, i.p., SID) for 5 consecutive days, followed by 2 days without drug for a total of 4 cycles. Animals received no vector in this experiment. 5-FC, without the presence of vector, has no effect on survival. (B) Hematologic analysis, including values for WBCs, LYMs, and NEUs from animals treated with 5-FC or 5-FU. Animals had established CT26 liver metastases but were not given Toca 511 vector. Error bars represent SEM.
Toca 511 and 5-FC Treatment in a Murine Model of Colorectal Liver Metastases Induced a Systemic Anti-tumor Immune Response
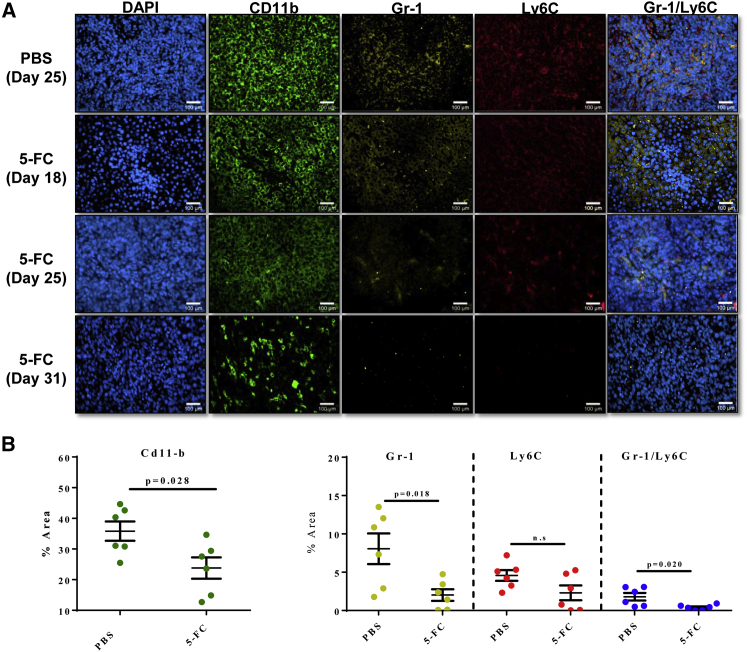
To investigate the potential role of MDSCs in Toca 511 and 5-FC mechanism of action, we measured MDSCs in Toca 511 and 5-FC and Toca 511 and PBS treatment groups in metastatic liver foci. CD11b, Gr-1, and Ly6C were used to define myeloid cell populations. CD11b, in general, defines cells of myeloid lineage. The antibody against Gr-1 binds to both Ly6C and Ly6G, which defines MDSCs because these cells exist as a heterogeneous population of cells that express markers of both monocytes (Ly6C) and neutrophils (Ly6G). Therefore, by staining cells with both antibodies against Gr-1 as well as Ly6C, we were able to define three subsets of cells. Gr-1+Ly6C+ cells are defined here as MDSCs, whereas Gr-1+Ly6C− cells are neutrophils and Gr-1−Ly6C cells are monocytes. CT26 liver metastatic foci were collected throughout the study. Myeloid cells (CD11b), MDSCs, and neutrophils were all significantly depleted with treatment. Monocytes were not significantly reduced here, albeit a trend toward reduction was observed (Figure 6B).
Figure 6.
Myeloid-Derived Suppressor Cells Decreased with Toca 511 and 5-FC Treatment in a Liver Metastasis Model of mCRC
Liver metastases treated with 5-FC visually show a decrease in MDSC populations. Mice were administered three cycles of 5-FC (500 mg/kg, i.p., BID) or PBS. (A) Immunofluorescence color staining of CD11b (myeloid cells), Gr-1 (monocytes and neutrophils), Ly6C (monocytes), and Gr-1/Ly6C (MDSCs) confirms decrease of expression with treatment of 5-FC. (B) Quantitative analysis of percentage area (n = 6) of CD11b, Gr-1, and Ly6C show decrease of MDSCs with treatment of 5-FC compared with PBS. *Statistical significance was defined as p < 0.05. Error bars represent SEM.
Toca 511 and 5-FC Prolonged Survival in a Brain Metastasis Model of mCRC
Toca 511 pre-transduced CT26 cells were implanted in the right hemisphere, followed by treatment, with a total of four cycles of 5-FC beginning on day 10 after tumor implantation. As expected, treatment of mice bearing colorectal brain metastases with 5-FC resulted in prolonged survival compared with PBS control (p = 0.001) (Figure 7A). Seven out of twelve (58%) tumor-bearing animals remained tumor-free 65 days after intracranial (i.c.) CT26 implantation. These tumor-free animals and age-matched naive mice were given a subcutaneous tumor challenge. Tumors engrafted and grew in all naive animals challenged with parental CT26 cells but not in mice that had cleared CT26 colorectal brain metastases after treatment with Toca 511 and 5-FC (Figure 7B). Splenocytes from mice that cleared colorectal brain metastases through treatment were used in adoptive transfer experiments in nude mice. Subcutaneous CT26 tumor progression was slower in animals receiving splenocytes from cured animals compared to control (p = 0.045) (Figure 7C). These results also support the conclusion that immune components play a role in maintaining long-term survival in mice treated with Toca 511 and 5-FC.
Figure 7.
Toca 511 and 5-FC Prolonged Survival and Promoted Long-Term Anti-tumor Immunity in a Brain Metastasis Model of mCRC
(A) Kaplan-Meier analysis. BALB/cJ mice received Toca 511 pre-transduced CT26 cells intracranially and were subsequently treated with cycles of 5-FC (500 mg/kg, i.p., BID) (n = 12) or PBS (n = 5). Treatments started at day 10 post cell inoculation and were for 7 days on and 7 days off for four cycles. Survival was examined out to 150 days. (B) Long-term survivors (n = 5) were rechallenged subcutaneously at day 65 post intracranial tumor implant with parental CT26 cells, and tumor growth was monitored over time. As a control, CT26 cells were implanted into naive, age-matched, BALB/cJ mice (n = 5) (*p = 0.0001; naive versus Toca 511+5-FC). (C) Recipient athymic animals received splenocytes from either cured or naive animals and were inoculated subcutaneously with parental CT26 cells and tumor growth was monitored over time (n = 6/group) (*p = 0.04). *Statistical significance was defined as p < 0.05. Error bars represent SEM.
Myeloid-Derived Suppressor Cells Decreased after Toca 511 and 5-FC Treatment in a Murine Model of Colorectal Brain Metastasis
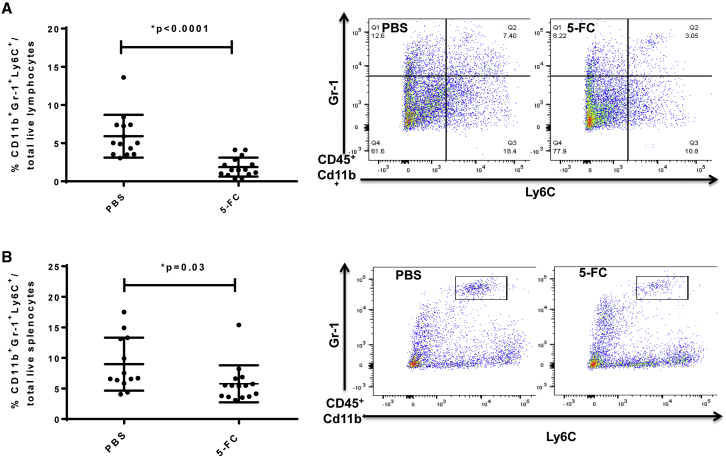
i.c. tumors, which were pre-transduced with Toca 511 prior to tumor implantation, were excised at 14 days post injection (after one cycle of 5-FC). The tumors were disaggregated to single-cell suspensions and the proportions of different immune cell types in the tumor were investigated by FACS analysis. MDSCs (CD11b+ Gr-1+ Ly6C+) were detected in CT26 brain tumors and spleens of tumor-bearing animals. MDSCs were significantly decreased in tumors pre-transduced with Toca 511 and treated with one cycle of 5-FC compared to PBS control (p < 0.0001; Figure 8A). In tumors, MDSCs were 5.9% ± 2.8% of total live lymphocytes, of which more were granulocytic (G-MDSCs, CD11b+ Grhi Ly6C−; 25.6% ± 2.9% of total MDSCs) than monocytic (Mo-MDSCs, CD11b+ Gr1lo Ly6C+; 5.5% ± 1.2% of total MDSCs) in the PBS treatment group. Treatment with 5-FC significantly decreased the infiltration of monocytic MDSCs (2.4% ± 0.5% of total MDSCs) (*p = 0.003 versus PBS) (Figure S4). Neutrophils were reduced in the Toca 511 treatment group after one cycle of 5-FC treatment compared to the PBS control group (p = 0.04) (Figure S5). A significant decrease in the proportion of MDSC in spleens was also observed in the Toca 511 treatment group after one cycle of 5-FC treatment compared to the PBS control group (p = 0.03; Figure 8B). In line with previous data that showed the concentration of therapeutic response at the site of the tumor, there was no significant impact on myeloid cell populations in the bone marrow (Figure S6), suggesting that Toca 511 and 5-FC does not induce myelosuppression in bone marrow, which is seen with traditional chemotherapeutic regimens.
Figure 8.
Myeloid-Derived Suppressor Cells Decreased with Toca 511 and 5-FC Treatment in a Brain Metastasis Model of mCRC
BALB/c mice received Toca 511 pre-transduced CT26 cells intracranially and were treated with 5-FC (500 mg/kg, i.p., BID) or PBS. Treatments started at day 10 post cell inoculation and were 7 days on and 7 days off for one cycle. (A and B) The tumor (A) (*p < 0.0001) and spleen (B) (*p = 0.03) were collected for flow cytometric analysis 14 days post cell implantation. Representative flow analysis from PBS and 5-FC treatment groups separating the populations of Cd11b+ Gr-1+ Ly6C+ cells in the tumor (A) and spleen (B). Error bars represent SEM.
Discussion
CRC is the third most common cancer in the Western hemisphere, with high metastatic incidences and poor overall survival.23, 24 First-line treatment failure results in local recurrence and distant metastases to vital organs, most commonly the liver.25 Additionally, although brain involvement is less common, improved treatment protocols are resulting in longer survival, which ultimately manifests in an observed increase in the incidence of metastasis to the brain.11, 13, 26, 27, 28 Elucidating the efficacy and underlying effects of treatment of metastatic disease requires a mouse model that recapitulates involvement of secondary organs. Therefore, we developed the metastatic models described here to investigate the efficacy of a novel therapeutic approach using a prodrug activating gene therapy: retroviral replicating vector encoding cytosine deaminase, Toca 511, and subsequent treatment with 5-FC.
Here, we utilized a mouse model that consistently gives rise to multifocal liver metastases in an immune-competent syngeneic host through intrasplenic implantation of a well-characterized mouse CRC cell line, CT26. We showed that i.v. administration of Toca 511 was as efficient a delivery modality as intrasplenic or intraportal administration and, followed by cycles of 5-FC treatment, achieved complete tumor regression. In addition, we modeled CRC brain metastasis by direct surgical implantation of CT26 cells into the right hemisphere of the brain because brain metastases from CRC often present as single cerebral lesions. Long-term survival was also achieved in this model after treatment with Toca 511, followed by cycles of 5-FC. Our current models provide important support for the utility of Toca 511 and 5-FC in the clinical treatment of CRC-derived metastases and potentially other solid tumor metastases as well.
Toca 511 in combination with 5-FC is designed to generate high levels of 5-FU locally in the tumor, without generating high levels of systemic 5-FU.1 Earlier, we reported that Toca 511 was distributed throughout the tumor when delivered i.v., which may enhance the potential for the vector to infect and spread to more tumor cells and for the bystander effect of 5-FU to be more widespread.4 Recently, we reported that 5-FU concentrations were significantly higher in the tumor compared to plasma in an intraperitoneal (i.p.) model using Toca 511 pre-transduced CT26 cells (200 μg/g and 0.48 μg/mL, respectively)29 and in a brain cancer model using Toca 511 pre-transduced F98 rat glioma cells (69.8 μg/g and 0.37 μg/mL, respectively). In our current work, pharmacokinetic analysis confirmed that concentrations of 5-FU were greater in the tumor than in the plasma in these models of mCRC. Because the desired cytotoxic effect is concentrated at the site of the tumor, 5-FU production after Toca 511 and 5-FC treatment spares tissues and cells that are typically affected with systemic 5-FU exposure.
A large body of evidence has shown that 5-FU systemic exposure is the major contributor to dose-limiting toxicities and, ultimately, tumor treatment failure.30, 31 In fact, studies have indicated that only 20%–30% of patients treated with a 5-FU-based regimen have 5-FU levels that are in the appropriate therapeutic range. Approximately 40%–60% of patients are underdosed, and 10%–20% of patients experience severe systemic toxicities.32 Importantly, treatment with 5-FU often causes hematologic toxicity, resulting in a narrow therapeutic index. As reported here, tumor-bearing animals treated with systemic 5-FU had lower blood counts compared to the control treatment and limited efficacy due to dose-limiting toxicities. However, Toca 511 and 5-FC treatment, with a known short half-life in blood,33 results in primarily intratumoral 5-FU production that resulted in modest lymphoid suppression relative to systemic 5-FU treatment,5, 29, 34 thus avoiding this limitation.
Immunosuppression mediated by MDSCs has been shown in several colorectal cancer models, and levels of MDSCs in colorectal cancers in patients correlate with poor outcomes.35 Further, inhibition or depletion of MDSCs has been shown to enhance anti-tumor immunity.17, 18, 36 Interestingly, some cytotoxic agents, such as gemcitabine, cisplatin, paclitaxel, and 5-FU, have been found to deplete MDSCs both systemically and in the tumor microenvironment.20, 37, 38, 39 Recently, in a subcutaneous Tu-2449 glioma model, we have demonstrated that tumor-associated macrophages (TAMs), tumor-associated monocytes, and MDSCs are significantly reduced shortly after Toca 511 and 5-FC treatment and continued to be reduced until tumor clearance. Moreover, those animals that cleared tumors through treatment with Toca 511 and 5-FC were protected from subsequent tumor rechallenge, suggesting that immune activation and the ensuing antitumor immunity was associated with depletion of immunosuppressive MDSCs.40 In the current study, MDSC populations were also monitored in tumor-bearing animals after Toca 511 and 5-FC treatment. As expected, MDSCs were significantly reduced after Toca 511 and 5-FC treatment in both liver and brain metastatic models. As a correlate, strong antitumor activity was observed after Toca 511 and 5-FC treatment.
Because MDSCs are a heterogeneous population of myeloid cells with a multitude of immune-suppressive capabilities, including several mechanisms by which they directly induce T cell suppression.15, 41, 42 MDSCs can be divided phenotypically into granulocytic (G-MDSC) and monocytic (Mo-MDSC) subgroups and each have demonstrated immunosuppressive properties.43, 44 The G-MDSCs produce high levels of ROS but only nominal amounts of NO, indicating that ROS are the primary mediators of their suppressive functions.45, 46 In contrast, Mo-MDSCs express high levels of NO and low levels of ROS, and they effectively suppress T cell function in both antigen-dependent and -independent manners, without requiring cell-cell contact.47 It has been suggested that the tumor microenvironment could have an impact, either favoring or repelling preferential migration of MDSC subtypes.48 In our brain metastatic model, G-MDSCs were the most prevalent subtype in the tumor microenvironment. However, Toca 511 and 5-FC treatment significantly reduced the Mo-MDSC population. Treatment with 5-FU has been shown to induce selective apoptosis of MDSCs, thereby decreasing the burden of these cells in the murine spleen and tumor environment, but without depleting host T cells, natural killer (NK) cells, dendritic cells, or B cells, and promoted T cell-dependent anti-tumor responses.20 Therefore, the elimination of MDSCs represents a promising approach in cancer therapy. Future studies are needed to determine suppressive activities of different subsets of MDSCs that could improve the development of novel interventions for cancer treatment in colorectal metastases.
5-FU has been used for more than 50 years in the treatment of CRC. To improve the clinical efficacy of 5-FU, combined therapies or changes in the schedule of administration of 5-FU from bolus to continuous infusion, or both, have been actively investigated.49 The most common U.S. Food and Drug Administration (FDA)-approved protocols for CRC treatment have been combined chemotherapies FOLFIRI (folinic acid, 5-FU, and irinotecan) or FOLFOX (folinic acid, 5-FU, and oxaliplatin).35 The introduction of drugs such as bevacizumab,50, 51 cetuximab,52 and panitumumab53 have improved the median survival of patients with advanced metastatic disease from 10 to 12 months to almost 24 months.7, 54 However, despite its limited therapeutic index, 5-FU has remained the main agent for the treatment of both advanced and early-stage CRC.
In conclusion, our data from disease-relevant in vivo tumor models suggest that Toca 511 and 5-FC is safe, efficacious, and represents a novel tumoricidal and immunotherapeutic approach for the treatment of liver and brain metastases for patients with mCRC. Importantly, this work demonstrates that Toca 511 in conjunction with 5-FC promotes both direct killing of tumor cells by local production of 5-FU and induction of a local and systemic immunotherapeutic response, resulting in long-term survival by selectively depleting a highly immunosuppressive population of cells, MDSCs. We believe that this platform may provide improved treatment outcomes for individuals with mCRC when translated into clinical trials (NCT02576665).
Materials and Methods
Drugs and Reagents
5-FC for in vivo assays was synthesized to order by a contract chemical supplier. 5-FU was purchased from Sigma-Aldrich (St. Louis, MO). D-Luciferin was purchased from Biotium (Hayward, CA).
Retroviral Replicating Vectors
A detailed description of Toca 511 vector design and modification has been previously published.55 Toca GFP is the same as Toca 511, with the GFP gene in place of the CD gene. Toca 511 (3.3 × 108 TU/mL) and Toca GFP (1.7 × 108 TU/mL) were used for all experiments.
Cell Lines
The mouse colon carcinoma cell line CT26 (CRL 2638) was purchased from American Type Culture Collection (ATCC) (Manassas, VA). CT26-Lluc was generated from the parental CT26 cell line by transduction with CMV-Luc-IRES-Neo lentivirus (University of California, Los Angeles, CA) encoding luciferase and a Geneticin resistance gene, followed by selection with Geneticin (G418) (Thermo Fisher Scientific, Waltham, MA). CT26 parental cells and CT26-Luc cells were each infected with either Toca 511 or Toca GFP vector to create CT26-T511, CT26-Luc T511, and CT26-GFP. All cell lines were cultured as described.1
Mice and In-Life Observations
Female BALB/cJ mice (aged ∼8 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME, or Sacramento, CA). Athymic nude mice were purchased from Harlan (Indianapolis, IN). Mice were acclimated for 7–14 days after arrival. Routine general health, in-life observations, and body weights were collected throughout the course of the study. In-life observations were scored on a 0–4 point system for severity of each symptom. Mice with a cumulative score of 5 were euthanized. Mice with body weight loss of more 20% for more than 2 days were euthanized. All animal protocols and experiments were approved by the Institutional Animal Care and Use Committee.
Bioluminescence Imaging
Tumor growth was measured using the IVIS Imaging system (PerkinElmer, Waltham, MA). Mice were anesthetized with isoflurane, and 10 min after i.p. administration with D-luciferin (126 mg/kg), bioluminescent signals were analyzed with a 45-s acquisition time.
Orthotopic Liver Metastasis Model of mCRC
The syngeneic cell line CT26 was used as a tumor model in BALB/cJ mice. Various vector delivery routes were examined to optimize vector delivery. On day 0, mice underwent intrasplenic implantation of 3.5 × 105 CT26-Luc666. On day 4, mice were injected with 200 μL Toca GFP or Toca 511 intrasplenically, intraportally, or i.v. infusion over a minute, followed by a hold of 2 min. 1 week post vector, tumors were excised into media containing 1x DNase (Sigma-Aldrich, St. Louis, MO) and 1x Collagenase (Sigma-Aldrich, St. Louis, MO) and placed on a shaker for 1 hr. After incubation, tumors were filtered through a 40-μM filter, centrifuged, and resuspended in DMEM media and analyzed by flow cytometry for GFP expression (BD FACS Canto II).
For therapeutic experiments, on day 0, mice underwent intrasplenic implantation of either 3.5 × 105 Toca 511 pre-transduced CT26-Luc or parental CT26-Luc cells. Splenectomy was done immediately after tumor cell inoculation. Starting on day 4, mice inoculated with CT26-Luc cells were injected with 200 μL i.v. Toca 511 for 5 consecutive days. Mice inoculated with Toca 511 pre-transduced CT26-Luc cells did not receive any vector injections. Starting on day 13, mice that received Toca 511 were treated with either PBS or 5-FC (500 mg/kg/dose) i.p. BID for 5 consecutive days, followed by 2 days without drug. Cycles of 5 days on, 2 days off of drug treatment were repeated.
Toxicity Studies in Multifocal Liver Metastasis
BALB/cJ mice bearing multifocal liver metastasis were given i.p. injections of 5-FC (500 mg/kg/dose BID) or 5-FU (20 mg/kg/day SID) treatments for 5 consecutive days, followed by 2 days without drug for every week, for a total of 4 weeks. Blood samples were collected and then transferred to Explora BioLabs (San Diego, CA) for hematology analysis. A complete blood count, including values for WBCs, LYMs, NEUs, and PLTs were obtained using an VetScan HM2 hematology analyzer (ABAXIS, Union City, CA) following the manufacturer’s instructions. LLN was determined based on strain and age of the animals tested.
Pharmacokinetic Analysis of 5-FU and 5-FC in Multifocal Liver Metastasis Model
Plasma and tumor pharmacokinetics of 5-FC and 5-FU were studied in a multifocal liver metastasis model. In the first study, mice inoculated with Toca 511 pre-transduced CT26-Luc were treated with either PBS or 5-FC (500 mg/kg i.p. BID) for 3.5 days. In the second study, mice inoculated with CT26-Luc cells received 5-day i.v. Toca 511 and were treated with one full cycle of 5-FC or PBS. During the second cycle of treatment, mice received 3.5 days of treatment. For both studies, 1 hr after the last injections, whole blood samples were collected into EDTA-treated tubes. Cells were removed from plasma by centrifugation for 2 min at 1,000–2,000 × g. Plasma samples were frozen into liquid nitrogen. Tumors were immediately excised, weighed, and directly frozen in liquid nitrogen.
Quantitative determination of 5-FU and 5-FC in samples was done using supported liquid extraction (SLE) and hydrophilic interaction chromatography with tandem mass spectrometry detection (LC-MS/MS). Analyses of the samples were performed by the Southern Research Institute (Birmingham, AL). 5-FC/5-FU was equally detectable in both plasma and tumor samples. Concentrations of incurred and quality control samples were calculated with the same regression analysis. Results were converted into μg 5-FU and 5-FC per g of tissue or plasma.
Immunofluorescence of MDSCs
Multiple liver metastases cells were detected for CD11b, Gr-1, and Ly6C. To examine MDSC populations, mice were perfused with 4% paraformaldehyde (PFA). Livers were collected and fixed in 4% PFA overnight, washed with PBS and 70% ethanol, tissue processed, and embedded into paraffin. MDSC immunostaining was performed for primary monoclonal rabbit antibody against CD11b (ab133357, Abcam) with 1:250 dilution, primary monoclonal rat antibody against Gr-1 (MAB1037, Novus Biologicals) with 1:50 dilution, and primary monoclonal mouse antibody against Ly6C (sc-271811, Santa Cruz Biotechnology) with 1:100 dilution. For each specimen section, three color images were acquired with a light digital microscope (Zeiss Instruments) running under Image Pro Plus v.7.0. for Windows.
Quantitative Immunohistochemistry of MDSCs
The area based on color sampling of fluorescence by immunochemical reaction was selected using the “eye dropper tool” in Image Pro Premier. The number of selected pixels was read from the histogram of color and the area percentage per section was then determined.
In Vivo Survival in Brain Metastasis Model
BALB/cJ mice underwent IC implantation of 1.4 × 104 Toca 511 pre-transduced CT26 cells using a sharp, gas-tight, 26G 10-μL Hamilton syringe inserted through the burr hole on day 0. The stereotaxic coordinates were anterior-posterior (AP) = 0.5 mm and medial-lateral (ML) = 1.8 mm (from bregma). Starting on day 10, mice were treated with either PBS or 5-FC (500 mg/kg/dose) i.p. BID for 7 consecutive days, followed by 7 days without drug to allow vector spread. After the first 5-FC cycle, tumors and spleens were collected for FACS analysis for MDSCs. For the survival study, animals received 3 more cycles of 5-FC or PBS treatments. Survival was monitored during and after cessation of 5-FC cycles.
Flow Cytometry Analysis
Spleens and tumor samples were collected from Toca 511 pre-transduced CT26 IC tumor-bearing mice. Single-cell suspensions were prepared from spleens. Brains tumors were diced with a razor blade before homogenizing in RPMI medium (Hyclone, Logan, UT) using a glass Tenbroeck homogenizer. Mononuclear cells were purified from brain tissue by centrifugation (600 × g) through a Percol step gradient (70%–30% Percol in PBS) for 20 min in 15-mL falcon tubes; mononuclear cells migrate to the interface between 30% and 70% Percol. The mononuclear cells on the interphase were removed and then washed with fresh media. Antibodies were purchased from eBioscience (San Diego, CA). Immune cells were labeled with antibodies in cell surface staining buffer (1% fetal bovine serum [FBS]) for flow cytometry analysis using a flow cytometer (BD FACS Canto II) and analyzed with FlowJo (Tree Star, Ashland OR) software.
Tumor Challenge and Adoptive Transfer Assays
Tumor challenge was performed on all cured mice from both multifocal liver and brain metastasis CT26 tumor long-term survival studies by subcutaneous implantation of parental 5 × 105 CT26 cells in 100 μL volume into each mouse. As a control, CT26 cells were implanted into age-matched naive BALB/cJ mice. Tumor measurements were performed three times a week.
For adoptive transfer studies, cured mice were used from i.c. brain metastasis CT26 tumor long-term survival studies. Recipient mice were administered an i.v. injection of 5 × 106 splenocytes from cured or naive donor mice. 4 days later, these animals were injected with 5 × 105 CT26 tumor cells on the right flank. Tumor measurements were performed three times a week.
Statistical Analyses
Survival data were plotted using the Kaplan-Meier method, and were compared by the log-rank test as noted. p values of < 0.05 were considered statistically significant in all analyses, which were done with Prism 5 statistical software (GraphPad Software).
Author Contributions
K.Y., J.M.R., and M.E.R.-A. designed the study. K.Y., M.E.R.-A. and T.T.M. performed the experimental work. F.L. and D.M. performed the animal surgeries and imaging. C.E.I. prepared Toca 511 and Toca GFP vectors. K.Y., M.E.R.-A., T.T.M., and J.M.R. analyzed and discussed the results. K.Y. wrote the manuscript. L.A.M., H.E.G., D.J.J., and J.M.R. edited the manuscript. N.K. provided scientific guidance.
Conflicts of Interest
K.Y., M.E.R.-A., F.L., T.T.M., D.M., L.A.M., C.E.I., N.K., H.E.G., D.J.J., and J.M.R. are employees and/or shareholders of Tocagen Inc.
Acknowledgments
We thank the Investors in Tocagen Inc., ABC2 Foundation (Washington, DC), the National Brain Tumor Society (Watertown, MA), the American Brain Tumor Association (Chicago, IL), the Musella Foundation (Hewlett, NY), and Voices against Brain Cancer (New York, NY) for financial support. This work was funded, in part, by a stipend to M.E.R.-A. from National University. We thank Nicholas A. Boyle, John Wood, and Asha Das at Tocagen Inc. for their critical reading of the manuscript.
Footnotes
Supplemental Information includes six figures and one table and can be found with this article online at https://doi.org/10.1016/j.omto.2017.12.001.
Supplemental Information
References
- 1.Ostertag D., Amundson K.K., Lopez Espinoza F., Martin B., Buckley T., Galvão da Silva A.P., Lin A.H., Valenta D.T., Perez O.D., Ibañez C.E. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro-oncol. 2012;14:145–159. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raval R.R., Sharabi A.B., Walker A.J., Drake C.G., Sharma P. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. J. Immunother. Cancer. 2014;2:14. doi: 10.1186/2051-1426-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang T.T., Hlavaty J., Ostertag D., Espinoza F.L., Martin B., Petznek H., Rodriguez-Aguirre M., Ibañez C.E., Kasahara N., Gunzburg W. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20:544–551. doi: 10.1038/cgt.2013.51. [DOI] [PubMed] [Google Scholar]
- 4.Huang T.T., Parab S., Burnett R., Diago O., Ostertag D., Hofman F.M., Espinoza F.L., Martin B., Ibañez C.E., Kasahara N. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum. Gene Ther. 2015;26:82–93. doi: 10.1089/hum.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yagiz K., Huang T.T., Lopez Espinoza F., Mendoza D., Ibañez C.E., Gruber H.E., Jolly D.J., Robbins J.M. Toca 511 plus 5-fluorocytosine in combination with lomustine shows chemotoxic and immunotherapeutic activity with no additive toxicity in rodent glioblastoma models. Neuro-oncol. 2016;18:1390–1401. doi: 10.1093/neuonc/now089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraoka K., Kimura T., Logg C.R., Kasahara N. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin. Cancer Res. 2006;12:7108–7116. doi: 10.1158/1078-0432.CCR-06-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hocking C.M., Price T.J. Panitumumab in the management of patients with KRAS wild-type metastatic colorectal cancer. Therap. Adv. Gastroenterol. 2014;7:20–37. doi: 10.1177/1756283X13498660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmathulla G., Toms S.A., Weil R.J. The molecular biology of brain metastasis. J. Oncol. 2012;2012:723541. doi: 10.1155/2012/723541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden A.D., Wen P.Y., Kesari S. Brain metastases. Curr. Opin. Neurol. 2005;18:654–661. doi: 10.1097/01.wco.0000191514.37498.2b. [DOI] [PubMed] [Google Scholar]
- 10.Cohen E.E., Kane M.A., List M.A., Brockstein B.E., Mehrotra B., Huo D., Mauer A.M., Pierce C., Dekker A., Vokes E.E. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2005;11:8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 11.Mongan J.P., Fadul C.E., Cole B.F., Zaki B.I., Suriawinata A.A., Ripple G.H., Tosteson T.D., Pipas J.M. Brain metastases from colorectal cancer: risk factors, incidence, and the possible role of chemokines. Clin. Colorectal Cancer. 2009;8:100–105. doi: 10.3816/CCC.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 12.Mege D., Ouaissi M., Fuks D., Metellus P., Peltier J., Dufour H., Regimbeau J.M., Dahan L., Sielezneff I., Sastre B. Patients with brain metastases from colorectal cancer are not condemned. Anticancer Res. 2013;33:5645–5648. [PubMed] [Google Scholar]
- 13.Kye B.H., Kim H.J., Kang W.K., Cho H.M., Hong Y.K., Oh S.T. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal Dis. 2012;14:e378–e385. doi: 10.1111/j.1463-1318.2012.02962.x. [DOI] [PubMed] [Google Scholar]
- 14.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilkovitch D., Lopez D.M. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69:5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundy-Bosse B.L., Young G.S., Bauer T., Binkley E., Bloomston M., Bill M.A., Bekaii-Saab T., Carson W.E., 3rd, Lesinski G.B. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4+ T cells from patients with GI malignancy. Cancer Immunol. Immunother. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesolowski R., Markowitz J., Carson W.E., 3rd Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J. Immunother. Cancer. 2013;1:10. doi: 10.1186/2051-1426-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baniyash M. Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer Immunol. Immunother. 2016;65:857–867. doi: 10.1007/s00262-016-1849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent J., Mignot G., Chalmin F., Ladoire S., Bruchard M., Chevriaux A., Martin F., Apetoh L., Rébé C., Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 21.Peters G.J., van der Wilt C.L. Thymidylate synthase as a target in cancer chemotherapy. Biochem. Soc. Trans. 1995;23:884–888. doi: 10.1042/bst0230884. [DOI] [PubMed] [Google Scholar]
- 22.Cloughesy T.F., Landolfi J., Hogan D.J., Bloomfield S., Carter B., Chen C.C., Elder J.B., Kalkanis S.N., Kesari S., Lai A. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016;8:341ra75. doi: 10.1126/scitranslmed.aad9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haraldsdottir S., Einarsdottir H.M., Smaradottir A., Gunnlaugsson A., Halfdanarson T.R. [Colorectal cancer - review] Laeknabladid. 2014;100:75–82. doi: 10.17992/lbl.2014.02.531. [DOI] [PubMed] [Google Scholar]
- 24.Zhu P., Zhao N., Sheng D., Hou J., Hao C., Yang X., Zhu B., Zhang S., Han Z., Wei L. Inhibition of growth and metastasis of colon cancer by delivering 5-fluorouracil-loaded pluronic P85 copolymer micelles. Sci. Rep. 2016;6:20896. doi: 10.1038/srep20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang Y.W., Gu X.D., Xiang J.B., Chen Z.Y. Brain metastases from colorectal cancer: microenvironment and molecular mechanisms. Int. J. Mol. Sci. 2012;13:15784–15800. doi: 10.3390/ijms131215784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos S., Davey P., Hird A., Pressnail B., Bilbao J., Aviv R.I., Symons S., Pirouzmand F., Sinclair E., Culleton S. Brain metastasis from an unknown primary, or primary brain tumour? A diagnostic dilemma. Curr. Oncol. 2009;16:62–66. [PMC free article] [PubMed] [Google Scholar]
- 27.Noura S., Ohue M., Shingai T., Fujiwara A., Imada S., Sueda T., Yamada T., Fujiwara Y., Ohigashi H., Yano M. Brain metastasis from colorectal cancer: prognostic factors and survival. J. Surg. Oncol. 2012;106:144–148. doi: 10.1002/jso.23055. [DOI] [PubMed] [Google Scholar]
- 28.Schouten L.J., Rutten J., Huveneers H.A., Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 29.Yagiz K., Mendoza D., Rodriguez-Aguirre M., Espinoza F.L., Ibanez C.E., Gruber H.E., Jolly D.J., Robbins J.M. Cytotoxic and immunotherapeutic effects of Toca 511 and 5-fluorocytosine in an intraperitoneal model of metastatic colorectal cancer. Mol. Ther. 2016;24(Suppl 1):S80. [Google Scholar]
- 30.Gamelin E., Delva R., Jacob J., Merrouche Y., Raoul J.L., Pezet D., Dorval E., Piot G., Morel A., Boisdron-Celle M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 31.Kline C.L., Schiccitano A., Zhu J., Beachler C., Sheikh H., Harvey H.A., Mackley H.B., McKenna K., Staveley-O’Carroll K., Poritz L. Personalized dosing via pharmacokinetic monitoring of 5-fluorouracil might reduce toxicity in early- or late-stage colorectal cancer patients treated with infusional 5-fluorouracil-based chemotherapy regimens. Clin. Colorectal Cancer. 2014;13:119–126. doi: 10.1016/j.clcc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Saif M.W., Choma A., Salamone S.J., Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J. Natl. Cancer Inst. 2009;101:1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 33.Vermes A., Guchelaar H.J., Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 34.Yagiz K., Rodriguez-Aguirre M.E., Espinoza F.L., Martin B., Huang T.T., Ibanez C., Ostertag D., Kasahara N., Gruber H.E., Jolly D.J. Intravenous delivery of Toca 511 gene therapy in combination with 5-fluorocytosine for intratumoral production of 5-fluorouracil in a colon cancer metastasis model. Mol. Ther. 2015;23(Suppl 1):S213. [Google Scholar]
- 35.Kanterman J., Sade-Feldman M., Biton M., Ish-Shalom E., Lasry A., Goldshtein A., Hubert A., Baniyash M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74:6022–6035. doi: 10.1158/0008-5472.CAN-14-0657. [DOI] [PubMed] [Google Scholar]
- 36.Otvos B., Silver D.J., Mulkearns-Hubert E.E., Alvarado A.G., Turaga S.M., Sorensen M.D., Rayman P., Flavahan W.A., Hale J.S., Stoltz K. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34:2026–2039. doi: 10.1002/stem.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki E., Kapoor V., Jassar A.S., Kaiser L.R., Albelda S.M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 39.Ko H.J., Kim Y.J., Kim Y.S., Chang W.S., Ko S.Y., Chang S.Y., Sakaguchi S., Kang C.Y. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell L.A., Lopez Espinoza F., Mendoza D., Kato Y., Inagaki A., Hiraoka K., Kasahara N., Gruber H.E., Jolly D.J., Robbins J.M. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro-oncol. 2017;19:930–939. doi: 10.1093/neuonc/nox037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monu N.R., Frey A.B. Myeloid-derived suppressor cells and anti-tumor T cells: a complex relationship. Immunol. Invest. 2012;41:595–613. doi: 10.3109/08820139.2012.673191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raychaudhuri B., Rayman P., Huang P., Grabowski M., Hambardzumyan D., Finke J.H., Vogelbaum M.A. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. J. Neurooncol. 2015;122:293–301. doi: 10.1007/s11060-015-1720-6. [DOI] [PubMed] [Google Scholar]
- 43.Ribechini E., Greifenberg V., Sandwick S., Lutz M.B. Subsets, expansion and activation of myeloid-derived suppressor cells. Med. Microbiol. Immunol. (Berl.) 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- 44.Raber P.L., Thevenot P., Sierra R., Wyczechowska D., Halle D., Ramirez M.E., Ochoa A.C., Fletcher M., Velasco C., Wilk A. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int. J. Cancer. 2014;134:2853–2864. doi: 10.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Movahedi K., Guilliams M., Van den Bossche J., Van den Bergh R., Gysemans C., Beschin A., De Baetselier P., Van Ginderachter J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 46.Youn J.I., Nagaraj S., Collazo M., Gabrilovich D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parker K.H., Beury D.W., Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv. Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umansky V., Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron. 2013;6:169–177. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyerhardt J.A., Mayer R.J. Systemic therapy for colorectal cancer. N. Engl. J. Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 50.McCormack P.L., Keam S.J. Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs. 2008;68:487–506. doi: 10.2165/00003495-200868040-00009. [DOI] [PubMed] [Google Scholar]
- 51.Razenberg L.G., van Gestel Y.R., de Hingh I.H., Loosveld O.J.L., Vreugdenhil G., Beerepoot L.V., Creemers G.J., Lemmens V.E. Bevacizumab for metachronous metastatic colorectal cancer: a reflection of community based practice. BMC Cancer. 2016;16:110. doi: 10.1186/s12885-016-2158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grigorean V.T., Ciuhu A.N., Rahnea Nita G., Strambu V., Straja D.N., Popescu M., Sandu A.M., Rahnea Nita R.A. Efficacy of cetuximab in metastatic colon cancer - case report. Chirurgia (Bucur.) 2014;109:383–389. [PubMed] [Google Scholar]
- 53.Price T.J., Peeters M., Kim T.W., Li J., Cascinu S., Ruff P., Suresh A.S., Thomas A., Tjulandin S., Zhang K. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569–579. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 54.Foubert F., Matysiak-Budnik T., Touchefeu Y. Options for metastatic colorectal cancer beyond the second line of treatment. Dig. Liver Dis. 2014;46:105–112. doi: 10.1016/j.dld.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Perez O.D., Logg C.R., Hiraoka K., Diago O., Burnett R., Inagaki A., Jolson D., Amundson K., Buckley T., Lohse D. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol. Ther. 2012;20:1689–1698. doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.