Abstract
Despite their essential roles in signal processing in the brain, the functions of interneurons currently remain unclear in humans. We recently developed a method using the prepulse inhibition of sensory evoked cortical responses for functional measurements of interneurons. When a sensory feature is abruptly changed in a continuous sensory stimulus, change-related cortical responses are recorded using MEG. By inserting a weak change stimulus (prepulse) before the test change stimulus, it is possible to observe the inhibition of the test response. By manipulating the prepulse–test interval (PTI), several peaks appear in inhibition, suggesting the existence of temporally distinct mechanisms. We herein attempted to separate these components through the oral administration of diazepam and baclofen. The test stimulus and prepulse were an abrupt increase in sound pressure in a continuous click train of 10 and 5 dB, respectively. The results obtained showed that the inhibition at PTIs of 10 and 20 ms was significantly greater with diazepam than with the placebo administration, suggesting increased GABAA-mediated inhibition. Baclofen decreased inhibition at PTIs of 40 and 50 ms, which may have been due to the activation of GABAB autoreceptors. Therefore, the present study separated at least two inhibitory mechanisms pharmacologically.
Introduction
The outputs of a neural circuit are influenced by the balance between excitation and inhibition produced by pyramidal cells and interneurons, respectively. Despite their crucial roles in signal processing, the functions of interneurons remain largely unknown in humans mainly due to methodological limitations associated with the observation of their activities. The recording of inhibitory postsynaptic potentials (IPSPs) or currents (IPSCs) in the brain slices of animals enables direct observations of the synaptic events mediated by interneurons via GABA. However, there is currently no method to record IPSPs in their pure form in humans. This recording in vivo is also challenging in animals. Since many diseases, such as schizophrenia [1–4] and developmental disorders [5,6], have been suggested to have deficits in inhibitory functions or excitation/inhibition imbalances [7,8], non-invasive methods for functional measurements of the inhibitory system are needed.
We recently developed a method for this purpose using the prepulse inhibition (PPI) of auditory evoked cortical responses [9–11]. It appears to serve as a tool to record IPSPs indirectly. When a sound feature is abruptly changed in a continuous sound, change-specific cerebral responses are evoked that are clearly recorded by electroencephalograms [12,13] or magnetoencephalograms (MEG)[14]. Change-related brain activity is used as a test response that reflects postsynaptic events in the change-detecting circuit in the auditory cortex [11]. Similar to standard paradigms for the PPI of startle reflexes, a weak change in a sound feature is inserted before the test stimulus, which results in the suppression of the test response despite the weak change stimulus (prepulse) itself evoking no or only a weak response [9,11]. This paradigm is advantageous because a weak prepulse may be precisely and repeatedly delivered. Change-related brain activity may be elicited by a change in any of the sound dimensions, i.e., intensity, location, and frequency [12], and a brief preceding sound with any feature change may be a prepulse. In our recent studies, the degree of PPI depended on the prepulse–test interval (PTI)[11] as well as the magnitude of the change in the prepulse [9]. When the magnitude of PPI was plotted against the PTI, the PPI curve showed several peaks at different PTIs: 10–30, 50–60, and 600 ms [11]. These findings suggest that the suppression of the test response reflects an active inhibitory process because the prepulse only evoked a weak response if present, and that the PPI curve plotted against the PTI appears to reflect the time course of IPSPs because the inhibitory process was not significantly confounded by changes in pyramidal cell-pyramidal cell transmission [11]. Given that the PPI curve against the PTI reflects the time course of IPSPs, several peaks at different PTIs suggests temporally distinct several mechanisms involved in this suppression.
In the present study, we attempted to separate these inhibitory mechanisms pharmacologically using diazepam and baclofen, a positive modulator of the GABAA receptor and agonist of the GABAB receptor, respectively. The results obtained showed early and late inhibition sensitive to diazepam and baclofen, respectively, confirming that the GABAergic system is involved in shaping the PPI of auditory evoked cortical responses and also indicating that this measure may be a clinical tool for functional assessments of the inhibitory system mediated by GABAA and GABAB receptors.
Methods
This study was approved in advance by the Ethics Committee of the National Institute for Physiological Sciences, Okazaki, Japan and conducted in accordance with the Declaration of Helsinki. Written consent was obtained from all subjects. The experiment was performed on ten (three females and seven males) healthy volunteers, aged 20–52 (33.2 ± 10.9) years. They were asked to refrain from alcohol, caffeine, and smoking for at least 12 hours prior to the experiment. None of the subjects had any history of mental or neurological disorders or substance abuse in the last two years. They were free of medication at testing. They had a hearing threshold lower than 30 dB at 1000 Hz, as assessed by an audiometer (AA-71, Rion, Tokyo, Japan).
Auditory stimuli
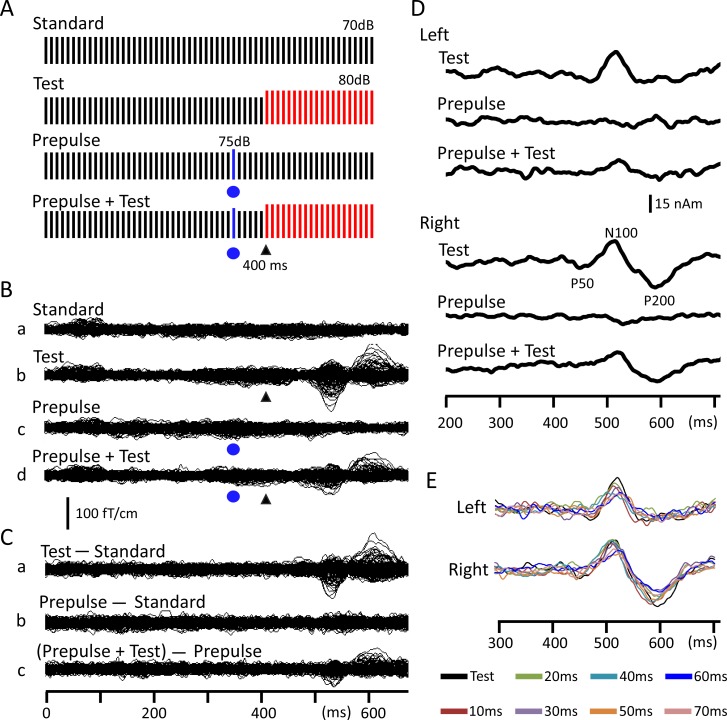
Repeats of a 1-ms sine wave click at 100 Hz [10] were used. There were four types of sound stimuli (Fig 1A): repeats of the same click at 70 dB SPL in sound pressure (Standard), repeats of standard clicks followed by 20 clicks of 80 dB (Test), the Test preceded by a click of 75 dB (Prepulse + Test), and the Standard with a Prepulse (Prepulse). Prepulses were presented 10, 20, 30, 40, 50, 60, or 70 ms before the Test presented at 400 ms in the click train. Therefore, there were 16 stimuli: Standard, Test, Prepulse alone with a prepulse at 330–390 ms, and Prepulse + Test with a prepulse at 330–390 ms. Sixteen stimuli were presented randomly at an even probability at a trial-trial interval of 1000 ms. A total of 120–125 artifact-free epochs were averaged for each stimulus. Sound stimuli were presented binaurally through ear pieces (E-A-Rtone 3A, Aero Company, Indianapolis, IN).
Fig 1. Change-related cortical response and its inhibition by a weak prepulse.
(A) Sound stimuli consisted of a train of 1-ms clicks at 100 Hz in repetitive frequency and 70 dB SPL in sound pressure. An abrupt increase of 10 dB in sound pressure was used to evoke the Test response, while that for the prepulse (5 dB) was used to inhibit the Test response. Each bar indicates a single click. (B) An example of prepulse inhibition of the change-related auditory response by a prepulse presented 60 ms before the Test stimulus in a representative subject. Recorded MEG waveforms in the Pre run of the Placebo session for the Standard (Ba), Test (Bb), Prepulse (Bc), and Prepulse + Test (Bd) stimuli are shown. The Test alone MEG response (Ca) was obtained by subtracting the waveform for the Standard stimulus (Ba) from that for the Test stimulus (Bb). The Prepulse alone response (Cb) was obtained by subtracting the waveform for the Prepulse stimulus (Bc) from that for the Standard (Ba). The Prepulse + Test response (Cc) was obtained by subtracting the waveform for the Prepulse stimulus (Bc) from that for the Test + Prepulse stimulus (Bd). (D) Time course of the source strength of dipoles obtained from MEG waveforms in C. (E) The source strength waveforms for all eight PTI conditions in this run. Filled blue circles and black arrow heads indicate the presentation of the prepulse and test stimulus, respectively.
MEG recordings
Magnetic signals were recorded using a 306-channel whole-head type MEG system (Vector-view, ELEKTA Neuromag, Helsinki, Finland), which comprised 102 identical triple sensor elements. Each sensor element consisted of two orthogonal planar gradiometers and one magnetometer coupled to a multi-superconducting quantum interference device (SQUID) and, thus, provided 3 independent measurements of the magnetic fields. In the present study, we analyzed MEG signals recorded from 204 planar-type gradiometers. These planar gradiometers are sufficiently powerful to detect the largest signal just over local cerebral sources. Signals were recorded with a bandpass filter of 0.1–330 Hz and digitized at 1000 Hz. An analysis was conducted from 100 ms before to 300 ms after the onset of the Test. Epochs with MEG signals larger than 2.7 pT / cm were rejected from averaging. Subjects sat in a chair and watched a silent movie on a screen 1.5 m in front of them throughout the experiments.
Procedures
All subjects participated in three experimental sessions: Placebo, Diazepam, and Baclofen, on different days spaced by more than one week. The order was randomized among subjects. In each session, there were two MEG measurement runs: Pre (baseline) and Post. At the beginning of the MEG recording, brain activity during a 2-min eye-closed rest period was recorded in order to assess the dominant alpha frequency of background activity. Thereafter, we recorded auditory evoked magnetic fields for the baseline run (Pre). After the Pre run, subjects were blindly administered a placebo, diazepam (5 mg), or baclofen (10 mg) tablet. The dosage of diazepam and baclofen was approximately one-third of the standard daily dosage. One hour (Placebo and Diazepam)[15,16] or 2.5 hours (Baclofen)[17] after drug administration, 2-min background activity was recorded again and the Post run was then conducted. At the end of each run, subjects reported their sleepiness during the run using the Stanford Sleepiness Scale questionnaire [18]. This was done because the two drugs often cause sleepiness but we did not know the effect of vigilance on PPI. The time needed to complete a run was approximately 40 min.
Analysis
Recorded MEG waveforms were subjected to band-pass filtering of 1–75 Hz and analyzed as previously reported [9–11]. In brief, the Test-alone response (Panel C, Part a in Fig 1) was obtained by subtracting the waveform for the Standard (Ba) from that for the Test alone stimulus (Bb). Similarly, the Prepulse + Test response (Cc) was obtained by subtracting the waveform for the Prepulse alone stimulus (Bc) from that for the Prepulse + Test stimulus (Bd). Using the subtracted Test-alone response waveform for the Pre run, an equivalent current dipole for the magnetic component at approximately 130 ms, Change-N1m, was estimated at the peak latency for each hemisphere using BESA (NeuroScan, Mclean, VA). A dipole model was obtained for the Test-alone response of all three sessions: Placebo, Diazepam, and Baclofen, and the orientation and location of the dipole for the Test-alone response were then averaged across three sessions. The dipole model obtained was applied to all subtracted MEG waveforms (Fig 1C) of all sessions, and the source strength waveform was used to measure the amplitude of Change-N1m. Fig 1D shows the source strength waveforms of the Pre run of the Placebo session in a representative subject. Fig 1E shows the superimposed test-evoked source strength waveforms of all eight PTI conditions.
The peak amplitude of Change-N1m was measured between the peak in Change-N1m within the period of 100–200 ms and the peak in the polarity-reversed earlier component within 50–100 ms after the change onset to minimize issues associated with a baseline shift [9,14]. The percent inhibition of the Change-N1m amplitude by the prepulse (%PPI) was defined as (Test alone response–(Prepulse + Test response)) / Test alone response * 100. The Change-N1m amplitude or degree of inhibition was compared among Test-evoked responses using a three-way repeated measures ANOVA with Prepulse, Hemisphere, and Drug as the independent variables. The effects of drugs on PPI were compared between placebo and diazepam, and between placebo and baclofen. Changes in %PPI between Pre and Post runs were calculated for each PTI of each drug, and the difference was compared using a three-way ANOVA (Hemisphere x Drug x PTI) followed by paired t-tests corrected with Bonferroni adjustments for multiple comparisons. In addition, paired comparisons for each PTI between placebo and diazepam and between placebo and baclofen were conducted using the bootstrapping resampling method (2000 times). We calculated confidence intervals of resampled data and tested whether they covered zero. IBM SPSS Statistics (version 21) was used for statistical analyses.
In order to assess the dominant alpha rhythm, a stable period of 2 min was selected and subjected to FFT. Twenty symmetric sensors in the occipital area were chosen and the peak frequency of background activity was averaged across 20 sensors [19]. The dominant alpha rhythm of the background activity and sleepiness score were compared between Pre and Post runs using paired t-tests. All statistical analyses were performed at the 0.05 level of significance. Data are expressed as the mean ± standard deviation (SD).
Results
Baseline responses
The test stimulus, an abrupt increase of 10 dB from the background (Fig 1A), evoked a clear change-related response (Fig 1B). As in previous studies [9,10,14], the generator of the N100 component (Fig 1D) was estimated to be located in and around the superior temporal gyrus. The peak amplitude of each condition is shown in Fig 2 and Table 1. Among the three baseline (Pre) runs before drug administration, the results of a three-way ANOVA (Hemisphere x Drug x PTI) showed that the peak amplitude was significantly different among PTIs (F7,63 = 17.0, p = 2.1 x 10−12), but not among drugs (F2,18 = 2.31, p = 0.13) or between hemispheres (F1,9 = 0.02, p = 0.89). Post hoc tests showed that all prepulses, except 10-ms PTI, significantly suppressed the test response (P < 0.002), confirming the strong effect of the prepulse. The greatest suppression was obtained by 40-ms PTI. Fig 1D shows an example of waveforms for the Test alone, Prepulse alone, and Prepulse + Test responses with a prepulse presented 60 ms before the test stimulus.
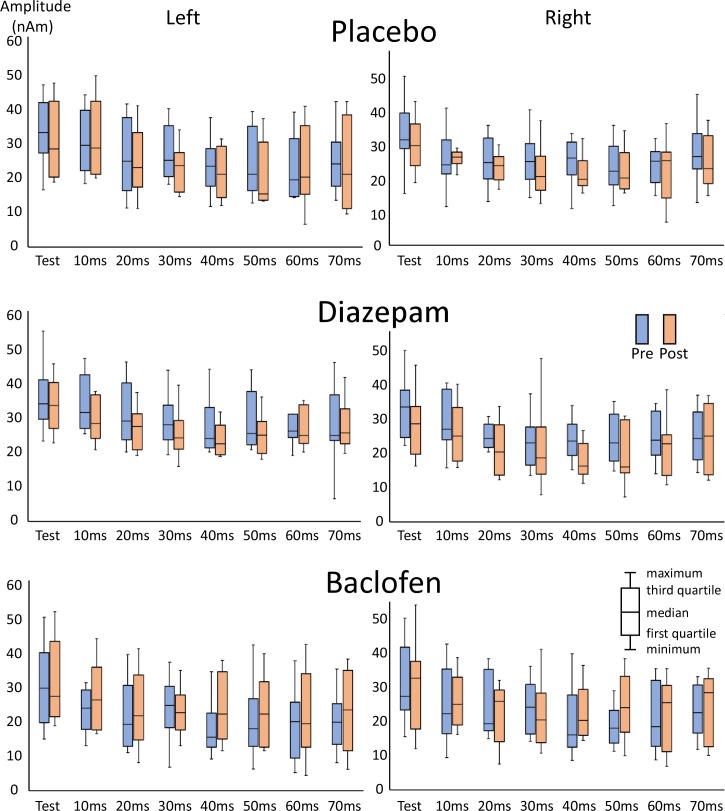
Fig 2. Amplitudes of the change-related response.
The mean amplitude of the test response across subjects for all PTI conditions is shown in box plots.
Table 1. The peak amplitude of the change-related auditory response.
| Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Pre | Post | Pre | Post | ||||
| Prepulse | Amplitude | %PPI | Amplitude | %PPI | Amplitude | %PPI | Amplitude | %PPI |
| Test alone | 33.3(9.3) | 30.0(10.7) | 31.4(8.9) | 28.7(7.2) | ||||
| 10ms | 29.9(8.9) | 9.4(12.4) | 30.4(10.3) | -2.9(16.3) | 24.7(8.2) | 21.8(11.1) | 25.4(3.7) | 8.5(17.0) |
| 20ms | 25.3(10.4) | 25.4(14.8) | 24.2(9.2) | 19.2(12.4) | 23.7(7.5) | 24.3(12.5) | 23.3(6.3) | 18.0(12.7) |
| 30ms | 26.6(7.8) | 18.0(17.7) | 22.5(6.3) | 22.4(13.8) | 24.4(7.6) | 21.6(12.1) | 20.7(6.8) | 28.2(11.9) |
| 40ms | 23.0(7.4) | 28.8(19.3) | 21.2(7.7) | 28.7(13.5) | 24.1(6.6) | 22.3(14.7) | 20.4(5.0) | 25.5(24.0) |
| 50ms | 23.7(9.3) | 27.8(19.9) | 20.5(8.9) | 32.1(11.3) | 22.3(7.2) | 29.1(8.4) | 21.5(6.2) | 23.5(18.5) |
| 60ms | 22.7(9.2) | 30.6(21.8) | 23.3(10.9) | 22.8(23.9) | 22.6(5.4) | 25.4(16.5) | 21.0(8.6) | 25.9(30.0) |
| 70ms | 24.5(8.8) | 23.9(24.2) | 24.0(12.8) | 23.1(23.5) | 26.8(9.2) | 15.2(11.6) | 24.0(7.7) | 14.0(26.3) |
| Diazepam | ||||||||
| Test alone | 30.9(11.0) | 28.9(8.7) | 32.3(8.5) | 27.5(9.0) | ||||
| 10ms | 29.1(9.0) | 2.5(20.4) | 23.8(7.3) | 16.3(16.1) | 28.9(7.9) | 6.5(30.8) | 25.6(8.1) | 5.6(11.7) |
| 20ms | 26.0(10.8) | 16.8(13.4) | 21.2(6.7) | 26.8(6.0) | 26.4(7.9) | 16.3(18.6) | 21.4(7.6) | 22.0(11.6) |
| 30ms | 23.8(9.3) | 23.0(10.9) | 19.2(7.7) | 33.1(18.0) | 22.7(7.3) | 29.4(15.2) | 21.5(11.9) | 25.2(19.3) |
| 40ms | 20.9(8.7) | 32.3(11.3) | 17.4(5.5) | 38.5(12.0) | 23.5(5.8) | 26.0(13.4) | 18.8(8.2) | 30.8(16.5) |
| 50ms | 23.4(10.2) | 24.4(16.0) | 18.9(7.3) | 33.2(19.8) | 23.7(7.2) | 26.0(15.2) | 19.6(8.3) | 28.3(21.6) |
| 60ms | 22.5(8.9) | 25.7(19.8) | 21.2(6.7) | 24.1(21.5) | 24.8(6.9) | 21.1(19.9) | 21.4(7.9) | 22.2(13.8) |
| 70ms | 22.8(13.4) | 30.6(32.2) | 22.0 (8.0) | 23.1(16.3) | 24.7(7.8) | 22.6(19.9) | 24.0(9.2) | 13.5(17.5) |
| Baclofen | ||||||||
| Test alone | 29.4(11.2) | 31.0(11.4) | 30.9(11.3) | 30.3(12.1) | ||||
| 10ms | 24.9(9.9) | 12.2(23.5) | 27.0(9.2) | 11.6(14.1) | 23.4(9.5) | 23.5(19.1) | 25.2(7.5) | 10.6(22.6) |
| 20ms | 21.9(10.3) | 26.7(14.0) | 23.0(10.7) | 26.4(22.5) | 23.7(9.1) | 19.9(26.2) | 21.9(8.2) | 26.0(15.8) |
| 30ms | 23.6(9.1) | 19.8(15.5) | 22.8(6.7) | 23.7(14.8) | 23.4(7.5) | 21.4(18.1) | 21.5(9.5) | 27.3(17.2) |
| 40ms | 18.5(9.7) | 37.4(16.3) | 23.0(9.5) | 26.5(10.7) | 18.9(9.3) | 39.8(12.3) | 22.2(7.4) | 21.9(20.2) |
| 50ms | 20.2(10.9) | 31.9(18.8) | 22.8(9.4) | 27.1(13.0) | 17.9(5.2) | 39.1(14.7) | 23.8(9.5) | 19.8(16.6) |
| 60ms | 19.1(9.9) | 36.2(19.8) | 21.5(12.2) | 33.2(24.9) | 19.7(8.3) | 36.8(9.9) | 21.5(10.2) | 30.1(16.7) |
| 70ms | 19.8(8.5) | 32.3(15.6) | 22.4(11.7) | 29.0(26.4) | 21.8(6.7) | 26.7(15.0) | 23.3(10.0) | 20.5(25.9) |
Amplitudes (nAm) are shown as the mean (SD)
When percent inhibition (%PPI) was compared among the three Pre runs, the results obtained were similar; PTI significantly affected the degree of suppression (F6,54 = 5.27, p = 2.5 x 10−4), whereas Hemisphere (p = 0.90) and Drug (p = 0.13) did not. Bonferroni’s post hoc tests showed that %PPI was significantly greater for 40-ms PTI than for 10-ms (p = 0.008) and 30-ms (P = 0.015) PTIs.
Effects of drugs
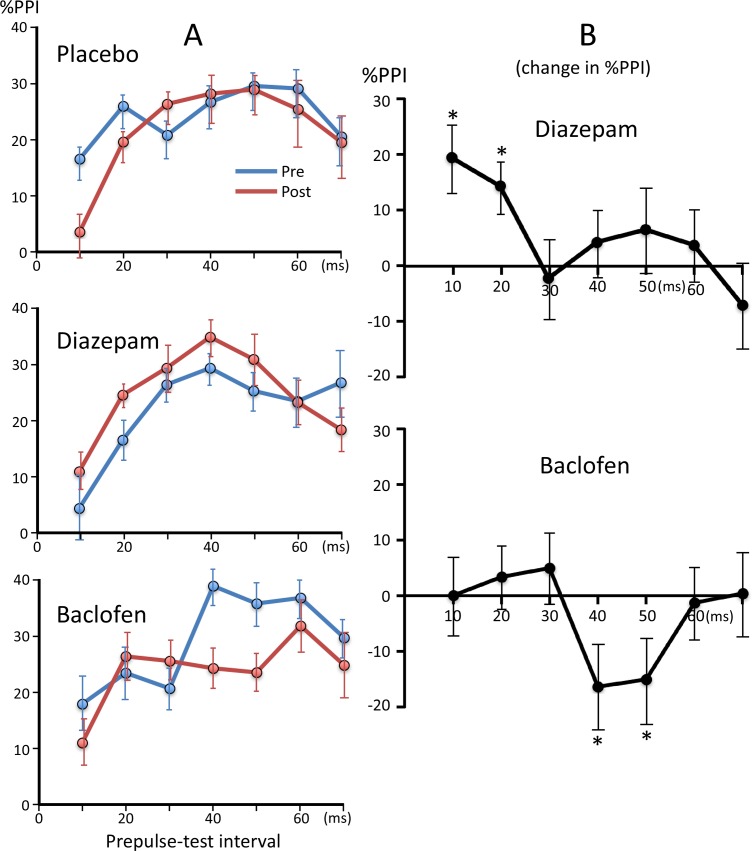
The results of paired t-tests showed that none of the three drugs significantly altered the amplitude of the test response for both hemispheres (p > 0.05, uncorrected for multiple comparisons). Fig 3 shows plots of %PPI against PTI before and after the drug treatment. In order to analyze the effects of drugs, we calculated differences in %PPI between Pre and Post runs and the difference was then subjected to a three-way ANOVA (Hemisphere, Drug, and PTI). When the difference in %PPI was compared between placebo and diazepam, the results of the ANOVA showed that none of the factors significantly affected %PPI (p > 0.13), while the Drug x PTI interaction was significant (F6,54 = 2.37, p = 0.042). The difference in %PPI for each PTI was compared between placebo and diazepam using paired t-tests with Bonferroni adjustments for multiple comparisons. The results obtained showed that the administration of diazepam significantly enhanced the suppression of 10- (p = 0.035) and 20-ms (p = 0.042) PTIs. Fig 3B shows changes in %PPI after diazepam, from which changes after the placebo were subtracted. In the comparison between placebo and baclofen, the Drug x PTI interaction was significant (F6,54 = 2.93, p = 0.015). As shown in Fig 3B, baclofen decreased the %PPI for 40- and 50-ms PTIs. Although uncorrected t-tests showed that the difference was significant for both conditions (p = 0.023 and 0.026, respectively), it was not after the Bonferroni correction.
Fig 3. Percent inhibition at each prepulse-test interval before and after drug administration.
The mean %PPI across subjects are shown. (A) Plots of %PPI against PTI before and after each drug administration. (B) Changes in %PPI following diazepam and baclofen administration after normalization by subtracting the values of the placebo session. *, p < 0.05 significantly different from the Placebo (paired t-test uncorrected for multiple comparisons). Error bars show ± the standard error.
As shown in Fig 2, fluctuations were observed in baseline PPI among sessions, which may affect statistical evaluations of drug effects. Therefore, we conducted an additional analysis for these four PTIs. The effects of drugs were compared between placebo and diazepam, and between placebo and baclofen using the bootstrapping resampling (2000 times) method. The results obtained showed a significant effect by diazepam at 10 (p < 0.00001) and 20 ms (p < 0.001), and by baclofen at 40 and 50 ms (p < 0.05).
Sleepiness and background alpha rhythm
The sleepiness score was similar between Pre and Post runs for placebo (2.2 ± 1.3 and 2.1 ± 1.1, respectively). Although diazepam (2.0 ± 1.1 and 2.9 ± 1.4) and baclofen (1.5 ± 0.8 and 2.0 ± 1.2) increased the score for the Post run, the difference was not significant (p = 0.12, uncorrected for multiple comparisons). While the background alpha rhythm was slightly slower for the Post run of all drugs (10.1 ± 1.1 and 9.8 ± 0.9 for placebo; 10.0 ± 0.8 and 10.0 ± 1.1 for diazepam; 10.3 ± 1.1 and 10.1 ± 1.0 for Baclofen), the difference was not significant (p = 0.28, 0.76, and 0.07, respectively; uncorrected for multiple comparisons).
Discussion
The present study confirmed the PPI of change-related cortical responses by a weak change stimulus that itself evoked no or only a weak response (Fig 1B). Suppression by such a weak leading stimulus indicates that this suppression is not due to passive events such as fatigue, but rather due to active inhibitory processes. The present study confirmed this, showing that the GABA system participated in PPI. By manipulating the PTI, a previous study suggested that at least three different mechanisms are involved in the PPI of the change-related cortical response showing peaks at PTIs of approximately 20–30, 50–60, and 500–600 ms [11]. The present study pharmacologically separated at least two mechanisms for short-latency inhibition.
The results of the present study showed enhanced PPI at 10- and 20-ms PTIs following the administration of diazepam, suggesting that GABAA receptors are involved in short-latency inhibition. Although PPI at the 10-ms PTI did not significantly suppressed the test response in the present study, the difference was significant in our previous study [11], suggesting that although the inhibitory effects of prepulses with short PTIs were weak, their actions became obvious in the presence of diazepam. However, the significant effect of diazepam at the 10-ms PTI depended on slightly decreased PPI for placebo and slightly increased PPI for diazepam. The difference was not significant without subtraction of the placebo effects. The GABAA receptor is ionotropic and when it is activated by its endogenous ligand GABA, the chloride ion-selective pore opens. This causes hyperpolarization of the membrane and, thus, decreases temporally overlapping EPSPs in target cells. Besides the active site for GABA, the GABA receptor has a number of other bindings sites, one of which, the benzodiazepine site, is the target of diazepam [20]. Such an allosteric modulator indirectly influences the effects of GABA. Diazepam augments effects through the GABAA receptor by increasing the frequency of channel openings [21]. In whole-cell recording studies, GABAA-mediated early IPSPs or IPSCs were enhanced by the bath application of diazepam [22–24].
Since the time course of PPI in the present study is considered to reflect that of IPSPs being negligibly confounded by EPSPs produced by a prepulse [11], the present results indicate that early IPSPs at 10–20 ms were induced through GABAA receptors. This is consistent with the peak latency of the early GABAA-mediated IPSPs reported in whole-cell studies of approximately 10–30 ms [25–28]. Although several types of interneurons elicit fast IPSPs [28], we considered parvalbumin-positive (PV) cells to be a possible candidate for early inhibition [11]. PV or fast spiking (FS) interneurons, the largest subclass of interneurons [29,30], play a role in fast inhibition [31]. While the present study was unable to identify the cell type, the results obtained indicate that at least one interneuron type sensitive to benzodiazepine participated in the present PPI.
Baclofen slightly suppressed PPI at PTIs of 40–50 ms, suggesting the involvement of GABAB receptors [32] in the present PPI. However, unlike diazepam, the interpretation of results is not straightforward. GABAB receptors are metabotropic and induce late inhibition through G-protein activation [33]. Baclofen is an agonist of GABAB receptors and induces late IPSPs in target cells. However, it has also been shown to block GABA release via presynaptic autoreceptors [34–37] (for a review, see Misgeld et al. [38]). In addition, GABAB receptors are located on glutamate fiber terminals [39]. Therefore, its overall effects on a circuit depend on which receptors are predominantly activated. In the present study, no significant differences were observed in the amplitude of the test response before and after the administration of baclofen, suggesting that its actions on the presynaptic GABAB receptors of glutamatergic cells did not play a major role. Baclofen appears to have suppressed GABA release via autoreceptors on the axon terminals of GABAergic neurons in the present study. Therefore, the present results suggest that GABAB-mediated inhibition peaked at 40–50 ms, which is consistent with GABAB-mediated IPSPs following GABAA-mediated fast IPSPs. Although the peak latency of GABAB-mediated IPSPs varied in whole-cell recording studies, it was always markedly longer than that of early IPSPs [26,27,40,41]. A previous study using the microapplication of glutamate to rat cortical slices showed late IPSPs sensitive to 2-hydroxysaclofen, a blocker of GABAB receptors, peaking at 45 ms [42], which is consistent with the present results.
In the present study, 10 mg of baclofen was used. Since baclofen does not readily penetrate the blood brain barrier [43], its brain concentration in the present study was expected to be very low. However, presynaptic GABAB receptors are more sensitive to GABA than postsynaptic receptors [44]. Baclofen inhibits GABA release from axon terminals at a low concentration at which it exerts no postsynaptic effect. Scholfield et al. showed in olfactory cortex slice preparations from guinea pigs that the bath application of 0.2 μM baclofen almost completely abolished IPSCs evoked by a stimulation of the lateral olfactory tract [45]. Guetg et al. [46] examined the roles of the GABAB receptor subtypes, GABAB(1a, 2) and GABAB(1b, 2), in the inhibition of transmitter release and demonstrated that 0.1 μM baclofen was exclusively effective for GABAB(1a, 2) receptors that were preferentially distributed on presynaptic sites. Since the cerebrospinal fluid concentration of baclofen was expected to be 0.07–0.13 μM in the present study [43,47], it is possible that baclofen under the present experimental conditions acted dominantly on presynaptic GABAB receptors and the inhibition of GABA release resulted in decreased PPI. Regarding GABAA-mediated inhibition, the present results showed that baclofen did not affect PPI at PTIs of 10 and 20 ms, whereas diazepam significantly increased inhibition, suggesting that GABAA-induced inhibition was not affected by presynaptic GABAB receptors. In patch clamp studies, these effects of baclofen were shown to be stronger for GAGAB-induced late IPSPs than GABAA-induced early IPSPs [35,41]. Connors et al. [41] reported that baclofen abolished late IPSPs, while leaving early IPSPs almost intact.
Therefore, the present results suggest that baclofen reduced GABA-mediated inhibition, which may be associated with seizures after the intrathecal application of baclofen [48] because hypofunction of the GABAB receptor is considered to cause epilepsy [49]. In support of this, Dugladze et al. [37] demonstrated that baclofen at a low concentration induced the hyperexcitability of hippocampal neurons in epileptic mice, and this was due to the enhanced inhibition of GABA release via presynaptic GABAB receptors.
GABA functions are considered to be related to susceptibility to epilepsy; many anticonvulsants act on GABA receptors, GABA receptor antagonists or negative allosteric modulators act as a convulsant, GABA receptor knockout animals develop epilepsy, many animal models of epilepsy show abnormalities in GABA functions, and GABA elicits the hyperpolarization of target neurons, making them fire less [38,50,51]. Therefore, the present measure may serve as a clinical test for susceptibility to epilepsy.
Besides epilepsy, GABA is considered to play a role in the pathophysiology of many diseases such as schizophrenia [1–4] and autism spectrum disorder (ASD)[5]. Due to the lack of appropriate methods for functional measurements of the GABA system, the present method appears to be a useful tool for understanding the pathophysiology of these diseases and estimating inhibitory functions in individuals. However, it currently remains unclear whether inhibition in a specific circuit in the present study reflects the fundamental function of the GABA system of the individual. In whole-cell recordings, the sequence of early IPSPs mediated by GABAA receptors and late IPSPs mediated by GABAB receptors is common across species [41], including humans [27], and brain areas [52,53]. Furthermore, FS or PV interneurons are known to be densely connected to nearby pyramidal cells in a non-selective manner across cortical areas and layers, suggesting their role in unspecific inhibition [52]. Similar unspecific dense connections to neighboring pyramidal cells, a blanket of inhibition, have also been reported for the interneurons responsible for long-latency inhibition [54]. Therefore, inhibition in the change-driven circuit of the auditory cortex in the present study may represent a basic inhibitory mechanism and the present method serves as a tool for translational studies.
ASD, for example, is considered to be associated with alterations in GABAergic signaling [5,55]. The high prevalence of epilepsy in ASD [56] further supports this idea and common mechanisms underlying both disorders have been investigated [57,58]. In vitro analyses of the post-mortem brain tissues of ASD patients showed reductions in GABA receptor density or glutamic acid decarboxylase (GAD) in various brain areas including the anterior cingulate cortex, fusiform gyrus, hippocampus, parietal cortex, and cerebellum [55]. In vivo studies using positron emission tomography (PET), single photon emission computed tomography (SPECT), and magnetic resonance spectroscopy (MRS) showed reduced levels of GABA receptors or GABA concentrations in the frontal lobe, perisylvian region, superior and medial frontal cortex, fronto-temporal cortex, auditory cortex, motor cortex, anterior cingulate cortex, and limbic areas (for a review, see Cellot & Cherubini [55]; Dickinson et al. [59]). These findings indicate alterations in the GABA system in various brain areas in ASD, thereby suggesting regional non-specific fundamental changes in the GABA system. However, the function of the inhibitory system in ASD is unknown. In animal models of ASD, PV interneurons are reduced in the neocortex across multiple models [60], suggesting common PV-circuit disruption across animals showing ASD behavioral phenotypes. In animals, unlike humans, IPSCs produced through GABA receptors may be observed using whole-cell recording techniques [61,62]. For example, Lo et al. [63] showed markedly reduced GABAA-mediated IPCSs in the barrel cortex of ASD model mice, confirming deficits in the GABA system. However, in vivo recordings of IPSPs are challenging and, to the best of our knowledge, this has not yet been conducted on ASD model animals. Measurements of the function of the GABA system in vivo are difficult in humans and animals. The present measure is easily performed using electroencephalograms [12] and, thus, may also be applied to animals, which enables comparisons of data between animals and humans. Similar measurements in animals are needed in the future.
Limitations
The test response in the present study was evoked by a sound feature change in a continuous click train. Since neurons in the auditory cortex easily adapt to a repetitive sound stimulus [64,65], the generation of change-related activity appears to be based on disinhibition of the change-detecting system. Although the generation mechanism of change-related brain activity is unclear, inhibitory circuits may be involved. A recent computational study on stimulus-specific adaptation by Nelken and Yarden [66] supported this view. Our results showed that diazepam and baclofen did not significantly alter the test response, suggesting that this was not the main mechanism responsible for the present results showing the significant impacts of these drugs on the test response. However, these drugs may have altered brain sensitivity to a change and, thus, affected the results obtained.
The systemic administration of drugs was problematic. Although the sleepiness score did not significantly differ among the three sessions, diazepam and baclofen caused slight sleepiness in some subjects. Since we did not know the effects of vigilance on PPI, the possibility of this effect on the results obtained cannot be denied. Furthermore, the anxiolytic effects of diazepam may have affected the present results. This may be problematic when these effects differ among inhibitory mechanisms.
To understand whether this method can be applied to pathological conditions, further studies are necessary. The baseline PPI showed fluctuations among the three sessions, which is important if the present method is used as a clinical test. For example, the present results for the PPI with the 10-ms PTI showed reduced inhibition after placebo administration and a slight increase after diazepam. Without normalization procedures, the effect of diazepam was not significant, which may hamper interpretation of the results. Therefore, the consistency of measurement across sessions is important. This was due, at least in part, to the low S/N ratio of the evoked response. Since there were eight conditions in one run, we had to use a minimum number of trials for averaging. The present results revealed some key PTIs for testing, and, thus, we may reduce the number of conditions. Future studies with fewer conditions, but with a greater amount of averaging will confirm if more stable data may be obtained. For applying to patients, test-retest reliability should be tested using electroencephalograms. Because clinical importance of PPI of startle reflexes is well known [67], research on the relationship between the conventional PPI and present PPI is also necessary. Since the sensory cortex is outside the conventional PPI pathway, the present results may suggest that the prepulse-induced suppression is not specific to startle pathways but represents a non-specific mechanism of sensory processing [11].
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number JP25351001 and the ImPACT Program (2015-PM11-23-01) of the Council for Science, Technology, and Innovation (Cabinet Office, Government of Japan) to K.I. The authors are grateful to Yasuyuki Takeshima for his technical support.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by JSPS KAKENHI (https://www.jsps.go.jp/j-grantsinaid/) Grant Number JP25351001 and the ImPACT Program (2015-PM11-23-01) of the Council for Science, Technology, and Innovation (Cabinet Office, Government of Japan) (http://www8.cao.go.jp/cstp/sentan/about-kakushin.html) to Koji Inui. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13:107–120. doi: 10.1038/nrn3155 [DOI] [PubMed] [Google Scholar]
- 2.Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol 2014; 26:22–26. doi: 10.1016/j.conb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol 2003; 23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5 [DOI] [PubMed] [Google Scholar]
- 4.Chiapponi C, Piras F, Piras F, Caltagirone C, Spalletta G. GABA system in schizophrenia and mood disorders: a mini review on third-generation imaging studies. Front Psychiatry 2016; 7:61 doi: 10.3389/fpsyt.2016.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord 2001; 31:247–248. [DOI] [PubMed] [Google Scholar]
- 6.Braat S, Kooy RF. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 2015; 86:1119–1130. doi: 10.1016/j.neuron.2015.03.042 [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2003; 2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med 2016; 15:146–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inui K, Tsuruhara A, Kodaira M, Motomura E, Tanii H, Nishihara M, et al. Prepulse inhibition of auditory change-related cortical responses. BMC Neurosci 2012; 13:135 doi: 10.1186/1471-2202-13-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inui K, Tsuruhara A, Nakagawa K, Nishihara M, Kodaira M, Motomura E, et al. Prepulse inhibition of change-related P50m no correlation with P50m gating. Springerplus 2013; 2:588 doi: 10.1186/2193-1801-2-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inui K, Nakagawa K, Nishihara M, Motomura E, Kakigi R. Inhibition in the human auditory cortex. PLoS One 2016; 11:e0155972 doi: 10.1371/journal.pone.0155972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inui K, Urakawa T, Yamashiro K, Otsuru N, Nishihara M, Takeshima Y, et al. Non-linear laws of echoic memory and auditory change detection in humans. BMC Neurosci 2010; 11:80 doi: 10.1186/1471-2202-11-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanahashi M, Motomura E, Inui K, Ohoyama K, Tanii H, Konishi Y, et al. Auditory change-related cerebral responses and personality traits. Neurosci Res 2016; 103:34–39. doi: 10.1016/j.neures.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 14.Inui K, Urakawa T, Yamashiro K, Otsuru N, Takeshima Y, Nishihara M, et al. Echoic memory of a single pure tone indexed by change-related brain activity. BMC Neurosci 2010; 11:135 doi: 10.1186/1471-2202-11-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Silva JA, Koechlin BA, Bader G. Blood level distribution patterns of diazepam and its major metabolite in man. J Pharm Sci 1966; 55:692–702. [DOI] [PubMed] [Google Scholar]
- 16.Mandelli M, Tognoni G, Garattini S. Clinical pharmacokinetics of diazepam. Clin Pharmacokinet 1978; 3:72–91. [DOI] [PubMed] [Google Scholar]
- 17.Wuis EW, Dirks MJ, Termond EF, Vree TB, Van der Kleijn E. Plasma and urinary excretion kinetics of oral baclofen in healthy subjects. Eur J Clin Pharmacol 1989; 37:181–184. [DOI] [PubMed] [Google Scholar]
- 18.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology 1973; 10:431–436. [DOI] [PubMed] [Google Scholar]
- 19.Otsuru N, Tsuruhara A, Motomura E, Tanii H, Nishihara M, Inui K, et al. Effects of acute nicotine on auditory change-related cortical responses. Psychopharmacology (Berl) 2012; 224:327–335. [DOI] [PubMed] [Google Scholar]
- 20.Johnston GA. GABAA receptor pharmacology. Pharmacol Ther 1996; 69:173–198. [DOI] [PubMed] [Google Scholar]
- 21.Study RE, Barker JL. Diazepam and (—)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A 1981; 78:7180–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal M, Barker JL. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol 1984; 52:469–487. doi: 10.1152/jn.1984.52.3.469 [DOI] [PubMed] [Google Scholar]
- 23.Pawelzik H, Bannister AP, Deuchars J, Ilia M, Thomson AM. Modulation of bistratified cell IPSPs and basket cell IPSPs by pentobarbitone sodium, diazepam and Zn2+: dual recordings in slices of adult rat hippocampus. Eur J Neurosci 1999; 11:3552–3564. [DOI] [PubMed] [Google Scholar]
- 24.Kang-Park MH, Wilson WA, Moore SD. Differential actions of diazepam and zolpidem in basolateral and central amygdala nuclei. Neuropharmacology 2004; 46: 1–9. [DOI] [PubMed] [Google Scholar]
- 25.Avoli M. Inhibitory potentials in neurons of the deep layers of the in vitro neocortical slice. Brain Res 1986; 370:165–170. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi Y. Receptor subtypes involved in callosally-induced postsynaptic potentials in rat frontal agranular cortex in vitro. Exp Brain Res 1992; 88:33–40. [DOI] [PubMed] [Google Scholar]
- 27.McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol 1989; 62:1018–1027. doi: 10.1152/jn.1989.62.5.1018 [DOI] [PubMed] [Google Scholar]
- 28.Tamás G, Buhl EH, Somogyi P. Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol 1997; 500:715–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 1997; 7:476–486. [DOI] [PubMed] [Google Scholar]
- 30.Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 1997; 7:347–358. [DOI] [PubMed] [Google Scholar]
- 31.Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist 2013; 19:228–237. doi: 10.1177/1073858412456743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, et al. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature 1980; 283:92–94. [DOI] [PubMed] [Google Scholar]
- 33.Thalmann RH. Evidence that guanosine triphosphate (GTP)-binding proteins control a synaptic response in brain: effect of pertussis toxin and GTP gamma S on the late inhibitory postsynaptic potential of hippocampal CA3 neurons. J Neurosci 1988; 8:4589–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutar P, Nicoll RA. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron 1988; 1:585–591. [DOI] [PubMed] [Google Scholar]
- 35.Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol 1990; 424:513–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert NA, Wilson WA. Heterogeneity in presynaptic regulation of GABA release from hippocampal inhibitory neurons. Neuron 1993; 11:1057–1067. [DOI] [PubMed] [Google Scholar]
- 37.Dugladze T, Maziashvili N, Börgers C, Gurgenidze S, Häussler U, Winkelmann A, et al. GABA(B) autoreceptor-mediated cell type-specific reduction of inhibition in epileptic mice. Proc Natl Acad Sci U S A 2013; 110:15073–15078. doi: 10.1073/pnas.1313505110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 1995; 46:423–462. [DOI] [PubMed] [Google Scholar]
- 39.Kulik A, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, et al. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci 2003; 23:11026–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satou M, Mori K, Tazawa Y, Takagi SF. Two types of postsynaptic inhibition in pyriform cortex of the rabbit: fast and slow inhibitory postsynaptic potentials. J Neurophysiol 1982; 48:1142–1156. doi: 10.1152/jn.1982.48.5.1142 [DOI] [PubMed] [Google Scholar]
- 41.Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol 1988; 406:443–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benardo LS. Separate activation of fast and slow inhibitory postsynaptic potentials in rat neocortex in vitro. J Physiol 1994; 476:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faigle JW, Keberle H. The metabolism and pharmacokinetics of Lioresal In: Spasticity—a topical Survey. Switzerland: Hans Huber Bern; 1972. pp. 94–100. [Google Scholar]
- 44.Yoon KW, Rothman SM. The modulation of rat hippocampal synaptic conductances by baclofen and gamma-aminobutyric acid. J Physiol 1991; 442:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholfield CN. Baclofen blocks postsynaptic inhibition but not the effect of muscimol in the olfactory cortex. Br J Pharmacol 1983; 78:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, et al. The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses. J Neurosci 2009; 29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nahar LK, Cordero RE, Nutt D, Lingford-Hughes A, Turton S, Durant C, et al. Validated method for the quantification of baclofen in human plasma using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Anal Toxicol 2016; 40:117–123. doi: 10.1093/jat/bkv125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kofler M, Kronenberg MF, Rifici C, Saltuari L, Bauer G. Epileptic seizures associated with intrathecal baclofen application. Neurology 1994; 44:25–27. [DOI] [PubMed] [Google Scholar]
- 49.Schuler V, Lüscher C, Blanchet C, Klix N, Sansig G, Klebs K, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 2001; 31:47–58. [DOI] [PubMed] [Google Scholar]
- 50.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol 1993; 33:109–147. doi: 10.1146/annurev.pa.33.040193.000545 [DOI] [PubMed] [Google Scholar]
- 51.Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci 2003; 26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210 [DOI] [PubMed] [Google Scholar]
- 52.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 2011; 31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kätzel D, Zemelman BV, Buetfering C, Wölfel M, Miesenböck G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat Neurosci 2011; 14:100–107. doi: 10.1038/nn.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron 2011; 69:1188–1203. doi: 10.1016/j.neuron.2011.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2014; 2:70 doi: 10.3389/fped.2014.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, et al. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS One 2013; 8:e67797 doi: 10.1371/journal.pone.0067797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frye RE, Casanova MF, Fatemi SH, Folsom TD, Reutiman TJ, Brown GL, et al. Neuropathological mechanisms of seizures in autism spectrum disorder. Front Neurosci 2016; 10:192 doi: 10.3389/fnins.2016.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacob J. Cortical interneuron dysfunction in epilepsy associated with autism spectrum disorders. Epilepsia 2016; 57:182–193. doi: 10.1111/epi.13272 [DOI] [PubMed] [Google Scholar]
- 59.Dickinson A, Jones M, Milne E. Measuring neural excitation and inhibition in autism: Different approaches, different findings and different interpretations. Brain Res 2016; 1648(Pt A):277–289. doi: 10.1016/j.brainres.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 60.Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 1, 172–181 (2009). doi: 10.1007/s11689-009-9023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banerjee A, García-Oscos F, Roychowdhury S, Galindo LC, Hall S, Kilgard MP, et al. Impairment of cortical GABAergic synaptic transmission in an environmental rat model of autism. Int J Neuropsychopharmacol 2013; 16:1309–1318. doi: 10.1017/S1461145712001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabanov V, Braat S, D'Andrea L, Willemsen R, Zeidler S, Rooms L, et al. Impaired GABAergic inhibition in the hippocampus of Fmr1 knockout mice. Neuropharmacology 2016; 116:71–81. doi: 10.1016/j.neuropharm.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 63.Lo FS, Erzurumlu RS, Powell EM. Insulin-independent GABAA receptor-mediated response in the barrel cortex of mice with impaired met activity. J Neurosci 2016; 36:3691–3697. doi: 10.1523/JNEUROSCI.0006-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brosch M, Schreiner CE. Time course of forward masking tuning curves in cat primary auditory cortex. J Neurophysiol 1997; 77:923–943. doi: 10.1152/jn.1997.77.2.923 [DOI] [PubMed] [Google Scholar]
- 65.Alves-Pinto A, Baudoux S, Palmer AR, Sumner CJ. Forward masking estimated by signal detection theory analysis of neuronal responses in primary auditory cortex. J Assoc Res Otolaryngol 2010; 11:477–494. doi: 10.1007/s10162-010-0215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yarden TS, Nelken I. Stimulus-specific adaptation in a recurrent network model of primary auditory cortex. PLoS Comput Biol 2017; 13:e1005437 doi: 10.1371/journal.pcbi.1005437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology 2008; 199:331–388. doi: 10.1007/s00213-008-1072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.