Abstract
Non-metastatic breast cancer patients often experience psychological distress which may influence disease progression and survival. Cognitive-behavioral stress management (CBSM) improves psychological adaptation and lowers distress during breast cancer treatment and long-term follow-ups. We examined whether breast cancer patients randomized to CBSM had improved survival and recurrence 8–15 years post-enrollment. From 1998 to 2005, women (N = 240) 2–10 weeks post-surgery for non-metastatic Stage 0–IIIb breast cancer were randomized to a 10-week, group-based CBSM intervention (n = 120) or a 1-day psychoeducational seminar control (n = 120). In 2013, 8–15 years post-study enrollment (11-year median), recurrence and survival data were collected. Cox Proportional Hazards Models and Weibull Accelerated Failure Time tests were used to assess group differences in all-cause mortality, breast cancer-specific mortality, and disease-free interval, controlling for biomedical confounders. Relative to the control, the CBSM group was found to have a reduced risk of all-cause mortality (HR = 0.21; 95 % CI [0.05, 0.93]; p = .040). Restricting analyses to women with invasive disease revealed significant effects of CBSM on breast cancer-related mortality (p = .006) and disease-free interval (p = .011). CBSM intervention delivered post-surgery may provide long-term clinical benefit for non-metastatic breast cancer patients in addition to previously established psychological benefits. Results should be interpreted with caution; however, the findings contribute to the limited evidence regarding physical benefits of psychosocial intervention post-surgery for non-metastatic breast cancer. Additional research is necessary to confirm these results and investigate potential explanatory mechanisms, including physiological pathways, health behaviors, and treatment adherence changes.
Keywords: Breast neoplasm, Survival, Recurrence, Cognitive therapy, Behavior therapy, Breast cancer
Introduction
Breast cancer is the most common cancer among women globally and is the second cause of cancer death in developed regions [1]. Five-year survival rates for breast cancer range from 40 % in low-income countries to 80 % in developed countries [2]. Women with breast cancer also have increased risk of psychological distress [3, 4]. A biopsychosocial model of cancer survival suggests that distress related to modifiable psychosocial factors, such as depressive symptoms, low social support, and psychological stress, may exacerbate metastatic processes, thereby influencing cancer progression and mortality [5–7]. Thus, researchers have examined whether psychological interventions aimed at modifying psychosocial factors can reduce recurrence and mortality in cancer. While one study showed such interventions could improve survival in metastatic breast cancer patients [8], efforts to replicate have had limited success, creating controversy [9]. A recent Cochrane review determined that psychological interventions were effective in improving survival at 12 months in metastatic breast cancer [9].
While most intervention studies were conducted in women with metastatic breast cancer, only one randomized controlled trial (RCT) in 2008 demonstrated beneficial effects of a psychosocial intervention on survival and recurrence in women with non-metastatic breast cancer [10]. At an 11-year median follow-up, women given a 12-month cognitive-behavioral intervention had significantly lower breast cancer-specific mortality (HR = 0.44, p = .016), all-cause mortality (HR = 0.51, p = .028), and breast cancer recurrence (HR = 0.55, p = .034) than control women [10].
We have found that a shorter (10-week) group-based cognitive-behavioral stress management (CBSM) program enhances physiological [11, 12] and psychological adaptation [13, 14] as women recover from surgery and undergo adjuvant treatments for non-metastatic breast cancer. The CBSM group also reported less depressive symptoms at 5- and 11-year (median) follow-ups [15, 16] and better emotional and physical well-being at the 11-year follow-up compared to the control group [16]. This secondary analysis asked whether women from that RCT who received CBSM also had reduced mortality or breast cancer recurrence at the 11-year follow-up (range 8–15 years). It is noteworthy that this study used an intervention similar in content but briefer than that of Andersen et al. [10] and had a similar patient population, sample size, and follow-up period.
Methods
Study design and patients
Participants were women with Stage 0–IIIb breast cancer who were 2–10 weeks post-surgery and enrolled in an RCT of CBSM between 1998 and 2005. The study was a single center, single blind, randomized, parallel assignment efficacy trial approved by the University of Miami (UM) Institutional Review Board (IRB; National Institutes of Health Clinical Trial NCT01422551) in 1998. Original study design is described in previous reports [14, 17]. Women were recruited from surgical oncology practices in South Florida through advertising and private physician referrals, and at the UM/Sylvester Cancer Center. Women were excluded if not between 21 and 75 years old, not fluent in English, had stage IV breast cancer or prior serious cancer (except minor skin cancers), had begun adjuvant treatment, had a major medical condition other than cancer, were previously psychiatrically hospitalized, or currently endorsed psychosis, suicidality, major depressive disorder, or panic disorder (see CONSORT diagram Fig. 1).
Fig. 1.
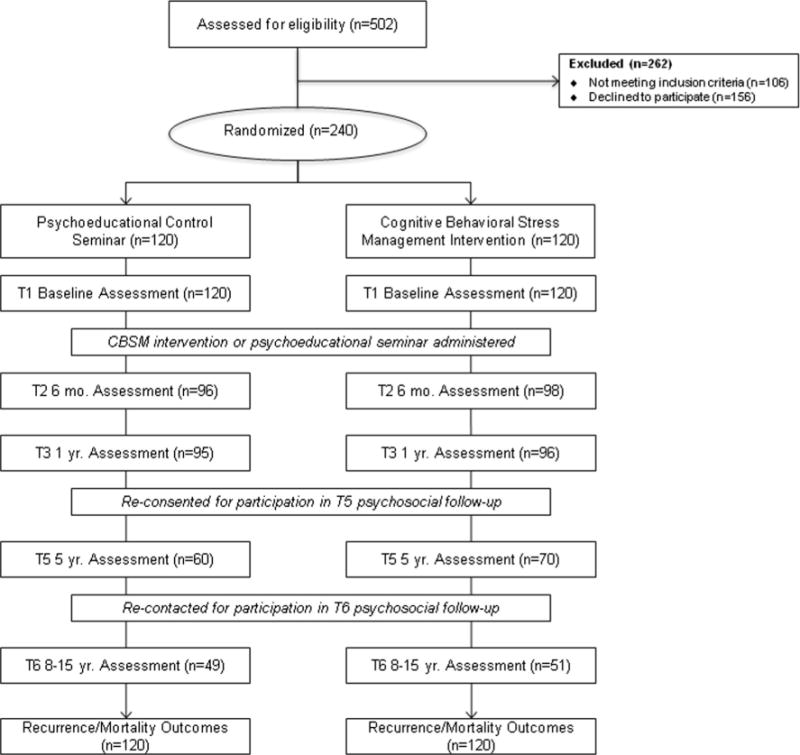
CONSORT diagram. Study flow illustrated in CONSORT diagram extending from recruitment for the original trial through the present follow-up
Procedures
Of 502 women screened, 240 signed informed consent, were enrolled, completed baseline assessments, and were randomized to CBSM intervention or a 1-day psychoeducational control group (Fig. 1). Randomization was performed on a 1:1 basis, with each cohort averaging approximately 14 participants. Randomization and assessment were conducted by blinded study coordinators. Assessments were repeated at 6, 12 months, and 5 years post-study enrollment. Women were re-contacted in 2013, 8–15 years post-study enrollment (11-year median), for participation in a new long-term follow-up study to assess medical status, which they had previously consented to. Study personnel obtained self-report information about participants’ disease status and conducted medical chart reviews to confirm recurrences and gather diagnostic- and treatment-related information. Vital statistics regarding participant death, cause, and date of death was obtained from the Florida Cancer Data System registry with approval from the Florida Department of Epidemiology and the Florida Department of Health IRB. Baseline self-reported demographic, medical, and treatment-related information was verified during medical chart reviews at the follow-up.
Intervention condition
Women randomized to CBSM [18] received a manualized intervention co-delivered by a Ph.D. level clinical psychologist and a doctoral student in clinical psychology. The group-based intervention was administered in 90-min sessions once per week for 10 weeks and aimed to improve coping and psychological adaptation as well as reduce stress and negative mood using cognitive-behavioral therapy (e.g., cognitive reframing, stress re-appraisal, effective coping skills training, assertiveness training, anger management, optimize use of social support) and relaxation training (e.g., progressive muscle relaxation, guided visual imagery, diaphragmatic breathing). Intervention components have been discussed in detail elsewhere [13, 14, 18].
Control condition
Women randomized to the control group participated in a 1-day psychoeducational “self-help” classroom seminar within the corresponding 10-week intervention period. Participants were provided with general information about breast cancer care and health. A condensed version of select portions of the CBSM modules was provided in handouts, but women were not given opportunities to practice these techniques.
Outcomes
Three clinical outcomes of interest were examined at follow-up. Time to all-cause mortality was computed as days elapsed from date of randomization to death. Time to breast cancer-specific mortality was computed as days elapsed from date of randomization to breast cancer-related death. Disease-free interval was computed as days elapsed from date of randomization to documented breast cancer recurrence (local or distant recurrence).
Statistical analysis
Time-to-event analyses were conducted in the Statistical Package for the Social Sciences (Version 21.0) and Statistical Analysis Software (Version 9.3). Chi-square tests, Fisher’s exact test, and one-way ANOVAs were conducted to determine baseline group differences. Cox Proportional Hazards Models [19] were conducted to test group differences in time to all-cause and breast cancer mortality at 8–15 year follow-up. The Proportional Hazards Assumption was met for all regressions [20]. Estimates for hazard ratios (including 95 % confidence intervals) were declared significant based on a two-sided alpha of 0.05. Data were censored for women who did not have a death or recurrence at the time of follow-up, were lost-to follow-up, or had previously dropped out, using the date of last study contact. For seven women who had unknown breast cancer recurrence dates, data on time to recurrence was interval censored between randomization and end of study/death. Weibull accelerated failure time (AFT) models [21] using interval censoring were conducted to estimate group difference in disease-free survival time.
All models examined the effects of group assignment over and above the effects of biomedical confounders. Covariate relationships were examined to limit number of covariates and avoid model overfitting. Prognostic and treatment factors known to influence disease endpoints were chosen apriori and included in addition to variables significantly associated with outcomes [22]. The final covariates—age at diagnosis, disease stage, tumor size, Her2/neu status, and hormonal treatment received—were manually entered into the regression model (rather than automated stepwise methods [22]). Due to a high correlation between hormonal treatment and ER/PR receptor status, hormonal treatment was chosen as a covariate to limit the number of covariates. Disease stage was determined with AJCC/UICC TNM grouping [23] and was categorized as stage 0 vs. I, II, and III. The assumption of linearity was met for all continuous variables except tumor size, which was subsequently classified as a categorical variable according to AJCC/UICC TNM grouping [23] Information on tumor grade was not available. Results were verified by independent replication analysis conducted by an NIH statistical consultant, Westat. Inclusion of appropriate study covariates and use of Weibull AFT was determined amongst authors, the NCI Network of Biobehavioral Pathways in Cancer committee, and Westat consultants.
Results
Participant characteristics
At the time of diagnosis and enrollment, women were an average of 50 (SD = 9.03) years old. Approximately 36 % self-reported being of a racial or ethnic minority (e.g., Black, Hispanic, Asian). See Table 1.
Table 1.
Means, standard deviations, and frequencies of demographic, medical, and treatment variables by study group
| Variable | n | Control | Intervention | p |
|---|---|---|---|---|
| Age at diagnosis (years) | 240 | 50.99 (9.06) | 49.69 (8.98) | .27 |
| Race/ethnicity | 239 | .64 | ||
| White non-Hispanic | 74 (61.7 %) | 78 (65.0 %) | ||
| Hispanic | 31 (26.1 %) | 30 (25.0 %) | ||
| African American | 10 (8.4 %) | 11 (9.2 %) | ||
| Asian | 4 (3.4 %) | 1 (.8 %) | ||
| Employment status | 240 | .38 | ||
| Not employed | 28 (23.3 %) | 34 (28.3 %) | ||
| Employed | 92 (76.7 %) | 86 (71.7 %) | ||
| Education (years) | 15.47 (2.26) | 15.69 (2.5) | .47 | |
| Income (thousands of dollars) | 213 | 78.85 (68.27) | 80.45 (66.11) | .86 |
| Partnered status | 240 | 1.00 | ||
| Not partnered | 45 (37.5 %) | 45 (37.5 %) | ||
| Partnered | 75 (62.5 %) | 75 (62.5 %) | ||
| Menopausal status | 240 | .90 | ||
| Premenopausal | 53 (44.2 %) | 54 (45.0 %) | ||
| Postmenopausal | 67 (55.8 %) | 66 (55.0 %) | ||
| Stage | 239 | .48 | ||
| 0 | 24 (20.0 %) | 18 (15.1 %) | ||
| I | 44 (36.7 %) | 39 (32.8 %) | ||
| II | 43 (35.8 %) | 48 (40.3 %) | ||
| III | 9 (7.5 %) | 14 (11.8 %) | ||
| Early vs. invasive stage | 239 | .41 | ||
| 0 | 23 (19.2 %) | 18 (15.0 %) | ||
| I, II, III | 97 (80.8 %) | 101 (84.2 %) | ||
| Positive lymph nodes | 1.56 (3.60) | 1.45 (2.97) | .79 | |
| Size of tumor | 122 | 1.65 (1.14) | 3.76 (12.93) | .19 |
| Size of tumor (log-transformed) | 122 | 0.23 (0.81) | 0.55 (0.90) | .04 |
| ER status | 198 | .17 | ||
| Positive | 78 (83.0 %) | 78 (75.0 %) | ||
| Negative | 16 (17.0 %) | 26 (25.0 %) | ||
| PR status | 178 | .75 | ||
| Positive | 55 (64.7 %) | 58 (62.4 %) | ||
| Negative | 30 (35.3 %) | 35 (37.6 %) | ||
| HER2/neu status | 119 | .24 | ||
| Positive | 10 (17.2 %) | 16 (26.2 %) | ||
| Negative | 48 (82.8 %) | 45 (73.8 %) | ||
| Procedure type | 240 | .07 | ||
| Lumpectomy | 68 (56.7 %) | 54 (45.0 %) | ||
| Mastectomy | 52 (43.3 %) | 66 (55.0 %) | ||
| Received chemotherapy | 230 | .06 | ||
| Yes | 57 (49.1 %) | 70 (61.4 %) | ||
| No | 59 (50.9 %) | 44 (38.6 %) | ||
| Received radiation therapy | 226 | .59 | ||
| Yes | 69 (61.1 %) | 65 (57.5 %) | ||
| No | 44 (38.9 %) | 48 (42.5 %) | ||
| Received endocrine therapy | 228 | .35 | ||
| Yes | 78 (67.8 %) | 83 (73.5 %) | ||
| No | 37 (32.2 %) | 30 (26.5 %) | ||
| Body mass index (BMI) | 134 | 26.60 (6.16) | 26.63 (5.26) | .97 |
| BMI categories | 134 | .30a | ||
| Underweight | 0 (0.0 %) | 1 (1.6 %) | ||
| Normal | 33 (45.25 %) | 30 (49.2 %) | ||
| Overweight | 28 (38.4 %) | 16 (26.2 %) | ||
| Obese | 12 (16.4 %) | 14 (23.0 %) |
ER estrogen receptor, PR progesterone receptor, HER2/neu human epidermal growth factor receptor
At the 8–15 year follow-up, 30 (12.5 %) of the 240 participants were deceased (CBSM = 15; Control = 15). The average number of years from study enrollment to death was 7.60 (SD = 3.71). Of these 30 deaths, 22 were breast cancer-related (CBSM = 12; Control = 10). For eight women whose death was not related to breast cancer, causes were as follows: unknown (N = 4), Alzheimer’s disease (N = 1), malignant neoplasm without site specification (N = 1), non-traumatic subarachnoid hemorrhage (N = 1), and ovarian cancer (N = 1). There were 150 with no breast cancer recurrence, 47 had a local or distant breast cancer recurrence (CBSM = 24; Control = 23), 39 were lost-to follow-up, and 4 were deceased with unknown breast cancer status. The average disease-free interval was 5.92 (SD = 3.91) years. The effective sample size in specific analyses varied as a function of the cases with available covariate information (Table 1).
Adjusted Cox proportional hazards models
All-cause mortality
Cox proportional hazards models determined differences between CBSM and control groups on time to all-cause mortality adjusting for age, disease stage, tumor size, Her2/neu status, and hormonal therapy receipt. Women older at diagnosis had longer survival (p = .025), and those who received hormonal therapy had longer survival (p = .045). Over and above the effects of covariates, assignment to CBSM was associated with longer survival (CBSM HR = 0.21 (95 % CI [0.05, 0.93]; p = .040; see Fig. 2). See Table 2 for hazard ratios.
Fig. 2.
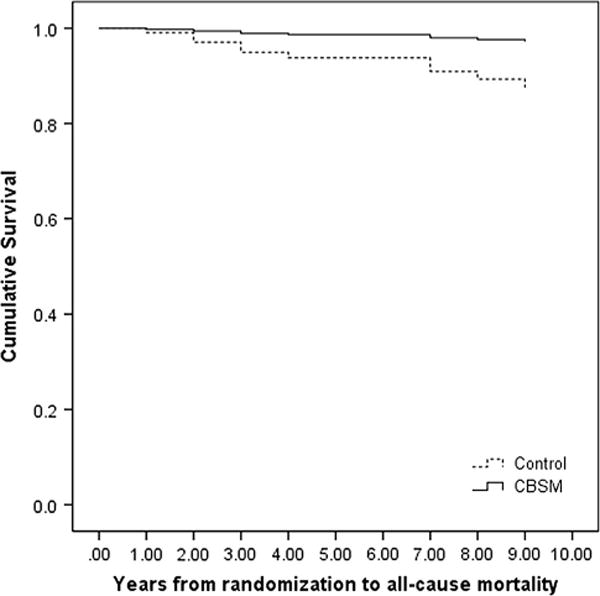
Overall survival difference in study groups. Differences between (CBSM vs. control) with Cox proportional hazards models on time to all-cause mortality controlling for covariates: age, stage of disease, HER2/neu, endocrine therapy, and tumor size
Table 2.
Intervention effects on clinical outcomes at 11-year (median) follow-up: multivariate Cox proportional hazards regressions and Weibull accelerated failure time models (N = 240)
| Variable | All-cause mortalityb
|
Breast cancer-specific mortalityb
|
Breast cancer recurrencec
|
|||
|---|---|---|---|---|---|---|
| HR (95 % CI) | p | HR (95 % CI) | p | HR (95 % CI) | p | |
| Study condition (CBSM) | 0.21 (0.05–0.93) | .040 | 0.25 (0.05–1.11) | .068 | 0.45 (0.17–1.18) | .083 |
| Age at diagnosis | 0.91 (0.84–0.99) | .025 | 0.91 (0.83–0.99) | .025 | 0.94 (0.89–1.00) | .023 |
| Her2/neu (positive) | 2.12 (0.53–8.33) | .288 | 1.70 (0.39–7.41) | .481 | 1.85 (0.70–4.91) | .199 |
| Tumor size | ||||||
| >T2 | 3.47 (0.40–30.13) | .259 | 4.81 (0.55–42.11) | .156 | 4.65 (0.85–25.43) | .057 |
| T1c | 1.79 (0.18–17.86) | .619 | 2.34 (0.23–23.89) | .474 | 2.89 (0.54–15.38) | .195 |
| <T1c | – | .444 | – | .306 | – | – |
| Endocrine therapy (yes) | 0.25 (0.06–0.97) | .045 | 0.29 (0.07–1.28) | .102 | 0.46 (0.16–1.29) | .121 |
| Stage (invasive) | 0.45 (0.03–7.60) | .578 | –a | –a | 0.64 (0.05–7.73) | .723 |
HR hazard ratio, 95 % CI 95 % confidence interval, HER2/neu human epidermal growth receptor, CBSM cognitive-behavioral stress management
Stage of disease not included in this analysis due to large standard error
Analyzed with Cox proportional hazards models
Analyzed with Weibull accelerated failure time models
Breast cancer-specific mortality
Cox proportional hazards models determined differences between CBSM and control groups on time to breast cancer-specific mortality adjusting for age, tumor size, Her2/neu status, and hormonal therapy receipt. Disease stage was removed from the model due to a large standard error that led to invalid statistical inferences. Women older at time of diagnosis had longer survival (p = .025). Assignment to CBSM tended to be associated with breast cancer survival over and above the effects of covariates (CBSM HR = 0.25 (95 % CI [0.05, 1.11]; p = .068; see Fig. 3). See Table 2 for hazard ratios.
Fig. 3.

Breast cancer-specific survival difference in study groups. Differences between study groups (CBSM vs. control) with Cox proportional hazards models on time to breast cancer-specific mortality controlling for covariates: age, HER2/neu, endocrine therapy, and tumor size
Breast cancer recurrence
Weibull AFT models were conducted to determine differences between CBSM and control groups on disease-free interval (time to breast cancer recurrence). Older age at diagnosis was associated with longer disease-free interval. There was a tendency for assignment to CBSM to be associated with a greater disease-free interval time beyond covariate effects (CBSM HR = 0.45; 95 % CI [0.17, 1.18]; p = .083; Fig. 4). See Table 2 for hazard ratios.
Fig. 4.
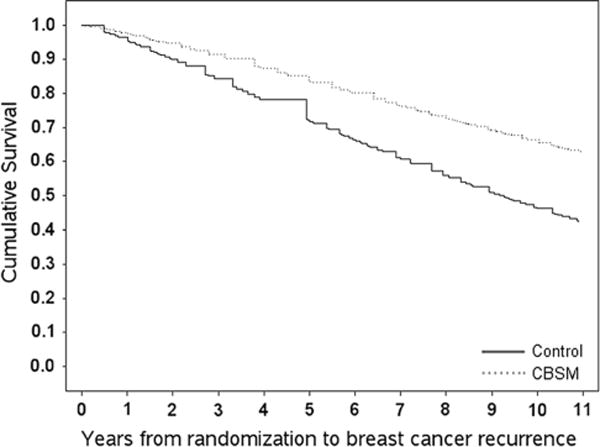
Disease-free interval difference in study groups. Differences between study groups (CBSM vs. control) with Weibull accelerated failure time models on disease-free interval controlling for covariates: age, stage of disease, HER2/neu, endocrine therapy, and tumor size. “Cumulative Survival” indicates disease-free interval
Effects in invasive cancer subsample
Because prior psychological intervention studies of survival and recurrence effects have restricted their samples to women with invasive cancer [8, 10] we reanalyzed our data for the 197 cases with Stage I–IIIb breast cancer. Once accounting for covariates in this subsample, the only cause of death was breast cancer; therefore, all-cause mortality was not included as an outcome. The average number of years from study enrollment to breast cancer-related death was 7.56 (SD = 3.76), and the average disease-free interval was 5.90 (SD = 3.96) years. Using the covariates noted previously, we found that participating in CBSM was associated with lower odds of breast cancer mortality (CBSM HR = 0.08; 95 % CI [0.01, 0.49]; p = .006) and greater disease-free interval (CBSM HR = 0.24; 95 % CI [0.07, 0.82]; p = .011). See Table 3 for hazard ratios.
Table 3.
Intervention effects on clinical outcomes at 11-year (median) follow-up multivariate Cox proportional hazards regressions and Weibull accelerated failure time model: invasive tumor subsample only (N = 197)
| Variable | Breast cancer-specific mortalitya
|
Breast cancer recurrenceb
|
||
|---|---|---|---|---|
| HR (95 % CI) | p | HR (95 % CI) | p | |
| Study condition (CBSM) | 0.08 (0.01–0.49) | .006 | 0.24 (0.07–0.82) | .011 |
| Age at diagnosis | 0.88 (0.79–0.97) | .010 | 0.94 (0.88–1.00) | .024 |
| Her2/neu (positive) | 4.33 (0.81–23.21) | .087 | 2.92 (0.97–8.77) | .039 |
| Tumor size | ||||
| >T2 | 7.47 (0.64–87.57) | .109 | 4.61 (0.82–25.82) | .062 |
| T1c | 3.61 (0.29–45.71) | .321 | 2.89 (0.67–22.18) | .111 |
| <T1c | – | .266 | – | – |
| Endocrine therapy (yes) | 0.28 (0.06–1.24) | .093 | 0.49 (0.17–1.43) | .174 |
| Stage (III vs. I and II) | 23.46 (3.65–150.62) | .001 | 4.03 (1.05–15.46) | .026 |
HR hazard ratio, 95 % CI 95 % confidence interval, HER2/neu human epidermal growth receptor, CBSM cognitive-behavioral stress management
Analyzed with Cox proportional hazards models
Analyzed with Weibull accelerated failure time models
Discussion
This secondary analysis found that women with Stage 0–IIIb breast cancer who were randomly assigned to a 10-week CBSM intervention 2–10 weeks post-surgery had longer survival, up to 11-years post-enrollment, compared to those in the control group, while accounting for disease- relevant characteristics.
These findings are consistent with that of Spiegel et al. [8] and Andersen et al. [10]. Our findings are particularly relevant given the controversial evidence regarding the influence of psychosocial interventions on cancer disease outcomes [9]. It is important to note that most studies, except Andersen et al. [10] investigated these associations in metastatic breast cancer samples. Importantly, there are notable commonalities among the current study, and those of Andersen et al. [10] and Spiegel et al. [8]. These three study interventions emphasized skills around stress management, coping, and symptom management. Interestingly, when we restricted our analyses to include only those diagnosed with invasive disease (Stage I–IIIb), a sample that is more comparable to that of Spiegel et al. [8] and Andersen et al. [10] we found significant intervention effects on breast cancer mortality and disease-free interval, independent of potential confounding factors. This is the first study to replicate the Andersen et al. [10] findings suggesting that psychosocial intervention administered post-surgery for non-metastatic breast cancer is associated with improved survival.
The findings from this sample of non-metastatic breast cancer patients highlight the potential for psychosocial interventions to influence disease outcomes in a non- metastatic cancer population. They suggest there may be opportunity to modify psychosocial factors in a way that reduces the risk of metastases before they begin or slows progression. There are multiple pathways by which a psychosocial intervention may influence disease outcomes. It is possible that the short- [13, 14] and long-term [15, 16] psychological improvements from the CBSM intervention mediated the effects on survival. CBSM decreases anxiety and depressive mood the first year of primary treatment, which parallel decreases in leukocyte pro-inflammatory and pro-metastatic gene expression over this period [12]. CBSM increases confidence in stress management skills such as relaxation and cognitive reframing in breast cancer patients [14, 24] which covary with parallel decreases in neuroendocrines, such as serum cortisol [11]. Women may continue to engage in relaxation techniques post-treatment, which could lower distress and circulating neuroendocrines mitigating inflammatory and metastatic processes via stress response pathways [5].
Skills-based aspects of the CBSM teach women cognitive restructuring to manage cancer-specific distress around fears of recurrence that persist post-treatment and cause ongoing emotional distress [4]. Components of CBSM address adaptive coping techniques and re-appraisals of harm and loss that contribute to depressive symptoms and poor QOL in breast cancer patients and survivors [25]. The effects of CBSM on depressive symptoms may be particularly important, given the strong association between depression and breast cancer survival [6, 7, 26]. Depression is an established risk factor for noncompliance with medical treatment [26]. Treatment noncompliance with long- term regimens such as endocrine therapy, and noncompliance with follow-up visits, in turn, may explain poorer clinical outcomes in breast cancer [6, 7]. Finally, group social dynamics may have influenced women’s participation in behaviors that increase or decrease cancer risk, such as alcohol consumption, physical activity, and diet [27].
Strengths and limitations
Results should be interpreted with caution. Survival and recurrence were not primary endpoints in this study at the time it was planned. Other limitations include a small sample size, a low number of observed deaths, and missing data. As this was a sample of women with non-metastatic breast cancer, only 12.5 % of the sample had died by the follow-up. While generalizability is increased by the fact that approximately one-third of the sample was of an ethnic minority (i.e., Black, Hispanic, Asian), it is limited by factors such as academic study setting, geographical location, and inclusion criteria. Given the recruitment strategies and stringent inclusion criteria, the findings may be more applicable to intervention-seeking patients, and those that are relatively mentally and physically healthy.
The number of covariates used in our analyses was high in relation to the number of clinical events [22]. However, the current study used an a priori list of covariates that was theoretically based and manually entered into the model to minimize overfitting [22]. We initially chose to conduct analyses on the original full sample of 240 women, given evidence that women with Stage 0 breast cancer report similar psychological distress when compared to women with later stages of disease [3]. However, the fact that women with Stage 0 are less likely to progress and recur should be noted when interpreting the results. The fact that CBSM effects were stronger in the subsample of women diagnosed with invasive disease suggests that future research should focus on examining intervention effects on clinical outcomes in women with invasive tumors. The use of a structured, manualized intervention [18] increases feasibility, implementation, and ease of future replication.
Clinical relevance
This study provides preliminary evidence that a stress management group intervention modifying psychological adaptation early on in treatment may have lasting effects over the course of the disease for women with breast cancer. Within the context of a biopsychosocial, multidisciplinary model of care, CBSM is a group-based, manualized, feasible intervention that can be implemented in clinical oncology settings and may provide women an opportunity to reap long-term health benefits in addition to improved QOL and less depressive symptoms.
Future research directions
Additional studies should evaluate long-term effects and underlying mechanisms of cognitive-behavioral interventions on clinical disease outcomes of survival and recurrence in non-metastatic breast cancer patients. This is an area in need of further exploration with clinical endpoints as primary outcomes and more rigorous study designs. Research should address whether intervention-related changes in neuroendocrine, immune, inflammatory, and other tumor-promoting processes [11, 12] mediate effects of CBSM on survival [26]. Future research should also examine whether improved adherence to long-term endocrine regimens or changes in health behaviors may be explanatory mechanisms [27, 28]. Because the effects of CBSM reported here may have been diluted by including both distressed and non-distressed patients, future research could screen and pre-select patients based on clinical mood/distress symptoms [29]. Future research should also investigate the effectiveness of CBSM in venues including oncology clinics and remote platforms, in order to reach the broadest number of patients.
Conclusions
With notable limitations, women with non-metastatic breast cancer who receive CBSM intervention 2–10 weeks post-surgery showed improved survival compared to women in a 1-day group psychoeducational control condition at an 11-year median follow-up. In a subsample of women with invasive disease, breast cancer-specific survival and disease-free survival was improved for those in CBSM. This research contributes evidence for the effects of psychosocial interventions on clinical health outcomes in breast cancer patients, and may have implications for clinical practice.
Acknowledgments
MA, SL, and CC were responsible for the original study conception and design. JS, MA, CC, and LB were responsible for the follow-up study conception and design. JS and MA obtained funding. JS, LB, LG, and DJ collected the data and provided administrative support. AD, BB, SL, GI, SG, and CC provided technical and material support. JS, MA, and QY conducted the statistical analysis. JS, LB, LG, DJ, AD, BB, SL, GI, SG, CC, QY, and MA interpreted the data. JS, LB, LG, DJ, SL, AD, BB, GI, SG, CC, QY, and MA drafted and reviewed the manuscript. JS, CC, SG, SL, GI, and MA provided critical revision of the manuscript for important intellectual content. We are grateful to all of the women who participated in this study. We are appreciative for study coordination efforts from Stephanie Montarroyos, Janny Rodriguez, and the NCI Network of Biobehavioral Pathways in Cancer Steering Committee.
Funding This project has been funded in whole or in part with Federal funds from the National Cancer Institute (NCI), National Institutes of Health, under Contract No. HHSN261200800001E and NCI grant R01-CA-064710. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The NCI Network of Biobehavioral Pathways in Cancer (under Contract No. HHSN261200800001E) was involved in the design of the study, provided funding for data collection, provided statistical support for data analysis and interpretation through Westat, reviewed and approved the manuscript and the decision to submit the manuscript for publication. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Compliance with ethical standards
Conflict of interest Dr. Antoni reports receiving publication royalties from a book he co-authored on cognitive-behavioral stress management. Dr. Glück is employed at Celgene Corporation. Other co-authors declare that they have no conflicts of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.11. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr, accessed 17 Sept 2014. [Google Scholar]
- 2.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9:730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 3.Lauzier S, Maunsell E, Levesque P, et al. Psychological distress and physical health in the year after diagnosis of DCIS or invasive breast cancer. Breast Cancer Res Treat. 2010;120:685–691. doi: 10.1007/s10549-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 4.Herschbach P, Keller M, Knight L, et al. Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;91:504–511. doi: 10.1038/sj.bjc.6601986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jorgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44:24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Giese-Davis J, Collie K, Rancourt KMS, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa M, Carson-Stevens A, Gillespie D, Edwards AG. Psychological interventions for women with metastatic breast cancer. Cochrane Db Syst Rev. 2013;6:CD004253. doi: 10.1002/14651858.CD004253.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen BL, Yang H, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips KM, Antoni MH, Carver CS, et al. Stress management skills and reductions in serum cortisol across the year after surgery for non-metastatic breast cancer. Cogn Therapy Res. 2011;35:595–600. [Google Scholar]
- 12.Antoni MH, Lutgendorf SK, Blomberg B, et al. Cognitive- behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoni MH, Wimberly SR, Lechner SC, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. The Am J Psychiatry. 2006;163:1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoni MH, Lechner SC, Kazi A, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagl JM, Antoni MH, Lechner SC, et al. Randomized controlled trial of cognitive behavioral stress management in breast cancer: a brief report of effects on 5-year depressive symptoms. Health Psychol. 2015;34:176–180. doi: 10.1037/hea0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up on a randomized controlled trial. Cancer. 2015;121:1873–1881. doi: 10.1002/cncr.29076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas S, Antoni MH, Carver CS, et al. Sleep quality and fatigue after a stress management intervention for women with early-stage breast cancer in Southern Florida. Int J Behav Med. 2014;21:971–981. doi: 10.1007/s12529-013-9374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoni MH. Stress management for women with breast cancer. American Psychological Association Press; Washington, DC: 2003. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc B Met. 1972;34:187–220. [Google Scholar]
- 20.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part IV: further concepts and methods in survival analysis. Br J Cancer. 2003;89:781–786. doi: 10.1038/sj.bjc.6601117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson KM. A nonproportional hazards Weibull accelerated failure time regression model. Biometrics. 1991;47:281–288. [PubMed] [Google Scholar]
- 22.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 23.Sobin L, Gospodarowicz M, Wittekind C, et al. TNM classification of malignant tumors. 7th. Wiley; Hoboken, NJ: 2009. [Google Scholar]
- 24.Phillips KM, Jim HSL, Small BJ, Tanvetyanon T, Roberts WS, Jacobsen PB. Effects of self-directed stress management training and home-based exercise on stress management skills in cancer patients receiving chemotherapy. Stress Health. 2012;28:368–375. doi: 10.1002/smi.2450. [DOI] [PubMed] [Google Scholar]
- 25.Bigatti SM, Steiner JL, Miller KD. Cognitive appraisals, coping and depressive symptoms in breast cancer patients. Stress Health. 2012;28:355–361. doi: 10.1002/smi.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissane D. Beyond the psychotherapy and survival debate: the challenge of social disparity, depression and treatment adherence in psychosocial cancer care. PsychoOncol. 2009;18:1–5. doi: 10.1002/pon.1493. [DOI] [PubMed] [Google Scholar]
- 27.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 28.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross L, Boesen EH, Dalton SO, Johansen C. Mind and cancer: does psychosocial intervention improve survival and psychological well-being? Eur J Cancer. 2002;38:1447–1457. doi: 10.1016/s0959-8049(02)00126-0. [DOI] [PubMed] [Google Scholar]


