Abstract
Purpose
Diet may influence the development of ovarian cancer. While inflammation has been shown to play an important etiologic role on ovarian carcinogenesis, little is known about the influence of the inflammatory potential of food consumption.
Methods
Data from a case-control study conducted in New Jersey (USA) were used to estimate the relation between a dietary inflammatory index (DII) and the risk of ovarian cancer. The study consisted of 205 cases with incident, histologically confirmed ovarian cancer, and 390 controls identified by random digit dialing, based on CMS (Centers for Medicare and Medicaid Service) lists, and area sampling. Computation of the DII was based on the intake of selected dietary factors assessed by a validated Food Frequency Questionnaire (FFQ). Logistic regression models were fit to estimate odds ratios (ORs) and 95% confidence intervals (CI) adjusted for potential covariates.
Results
Although there was no significant association observed in pre and peri-menopausal women, a significant association was observed between the most pro-inflammatory DII scores and ovarian cancer among post-menopausal women (ORQuartile4vs1=1.89, 95 % CI, 1.02–3.52; Ptrend=0.03).
Conclusion
Our finding suggests that a pro-inflammatory diet may increase ovarian cancer risk among post-menopausal women, and warrants further study to confirm this association.
Keywords: diet, inflammatory index, ovarian cancer
INTRODUCTION
Among gynecological cancers ovarian cancer has the highest mortality rates, with dismal five-year survival rates (46% for all stages combined; 28% for advanced stage, in which 62% of the cases are diagnosed) [1]. The American Cancer Society estimates 22,440 new cases and 14,080 deaths from ovarian cancer in the United States in 2017 [1]. Risk factors for ovarian cancer include increasing age, family history of the disease (specifically mutations in BRCA1 and BRCA2 genes), obesity and nulliparity, while oral contraceptive use, higher parity, and tubal ligation have been shown to reduce risk [2,3]. Several studies have been conducted exploring the association between dietary factors and ovarian cancer with inconsistent results [4]. While there is growing evidence linking inflammation to ovarian carcinogenesis [5,6], to date there have been only two studies that have explored the role that inflammatory potential of diet plays in ovarian cancer risk [7,8]; one of them was conducted exclusively among African American women [7] and the other was among Italian women [8].
A literature-derived, population-based dietary inflammatory index (DII) was recently developed to assess the inflammatory potential of an individual’s diet [9]. A pro-inflammatory diet is high in foods rich in saturated fat and carbohydrates, and low in foods rich in poly-unsaturated fatty acids, flavonoids, and other dietary components, include a variety of vitamins and minerals [10]. The DII has been validated in a variety of longitudinal and cross-sectional studies with various inflammatory markers, including C-reactive protein [11,12], interleukin-6 [13,14], and tumor necrosis factor-α [14]. The DII has been associated with risk of colorectal cancer in case-control studies in Spain and Italy [15,16] and in 3 cohort studies in the USA [10,17,18], and risk of pancreatic, prostate and endometrial cancers in case-control studies in Italy [19–22]. In this study we evaluate the impact of a pro-inflammatory diet, as indicated by a high DII on ovarian cancer risk in a New Jersey population.
METHODS
We evaluated the association between DII and ovarian cancer in the NJ Ovarian Cancer Study, described in detail elsewhere [23–26]. In brief, our study included 205 newly diagnosed, histologically confirmed cases of invasive epithelial ovarian cancer identified through rapid case ascertainment implemented by the New Jersey State Cancer Registry (NJSCR) staff. Women older than 21 years, able to understand English or Spanish, and residing in one of six New Jersey counties (Bergen, Essex, Hudson, Middlesex, Morris, and Union) were eligible to participate. Controls (n=390) had the same eligibility criteria as the cases except that women with a history of hysterectomy and/or bilateral oophorectomy were excluded from the analysis. Controls were identified through random digit dialing for women <65 years of age and through random selection of Center for Medicare and Medicaid Services lists, complemented with area sampling for women ≥65 years of age.
After obtaining informed consent, a telephone interview was scheduled, during which information was collected on established and suspected risk factors for ovarian cancer as well as on demographic characteristics. Dietary data were collected using the Block 98.2 Food Frequency Questionnaire (FFQ), which included questions about usual intake during six months before diagnosis for cases or on the date of interview for controls. The Block 98.2 FFQ (NutritionQuest, Berkeley, CA) was developed from the National Health and Nutrition Examination Survey III dietary recall data includes 110 food and beverage items and queries on frequency and portion size for each item. Pictures were provided to enhance accuracy of estimation of portion size.
Dietary Inflammatory Index (DII)
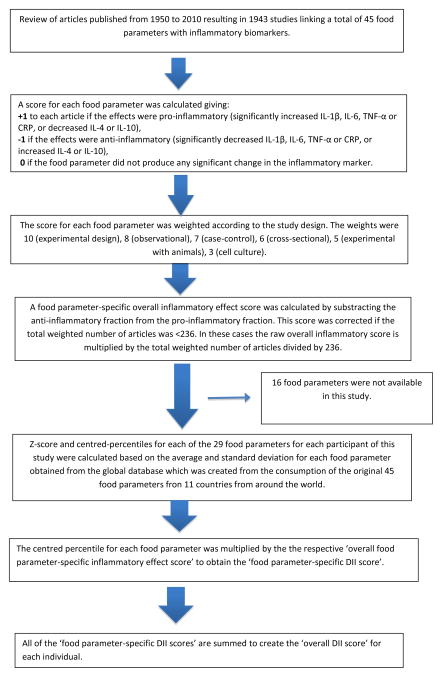
FFQ-derived dietary data were used to calculate the DII for each subject. A complete description of the DII is available elsewhere [9]. Briefly, dietary data were first linked to a regionally representative global database that provided a robust estimate of the mean and the standard deviation for each food parameter included in the DII. These parameters then became the multipliers to express an individual’s exposure relative to the “standard global mean” as a z-score. This was achieved by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing,” this value was then converted to a centered (on zero) percentile score (by taking the percentile ranking of the z-score, multiplying by 2 and subtracting 1). The centered percentile score for each food parameter for each subject was then multiplied by the corresponding food parameter effect score in order to obtain a food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for each subject. The DII was calculated from foods and supplements. To control for total energy intake, the DII was calculated per 1,000 calories of food consumed, which requires using the energy-standardized version of the global database. This study had data on 29 of the 45 food parameters studied for DII development; food parameters that are available and that are unavailable in this study are shown in Appendix 1. Steps involved in calculating the DII score are described in Figure 1.
Figure 1.
Sequence of steps in creating the dietary inflammatory index in the New Jersey Ovarian cancer case-control study
Statistical Analyses
DII scores were analyzed by quartiles of exposure in controls. Body mass index (BMI) was calculated as weight (in kg) divided by height (in meters) squared and was categorized as: underweight (<18.5 kg/m2); normal weight (BMI 18.5– 24.9 kg/m2); overweight (25.0 kg/m2 ≤ BMI <30.0 kg/m2); and obese (BMI ≥ 30.0 kg/m2). Age-adjusted means were calculated for cases and controls for pro-inflammatory food parameters (protein, saturated fat, cholesterol and carbohydrates) and anti-inflammatory food parameters (vitamin B1, Niacin, Folate, Vitamin C and dietary fiber) and compared using analysis of covariance. Odds ratios (OR) and the corresponding 95% confidence intervals (95% CI) were estimated using logistic regression models, adjusting only for age as a continuous variable and additionally adjusting for education, race, age at menarche, menopausal status, parity, oral contraceptive use, hormone therapy use, tubal ligation, BMI categories, physical activity (in metabolic equivalents (or METs) for reported average hours per week of strenuous or moderate recreational activities), and smoking status. Effect modification by menopausal status and BMI categories was evaluated. Testing for heterogeneity was carried out by including the interaction terms in the model. Tests for trend were computed by assigning the median value to each quartile. All analyses were completed using SAS® version 9.3 (SAS Institute, Cary NC).
RESULTS
Participants in the New Jersey Ovarian Cancer Study were primarily White (87.3% of cases and 88.4% of controls) and approximately 25% of cases and controls had a graduate school education [23] (data not shown). Mean values of selected pro- and anti-inflammatory food parameters for cases and controls are shown in Table 1. Mean DII value among cases was 1.1 (SD= ±0.2) and among controls was 0.8 (SD=±0.1) indicating a slightly more pro-inflammatory diet for cases (p=0.18). For pro-inflammatory food parameters, cases had slightly higher intakes of saturated fat and carbohydrates and for anti-inflammatory food parameters, cases had significantly lower intakes of niacin and slightly lower levels of vitamin B1, folate, vitamin C and dietary fiber compared to controls.
Table 1.
Means for cases and controls for the Dietary Inflammatory Index (DII) and some of its components
| Variablea | Cases (n=205) | Controls (n=390) | P-value |
|---|---|---|---|
|
| |||
| Mean (SE) | Mean (SE) | ||
| DII | 1.1 (0.2) | 0.8 (0.1) | 0.18 |
| Pro-inflammatory food parametersb | |||
| Protein (g) | 41.3 (2.23) | 42.5 (3.5) | 0.27 |
| Saturated fat (g) | 13.2 (1.82) | 12.9 (1.34) | 0.72 |
| Cholesterol (mg) | 114.9 (3.6) | 115.0 (2.6) | 0.98 |
| Carbohydrates(g) | 122.6 (1.7) | 119.8 (1.2) | 0.17 |
| Anti-inflammatory food parametersc | |||
| Vitamin B1 (mg) | 0.78 (0.01) | 0.80 (0.01) | 0.26 |
| Niacin (mg) | 10.8 (0.20) | 11.5 (0.14) | 0.006 |
| Folate (mcg) | 221.5 (4.5) | 222.5 (3.2) | 0.86 |
| Vitamin C (mg) | 77.8 (3.0) | 78.9 (2.1) | 0.76 |
| Dietary fiber (g) | 9.9 (0.30) | 10.4 (0.2) | 0.16 |
Density measure calculated as daily intake in respective units per 1,000 kcal
As indicated by the positive inflammatory effect scores in the DII development manuscript (38)
As indicated by the negative inflammatory effect scores in the DII development manuscript (38)
OR and 95% CI of ovarian cancer according to quartiles of DII are shown in Table 2. No significant associations were observed between DII and overall ovarian cancer (i.e., across all ages). In age-adjusted models results suggestive of a positive association were observed for DII with ovarian cancer (ORQuartile4vs 1= 1.38, CI= 0.85–2.26, Ptrend=0.27). Similarly, for multivariable analyses, suggestive positive associations were observed, with ORQuartile4vs 1 of 1.39 (95% CI=0.82–2.35, Ptrend=0.26). When stratified by menopausal status, a significant association was observed among post-menopausal women consuming the most pro-inflammatory diet (ORQuartile4vs1=1.89, 95 % CI, 1.02–3.52; Ptrend=0.03) (Table 3). P-value for interaction was nearly significant with menopausal status (P-value=0.08). In analyses stratified by BMI, the association appeared to be stronger in overweight and obese women, but the confidence interval for both the categories included the null (p for interaction=0.46).
Table 2.
Odds ratios (OR) of ovarian cancer and corresponding 95% confidence intervals (CI) for quartiles of DII among 205 cases and 390 controls. New Jersey, 2004–2008.
| DII quartiles | Ptrend | ||||
|---|---|---|---|---|---|
|
| |||||
| < −1.28 | −1.28–0.68 | 0.69–2.24 | >2.24 | ||
| Cases/Controls | 53/98 | 44/97 | 41/98 | 67/97 | |
| Model 1b | 1 a | 0.84 (0.50, 1.41) | 0.71 (0.42, 1.20) | 1.38 (0.85, 2.26) | 0.27 |
| Model 2c | 1 a | 0.86 (0.49, 1.49) | 0.83 (0.47, 1.46) | 1.39 (0.82, 2.35) | 0.26 |
Reference category.
Model 1 adjusted for age
Model 2 adjusted for age (continuous), education (high school or less, college, graduate school), race (White, Black, Other, Hispanic), age at menarche (continuous), menopausal status (pre-/peri-menopausal, postmenopausal), parity (0–1, 2, 3–4), oral contraceptive use (ever, never), HT use (never, unopposed estrogen only, any combined HT), tubal ligation (no, yes), BMI (continuous), smoking status (never, past, current).
Table 3.
Odds ratios (OR) of ovarian cancer and corresponding 95% confidence intervals (CI) for quartiles of DII among 205 cases and 390 controls. New Jersey, 2004–2008.
| Cases/Controls | DII quartilesa | Ptrend | Pinteraction | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| < −1.28 | −1.28,0.68 | 0.69,2.24 | >2.24 | ||||
| Menopausal status | |||||||
| Pre/Peri | 71/49 | 1b | 0.62 (0.14, 2.67) | 0.12 (0.03, 0.58) | 0.74 (0.19, 2.84) | 0.41 | |
| Post- | 134/338 | 1b | 0.91 (0.47, 1.75) | 1.30 (0.68, 2.52) | 1.89 (1.02, 3.52) | 0.03 | 0.08 |
| BMI (kg/m2) | |||||||
| <25 | 91/180 | 1b | 1.29 (0.60, 2.81) | 1.11 (0.47, 2.60) | 1.29 (0.58, 2.86) | 0.60 | |
| ≥25 | 112/203 | 1b | 0.57 (0.24, 1.31) | 0.61 (0.27, 1.37) | 1.60 (0.75, 1.37) | 0.19 | 0.46 |
Model adjusted for age (continuous), education (high school or less, college, graduate school), race (White, Black, Other, Hispanic), age at menarche (continuous), menopausal status (premenopausal, postmenopausal), parity (0–1, 2, 3–4), oral contraceptive use (ever, never), HT use (never, unopposed estrogen only, any combined HT), tubal ligation (no, yes), BMI (continuous), smoking status (never, past, current).
Reference category.
DISCUSSION
In this case-control study conducted in New Jersey, we found some evidence of elevated risk associated with higher DII only among postmenopausal women. No association with ovarian cancer was found in earlier reports in the same case-control study with the Healthy Eating Index or with total antioxidant capacity [24,25], while selenium from food sources reduced the risk [25] and there was suggestion of decreased risk with increased phytoestrogen consumption [23]. Results from other studies exploring dietary components that contribute to the DII score and ovarian cancer have been inconsistent. In the NIH-AARP cohort study, sugar consumption was inversely associated with ovarian cancer [27], whereas no association was observed with sugar in this NJ case-control study [26] and in a cohort study conducted in Canada, glycemic index and carbohydrate were not associated while glycemic load increased risk of ovarian cancer [28]. In an Italian multicenter case-control study, fiber intake was associated with reduced the risk [29]. No association was observed with dietary phytoestrogens in two Australian case-control studies [30]. In relation to the DII, fiber has an anti-inflammatory effect score while simple carbohydrates have a pro-inflammatory effect score [9]. Though phytoestrogens, especially flavonoids have anti-inflammatory scores, data on flavonoids were not available in this study; hence, they could not be used for DII calculation. The DII has been shown to be associated with ovarian cancer in one study in Italy; subjects in the highest quartile of DII scores (i.e., with the most pro-inflammatory diets) had a higher risk of ovarian cancer compared to subjects in the lowest quartile (i.e., with an anti-inflammatory diet) (ORQuartile4vs1 1.47, 95% confidence interval, CI, 1.07, 2.01; p trend = 0.009) [8]. Similarly, in a study conducted in the US African-American women consuming the most pro-inflammatory diet had a statistically significant increased ovarian cancer risk in comparison to the most anti-inflammatory diet (ORQuartile4/Quartile1 3=31.72; 95% CI3=31.18–2.51) [7,8].
We did not observe significant association between DII and ovarian cancer among pre-menopausal women, similar results were seen in the previous two studies [7,8]. The absence of an association between DII scores and ovarian cancer among pre-menopausal women in this study could be explained by the fact that there are strong hormonal and reproductive factors which play a more important role in the development of ovarian cancer at younger ages when the ovaries are fully functional [31,32]. By contrast, inflammation may represent relatively more important influences in in post-menopausal women. Furthermore, the pre-menopausal group may have a different type of ovarian cancer that has developed secondary to germline alterations independent of any dietary factors. For example, women with germline BRCA1/2 deleterious mutations tend to develop ovarian and other cancers at an earlier age and thus are more likely to be pre-menopausal [33]. In contrast, the postmenopausal group may develop cancer as a result of somatic mutations that happen over time and as a response to environmental factors such as exposure to an inflammatory diet.
Certain limitations of this study should be noted. Our sample size was relatively small, which may have affected our statistical power to detect associations. Additionally, the study was subjected to the limitations of case–control studies, such as recall and selection biases. However, the distribution of risk factors such as parity, tubal ligation, and oral contraceptive use of cases and controls in this study [23], is similar to that reported in other studies [3]which gives us reassurance in the validity of our data. Another limitation is the use of the FFQ, which may lead to measurement error, even in healthy individuals [34,35] and may be associated with disease- differential reporting biases [36,37]. With respect to the DII, no information was available on 16 16 food parameters. DII calculated from the 29 available food parameters has not been validated with inflammatory markers, though we have found little drop off in predictability in other studies, such as the SEASONS Study [10] and the Women’s Health Initiative [17], which used essentially the same FFQ as in this study.
In conclusion, our study provided suggestive evidence that a pro-inflammatory diet, as shown by higher DII scores, increased risk of ovarian cancer in postmenopausal women. However, this finding requires replication in larger studies, including prospective cohorts, which may provide more definite evidence regarding the possible role of diet-related inflammation on ovarian cancer etiology and possible effect modification by menopausal status and body mass index.
Supplementary Material
Highlights.
Ovarian cancer has been linked to chronic inflammation and diet. Yet, the impact of an inflammatory diet on ovarian cancer risk is unclear.
In this study, we assessed the association between dietary inflammation and risk for ovarian cancer.
Proinflammatory diets (as indicated by dietary scores) are associated with increased ovarian cancer risk among post-menopausal women.
Acknowledgments
Funding: NIH R44DK103377, R01CA83918, P30CA072720, and Rutgers Cancer Institute of New Jersey. The New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey Department of Health, is funded by the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute under contract HHSN261201300021I, the National Program of Cancer Registries (NPCR), Centers for Disease Control and Prevention under grant 5U58DP003931-02 as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey. Memorial Sloan Kettering Cancer Center is supported by P30CA008748 (C. Thompson, PI).
Footnotes
Disclosure Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2017. [Google Scholar]
- 2.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. European journal of cancer. 2007;43(4):690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer biology & medicine. 2017;14(1):9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report Summary. Food, Nutrition, Physical Activity, and the Prevention of Ovarian Cancer 2014 [Google Scholar]
- 5.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 6.Ose J, Schock H, Tjonneland A, Hansen L, Overvad K, Dossus L, Clavel-Chapelon F, Baglietto L, Boeing H, Trichopolou A, Benetou V, Lagiou P, Masala G, Tagliabue G, Tumino R, Sacerdote C, Mattiello A, Bueno-de-Mesquita HB, Peeters PH, Onland-Moret NC, Weiderpass E, Gram IT, Sanchez S, Obon-Santacana M, Sanchez-Perez MJ, Larranaga N, Castano JM, Ardanaz E, Brandstedt J, Lundin E, Idahl A, Travis RC, Khaw KT, Rinaldi S, Romieu I, Merritt MA, Gunter MJ, Riboli E, Kaaks R, Fortner RT. Inflammatory Markers and Risk of Epithelial Ovarian Cancer by Tumor Subtypes: The EPIC Cohort. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(6):951–961. doi: 10.1158/1055-9965.EPI-14-1279-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peres LC, Bandera EV, Qin B, Guertin KA, Shivappa N, Hebert JR, Abbott SE, Alberg AJ, Barnholtz-Sloan J, Bondy M, Cote ML, Funkhouser E, Moorman PG, Peters ES, Schwartz AG, Terry PD, Camacho F, Wang F, Schildkraut JM. Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. International journal of cancer Journal international du cancer. 2017;140(3):535–543. doi: 10.1002/ijc.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivappa N, Hebert JR, Rosato V, Rossi M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer causes & control: CCC. 2016;27(7):897–906. doi: 10.1007/s10552-016-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivappa N, Prizment AE, Blair CK, Jacobs DR, Jr, Steck SE, Hebert JR. Dietary inflammatory index and risk of colorectal cancer in the Iowa Women’s Health Study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(11):2383–2392. doi: 10.1158/1055-9965.EPI-14-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public health nutrition. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2015;45(1):177–183. doi: 10.1111/cea.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, Park HL, Rosal MC, Shikany JM, Shivappa N, Ockene JK, Hebert JR. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of epidemiology. 2015;25(6):398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, Hebert JR, Moreno V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case-control study. Genes & nutrition. 2015;10(1):447. doi: 10.1007/s12263-014-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, Hébert JR. Inflammatory potential of diet and risk of colorectal cancer: a case–control study from Italy. British Journal of Nutrition. 2015;114(01):152–158. doi: 10.1017/S0007114515001828. [DOI] [PubMed] [Google Scholar]
- 17.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, Wactawski-Wende J, Ockene JK, Hebert JR. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer causes & control: CCC. 2015;26(3):399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. The British journal of nutrition. 2015;113(11):1819–1827. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR. Association between dietary inflammatory index and prostate cancer among Italian men. The British journal of nutrition. 2014:1–6. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. The British journal of nutrition. 2014:1–7. doi: 10.1017/S0007114514003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivappa N, Hebert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, Rossi M. Dietary inflammatory index and endometrial cancer risk in an Italian case-control study. The British journal of nutrition. 2016;115(1):138–146. doi: 10.1017/S0007114515004171. [DOI] [PubMed] [Google Scholar]
- 22.Antwi SO, Oberg AL, Shivappa N, Bamlet WR, Chaffee KG, Steck SE, Hebert JR, Petersen GM. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking and longstanding diabetes. Carcinogenesis. 2016;37(5):481–490. doi: 10.1093/carcin/bgw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC women’s health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandran U, Bandera EV, Williams-King MG, Paddock LE, Rodriguez-Rodriguez L, Lu SE, Faulkner S, Pulick K, Olson SH. Healthy eating index and ovarian cancer risk. Cancer causes & control: CCC. 2011;22(4):563–571. doi: 10.1007/s10552-011-9728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gifkins D, Olson SH, Paddock L, King M, Demissie K, Lu SE, Kong AN, Rodriguez-Rodriguez L, Bandera EV. Total and individual antioxidant intake and risk of epithelial ovarian cancer. BMC cancer. 2012;12:211. doi: 10.1186/1471-2407-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King MG, Olson SH, Paddock L, Chandran U, Demissie K, Lu SE, Parekh N, Rodriguez-Rodriguez L, Bandera EV. Sugary food and beverage consumption and epithelial ovarian cancer risk: a population-based case-control study. BMC cancer. 2013;13:94. doi: 10.1186/1471-2407-13-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasevska N, Jiao L, Cross AJ, Kipnis V, Subar AF, Hollenbeck A, Schatzkin A, Potischman N. Sugars in diet and risk of cancer in the NIH-AARP Diet and Health Study. International journal of cancer Journal international du cancer. 2012;130(1):159–169. doi: 10.1002/ijc.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvera SA, Jain M, Howe GR, Miller AB, Rohan TE. Glycaemic index, glycaemic load and ovarian cancer risk: a prospective cohort study. Public health nutrition. 2007;10(10):1076–1081. doi: 10.1017/S1368980007696360. [DOI] [PubMed] [Google Scholar]
- 29.Pelucchi C, La Vecchia C, Chatenoud L, Negri E, Conti E, Montella M, Calza S, Dal Maso L, Franceschi S. Dietary fibres and ovarian cancer risk. European journal of cancer. 2001;37(17):2235–2239. doi: 10.1016/s0959-8049(01)00291-x. [DOI] [PubMed] [Google Scholar]
- 30.Neill AS, Ibiebele TI, Lahmann PH, Hughes MC, Nagle CM, Webb PM Australian Ovarian Cancer Study G, Australian National Endometrial Cancer Study G. Dietary phyto-oestrogens and the risk of ovarian and endometrial cancers: findings from two Australian case-control studies. The British journal of nutrition. 2014;111(8):1430–1440. doi: 10.1017/S0007114513003899. [DOI] [PubMed] [Google Scholar]
- 31.Kotsopoulos J, Lubinski J, Gronwald J, Cybulski C, Demsky R, Neuhausen SL, Kim-Sing C, Tung N, Friedman S, Senter L, Weitzel J, Karlan B, Moller P, Sun P, Narod SA the Hereditary Breast Cancer Clinical Study G. Factors influencing ovulation and the risk of ovarian cancer in BRCA1 and BRCA2 mutation carriers. International journal of cancer Journal international du cancer. 2014 doi: 10.1002/ijc.29386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin JR, Risch HA, Lubinski J, Moller P, Ghadirian P, Lynch H, Karlan B, Fishman D, Rosen B, Neuhausen SL, Offit K, Kauff N, Domchek S, Tung N, Friedman E, Foulkes W, Sun P, Narod SA Hereditary Ovarian Cancer Clinical Study G. Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. The Lancet Oncology. 2007;8(1):26–34. doi: 10.1016/S1470-2045(06)70983-4. [DOI] [PubMed] [Google Scholar]
- 33.Russo A, Calo V, Bruno L, Rizzo S, Bazan V, Di Fede G. Hereditary ovarian cancer. Crit Rev Oncol Hematol. 2009;69(1):28–44. doi: 10.1016/j.critrevonc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Hebert JR, Ma Y, Clemow L, Ockene IS, Saperia G, Stanek EJ, 3rd, Merriam PA, Ockene JK. Gender differences in social desirability and social approval bias in dietary self-report. American journal of epidemiology. 1997;146(12):1046–1055. doi: 10.1093/oxfordjournals.aje.a009233. [DOI] [PubMed] [Google Scholar]
- 35.Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L. Systematic errors in middle-aged women’s estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Annals of epidemiology. 2002;12(8):577–586. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 36.Holmberg L, Ohlander EM, Byers T, Zack M, Wolk A, Bruce A, Bergstrom R, Bergkvist L, Adami HO. A search for recall bias in a case-control study of diet and breast cancer. International journal of epidemiology. 1996;25(2):235–244. doi: 10.1093/ije/25.2.235. [DOI] [PubMed] [Google Scholar]
- 37.Hebert JRMY, Ebbeling CB, et al. Self-report data. In: Ockene IS, Burke LE, editors. Compliance in healthcare and research. Futura Publishing Company; Armonk, NY: 2001. pp. 163–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.