Abstract
The neural circuitry mediating sensory and motor representations is adaptively tuned by an animal's interaction with its environment. Similarly, higher order representations such as spatial memories can be modified by exposure to a complex environment (CE), but in this case the changes in brain circuitry that mediate the effect are less well understood. Here we show that prolonged CE exposure was associated with increased selectivity of CA1 “place cells” to a particular recording arena compared to a social control (SC) group. Furthermore, fewer CA1 and DG neurons in the CE group expressed high levels of Arc protein, a marker of recent activation, following brief exposure to a completely novel environment. The reduced Arc expression was not attributable to overall changes in cell density or number. These data indicate that one effect of CE exposure is to modify high-level spatial representations in the brain by increasing the sparsity of population coding within networks of neurons. Greater sparsity could result in a more efficient and compact coding system that might alter behavioural performance on spatial tasks. The results from a behavioural experiment were consistent with this hypothesis, as CE-treated animals habituated more rapidly to a novel environment despite showing equivalent initial responding.
Keywords: enriched environment, place cell, spatial representation, sparsity, immediate early gene
It is well known that environmental stimuli and response contingencies shape the structure and function of the relevant neural circuitry. This has been particularly well studied for sensory representations, which are readily modified during development by manipulations of the available sensory stimuli (Hubel et al., 1977). As a result of these changes the cells participating in these representations become more tuned to particular characteristics of the environment and the representations themselves become more sparse, with fewer cells activated at any time, improving energy efficiency and the ease with which structure in the input data can be extracted (Olshausen and Field, 2004). Higher order functions are also shaped by environmental stimuli and for more than 40 years it has been recognised that exposure to a complex environment (CE) facilitates memory function (Greenough et al., 1973; Tees, 1999; Teather et al., 2002; Schrijver et al., 2004). For example, when compared with those reared in social or isolated environments, rats reared in complex environments display superior performance in the water maze, a test of spatial information processing and memory that requires an intact hippocampus (Morris et al., 1982; Tees, 1999). While CE exposure has previously been shown to modify neural architecture in brain regions such as the hippocampus (Globus et al., 1973; Greenough et al., 1973, 1985; Kempermann et al., 1997; van Praag et al., 2000), it is not clear how such changes support improved spatial performance.
Activity in hippocampal pyramidal place cells appears to be fundamental to this region's role in spatial memory, as their firing occurs primarily in a sub-region of the environment (the cell's “place field”) that is specific for each cell (O'Keefe and Dostrovsky, 1971; Muller, 1996; Poucet et al., 2004). Place cell firing appears to reflect neural processing in a high-level, environment-centered representation of the animal's location in space (O'Keefe and Nadel, 1978) and it has been proposed that a network of place cells could represent a particular environmental context (O'Keefe and Nadel, 1978; Wilson and McNaughton, 1993; Jeffery et al., 2004). Changes in the way that the hippocampus represents spatial contexts could have marked effects on an animal's ability to discriminate one context from another, or to discriminate novel from familiar ones, and to determine which behaviors are appropriate in each context (Karlsson and Frank, 2008). With these considerations in mind, we hypothesised that the improvement in spatial memory performance that follows CE exposure may in part be due to changes in the way that hippocampal place cells process or represent spatial information, potentially through an increase in the sparsity of the spatial representations. In principle this could involve alterations in the synaptic, firing or network properties of hippocampal place cells. In the present study we found that CE exposure led to network-level changes in spatial representations within the hippocampus. These changes included a reduction in the number of place cells active following brief exposure to a novel environment, as measured both from levels of Arc protein, a marker of recent activation, and an increase in the number of cells that change state from activity to quiescence (or vice versa) when the animal shifts between recording rooms. These changes are consistent with an increase in the sparsity of spatial representations within the hippocampus of CE animals.
Materials and Methods
Male Sprague-Dawley rats (aged postnatal days 30-36) were randomly assigned to one of two living conditions: standard group housing in plastic cages (n=13, 4 rats per cage; social control, SC) or group housing (n=13, 4 rats per box) in a complex environment (CE). The standard cage was a clear plastic box with a 30 × 50 cm floor and 30cm high walls. The enriched environment was a large opaque box (1m × 1m × 80cm) filled with an assortment of objects (tunnels, ladders, toys, etc.) that were changed twice per week. Rats continuously inhabited one of these environments for at least 3 months prior to electrode implantation, and then throughout the recording procedure. During this time they were maintained on a twelve hour standard light-dark cycle. Surgery, electrophysiological and behavioral testing procedures took place during the light phase of the cycle.
Place cell procedures
Rats were chronically implanted in the hippocampal CA1 region with miniature, moveable microdrives containing either a bundle of eight electrodes or two tetrodes. Procedures were as we have described previously (Muir and Bilkey, 2001; Russell et al., 2003; Liu et al., 2004; Kyd and Bilkey, 2005) The recording electrode type was matched across the two groups and at least one week (matched across groups) elapsed between surgery and initial exposure to the recording environment. For all animals, recordings of each CA1 neuron encountered were made in a 75 cm diameter round tub with 56 cm high walls as each rat foraged freely for food reward (chocolate sprinkles) scattered randomly on the floor. The room was dimly lit and the animal had a limited view of any extra-apparatus room cues. The recording duration was 10 minutes. For a subset of animals (SC, n=8; CE, n=7), further recordings were also made in second recording room where a 75 cm diameter low-walled (5 cm high) circular arena was located (70 cm above the floor). When placed in this apparatus, which was also dimly lit, the rats had a clearer view of room cues. These animals were systematically shifted between the two recording rooms on a day to day basis so that, as their electrodes were lowered into the hippocampus, the search for place cells took place equally in each room with order counterbalanced across animals. Electrode placement in the CA1 cell layer was identified by careful monitoring of electrode depth from the dura and by the characteristic electrophysiology. Once a cell had been isolated in one of the rooms, a 10 minute recording session was conducted in that apparatus. Then, after a delay of either 4 or 20 hours (counterbalanced across groups), a second 10 minute recording session was conducted in the opposite room. Immediately after the second session a third 10 minute recording was made in the original apparatus in a counterbalanced ABA (or BAB) design. This procedure allowed us to determine how cells responded to a change of recording rooms and arenas. At the end of this procedure the electrode Microdrive was advanced by approximately 40 microns. The following day the recordings were examined again for evidence of neuronal activity.
Extracellular spike activity was recorded in the hippocampus and buffered via a Field Effect Transistor source-follower located in a head-stage mounted at the end of the recording cable. An electrode without single unit activity was used as an indifferent. The output signals were filtered at 300 Hz and 5 kHz, and amplified 10,000 times (Barc Neuro 8 Amplifier) before being digitized at 25 kHz by a Digidata 1200 series interface (Axon Instruments) under the control of Axoscope (Axon Instruments) or, in later recordings, an Axona acquisition system with amplification of 100 times, filtering at 360 Hz and 7 kHz and acquisition at 48 kHz. The change in recording systems occurred simultaneously across our lab such that both before and after the change the recording equipment in the two rooms was identical for any given cell. In all cases single unit signals were digitized when a spike on any channel exceeded a pre-determined threshold set above the background noise levels and were stored on a personal computer for off-line analysis. The position of the rat's head was simultaneously monitored by a tracking system connected to a video camera on the ceiling above the arena. This device tracked the position of three infrared light-emitting diodes (LEDs) mounted on the head stage. The LEDs were positioned in a triangular formation 1 cm apart and centered on the crown of the head. Head position was sampled at 10 Hz and this information was made available to the acquisition system.
Single unit data were isolated manually by template-matching software (PF 2000) or the cluster cutting software TINT (Axona, tetrode data) using spike height and spike width as the primary discriminating characteristics. Place cells were classified as such if the spike width (peak-to-trough) was greater than 400 μs, and the mean firing rate was lower than 5 Hz but higher than 0.1 Hz in one of the two environments. These cells were also identified on the basis of their typical ‘complex-burst’ discharges, consisting of two to five spikes in a series that decreased in amplitude with an interspike interval of 5-10 ms. Complex bursts were identified with an autocorrelation function that calculated the time between all spike pairs. The autocorrelation functions also allowed the experimenter to identify the post-spike refractory period as a guide to cell isolation and to distinguish between place cell and theta cell (putative interneuron) firing patterns (Fox and Ranck, 1981). Data recorded from theta cells were not included in the current analysis.
Firing fields (rate maps) were generated by dividing the whole apparatus into a 20 × 20 pixel grid. The number of spikes that were fired within each pixel was divided by the dwell time in that pixel to generate the firing rate (FR). When an animal had spent less than 200 ms in any pixel, that pixel was considered to be undersampled and it was removed from subsequent analysis. The place field of a cell was deemed to be those pixels in which firing was greater than the mean firing rate calculated for the whole apparatus where these pixels were adjacent to at least two other “in field” pixels. Data collected from each recording session were analyzed to obtain the mean firing rate inside and outside the place field. For repeated measure ANOVA comparisons of place field size and firing rate across environments cells that had a zero firing rate (no place field) in one environment were treated as having missing values for that environment and their missing data were replaced by linear interpolation. To provide measures of spatial firing that do not require characterization of the place field, information content, cell-firing sparsity (Skaggs and McNaughton, 1996) and spatial coherence (Kubie et al., 1990) measures were calculated. The information content is a quantitative measure of the amount of information (in bits) about animal position provided by each spike that a cell generates. A value of 0 indicates that no spatial information is conveyed. In contrast, a typical place cell will normally generate around 1 or more bits of information per spike. The spatial coherence measure was used to establish the local smoothness of the place fields (Kubie et al., 1990). This is an estimate of the strength of the spatial signal by means of a 2-D spatial autocorrelation. A positive value indicates the presence of a spatial determinant of firing. Coherence scores were normalized by computing z-scores before statistical analysis was performed (Fisher r-to-Z transform). The firing-sparsity measure describes the relative proportion of the apparatus in which the cell fired (Skaggs et al., 1996). A low value indicates that the cell was active in a small area of the environment, whereas a high value indicates more diffuse spatial firing. We use the term “firing-sparsity” here to differentiate this measure from later discussion of network or population sparsity (Willmore and Tolhurst, 2001).
In order to determine the distance that place fields shifted between multiple recording sessions, the first order moment of the place field was calculated to determine its centre (analogous to a calculation of centre of mass; COM). The shift in the COM between recordings was determined by calculating the distance (cm) between the two COM values. The COM measure was utilized on the basis of a previous study (where it was described as centroid) (Fenton et al., 2000), which compared different methods of measuring how fields shift after a manipulation. The coordinates (x and y) of the COM are defined by an equation where the mean x and y position of the pixels in the field are weighted by the firing rate in the pixel.
Arc immunocytochemistry
Behavioral procedure
For these experiments, new sets of rats were given either CE (n=7) or SC (n=8) treatment. After three months of differential housing, half of the rats in each group underwent a novel exploration procedure similar to that used previously (Ramirez-Amaya, 2005) while the other half remained in their home cage. Thus, the 4 conditions in the experiment were: SC caged (n=4), CE caged (n=3, one rat died during the experiment), SC exploration (n=4), CE exploration (n=4). For the novel environment exploration, after 5 days of pre-testing handling (3 min) each rat was carried to a dimly lit room that it had never experienced before. The room contained numerous visual cues including curtains, white board and bookshelves; quiet music was also played. In the center of the room was a wooden box (80 cm × 80 cm × 20 cm) with a grey floor and black walls. The rat was placed at a random position in the box and allowed to explore for 5 min. To ensure full exploration, the rat was gently moved to a new position in the box every 15 s. At the end of the 5-min exploration, the rat was returned to its home cage (either CE or SC) for one hour before being perfused, as this interval maximizes Arc protein expression following this behavioral treatment (Ramirez-Amaya, 2005).
Histology
Rats were deeply anesthetized with halothane and perfused through the heart with phosphate-buffered saline at room temperature followed by cold 4% paraformaldehyde in phosphate buffer. Brains were removed and post-fixed for 24 h in paraformaldehyde and then cryoprotected in phosphate-buffered sucrose. Coronal sections were cut from one hemisphere at 30 μm on a freezing microtome and collected in phosphate buffer. Sample sections were checked to verify electrode placement in CA1.
Fluorescent immunocytochemistry was performed on free-floating sections and each well contained two sections from one animal taken from each of the four conditions (SC caged, CE caged, SC exploration, CE exploration). Two wells were run for each set of sections, thus each rat had a total of four sections reacted. The sections were incubated in Arc and NeuN primary antibodies simultaneously for 48 h at 4° C (anti-Arc rabbit polyclonal supplied by the Worley laboratory, 1:1000 dilution; anti-NeuN mouse monoclonal from Chemicon, 1:2000 dilution). The sections were then incubated in fluorescent secondary antibody for 2 h at room temperature (Alexa-Fluor 546 for Arc and Alexa-Fluor 488 for NeuN, both 1:500 dilution). The sections were mounted onto slides and coverslipped with anti-fade mounting medium (Vector). Each slide contained the contents of one well, i.e., 2 sections from each of the 4 different conditions.
Confocal microscopy
For each rat, four z-stacks were collected from the two different slides (1 stack per section; 2.0 μm optical section, 20× objective) using a Zeiss LSM 510 laser scanning confocal microscope. For each slide, the microscope settings for brightness and contrast were optimized for the SC caged section and then these settings were held constant while collecting the stacks from all of the other sections on that slide. This procedure ensured that the immunoreactions and the fluorescence measurements were performed consistently across groups.
Image analysis
For each z-stack, the maximum brightness projection of the middle three optical sections was used for analysis. In the dentate gyrus, we obtained separate measures for the dorsal and ventral blades. Identifying Arc-positive neurons in the dentate was relatively easy as basal expression was very low. Because of this low basal expression, we used the raw count of positive neurons as our measure of Arc expression in each blade. In CA1, however, we observed a relatively constant basal level of Arc expression, and therefore we used a strict threshold criterion for determining what constituted an Arc-responsive neuron. For each slide, the average brightness of the fluorescence in the cell body layer of the SC caged animal was calculated and then a value 3 standard deviations brighter than this was set as the threshold for determining an Arc-responsive neuron. For both CA1 and the dentate gyrus, counting of Arc-responsive neurons was done while viewing an overlay of the Arc and NeuN to ensure that only neurons were counted. All analyses were performed on coded sections so that the investigators performing the analyses were blind to the group identity of the sections.
Behavioral testing procedures
After three months of differential housing, naïve rats from SC and CE groups were placed individually into a square white box measuring 60 cm by 60 cm, with a 20 cm high wall, for a series of 8 sessions. The box and the room in which it was located were initially novel. Each session in the box was 4 min long, with an inter-session interval of 40 min. Rats explored freely during each session. Forty minutes after the end of the last session the chamber was relocated into another unfamiliar room for a further 4 min session. Exploration of the environment was determined by counting the number of times the animal stood up on its hind legs (reared) and the total amount of time spent rearing. These data were recorded during the animals' first exposure to an environment (session 1 and 9) and on the last exposure to the first environment (session 8) (Lever et al., 2006).
Results
Basic place cell properties are not affected by environment treatment
A total of 217 well-isolated CA1 place cells were recorded while the animals were foraging in the high-walled cylinder. One hundred and thirteen of these cells were recorded from 13 animals that had experienced the social control environment for at least 3 months. One hundred and four cells were recorded from 13 animals that had experienced the enriched condition for at least 3 months. Within each group, 8 animals contributed more than 6 cells to the group total ensuring that data was well spread across individual rats. There was no between-group difference in the mean running speed of the animals or the mean area of the environment explored during recording (both p > 0.1). An analysis of the basic firing properties of these cells, based on data recorded as they foraged freely in the high-walled tub environment revealed that the two groups were not significantly different from each other on any measure (individual t-tests all p > 0.1). This included basic properties such as mean firing rate and spatial properties such as place field size and information content (Table 1).
Table 1.
Place cell firing properties of cells recorded in the high-wall arena in animals raised in social control (SC) or enriched (CE) conditions (mean ± s.e.m.). All between-group comparisons are p > 0.1. FR = firing rate.
| SC | CE | |
|---|---|---|
| Number of cells | 113 | 104 |
| Overall FR (Hz) | 1.5 ± 0.1 | 1.5 ± 0.1 |
| Information content (Bits per spike) | 1.01 ± 0.06 | 1.10 ± 0.08 |
| Spatial coherence | 0.55 ± 0.03 | 0.58 ± 0.03 |
| Firing-Sparsity | 0.42 ± 0.01 | 0.42 ± 0.02 |
| Place field size (% of chamber) | 27.8 ± 0.8 | 26.1 ± 0.9 |
| Infield FR (Hz) | 3.8 ± 0.2 | 3.6 ± 0.2 |
| Outfield FR (Hz) | 0.6 ± 0.04 | 0.4 ± 0.05 |
| Infield/Outfield FR ratio | 27.8 ± 0.8 | 26.1 ± 0.9 |
Remapping occurs with a higher probability in CE animals
Previous studies have shown that place cells respond to changes in the recording environment in a process called ‘remapping’. Remapping can occur in several forms, such as marked changes in place cell firing rate, including the complete cessation of firing, occurring when an animal is switched between environments (Muller and Kubie, 1987). To determine whether environmental enrichment altered the way in which these remapping responses occurred, cells were recorded in two different rooms, each containing a different type of recording arena (high or low wall). During this procedure, 83 cells were recorded from 8 animals that had lived for at least 3 months in the SC condition. Eighty seven cells were recorded from 7 animals that had lived in the CE condition. The mean number of exposures to the environments that rats had before the first cells were recorded was 11.8 + 2.1 for SC animals and 11.3 + 2.4 for the CE group (p > 0.5). This included four habituation exposures (two in each arena). The mean total number of exposures to the environments across all recording sessions was also similar for the two groups (p > 0.4) and on average the SC animals received 4.8 + 0.6 full ABA environment manipulations while the CE group received 4.6 + 0.9 of the same manipulations (p > 0.5).
A two-way ANOVA comparing mean firing rates for cells in each group in both of the recording rooms revealed that there was a main effect of room (F(1,168)=18.34, p = 0.001), with the rate being higher in the room with the high-walled arena. There was, however, no effect of group (F(1,168)= 0.05, p = 0.82) and no group by room interaction (F(1,168)= 0.351, p = 0.55). A similar comparison of place field size (% of environment) revealed a significant main effect of room (F(1,168)= 22.18, p=0.0001) with place fields being larger in the high-walled arena but no group effect (F(1,168)= 2.91, p = 0.09) and no interaction (F(1,168)= 0.052, p = 0.82) (Table 2). Further consideration of these data revealed, however, that a subgroup of cells were remapping between recording rooms by switching off or switching on their firing (Fig. 1). The form of remapping wherein a subset of cells turn on or off in different locations has previously been called ‘subset switching’ (Muller and Kubie, 1987; Kubie and Muller, 1991), and is a component of ‘global remapping’ (Leutgeb et al., 2005a; Colgin et al., 2008). These terms capture the notion that across the population of cells, different subsets encode different recording locations.
Table 2.
Place cell firing properties of cells in animals raised in social control (SC) or enriched (CE) conditions when recorded sequentially in a high wall and low wall arena (mean ± s.e.m.).
| High Wall | Low Wall | |||
|---|---|---|---|---|
| SC | CE | SC | CE | |
| Number of cells | 83 | 87 | 83 | 87 |
| Overall firing rate (Hz)* | 1.31±0.09 | 1.39±0.13 | 0.98±0.09 | 0.95±0.09 |
| Place field size (%)* | 27.0±0.9 | 24.9±1.0 | 22.7±1.0 | 20.9±1.1 |
significant difference between High Wall and Low Wall arena (p<0.0001)
Figure 1.
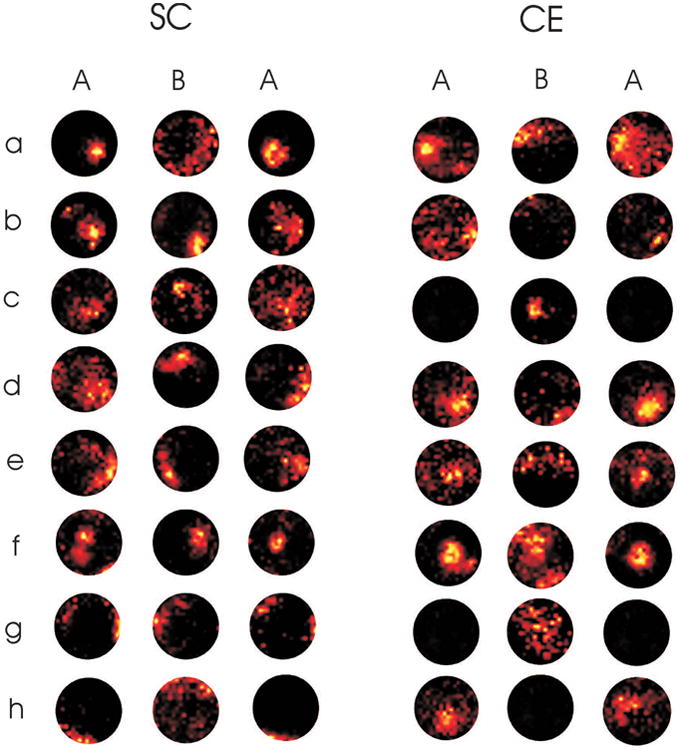
Example place fields of 16 different place cells recorded from animals maintained in a complex environment (CE; n= 8, cells a-h) or social control (SC; n=8, cells a-h) cage. Recordings were made as animals foraged freely in three recordings made in two separate recording environments (A and B). Order of room exposure was counterbalanced as either ABA or BAB although for clarity all changes are represented here as ABA. The hotter coloured pixels correspond to regions where the cell fired at a higher rate. All firing rates were normalised to the maximum rate in that recording. Note that all these fields were stable in repeated recordings in the same environment in that they generated similar place fields during the first and third recording. The firing rate in several place cells in the CE group, however, dropped to near zero in one of the environments, (e.g. cell c, g and h). Overall, although this effect occurred in both groups, it was more common in cells recorded from CE animals compared to those in the SC group.
Although global remapping was evident in cells recorded from both groups of animals and in both environments, the proportion of cells that exhibited this behaviour was higher in the CE group. To quantify this remapping behaviour, we categorized cells into those that remapped by subset-switching and those that didn't by setting 0.1 Hz as the lower bound of the ‘active’ threshold and 0.05 Hz as the upper bound of the ‘inactive’ threshold. This ensured that any cells that did not clearly change firing rates across these boundaries were treated as being ‘indeterminate’ and so were not counted as having remapped. Using these criteria we found that 13 out of 83 cells (15.7%) in the SC group turned on or off when the recording room was changed. By comparison, 35 of 87 (40.2%) cells in the CE group changed state when the recording room was changed (Fig. 2 A). This difference was statistically significant (χ2 = 12.6, p<0.001), indicating that place cells in the CE animals were more likely to globally remap by subset switching following a change in the recording room than were cells in SC animals. This effect was not critically dependent on the thresholds chosen and remained statistically significant when active/inactive thresholds were either 0.1/0.1 Hz (χ2 = 7.1, p<0.01) or 0.05/0.05 Hz (χ2 = 13.6, p< 0.01).
Figure 2.
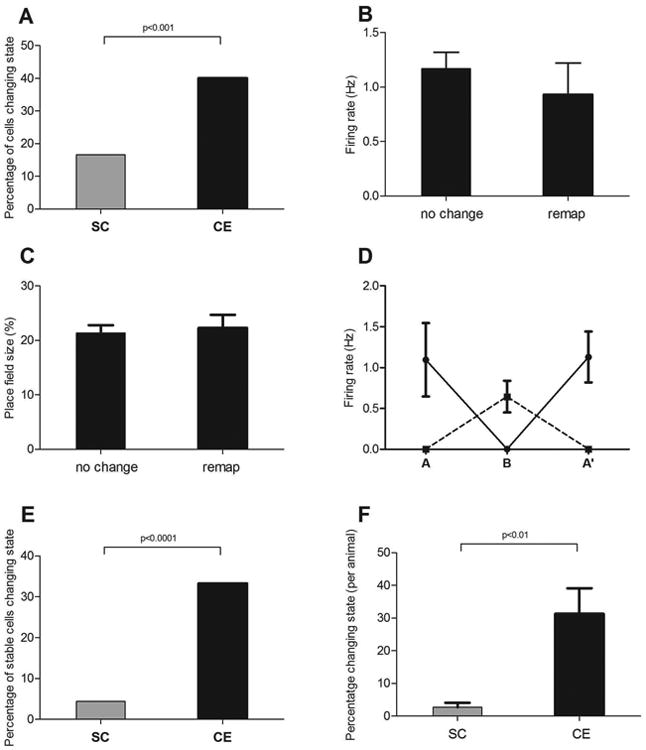
A) The percentage of hippocampal place cells that changed firing state from on to off, or vice versa, when animals were moved from a recording arena in one room into a different arena located in a different room, was significantly greater for the animals raised in a complex environment (CE group; n=87 cells) compared to cells recorded from animals in the social control (SC; n=83 cells) groups. This difference in cell response was not reflected in differences in basic firing behaviour as firing properties of the CE-group cells that changed state between recording environments were no different to those that did not change, for measures such as B) firing rate and C) place field size. D) The analysis was then restricted to only those cells that returned to the same firing state from A to A′ in an ABA′ manipulation (off/on/off = dashed line, on/off/on = solid line; SC n= 68; CE n = 57). E) For this subgroup of cells, CE-group cells were still more likely to change state than SC-group cells. F) This effect was not due to effects occurring in only one or two animals as the same pattern emerged when the analysis was conducted on a per animal basis (CE, n=7 animals; SC, n=8 animals) (error bars ± sem).
Because we ran an ABA (BAB) procedure, we were able to compare how many cells were in the same firing state (either active or inactive) in both the first and second recording in the initial recording room. We found that 82% of cells in SC animals did not change state (either remaining active or remaining inactive) across these recordings, while 66% of cells in CE animals were in the same state. This difference was statistically significant (χ2=5.87, p < 0.05). To address the possibility that cells in the enriched group were artefactually less stable across all recordings because the recording quality was poorer, we undertook several analyses. First an analysis of spike height and spike width changes, which might indicate poor recordings, indicated that there was no between-group difference in these variables. Second, poorer recording quality in one group would have likely resulted in larger place field size, but the data presented in Table 1 and 2 suggest this was not the case. Furthermore, there were no significant differences in mean firing rate, place field size, information content or coherence between those cells in the CE group that remapped compared to those that did not (all p >0.1; Fig. 2 B &- C; data were obtained from the cells in their ‘active’ recording room). Since some of our recordings had been conducted with single wires and some with tetrodes it was also possible that poorer cell isolation in the single wire recordings had contributed to the results. Again, this was not the case, as the proportion of cells that remapped was similar for the two recording techniques. Further, since similar numbers of cells from each group were recorded with each technique, both groups would have been equally affected by any such difference. These data indicate, therefore, that the enhanced responsiveness to room change that we observed in CE group cells cannot simply be accounted for by poorer recording quality in that group.
To test whether the increased subset switching we observed in enriched group cells might only be occurring in those cells that were more generally ‘unstable’, as measured by a change in state from A to A′, we limited the analysis of the A to B change to those ‘stable’ cells that remained in the same state in the A to A′ comparison (Fig. 2D). There were 68 cells in the SC group that met this criterion. Three of these cells (4%) subset-switched in the B condition. By comparison 57 cells in the enriched group were stable across A to A′ and 19 (33.3%) of these cells switched in the B condition. This difference was statistically significant both at the level of single cells (χ2= 17.8. p < 0.0001; Fig. 2E) and when analysed at the level of individual animals (Mann-Whitney U= 5.5, p < 0.01; Fig. 2F). This further supports our finding that cells recorded from the CE group animals are more likely to change state when a major change in recording location occurs, even when those cells are stable over repeated exposures to the same location.
Differences in remapping cannot be explained by changes in movement speed
Since changes in movement characteristics could produce changes in place cell firing rates (McNaughton et al., 1983), we analysed the average movement speed for a subset of data from the three recording sessions. An ANOVA revealed a significant effect of recording (F(2,124) = 5.01, p < 0.01) and a significant recording by group interaction (F(2,124) = 3.42, p <0.05). A subsequent Newman-Keuls test revealed that this effect was due to the SC animals increasing their speed during the second recording session of the triad (where the environment changed). Importantly, the CE animals did not change their movement speed significantly across recording rooms; therefore a loss of firing could not be explained by a sudden slowing or termination of movement in one of the recording environments.
Other forms of remapping are not affected
Remapping in response to a change in recording room can occur not only as a gross on/off change in activity state as shown here but also as a more subtle change wherein the cell continues to fire in each location, but at different firing rates (Leutgeb et al., 2005b). We assessed whether there was a between-group difference in the magnitude of firing rate change that occurred between the A and B recording for those cells that were active in both arenas. A t-test revealed, however, that there was no significant difference in the absolute change in firing rate (p > 0.1). A further form of remapping is a change in place field position. We examined the data for this type of change by measuring the shift in the centre of mass of the place field position (Muir and Bilkey, 2001; Kyd and Bilkey, 2005) between the A and A′ recordings for all those cells that were active in both. Again there was no significant between-group difference in this measure (p > 0.1), indicating that CE treatment did not affect overall place field stability.
Differences in remapping could result from changes in the hippocampal representation of an environment
These place field results indicate that exposure to an enriched environment does not change the basic firing properties of single neurons in the hippocampus. It does, however, increase the likelihood that a cell will change its firing state (on or off) when the animal is shifted from one recording location to another. Previous data suggest that the representation of a particular place depends on the pattern of firing within an active subset of hippocampal place cells (Wilson and McNaughton, 1993; Knierim, 2002). The finding that the firing of individual place cells varied more between two separate recording rooms for the CE animals than for the SC animals suggests that there is greater switching between these subsets of place cells in the CE animals. A consequence of this is that the hippocampal representations of the different places will be more orthogonal in the CE animals compared to the SC group. This altered orthogonality could potentially result from two different kinds of change in the hippocampal network of the CE animals. First, individual place cells in the CE animals could be more sensitive to particular characteristics of each recording room, perhaps through a fine tuning of inputs. As a result, place cells might be more likely to start or stop firing when that particular feature is altered as a result of a room change. Second, there could be increased network sparsity in the subset of place cells that represent each room within the hippocampus of CE animals. This would mean fewer cells involved in the representation of any one room. As a consequence, once a cell had been located during a recording in one room, the probability that the cell would also fire in another room would be reduced. Note that these two explanations are not mutually exclusive.
Arc protein expression is reduced in novelty-exposed CE animals
To test the network sparsity hypothesis, we investigated whether the number of cells activated in a novel environment was different for SC and CE animals by using fluorescence immunocytochemistry to determine the expression of Arc protein, an indicator of recent neural activity, in the CA1 region of the hippocampus (Lyford et al., 1995; Vazdarjanova et al., 2002). After three months of exposure to either SC (n=8) or CE (n=7) conditions, rats either underwent a 5 min exposure to a completely novel chamber (Exploration) or remained in their home cage (Caged). One hour later, at the time of maximal protein expression response (Ramirez-Amaya, 2005), the animals were perfused and the brain tissue was double-immunolabelled for Arc and NeuN expression. Consistent with previous reports (Ramirez-Amaya, 2005; Marrone et al., 2008), spatial exploration induced robust Arc expression above basal levels in CA1 neurons of normally housed SC rats (Fig. 3A). On average, 36 ± 6.3% of neurons strongly expressed Arc protein following novel environment exposure, compared to only 0.3 ± 0.1% of neurons in the caged control SC animals (p < 0.001; Fig. 3B). Remarkably, although there was also an increase in Arc expression following exploration in CE rats, it was significantly less than that observed in the SC rats (Fig. 3A), as predicted by the hypothesis of increased network sparsity. In CE rats, highly expressed levels of Arc protein were detected in 10% ± 4.2% of CA1 neurons following exploration, compared to the 36% observed in the SC rats (F(1,11)=10.77, p < 0.01; Fig. 3B).
Figure 3.
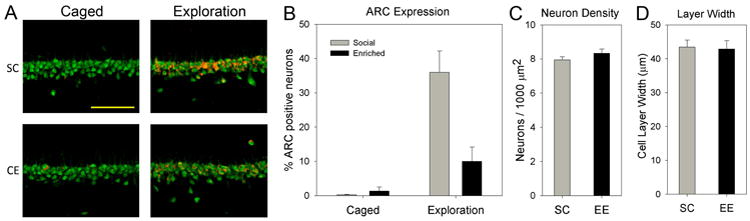
Environmental enrichment reduces novel exploration-induced Arc expression in CA1. A, Confocal images showing Arc expression (red) in the different groups (NeuN is green). Exploration caused a robust increase in Arc expression for SC rats that was much more widespread than that observed for CE rats. B, Quantification of Arc-responsive neurons (see Methods). Exploration-induced Arc expression occurred in significantly more neurons in SC rats than in CE rats. C, Cell density in CA1 was not different between CE and SC rats. D, Cell layer width was also not different between the groups.
One possible explanation of this effect is that the prolonged environmental enrichment caused a change in the basal level of Arc expression, but this was not the case. Caged CE rats showed a similar average number of Arc-positive neurons as caged SC rats (1.3% vs. 0.2% respectively, p > 0.05). We were also concerned that prolonged enrichment may have altered cell density or cell-layer width in CA1, which could affect the counts of Arc-positive neurons. However, neither density (Fig. 3C) nor layer width (Fig. 3D) were significantly different between the two groups. Finally, to ensure that our results were not some consequence of the brightness-threshold levels that we used to categorise neurons as Arc-responsive or not, we reanalysed the data without thresholding. The results are illustrated in Fig. 4 and show that the effects we observed were not dependent on thresholding.
Figure 4.
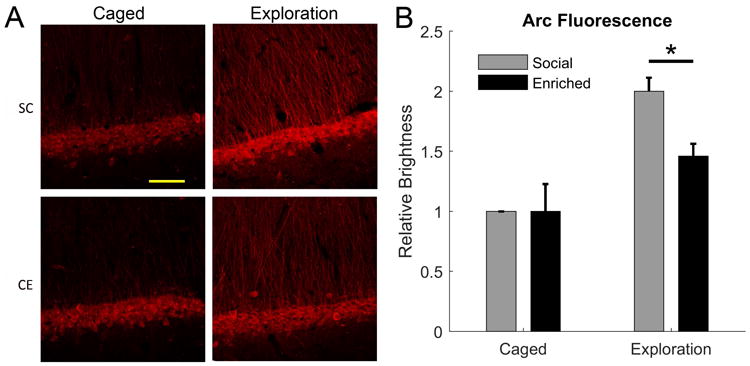
Effects of CE treatment and exploration on Arc expression in CA1 without thresholding. A, Representative images of immunofluorescence labelling of Arc expression for SC versus CE animals in either home cage or exploration conditions. B, Quantification of the brightness of the CA1 pyramidal cell layer, normalised to the SC caged control condition on each slide. CE caused no change in basal Arc immunoreactivity in the home cage but, in the exploration condition, there was less of an increase in the Arc immunoreactivity for the CE group compared to the SC group. *, p <0.05.
We also examined Arc expression in the dentate gyrus of the same animals, and observed a similar pattern of effects. The mean number of Arc-positive neurons per section/animal for the SC group increased from a home cage level of 3.4 ± 0.6 to 6.3 ± 0.8 following novel environment exposure (p = 0.03). In contrast, the CE group showed no change in the number of Arc-positive neurons between the caged and novel environment conditions (4.7 ± 1.4 and 3.5 ± 0.6, respectively). Overall this meant that there was significantly less Arc expression in the CE group compared to the SC group in the novel environment condition (p < 0.05).
Further analysis revealed, however, that these treatment effects were expressed selectively in the dorsal blade of the dentate gyrus, similar to that described previously for non-CE rats (Chawla et al., 2005). Thus, while caged CE and SC rats had similar basal numbers of Arc-positive neurons in the dorsal blade (6.8 ± 2.5 and 4.1 ± 0.6, respectively; p > 0.05; Fig. 5A), following novel environment exposure, CE rats had significantly fewer Arc-positive neurons than SC rats (3.6 ± 1.1 and 8.8 ± 1.2, respectively; F(1,11)=8.48, p = 0.014; Fig. 5B). In contrast, Arc expression in the ventral blade was not statistically different between groups or treatment conditions (Fig. 5C,D).
Figure 5.
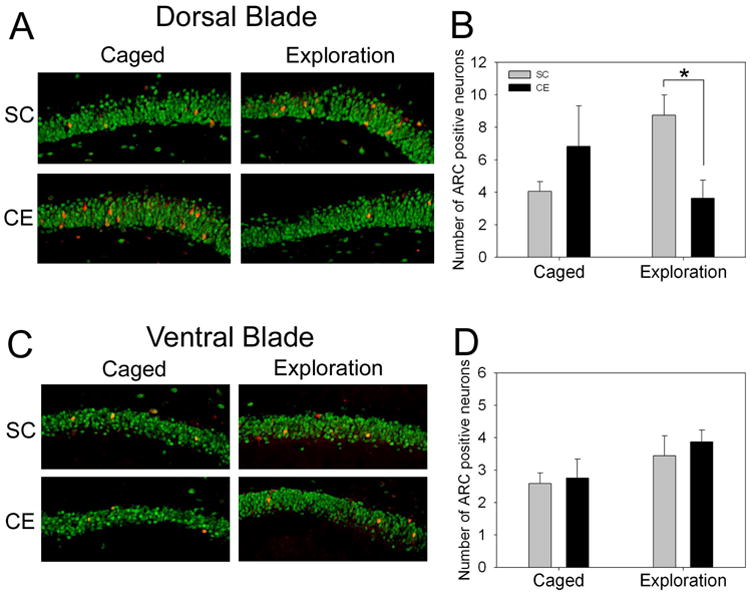
Environmental enrichment reduces novel exploration-induced Arc expression in the dorsal blade of the dentate gyrus, but not the ventral blade. A, Confocal images showing scattered Arc-positive neurons (red) in the dorsal blade (NeuN is green). CE caged rats showed a trend towards having more cells expressing Arc, but this was not significant. Exploration caused an increase in Arc-positive neurons in SC rats, and a decrease in CE rats. B, Quantification of Arc-positive neurons in the dorsal blade. Exploration induced Arc expression in significantly more neurons in SC rats compared to CE rats. *, p<0.05. C, Confocal images showing Arc-positive neurons in the ventral blade. CE and SC rats had similar numbers of Arc-positive neurons in both the caged and exploration conditions. D, Quantification of Arc-positive neurons in the ventral blade. Both CE and SC rats showed a modest increase in the number of Arc-positive neurons following novel exploration, but no significant differences were observed in any condition.
CE animals respond differently to a novel environment
To test whether our CE condition actually altered behavioural responses to a spatial environment, we compared responding in a simple, hippocampus-dependent learning paradigm, i.e., exposure to a novel environment with rearing as the dependent variable. The rearing response has previously been shown to be related to spatial novelty and exploration, and to be dependent on hippocampal function (Lever et al., 2006). Rearing decreases with time in a new environment as the animal learns about and becomes familiar with its surroundings. We predicted that if the surroundings were being represented differently in CE animals, then this learning behaviour should be altered. In particular, if the hippocampal representation of place is more sparse in CE animals, then fewer cells are active at any particular location within the environment. This results in a more condensed and/or efficient representation (Willshaw et al., 1969; Rolls and Treves, 1990; Olshausen and Field, 2004; Karlsson and Frank, 2008) which, in previous studies has been linked to more rapid learning (Fiete, 2004). For this test we repeatedly placed a new cohort of animals from each group into what was initially a novel environment (4 min sessions) and recorded the amount of rearing behaviour on the first and last trial (Fig. 6). An ANOVA revealed a significant effect of session (F(1,18) = 31.8, p < 0.0001) and a significant session x group interaction (F(1,18) = 5,62, p < 0.05) as a result of the number of rears in the CE group decreasing at a greater rate across trials than in the SC group. Consistent with this, a post-hoc test revealed no significant between-group difference in rearing on first exposure (p >0.1), but a significant effect for the last exposure (t(18) =2.89, p <0.01). A similar pattern was observed for the time spent rearing, with a significant effect of session (F(1,18) = 8.73, p < 0.01), a significant session by group interaction (F(1,18) = 10.1, p <0.01) and a significant between-group difference at the last exposure (t(18)=2.95, p < 0.01). When the animals were placed into a second novel environment (retest), the time spent rearing increased for both groups and there was no significant between-group differences in the novelty response for either measure (both p >0.1).
Figure 6.
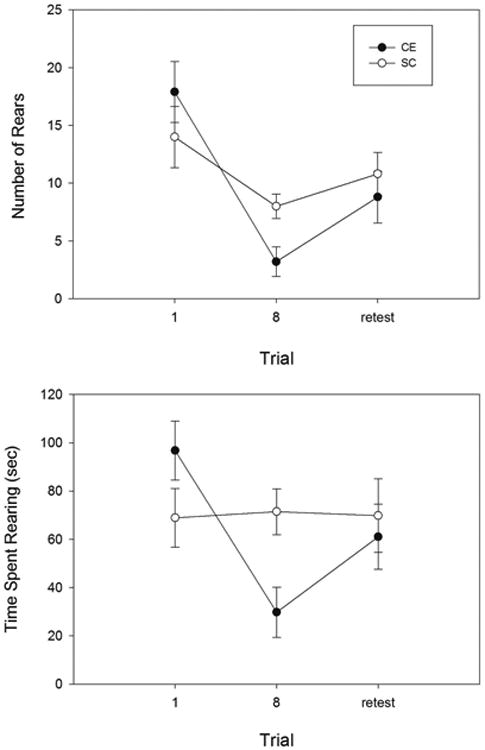
The amount of rearing, as measured by both the average number of exploratory rears (top) and the average time spent rearing (bottom) was similar in CE and SC groups on initial exposure to a novel location. Rearing decreased more rapidly in CE animals, however, after 8 brief habituation trials. When the animals were transferred to a second novel environment (retest), CE animals again showed a similar response to SC animals.
Discussion
Exposure to an enriched environment has widespread effects on brain anatomy and physiology, with concomitant improvements in memory and learning (Globus et al., 1973; Greenough et al., 1973, 1985; Kempermann et al., 1997; van Praag et al., 2000; Bengoetxea et al., 2012). It has not been clear, however, what physiological changes at the neural level account for the improvements observed at the behavioral level. In a previous report, we found that the enrichment procedures used here improved spatial watermaze learning but produced no discernable effects on basal excitatory synaptic transmission in either stratum radiatum or stratum oriens of area CA1, or for perforant path inputs to the dentate gyrus, perhaps reflecting homeostatic adjustments in global synaptic efficacy during long-term CE exposure (Eckert and Abraham, 2010). On the other hand, changes in recurrent inhibition and LTD/depotentiation were observed, suggesting a change in the operation of the CA1 network. Accordingly we sought to determine whether there were changes in the real-time processing of information in the CA1 network that might further our understanding of the neural basis for environmental effects on hippocampus-dependent memory. The data from the present study indicate for the first time that prolonged exposure to a complex environment changes spatial representations within hippocampal networks.
The single unit studies confirmed that long-term environmental enrichment does not affect the basic spatial firing properties of hippocampal place cells in freely moving animals. This was consistent with our failure to observe changes in average basal excitatory synaptic transmission using field potential analysis (Eckert and Abraham, 2010). Therefore, CE-associated improvements in performance cannot be attributed to a change in the resolution with which single place cells represent their associated place field (Zhang and Sejnowski, 1999). When the recording location was manipulated, however, there were clear differences in responding. Place cells recorded in CE animals were more likely to change state from being active to inactive or vice versa when the recording room was changed. This effect could not be explained by factors such as a difference in the movement speed of the animals or a difference in the quality of recordings between the two groups.
It has been shown previously that place cells can change their firing behaviour when the animal is placed into a new environment or if some salient feature of the environment is changed. This ‘remapping’ can take several forms. One form of remapping involves place cells responding to changes in the environment by continuing to fire in the same position but changing their firing rate. Leutgeb et al (Leutgeb et al., 2005b) have suggested that this ‘rate remapping’ might permit the generation of representations of unique episodes of experience while maintaining the integrity of the code for spatial location. In a second form of remapping, the position of a cell's place field can shift in response to various changes in the environment (O'Keefe and Nadel, 1978; Sharp, 2006; Fyhn et al., 2007), including colour (Anderson and Jeffery, 2003) and size (Muller and Kubie, 1987; Sharp, 1999). Furthermore, while some cells change their firing position in response to a change in environment and, therefore continue firing in each condition, a subset of cells display a place field in only one of the two conditions and are inactive in the other (O'Keefe and Nadel, 1978; Muller and Kubie, 1987; Thompson and Best, 1989; O'Keefe and Burgess, 1996; Sharp, 1997, 1999; Anderson and Jeffery, 2003; Leutgeb et al., 2005b; Colgin et al., 2008). This third form of remapping has been called ‘subset switching’ (Muller and Kubie, 1987; Kubie and Muller, 1991), and is a form of ‘global remapping’ (Leutgeb et al., 2005a; Colgin et al., 2008). It implies that different ensembles of cells are engaged in the representation of different environments. Leutgeb et al (Leutgeb et al., 2005a) suggest that global remapping allows for the distinction between similar experiences that occur in different spatial contexts.
An analysis of the firing behaviour of the cells in the current study suggests that there was no difference between the enriched and control groups in the amount of rate remapping that occurred when rats were switched between two different recording arenas. Furthermore, although it was not possible to directly compare place field positions across the two different arenas, as the fields tend to remap position randomly when the environment is changed markedly (Muller and Kubie, 1987) and there was no reference point to allow comparison, we were able to test for any changes in place field position from their original locations when the animals were placed back into their original recording environment after a delay period during which they had experienced another environment. There was no evidence to suggest that place cell positions changed differentially under these circumstances indicating that the underlying stability of the place fields in the two groups was similar across time. Thus we concluded that global remapping of place cells was taking place and that this was more likely to occur in the CE animals than in the SC animals as evidenced by the greater number of cells that turned on or turned off with the change in recording room.
Theoretically, a change in the likelihood that any one cell would terminate firing in a new environment could be the result of either a change in the sensitivity of the cell or a change in the sparsity of the neural network representation of the two rooms. We tested for changes in sparsity in an experiment that analysed levels of Arc protein, a marker of recent neural activation (Lyford et al., 1995; Vazdarjanova et al., 2002). This showed that a brief exposure to a novel environment produced activation in far fewer CA1 neurons in the CE group compared to their controls. A similar pattern of results was observed for dentate granule cells in the dorsal blade. These effects could not be explained simply as a reduced response to novelty or reduced attention to the environment in the CE group as our behavioral data showed that there was no significant difference between SC and CE animals in initial exploration of a novel arena, and in fact that CE animals tended to have larger, rather than smaller, behavioral responses. Although one could also predict that the increased sparsity should reduce the home cage Arc activity of CE animals compared to their controls, these levels were heavily influenced by floor effects and the hippocampal activity observed in a very familiar small space may not reflect what occurs in a more novel situation (Ludvig, 1999). A sparsity interpretation of these data requires that exposure to the novel environment resulted in ARC-activation in the whole representation of the new environment, rather than only in those cells that were newly activated (turned on) by the experience. Previous data indicates that this is the case, and that when an animal is transferred from one environment to another, ARC is activated in the whole CA1 representation, and not just in those cells that only became active in the second environment (see fig. 4, Guzowski et al., 1999). The Arc data were, therefore, consistent with the hypothesis that the difference between CE and SC animals is a result of a more sparse representation of space in the hippocampus of the enriched group. This effect is similar in principle to the experience-dependent refinement of representations that occurs in sensory areas, although a key difference is that, in the hippocampus, network properties are altered with no apparent changes in single cell representations such as the degree of “tuning” of place fields.
The neural code within the hippocampus that represents spatial aspects of the environment is distributed across a network of cells. It has been estimated using electrophysiological procedures that in any particular environment, at least 60% of the hippocampal place cells are inactive (Thompson and Best, 1989). This indicates that there is normally a high degree of sparseness in the representation. More recent analysis of data from the human medial temporal lobe is consistent with this model (Waydo et al., 2006; Hulme et al., 2014). The sparseness of the representation will have some impact on the function of the system. Increasing network sparsity has the advantage of allowing for efficient storage of information using local learning rules and greater energy efficiency (see (Olshausen and Field, 2004) for a review). This has the potential to increase the orthogonality between any two representations and to speed up learning (Fiete, 2004). Our behavioral procedure investigated this by using the fact that when a naive rat is placed into a new environment, exploratory activity, including rearing, is initially high but decreases over time as the animal habituates to that environment, a response that is hippocampus-dependent (Lever et al., 2006). The results from our behavioral experiment showed that there were differences in the way that CE and SC animals responded to the environment. CE-exposed animals habituated more rapidly to the novel environment than the SC control animals. One interpretation of this finding is that the network underlying environment representation in the CE animals is capable of more rapid encoding of information, or has better memory for previous sessions.
The mechanisms underlying the increased sparsity of place cell firing are not clear. One possibility is increased synaptic connectivity that allows for more latent links between neurons that can then be tuned for efficiency. Previously, however, we did not observe any change in basal synaptic transmission or LTP of the Schaffer collateral inputs to CA1 in CE-exposed hippocampus, as assessed through bulk stimulation of afferent fibres (Eckert and Abraham, 2010). However, this technique may not be sensitive to subtle changes, or may miss larger scale changes that occurred early during the CE experience (Foster and Dumas, 2001; Irvine and Abraham, 2005) that were homeostatically reset later during the exposure period. On the other hand, we observed a subtle reduction in recurrent inhibition and a decrease in LTD induction in the CE group that may contribute to the altered nature of spatial representations in CA1. Alternatively, there may have been changes to the dentate gyrus in terms of its pattern separation capabilities (Treves and Rolls, 1994; Morris et al., 2012; Rolls, 2013; Santoro, 2013; Chavlis et al., 2017) or to the perforant path input to the CA1 pyramidal cells. The effect of environmental enrichment on the perforant path input to region CA1 have not yet been investigated while no change has been observed in the perforant path inputs to the dentate gyrus (Eckert and Abraham, 2010).
We know that for sensory systems experience exerts a strong influence on the network properties of visual representations through processes such as pruning of axonal arborisations, synaptic plasticity and other forms of synaptic reorganisation (Wiesel, 1982; Diamond et al., 1993; de Villers-Sidani and Merzenich, 2011) which may lead to the ultimately more sparse representations that have been observed (Rochefort et al., 2009). It is possible that such changes are not confined to sensorimotor representations, but also occur in higher centers such as the hippocampus and its afferent structures during prolonged CE exposure, resulting in an improved efficiency of higher order representations such as spatial information processing. This apparent change in network connectivity is supported by the finding in aged mice that long-term environmental enrichment produced spatial memory enhancement that was correlated with decreased synaptophysin levels in the hippocampus (Bennett et al., 2006) indicative of a refinement of synaptic connectivity. The goal of this network level refinement may be to optimize the selection of cells that will participate in the storage of a particular experience. Past work has shown that although the spike times of a place cell can be predicted to a certain extent by the animal's position, the accuracy of the prediction is improved if population level activity is taken into account (Harris et al., 2003). This suggests that network level activity determines to some extent which place cells are active. Indeed, more recent studies have shown that most if not all principal cells in CA1 have the ability to form place fields in a given environment(Lee et al., 2012). Thus part of the function of CA1 may be to select which place cells are active in a given environment, and this function may be refined by the demand on the network such that ensemble selection in enriched rats is sparse.
Acknowledgments
This work was supported by The Marsden Fund of New Zealand and R35NS097966 to PW. We thank Desiree Dickerson for pilot work.
References
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoetxea H, Ortuzar N, Bulnes S, Rico-Barrio I, Lafuente JV, Argandoña EG. Enriched and deprived sensory experience induces structural changes and rewires connectivity during the postnatal development of the brain. Neural Plast. 2012;2012:305693. doi: 10.1155/2012/305693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Chavlis S, Petrantonakis PC, Poirazi P. Dendrites of dentate gyrus granule cells contribute to pattern separation by controlling sparsity. Hippocampus. 2017;27:89–110. doi: 10.1002/hipo.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci U S A. 1993;90:2082–2086. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MJ, Abraham WC. Physiological effects of enriched environment exposure and LTP induction in the hippocampus in vivo do not transfer faithfully to in vitro slices. Learn Mem Cold Spring Harb N. 2010;17:480–484. doi: 10.1101/lm.1822610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Csizmadia G, Muller RU. Conjoint Control of Hippocampal Place Cell Firing by Two Visual Stimuli. J Gen Physiol. 2000;116:191–210. doi: 10.1085/jgp.116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR. Temporal Sparseness of the Premotor Drive Is Important for Rapid Learning in a Neural Network Model of Birdsong. J Neurophysiol. 2004;92:2274–2282. doi: 10.1152/jn.01133.2003. [DOI] [PubMed] [Google Scholar]
- Foster TC, Dumas TC. Mechanism for increased hippocampal synaptic strength following differential experience. J Neurophysiol. 2001;85:1377–1383. doi: 10.1152/jn.2001.85.4.1377. [DOI] [PubMed] [Google Scholar]
- Fox SE, Ranck JB. Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41:399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Hwang HM, Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985;82:4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsáki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hulme OJ, Skov M, Chadwick MJ, Siebner HR, Ramsøy TZ. Sparse encoding of automatic visual association in hippocampal networks. NeuroImage. 2014;102 Pt 2:458–464. doi: 10.1016/j.neuroimage.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Irvine GI, Abraham WC. Enriched environment exposure alters the input-output dynamics of synaptic transmission in area CA1 of freely moving rats. Neurosci Lett. 2005;391:32–37. doi: 10.1016/j.neulet.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Anderson MI, Hayman R, Chakraborty S. A proposed architecture for the neural representation of spatial context. Neurosci Biobehav Rev. 2004;28:201–218. doi: 10.1016/j.neubiorev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Network Dynamics Underlying the Formation of Sparse, Informative Representations in the Hippocampus. J Neurosci. 2008;28:14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Knierim JJ. Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci. 2002;22:6254–6264. doi: 10.1523/JNEUROSCI.22-14-06254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubie JL, Muller RU. Multiple representations in the hippocampus. Hippocampus. 1991;1:240–242. doi: 10.1002/hipo.450010305. [DOI] [PubMed] [Google Scholar]
- Kubie JL, Muller RU, Bostock E. Spatial firing properties of hippocampal theta cells. J Neurosci. 1990;10:1110–1123. doi: 10.1523/JNEUROSCI.10-04-01110.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Hippocampal Place Cells Show Increased Sensitivity to Changes in the Local Environment Following Prefrontal Cortex Lesions. Cereb Cortex. 2005;15:720–731. doi: 10.1093/cercor/bhh173. [DOI] [PubMed] [Google Scholar]
- Lee D, Lin BJ, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012;337:849–853. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005a;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps and the population code for memory. Curr Opin Neurobiol. 2005b;15:738–746. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci. 2006;17:111–133. doi: 10.1515/revneuro.2006.17.1-2.111. [DOI] [PubMed] [Google Scholar]
- Liu P, Jarrard LE, Bilkey DK. Excitotoxic lesions of the pre- and parasubiculum disrupt the place fields of hippocampal pyramidal cells. Hippocampus. 2004;14:107–116. doi: 10.1002/hipo.10161. [DOI] [PubMed] [Google Scholar]
- Ludvig N. Place cells can flexibly terminate and develop their spatial firing. A new theory for their function. Physiol Behav. 1999;67:57–67. doi: 10.1016/s0031-9384(99)00048-7. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Schaner MJ, McNaughton BL, Worley PF, Barnes CA. Immediate-early gene expression at rest recapitulates recent experience. J Neurosci. 2008;28:1030–1033. doi: 10.1523/JNEUROSCI.4235-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- Morris AM, Churchwell JC, Kesner RP, Gilbert PE. Selective Lesions of the Dentate Gyrus Produce Disruptions in Place Learning for Adjacent Spatial Locations. Neurobiol Learn Mem. 2012;97:326–331. doi: 10.1016/j.nlm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muir GM, Bilkey DK. Instability in the place field location of hippocampal place cells after lesions centered on the perirhinal cortex. J Neurosci. 2001;21:4016–4025. doi: 10.1523/JNEUROSCI.21-11-04016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. A quarter of a century of place cells. Neuron. 1996;17:813–822. doi: 10.1016/s0896-6273(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J NadelL. The hippocampus as a cognitive map. Oxford; New York: Clarendon Press; Oxford University Press; 1978. [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Poucet B, Lenck-Santini PP, Hok V, Save E, Banquet JP, Gaussier P, Muller RU. Spatial navigation and hippocampal place cell firing: the problem of goal encoding. Rev Neurosci. 2004;15:89–107. doi: 10.1515/revneuro.2004.15.2.89. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V. Spatial Exploration-Induced Arc mRNA and Protein Expression: Evidence for Selective, Network-Specific Reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Garaschuk O, Milos RI, Narushima M, Marandi N, Pichler B, Kovalchuk Y, Konnerth A. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc Natl Acad Sci U S A. 2009;106:15049–15054. doi: 10.1073/pnas.0907660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci [Internet] 2013;7 doi: 10.3389/fnsys.2013.00074. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3812781/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Treves A. The relative advantages of sparse versus distributed encoding for associaive neuronal networks in the brain. Network. 1990;1:407–421. [Google Scholar]
- Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J Neurosci. 2003;23:6490–6498. doi: 10.1523/JNEUROSCI.23-16-06490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A. Reassessing pattern separation in the dentate gyrus. Front Behav Neurosci [Internet] 2013;7 doi: 10.3389/fnbeh.2013.00096. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3726960/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijver NCA, Pallier PN, Brown VJ, Würbel H. Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav Brain Res. 2004;152:307–314. doi: 10.1016/j.bbr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Sharp P. Subicular place cells generate the same “map” for different environments: Comparison with hippocampal cells. Behav Brain Res. 2006;174:206–214. doi: 10.1016/j.bbr.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav Brain Res. 1997;85:71–92. doi: 10.1016/s0166-4328(96)00165-9. [DOI] [PubMed] [Google Scholar]
- Sharp PE. Subicular place cells expand or contract their spatial firing pattern to fit the size of the environment in an open field but not in the presence of barriers: comparison with hippocampal place cells. Behav Neurosci. 1999;113:643–662. doi: 10.1037//0735-7044.113.4.643. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Teather LA, Magnusson JE, Chow CM, Wurtman RJ. Environmental conditions influence hippocampus-dependent behaviours and brain levels of amyloid precursor protein in rats. Eur J Neurosci. 2002;16:2405–2415. doi: 10.1046/j.1460-9568.2002.02416.x. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci. 1989;9:2382–2390. doi: 10.1523/JNEUROSCI.09-07-02382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Merzenich MM. Lifelong plasticity in the rat auditory cortex: basic mechanisms and role of sensory experience. Prog Brain Res. 2011;191:119–131. doi: 10.1016/B978-0-444-53752-2.00009-6. [DOI] [PubMed] [Google Scholar]
- Waydo S, Kraskov A, Quian Quiroga R, Fried I, Koch C. Sparse Representation in the Human Medial Temporal Lobe. J Neurosci. 2006;26:10232–10234. doi: 10.1523/JNEUROSCI.2101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Willmore B, Tolhurst DJ. Characterizing the sparseness of neural codes. Netw Bristol Engl. 2001;12:255–270. [PubMed] [Google Scholar]
- Willshaw DJ, Buneman OP, Longuet-Higgins HC. Non-holographic associative memory. Nature. 1969;222:960–962. doi: 10.1038/222960a0. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. Neuronal tuning: To sharpen or broaden? Neural Comput. 1999;11:75–84. doi: 10.1162/089976699300016809. [DOI] [PubMed] [Google Scholar]


