Abstract
Activity dependent plasticity is a key mechanism for the central nervous system (CNS) to adapt to its environment. Whether neuronal activity also influences axonal regeneration in the injured CNS, and whether electrical stimulation (ES) can activate regenerative programs in the injured CNS remains incompletely understood. Using KCl-induced depolarization, in vivo ES followed by ex-vivo neurite growth assays and ES after spinal cord lesions and cell grafting, we aimed to identify parameters important for ES-enhanced neurite growth and axonal regeneration. Using cultures of sensory neurons, neurite growth was analyzed after KCl-induced depolarization for 1–72h. Increased neurite growth was detected after short-term stimulation and after longer stimulation if a sufficient delay between stimulation and growth measurements was provided. After in vivo ES (20Hz, 2× motor threshold, 0.2ms, 1h) of the intact sciatic nerve in adult Fischer344 rats, sensory neurons showed a 2-fold increase in in vitro neurite length one week later compared to sham animals, an effect not observed one day after ES. Longer ES (7h) and repeated ES (7 days, 1h each) also increased growth by 56–67% one week later, but provided no additional benefit. In vivo growth of dorsal column sensory axons into a graft of bone marrow stromal cells 4 weeks after a cervical spinal cord lesion was also enhanced with a single post-injury 1 h ES of the intact sciatic nerve and was also observed after repeated ES without inducing pain-like behavior. While ES did not result in sensory functional recovery, our data indicate that ES has time-dependent influences on the regenerative capacity of sensory neurons and might further enhance axonal regeneration in combinatorial approaches after SCI.
Keywords: Electrical stimulation, spinal cord injury, CNS plasticity, axonal regeneration, dorsal root ganglion
INTRODUCTION
Axonal regeneration in the injured mammalian spinal cord is extremely limited. Consequently, spinal cord injury (SCI) results in substantial disability, putting a considerable burden on the life of affected individuals and on the health systems (Ackery et al., 2004). The insufficient activation of regenerative programs in injured neurons is one key factor responsible for the lack of regeneration. In contrast, neuron-intrinsic growth programs are activated in dorsal root ganglia (DRGs) after peripheral nervous system (PNS) lesions and contribute to axonal regeneration after PNS injuries. When lesions of the central branch of DRG neurons in the spinal cord are preceded by peripheral injuries, some regeneration can also be observed in the spinal cord (Hoffman, 1989, 2010; Smith and Skene, 1997). This so-called peripheral “conditioning lesion” (CL) typically induces extension of long sparsely branched neurites from DRG neurons that can grow into a central injury site or a cellular graft (McQuarrie and Grafstein, 1973; Neumann and Woolf, 1999; Richardson and Issa, 1984; Smith and Skene, 1997). In addition, CLs remain effective when performed briefly after a dorsal column lesion or in animal models of chronic SCI suggesting that the timeline for activating gene expression would not be a barrier for translation (Blesch et al., 2012; Kadoya et al., 2009). However, CLs are impractical as treatment and other means to enhance the neuron-intrinsic growth potential are needed. One clinically relevant approach to enhance the neuron-intrinsic growth capacity might be to induce neuronal activity by electrical stimulation (ES). Studies in the PNS strongly support beneficial effects of ES on regeneration of sensory and motor axons (reviewed in (Gordon et al., 2008)). A single stimulation for 1h at 20 Hz of injured peripheral nerves was found to enhance regeneration of motor neurons to their peripheral targets, shorten the time of reinnervation of the femoral nerve (Al-Majed et al., 2000b) and increase the percentage of sensory fibers returning to muscles and skin (Brushart et al., 2005). ES-induced sprouting of spared fibers has also been shown for corticospinal axons after unilateral injuries in rodents (Brus-Ramer et al., 2007; Carmel et al., 2010), and ES in the periphery for 1h has been shown to promote sensory fiber growth in vitro and in vivo after SCI (Udina et al., 2008). Thus, activity-mediated effects on neurite growth do not seem to be restricted to a specific neuronal population. Additionally, in spared corticospinal tract (CST) fibers, enhanced plasticity and sprouting, as well as expression of growth associated proteins and second messengers was induced either by ES, magnetic stimulation or a rehabilitative training (Brus-Ramer et al., 2007; Hou et al., 2014), suggesting a general positive effect of neuronal activity on axonal growth.
In DRG neurons, increases in neurite growth have been found in embryonic DRGs exposed to an electrical field (Wood and Willits, 2006), and in electrically stimulated adult neurons in peripheral and central regeneration (Geremia et al., 2007; Udina et al., 2008). However, electrical activity has also been associated with neurite growth inhibition after K+ induced depolarization of adult DRG cultures (Enes et al., 2010). In fact, it has been proposed that a lack of activity is a prerequisite for regeneration observed after CLs, because acute silencing of neurons induced by peripheral lesions correlates with the increased regeneration following such a lesion (Enes et al., 2010). However, increased excitability of DRG neurons has also been reported at more delayed time points after axotomy (Abdulla and Smith, 2001; Andre et al., 2003; Sleeper et al., 2000; Zhang et al., 1997) suggesting that the temporal relation and any causality between peripheral lesions, activity and regeneration are more complex.
The mechanisms of CLs include the upregulation of various transcription factors (TF), trophic factors and cytokines, while genes involved in neurotransmission are downregulated (Bareyre and Schwab, 2003; Hoffman, 2010). Peripheral ES has been associated with increased expression of growth-associated genes such as GAP-43 (Al-Majed et al., 2000a; Al-Majed et al., 2000b), increased expression of neurotrophic factors such as BDNF (Geremia et al., 2007) and GDNF in DRGs (Cobianchi et al., 2013), and second messengers such as cAMP (Udina et al., 2008), suggesting an overlap between the mechanisms of ES and CLs. Nevertheless, the pro-regenerative effects of ES in peripheral and central regeneration and the parameters influencing these effects are poorly understood.
In the current study, we investigated whether depolarization of DRG neurons, and short-term, repeated or extended ES applied to the sciatic nerve can activate the intrinsic growth potential of sensory neurons using in vitro neurite growth assays and in vivo axonal regeneration of dorsal column axons, and compared histological and functional outcomes to CL. Our data indicate that depolarization and ex vivo neurite growth after in vivo ES have a time-dependent stimulatory effect on DRG neurons, and that ES in vivo can stimulate in vivo axon extension into a graft of bone marrow stromal cells after a C3 dorsal column lesion without inducing adverse effects.
MATERIALS AND METHODS
Isolation and cultivation of adult DRGs
For in vitro depolarization, cervical, thoracic and lumbar DRGs from naïve adult male and female Fischer 344 rats were isolated and pooled as previously described (McCall et al., 2012). Rats were killed by an overdose of anesthesia mixture (375 mg/kg ketamine, 19.05 mg/kg xylazine, 3.75 mg/kg acepromazine; i.p.) and decapitated. During dissection, DRGs were collected in ice-cold Hibernate A medium, washed with HBSS and digested (Collagenase XI, 2.5 mg/ml, 1200 units/mg and Dispase I, 5 mg/ml, 4 units/mg; Worthington Biochemical in HBSS) at 37°C for 30 min, mixing every 10 min. Digestion was stopped by washing with 10% fetal bovine serum (FBS) in 1 ml complete medium (DMEM/F12, 1×B27, 2mM L-glutamine, and Pen-Strep 10 mg/ml) followed by a wash with 1 ml serum-free complete medium and trituration with a fire-polished Pasteur pipette. Larger debris was allowed to settle and cell suspensions were plated. In animals that underwent ES, sham electrode implantation, CLs of the sciatic and naïve control animals (see below), only L4-L6 DRGs were dissected for in vitro neurite outgrowth assays. DRGs from left and right side were collected independently 7 days after treatment, when maximum effects of CLs on neurite outgrowth and changes in gene expression are expected (Richardson and Issa, 1984; Smith and Skene, 1997). DRGs were isolated using a posterior approach by exposing the lumbar vertebral column and removing the posterior processes with a rongeur. L4-L6 DRGs were identified and digestion and dissociation was performed in a smaller volume (200–300μl) as described above.
For cultivation of dissociated DRGs, plates were coated and medium was freshly prepared on the day of DRG isolation. Six-well polystyrene plates (35 mm wells) were first coated with 16.67 μg/ml poly-D-lysine (PDL; 1.5 ml/well; Sigma) in sterile H2O for 1 h at room temperature (RT). The plates were washed with sterile H2O and coated with laminin (0.5 μg/ml or 5 μg/ml as indicated; Sigma) diluted in Dulbecco’s PBS (DPBS) for 3 h at RT. After laminin coating, plates were gently rinsed with DPBS, filled with 2 ml/well of complete medium and placed at 37°C until cell plating.
In vitro depolarization
Freshly isolated DRGs were plated at a density of 25.000 cells/35 mm2 well in complete media with or without KCl (40mM). The concentration was chosen based on previous studies showing an effect on neurite growth without affecting cell viability (Enes et al., 2010). As a control for osmolarity, 40mM NaCl was added to control wells. Cells were incubated for the indicated time, media was replaced in all wells and in some cases, cells were replated in normal media, fixed and immunolabeled at the time indicated.
To reinitiate growth, DRGs were replated at a certain time point as described (Saijilafu et al., 2013). Briefly, DRG neurons were cultivated for 1 h to 7 days as indicated, media was removed and cells were incubated with 0.125% Trypsin/EDTA for 5 min at 37°C. DRGs were collected by centrifugation (5 min at 800 rpm), suspended in serum-free complete medium and replated in newly coated plates to reinitiate axon growth. DRGs were fixed 24 h or 48 h after replating, or less, as specified.
Immunocytochemistry
Cultured DRG neurons were fixed with ice-cold 4% paraformaldehyde (PFA) for 30 min, blocked with tris-buffered saline (TBS), 0.1% Triton X-100, 5% donkey serum for 1 h at RT, then primary antibody (mouse anti-ßIII-tubulin; 1:1000; Promega; to identify neurons and their neurites) was added in TBS with 0.1% Triton X-100 (TBST), 1% donkey serum at 4°C overnight. Plates were washed 3× with TBS and secondary antibodies (donkey anti-mouse IgG coupled to Alexa Fluor-488 or -594;1:1000; Invitrogen) were added in TBST, 1% donkey serum for 3h at RT in the dark. DAPI (0.25 μg/ml) was added for 10 min in the first washing step, followed by 2 washing steps with TBS. Plates were maintained at 4°C in TBS, imaged, then covered with Fluoromount G (SouthernBiotech) and stored at RT in the dark.
Neurite growth assessment
Neurons and their neurites labeled for ßIII-tubulin were automatically imaged using an Olympus IX81 inverted microscope equipped with a motorized stage and controlled by Olympus Cell-P Software. Twelve 4×5 images were captured at 40× magnification and automatically stitched into non-overlapping composites per 35 mm2 well (72 images per 6-well plate). In some cases, 100× images were captured, resulting in 4×5 automatically stitched non-overlapping composites, 80% compressed for storage purposes (264 images per 6-well plate). Image files were converted in NIH ImageJ to 8-bit RGB format, opened and neurons were identified by ßIII-tubulin labeling. Longest neurite outgrowth assessments were done by semi-automatically tracing the longest neurite of each neuron using the NeuronJ plug-in, starting at the base of the neurite at the edge of the cell soma. The lengths in pixels were averaged for each well. The average longest neurite growth for each condition was then normalized to data from sham animals in the same experiment, to exclude possible influences of plate coating and isolation conditions, as well as potential surgery-related effects.
Cell classification by longest neurite length
To determine if the overall differences in average longest neurite values are due to increased/decreased neurite elongation or initiation, neurons were classified by the length of the longest neurite. The number of cells in each category was expressed as percentage of the total number of neurons. For replated cells with longer neurites, we used the categories <50, 50–250, 250–500 and >500μm, while for 24h growth immediately after isolation we used <20, 20–40, 40–100 and >100μm. Values shorter than the minimum diameter of a cell body (15–20μm) correspond to cells without neurites and were in some case separately analyzed.
Branching analysis
From all neurons analyzed for neurite growth, neurons with neurites longer than 100 μm were identified. Branch points of the longest neurite were manually counted. In some samples, additional images from the same wells were added to the analysis as the number of qualified neurons (neurites longer than 100 μm) was too low. On average, 30 neurons were analyzed per sample (total of 921 neurons). In one sample of the naive group, no neurons qualified for this analysis (all neurites shorter than 100 μm) and this sample was excluded, resulting in n=8 for ES, CL and sham conditions, n=7 for naïve animals. Data is expressed as the average number of branches per 100 um of neurite length for the longest neurite.
Surgeries
A total of 107 adult (8–14 weeks) female Fischer 344 rats (150–200g, Charles River Deutschland, Sulzfeld, Germany) were used for electrical stimulation, conditioning lesions and appropriate controls following European Commission Council Directives. All animal procedures were approved by institutional and state regulators. Animals had ad libitum access to food and water throughout the study. Within the same experiment, all groups received the same anesthesia. Isoflurane anesthesia (3–4% induction, up to 5 min and 1–1.5% maintenance) with an inhalator (FMI, Germany) was used for minimally-invasive surgeries including ES in chronically implanted animals, injection of the tracer cholera toxin ß-subunit (CTB) into the sciatic nerve (see below) and when anesthesia for extended time periods was required (ES for 7h). For surgeries involving dorsal column lesion (DCL) or cuff electrode implantation, anesthesia mix (62.5 mg/kg ketamine, 3.175 mg/kg xylazine, 0.625 mg/kg acepromazine) was administered intramuscularly (i.m.).
Postoperative pain was treated by subcutaneous (s.c) buprenorphine (Temgesic) injection (0.03–0.05 mg/kg twice a day) for 2 days followed by Carprofen (4–5 mg/kg once daily) as needed. Prophylactic antibiotics were delivered s.c. for 2 days post-surgery (ampicillin 167 mg/kg, twice daily). Control animals received either no manipulation (naïve) or implantation of electrodes without stimulation (sham). As a positive control, rats underwent CL of the sciatic nerve (crush at mid-thigh level for 20 s with a jeweler’s forceps). Cuff electrodes were placed on the sciatic nerve in the mid-thigh area in anesthetized rats, after exposing the nerve and gently dissecting the connective tissue, and ES was performed as described below. The electrodes were removed after ES, or remained in place for chronic/repeated ES, with a subcutaneous connector between the shoulder blades. Implants were left for up to 7 d for in vitro growth experiments and up to 28 d for in vivo growth experiments with no displacement or obvious adverse effects. Within each experiment, sham cuff electrodes were left in place for the same time as stimulating electrodes, to control for potential nonspecific effects due to the cuffs. In 2 pilot experiments, we performed ES, CL or sham unilaterally, to check for a potential influence of unilateral ES on the contralateral side. We found no indication of such effects in neurite growth assay. Therefore, right and left side of an animal were assessed independently in subsequent experiments. To minimize the influence of factors such as growth medium and laminin batch in in vitro growth assays, an equal number of sham animals were included in all experimental cohorts on the same day and data were normalized to sham values of the respective experiment.
Cuff electrodes were manufactured from silicone tube (8079, Science-Products, 1.574 mm inner diameter; 2.413 mm outer diameter), Teflon coated steel wire single strand annealed (SS-2TA, Science-Products) and connectors embedded in silicone non-toxic glue matching the output connector manufactured for the Iso-Flex stimulus isolator (A.M.P.I.). The silicone tube was cut in 3 mm cylinders with a longitudinal opening to insert the nerve into the cuff. The wires were fixed on one side of the cuff with knots, shaped as circular electrodes and isolation was removed on the inner surface of the cuff. Silicone glue was used, if necessary, to better fix or to isolate the wires outside the cuff. Resulting anode and cathode were 2–2.5 mm apart in the silicone cuffs. Final inner diameter of the cuffs was 1.2–1.4mm, resulting in loose enclosure of the sciatic nerve to avoid pressure to the nerve (Fouad and Pearson, 1997).
ES set-up and parameters
ES was applied to the intact nerve using Iso-Flex Stimulus Isolators controlled by a Digidata 1440 (Axon Instruments). The standard ES parameters used were: 20 Hz, constant current stimulation, pulse duration 0.2 ms for 1 h. These settings have been shown to be most effective for promoting DRG peripheral axon outgrowth (Geremia et al., 2007) and to also promote central sprouting (Udina et al., 2008). Stimulation intensity was set to 2 times motor threshold, assessed by the first visible movement of the hind-paw, activating both afferent and efferent large myelinated Aß-fibers and motor fibers. The motor threshold was determined independently for every nerve prior to every stimulation session, and intensity set accordingly. In 7 h ES experiments, polarity was switched after every hour, to make the stimulation as similar as possible to ES for 1h, while at the same time minimizing cumulative chemical changes. Stimulated sciatic nerves showed some variability in the intensity needed to evoke movements across subjects and experiments. To check for possible confounding factors from absolute stimulation intensity or different anesthesia used (described above), motor thresholds for sciatic nerves stimulated with ES 20Hz, 0.2ms, constant current stimulation, from all experiments were analyzed (anesthesia mix, n=28; anesthesia mix+dorsal column lesion, n=26; isoflurane, n=70). In case of repeated stimulation, only the threshold from the first stimulation session was included. Motor thresholds across all subjects were similar and not significantly influenced by anesthesia or dorsal column lesions (ANOVA p>0.05, data not shown). A few outliers from the value of the mean motor threshold were identified (ROUT test, Q<1%, p<0.05) in some experiments, likely due to loose cuffs, but were included in the neurite growth analysis.
Electrode impedance for cuff electrodes can vary considerably immediately after implantation, due to trapped air bubbles, which are slowly replaced by extracellular fluid (Loeb and Peck, 1996). Threshold measurements and stimulation were therefore initiated 5–10 min after implantation. Additionally, ES was done in a constant current regime, thus remaining effective irrespective of impedance changes (Clark, 2006).
In vivo growth of sensory neurons after central lesion and cell injection
Fischer 344 rats were anesthetized with anesthesia mix as described above and underwent a dorsal column lesion (DCL) and injection of bone marrow stromal cells (BMSCs) to fill the lesion, followed by either ES (n=7), or cuff electrodes implantation without stimulation (n=6). Cuffs were immediately removed after ES. Additional animals received either no manipulation of the sciatic (lesion only group) or CLs (n=6 each). To test if repeated ES could further increase growth in an in vivo setup, two additional groups with chronic implants with or without stimulation were examined, and a 1 h ES was repeated 7 days after the initial ES (n=6 each).
Dorsal column wire-knife lesion
Anesthetized animals were placed in a stereotaxic unit, the skin was incised longitudinally between C3 and C7 and the posterior arch of the vertebrae was exposed. A posterior laminectomy was performed at the C4 vertebra using a small rongeur and the spinal cord was exposed. A wire-knife (McHughMilieux) was first calibrated for depth of insertion and fixed into a stereotaxic micromanipulator. The spinal cord was punctured with a 30-gauge needle to insert the wire-knife 1.4 mm lateral to the midline, the wire was lowered to a depth of 1.1 mm ventral to the dorsal cord surface and extruded to form a 2.8 mm wide arc with the tip visible on the contralateral side of the posterior spinal cord just beneath the dura. The knife was slowly raised while pressing the spinal cord with a blunt tip, to ensure complete transection and was slowly retracted such that it did not penetrate the dura on the contralateral side. This lesion interrupts all dorsal column sensory fibers, leaving the dura and superficial blood vessels mostly intact (Blesch et al., 2012; Kadoya et al., 2009). Immediately following the lesion, 2 μl of BMSCs (60.000 cells/μl in PBS with 1% glucose; passage 2–4) were injected through pulled glass micropipettes into the center of the lesion at a depth of 0.5 mm using a Picospritzer at a rate of 1 μl/min to provide a cellular substrate for axonal growth. Pipettes were left in place for 1 min after the injection to prevent extrusion of cells and then slowly withdrawn. Overlying muscle layers were sutured and the skin was stapled. BMSCs were isolated from of 2–4 month old GFP transgenic Fischer 344 rats (F344-Tg(UBC-EGFP)F455Rrrc; Missouri Rat Resource Center) ubiquitously expressing green fluorescent protein (GFP) under the ubiquitin C promoter to identify grafted cells by GFP fluorescence as previously described (Dobson et al., 1999; Sandner et al., 2016). Briefly, bone marrow was harvested from tibias and femurs and cells were mechanically dissociated in Minimum Essential Medium alpha (α-MEM) (Merck Millipore) and recovered by centrifugation. Cell pellets were resuspended and cultivated in α-MEM containing 10% fetal bovine serum (αMEM-10% FBS) (PAA/GE Healthcare) and 100 U/ml penicillin/0.1 mg/l streptomycin (Invitrogen). After 3 days, the medium was changed and non-adherent cells were discarded. Adherent cells were incubated in fresh αMEM-10% FBS until cells were confluent.
CTB tracing
To visualize ascending dorsal column sensory fibers, animals were injected with the transganglionic tracer, cholera toxin ß-subunit (CTB; List Biologicals; 1%; 2 μl per side) bilaterally into the sciatic nerves, 3 days prior to perfusions. Rats were anesthetized with isoflurane, the sciatic nerve was exposed and the CTB was gently injected into the nerve with a Hamilton syringe, proximally to the crush or cuff location, as previously described (Blesch and Tuszynski, 2003). After 3 days (4 weeks post-lesion), rats were deeply anesthetized, transcardially perfused with ice cold saline followed by 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB). The brain and spinal cord were isolated and post-fixed in 4% PFA for 24 h followed by cryoprotection in 30% sucrose.
Histology and immunohistochemistry
Cervical spinal cords including the lesion site were cut into 35 μm sagittal sections using a cryostat (CM 3000, Leica, Germany) and 8 free-floating series were collected. Sections were rinsed 3× with TBS, incubated with 0.6% H2O2 for 15 min, followed by 2 washes in TBS and blocking in 5% horse serum in TBST (TBS, 0.25% Triton X-100) for 1 h. Sections were incubated in goat anti-CTB antibody (List Biological, 1:80.000 in TBST, 1% horse serum) for 72 h at 4 °C, washed 3× in TBS with 1% horse serum, and incubated with biotinylated horse anti-goat secondary antibody (Vector, 1:300 in TBS/1% horse serum) and avidin–biotin–peroxidase complex (ABC, Vector Laboratories) in TBS. The sections were treated with DAB substrate (Vector DAB kit, SK4100, Vector laboratories) for 3 min. After 3 washes, sections were blocked in 5% donkey serum for 1.5 h and incubated overnight at 4°C with antibodies against GFAP (Ms, Milipore, 1:1000) and GFP (Rb, Life Technologies, 1:750) diluted in TBST, 1% donkey serum. Sections were washed 2× with TBS and incubated with secondary antibody for 2.5 h at RT (IgG Alexa Fluor 594 Invitrogen, Ms, 1:300, IgG 488 Molecular Probes, Rb, 1:1000), followed by 3 washes in TBS. Sections were mounted on Superfrost slides, dried for 30–60 min and coverslipped with Cytoseal.
Quantification of axon growth within grafts
Spinal cord sections (1 out of 8 series) were screened for CTB-labeled axons and visualized under epifluorescent illumination (Olympus BX53) to identify the lesion border by GFAP (astrocytes) and GFP (cell graft) immunolabeling. The host/graft interface was identified as a virtual line rostral to any GFAP positive cell bodies and the number of CTB-labeled axons encountered along the rostral host/graft interface and at distances of 50 μm, 100 μm, 250 μm and 500 μm rostral to the interface were counted using a 10× ocular with a calibrated grid and a 40× objective. All measurements from the same animal were summed for each category. For quantification of growth cones and retraction bulbs in graft or host tissue, sections were imaged on an upright microscope both in light level for CTB and in epifluorescence for GFAP (astrocytes) and GFP labeling (transplanted cells/cell graft), at 200× magnification and image composites were assembled in CellO (Olympus), to cover the entire lesion area and CTB positive fibers with retraction bulbs in the caudal host tissue. The distance of all axon endings from the caudal GFAP-GFP border (retraction bulbs) and within the graft (growth cones/retraction bulbs) were measured using ImageJ. Counts were stacked into cumulative intervals for analysis (sum of the number of regenerated axons in 25μm or 50μm steps).
Sensory testing
Animals underwent sensory testing prior to dorsal column lesions and 4, 7, 14 and 24 days post-injury.
Von Frey filaments
Animals were habituated for 1 h/day for 3 days prior to the first sensory testing and for 30 min immediately before each testing in individual Plexiglas enclosures on an elevated grid platform (Ugo Basile). Responses to von Frey filaments (Ugo Basile) were assessed by stimulating the plantar center of the hind paws. A positive response was noted when the paw was withdrawn from the filament. After testing of a paw, a brief rest period of 2–3 min was allowed before testing the other paw with the same filament. We used 5 filaments of different strengths with an average response rate of ~10% for the thinnest filament and ~90% for the thickest filament in normal animals. Each filament was applied 5× per paw and the response rate was reported as percent response of all applications. If animals moved immediately after applying a filament instead of lifting the paw, the response was not considered and the filament was later reapplied.
Thermal sensitivity – Plantar Heater test (Hargreaves)
Animals were habituated for 1 h/day for 3 days prior to the first sensory testing and for 30 minutes immediately before every testing on an elevated glass platform in individual Plexiglas enclosures. Latency of hindlimb withdrawal in response to stimulation with a radiant heat source (Ugo Basile) was measured. The generator automatically stops upon paw withdrawal from the heat beam and the latency is electronically reported. We used an intensity of 60 units, corresponding to the upper mid emission range. If no paw withdrawal occurred after 30 seconds, the radiant source automatically stopped to prevent tissue damage and the animal was assigned the maximal withdrawal latency of 30 seconds. Each paw was assessed 4 times and the values were averaged. Testing was alternated between left and right foot with a delay for an individual paw of 10–12 minutes to prevent sensitization. If the enclosing had to be cleaned during testing, animals were allowed 5 more minutes of habitation before resuming measurements.
Data analysis and statistics
All samples from the same experiment were run in parallel, labeled and imaged together and assessed by an observer blinded to the nature of the experimental manipulation. Technical replicates were averaged. For in vitro neurite growth assays, data were normalized to values of sham-treated or naïve animals processed on the same day. At least 2 sham conditions were performed per group of animals/day.
Statistical comparisons were performed using GraphPad 7.0.1 software. Comparisons between 2 groups were performed by Student’s t-test, and by one-way or two-way analysis of variance (ANOVA) for comparisons of multiple groups followed by post-hoc tests/comparisons as indicated. For analysis of branching, a Kruskal-Wallis test followed by Dunn’s test was conducted due to unequal variances between groups. Statistical significance was accepted at p<0.05. Data are presented as mean ± standard error of the mean (SEM), unless otherwise indicated.
RESULTS
KCl-induced depolarization can inhibit or enhance neurite outgrowth of DRG neurons
Depolarization of adult neurons including DRG neurons with KCl has been reported to inhibit neurite growth (Enes et al., 2010). In contrast, short-term in vivo depolarization by electrical stimulation has been associated with increased neurite growth (Geremia et al., 2007; Udina et al., 2008). To resolve these contradicting data, we used KCl-mediated depolarization to mimic the cellular effects of neuronal activity. First, we were able to confirm neurite growth inhibition when DRG were incubated with KCl for 24h (One-way ANOVA, Sidak´s posthoc test, p<0.01) (Enes et al., 2010) (Fig.1.A–D). However, a shorter, physiologically more relevant depolarization for 1h, followed by a change to regular medium and growth for 48h mildly stimulated growth (~11%; p<0.05) compared to cells in control media or DGRs incubated with NaCl as osmolarity control (Fig.1.E–H).
Figure 1. KCl depolarization can inhibit or enhance DRG neurite growth.
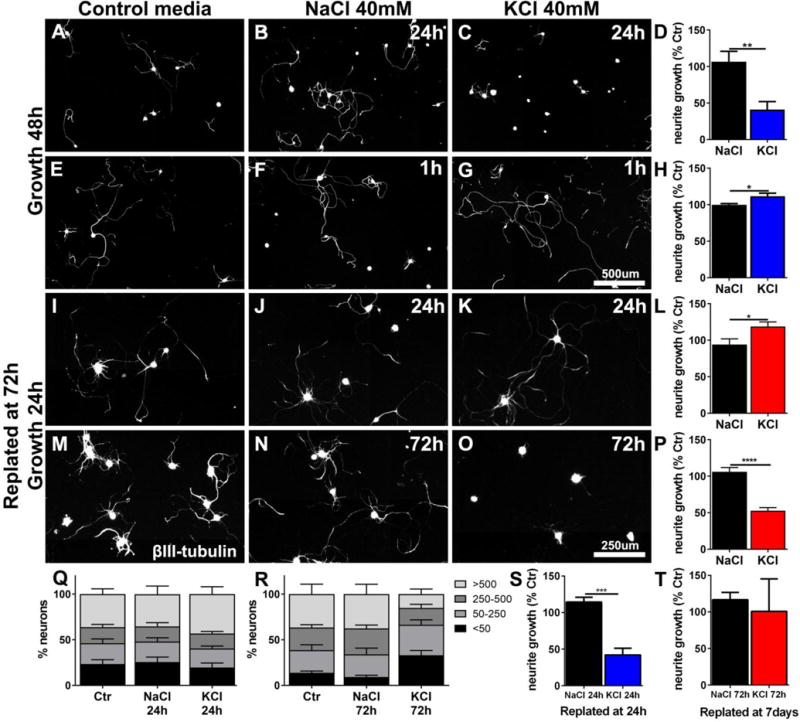
Naïve DRGs were isolated and cultured on PDL/Laminin (0.5μg/μl) for either (A-H) 48h, or (I-R) were replated at 72h and cultivated for an additional 24h. KCl was added to the culture medium, at the beginning of incubation, for (C) 24h or (G) 1h, and (K) 24h and then washed out and replaced with regular medium. (O) DRGs were kept in media with KCl for 72h and then immediately replated. Controls wells were incubated in (A, E, I, M) media only or (B, F, J, N) received NaCl (40mM) for osmolarity control for the same time as the KCl-treated DRGs. The longest ßIII-tubulin-labeled neurite was quantified. Average neurite length is expressed as % of control (176±80 neurons/well) (A-D) Growth is inhibited when DRGs are incubated in KCl for 24h (n=5–6 wells, 2 independent experiments). However, if (E-H) incubation is shorter (1h), total growth is enhanced (n=9–10 wells, from 3 independent experiments). (I-L) If DRGs are exposed to KCl for 24h then changed to normal media and replated at 72h in normal media, growth is enhanced (4 independent experiments). (M-P) If DRGs are exposed to KCl for 72h then replated in normal media, growth is inhibited (3 independent experiments). (Q) Neurons exposed to KCL for 24h and replated at 72h were classified by their longest neurite length (<50μm, 50–250μm, 250–500μm and >500μm) and expressed as % of the total number of neurons per condition. (R) The same classification for neurons exposed to KCl for 72h and immediately replated shows an increase in the percentage of neurons with very short neurites. (S) Growth is inhibited when 24h KCl exposed neurons were replated directly (n=3, 108±24neurons/well, 1-way ANOVA) (compare to L), (T) 72h KCl exposure is no longer inhibitory when cells are replated at 7 days (n=3, 159±35neurons/well) (compare to P). Bars represent means+SEM, One-way ANOVA, Sidak´s posthoc test, ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001.
To determine whether the inhibition of neurite growth after KCl exposure for 24h is due the length of exposure or a transient inhibition during KCl exposure, DRGs were incubated in KCl for 24h, the medium was changed to normal medium and cells were replated at 72h to reinitiate growth in normal media for 24h. Under these conditions, 24h of depolarization stimulated neurite growth (~25%; p<0.05) compared to NaCl-treated control neurons (Fig.1.I–L). However, if depolarization was maintained for the full 72h before replating in standard media, neurite growth was inhibited by ~53% after replating (p<0.001; Fig.1.M–P).
To investigate the type of growth response observed after KCl exposure and replating, neurons were classified by their maximum neurite length (<50 μm, 50–250 μm, 250–500 μm and >500 μm) (Fig.1.Q, R). The increase in overall neurite growth after 24h incubation in KCl and replating (Fig.1I–L) could be attributed to more DRG neurons with long neurites (>500μm) (Fig.1.Q). In contrast, growth inhibition by 72h incubation with KCl after replating in normal media was due to a larger percentage of DRGs without or only short neurites (<50μm) and a reduction in the percentage of cells with long neurites (>500μm) (Fig.1.R), suggesting reduced neurite growth initiation and reduced overall growth.
The previous experiments show that 24h incubation with KCl can be inhibitory during depolarization, or stimulating after delayed replating, while 72h incubation appears to be inhibitory also after the re-initiation of growth. To determine whether the actual duration of depolarization or the delay from KCl washout until the re-initiation of growth is the critical factor, we replated cells incubated for 24h with KCl immediately after the exposure. Under these conditions, neurite growth was inhibited (p<0.001) suggesting that a delay is needed to switch neurons to a growth-permissive state (Fig.1 S). Concomitantly, exposure of cells to KCl for 72h was no more inhibitory, if cells were switched to regular medium for 4 days and then replated (Fig.1 T). Thus, even extended periods of KCl-mediated depolarization have no long-term inhibitory effects, but rather stimulate neurite extension. Inhibitory effects are mainly due to a lack of axon growth during depolarization, and if sufficient time has passed following depolarization, detrimental effects on neurite growth are turned into increased neurite extension.
Electrical stimulation (ES) enhances ex-vivo growth of DRG neurons
To apply ES in vivo under standardized conditions, we used cuff electrodes placed around the rat sciatic nerves of both hindlimbs at mid-thigh level. ES was initially applied for 1h, while sham animals received cuff electrodes without stimulation for the same duration. For comparison, one group of naïve animals and one group of animals that underwent CLs was included. Lumbar (L4-L6) DRG neurons that were isolated 7 days after a single 1h ES and cultivated in vitro for 24h showed a nearly 2-fold increase in neurite growth compared to sham animals (ANOVA p<0.0001, Tukey post-hoc test p<0.01; Fig.2 E, F). Cuff electrodes in sham animals had no influence on neurite extension compared to naïve animals, and as expected, CLs resulted in the largest effect, significantly increasing neurite growth more than 4-fold compared to naïve animals (p<0.0001; Fig.2 B, F). When considering only neurite bearing cells, CLs increased neurite length by 374±44 μm compared to naive animals and ES by 137±44 μm compared to sham animals.
Figure 2. In vitro neurite growth 7 days after in vivo ES of the sciatic nerve.
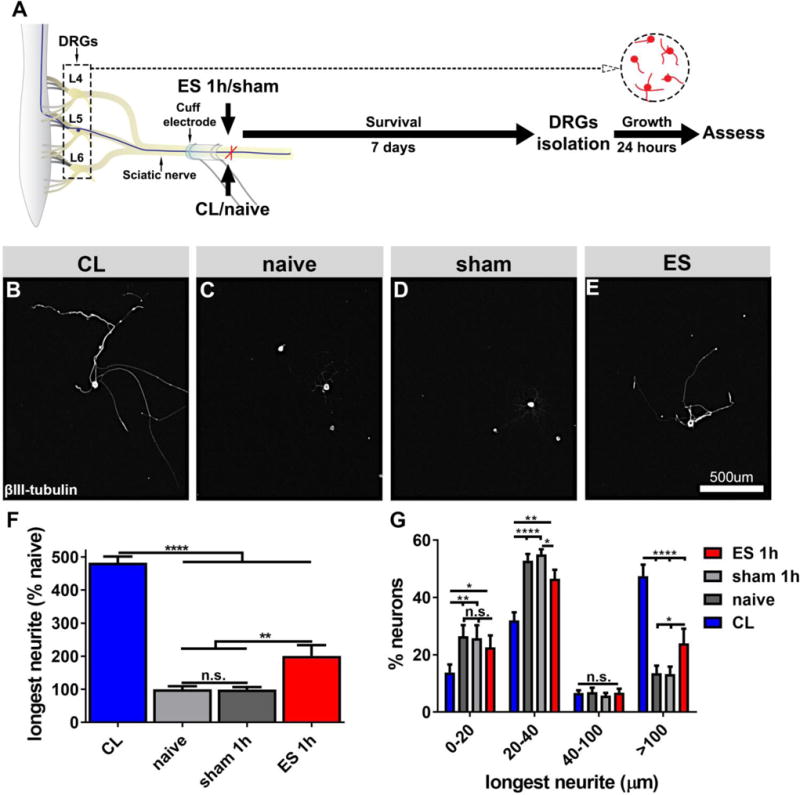
(A) Experimental design. DRGs (L4-L6) were isolated from (B) animals 7 days after CL, (C) naïve animals, (D) 7 days after sham or (E) after electrical stimulation. Cells were grown on PDL-laminin (5μg/ml) coated plates for 24 hours and labeled for βIII-tubulin. (F) Quantification of neurite growth demonstrates a strong effect of conditioning lesions, but 1h ES 7d prior to isolation is also growth-promoting (n=8/group, ANOVA p<0.0001, Tukey´s posthoc test). (G) Neurons classified by their longest neurites reveal that conditioning lesions enhance neurite initiation and extension, whereas electrical stimulation primarily enhances neurite extension (n=8/group, ANOVA p<0.0001, Tukey´s posthoc test). Mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
More detailed analyses showed that CL decreased the percentage of cells without or with very short neurites (<20μm and 20–40μm) and increased the percentage of cells with long neurites (>100μm). In contrast, ES did not influence neurite initiation (no change in the % of cells without neurites or neurites <20 μm; Fig.2 G), but increased the % of cells with neurites >100 μm. Similar to conditioned neurons, growth after ES was characterized by long, sparsely branched neurites (Suppl. Fig. 1). Neurons isolated from animals with CLs and 1h ES showed a reduction of branching by about 50% compared to naïve and sham animals (Kruskal-Wallis p=0.0001; Dunn’s test p<0.01 and p<0.05, respectively). Thus, ES seems to promote growth primarily through enhanced neurite extension similar to effects observed after KCl-mediated depolarization and delayed replating in vitro (Fig.1 Q).
Timing and duration of ES
To determine whether a delay between in vivo ES and DRGs isolation is necessary for enhanced in vitro neurite growth (as in the in vitro depolarization assays), neurons were isolated 1 day instead of 7 days after ES. With this shortened delay, no difference between ES and sham control animals in in vitro neurite growth was observed (Fig.3 A), indicating that a time delay to activate an intrinsic growth programs is necessary for the observed enhancement of neurite growth.
Figure 3. A delay longer than 1 day is necessary, while increased or repeated stimulation do not have additional growth-promoting effects.
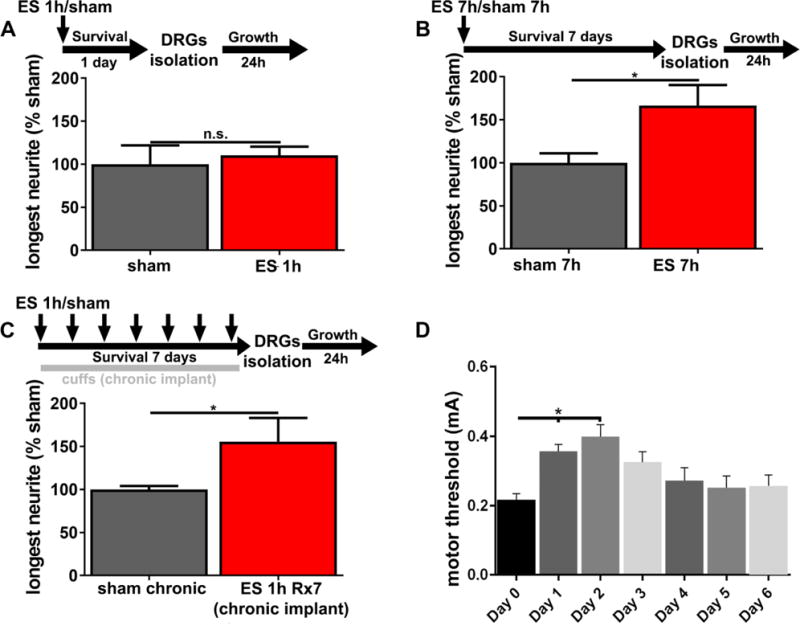
(A) Neurite growth of DRGs (L4-L6) isolated 1 day after ES is not enhanced (n=6, t-test p=0.68, mean ± SEM). DRGs (L4-L6) isolated (B) 7 days after a single 7h stimulation or (C) after daily 1h stimulations for 7 days show significantly increased neurite growth (n=5–8, t-test *p<0.05; mean ± SEM), but the effect size is similar to single 1h stimulation (compare to Fig.2). (D) For repeated stimulations (n=7, ANOVA p<0.01, Dunnett’s multi comparison test, *p<0.05), motor thresholds were determined on a daily basis, noticing a significant increase in the first 2 days post-surgery, with a return to baseline at later time points.
Because CL results in long-term de-afferentiation compared to a single 1h manipulation, and because a CL at the time of SCI followed by a second peripheral lesion one week later further increases neurite growth (Neumann et al., 2005), we further investigated whether repeated 1h stimulations on 7 consecutive days or a single continuous stimulation for 7h had more profound effects on neurite growth. Both, a single 7h stimulation session (Fig.3 B) and repeated stimulation (Fig.3 C) enhanced in vitro neurite growth compared to sham animals by 67% and 56%, respectively, an increase that did not exceed the effect size observed after a single 1h stimulation (compare to Fig.2).
Absolute stimulation intensity, motor threshold and anesthesia do not influence outcomes
To investigate whether other parameters might influence in vitro growth, we analyzed whether motor thresholds, and thus stimulation intensities correlate with the relative increase in neurite growth in animals that underwent a single 1h stimulation. Linear regression analysis did not indicate a correlation between stimulus intensity (motor threshold) and neurite growth (n=8, p=0.81, data not shown, ES animals from Fig. 2).
Motor thresholds of animals with repeated stimulation for 7 days indicated that the motor threshold significantly increased on days 1–2 after electrode implantation compared to Day 0 (n=7, ANOVA p<0.01, Dunnett’s multi comparison test, p<0.05, Fig.3 D), coinciding with the administration of postoperative analgesia (buprenorphine) during the first 2 days after implantation of cuff electrodes. On subsequent days, thresholds returned to baseline values, showing that electrodes were stable and functional for at least 1 week (Fig.3 D).
In vivo sensory axon growth after dorsal column lesions is enhanced by ES
To test the effects of ES on dorsal column sensory axon growth in vivo, animals underwent a C4 dorsal funiculus wire-knife lesion. Syngeneic GFP+ bone marrow stromal cells were injected into the lesion to provide a cellular substrate and ascending sensory axons were labeled with the transganglionic tracer cholera toxin ß (CTB) injected into the sciatic nerve after 4 weeks. As expected, animals with a CL showed a higher number of CTB-labeled axons extending beyond the caudal lesion border into the graft compared to animals that underwent only a spinal cord lesion and cell transplantation (Fig.4 A–F, M). A significantly higher number of fibers were found in animals that underwent CL at 0 μm, 50 μm and 100 μm compared to all other conditions (Fig.4 M, N). Naïve animals and animals with sham electrodes only exhibited a small number of CTB labeled axons in the lesion (Fig.4 D–F, G–I, M). Importantly, ES increased the number of injured sensory axons extending into the lesion compared to animals with sham electrodes (Fig.4 G–L, M) at 0 μm, 50 μm and 100 μm within the lesion compared to sham animals and the lesion only group. Overall axon growth (Fig.4 N) expressed as area under the curve, also indicated ES-induced increases in axonal growth, but to a lower degree than CLs.
Figure 4. Axonal growth 4 weeks after dorsal column lesions (DCL) and single or repeated 1h ES.
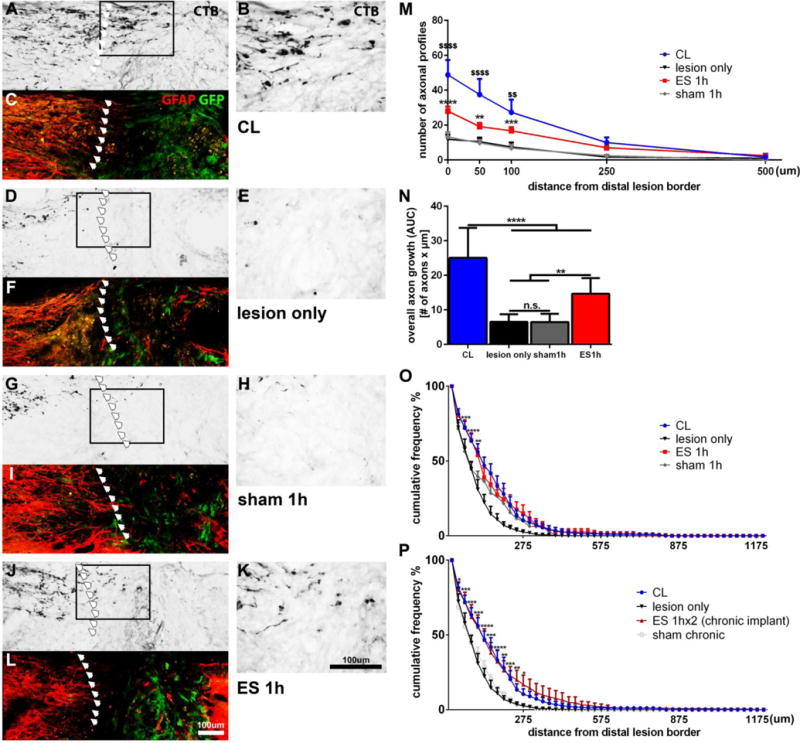
Following transection of ascending sensory axons at C4 and injection of GFP+ BMSCs into the lesion, animals received (A-C) bilaterally conditioning lesions, (D-F) no other manipulation, (G-I) electrodes with no stimulation or (J-L) electrical stimulation. (C, F, I, L) CTB-labeled axons were quantified at the caudal GFAP border (red) and within the lesion site filled with GFP+ BMSCs (green). Arrows mark the caudal lesion border. (B, E, H, K) Higher magnifications of boxed areas shown in (A, D, G, J). (M) The number of axonal profiles at different distances from the lesion site was quantified in 1 out of 8 serial sagittal sections. Significant differences between CL and lesion only animals ($$p<0.01, $$$$ p<0.0001), and between ES and sham animals (**p<0.01, ***p<0.001, ****p<0.0001) are indicated. (N) Overall axon growth (area under the curve; AUC) into the graft shows the highest growth in animals with CLs and a smaller but significant effect after ES. (O, P) The distance of CTB-labeled growth cones/axon tips within the graft was quantified from the caudal GFAP/GFP border. (O)The percentage of axons extending for a longer distance into the graft is significantly increased in ES compared to lesion only and sham animals. Sham 1h also shows an effect compared to lesion only. Significance shown for ES 1h versus sham 1h. (P) The percentage of axons that achieve growth over a longer distance into the graft is significantly increased in ES 1hx2 compared to lesion only and sham chronic. n=6–7/group, 2-way ANOVA, Tukey´s posthoc test. Mean ± SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. For comparison, naïve and CL animals shown in (O) are also shown in (P).
To further validate the growth-stimulating effects of a single session of ES, we also quantified the distance of axon tips (growth cones/retraction bulbs) within the graft from the caudal host/graft border (Fig.4 O). Because axons and their endings may not be present in the same section, this method samples differently. Increased growth in the ES group compared to the sham group were also detected up to 100 μm into the lesion site. However, sham animals also demonstrated significant differences to naïve animals between 125–200 μm (Fig.4 O) indicating that the cuff electrode implantation itself can influence outcomes.
Taken together, in vivo effects of ES are comparable to in vitro findings, including the effect size of ES in relation to CL.
Chronic implants and repeated stimulation enhances in vivo axon growth distance
Although repeated stimulation (7× 1h on 7 consecutive days) did not further increase neurite length compared to a single stimulation in in vitro growth assays (Fig. 2, 3C), other studies have indicated that repeating a CL in vivo 7 days after an initial CL has superior effects than a single peripheral lesion (Neumann et al., 2005). We therefore tested whether ES immediately post-lesion followed by a stimulation 7 days later for 1h would further increase growth examined 4 weeks post-lesion. Animals underwent spinal cord lesions, received cell grafts as described above, and chronic implantation of cuff electrodes. Animals with 2 stimulation sessions (immediately following spinal cord lesions and 7 days later; n=6) were compared to control animals that received chronic sham electrodes (n=6) and animals without electrode implantation.
Although repeated stimulation increased the number of fibers at short distances within the lesion site compared to animals without electrodes, (2-way ANOVA p<0.0001; Tukey’s multi comparison test: 0 μm, p<0.01; 50 μm, p<0.05), no difference was detected in comparison to chronic sham control animals (p>0.05). Chronic sham animals were more variable and also showed significantly more fibers extending into the lesion compared to the lesion only group (p<0.05) masking the effects of ES (data not shown). However, quantifying the cumulative % of axon tips (growth cones/retraction bulbs) within the graft from the caudal host/graft border of axons at different distances within the graft, a significantly larger proportion of axons extended for up to 275 μm in animals with repeated ES compared to sham animals (Fig.4 P) (n=6–7, 2-way ANOVA p<0.0001, Tukey’s p<0.05). Thus, repeated ES in animals with chronically implanted cuff electrodes resulted in a similar number of axons extending in the graft as chronic sham animals, but axons seem to extend for longer distances after ES.
All chronic electrodes remained functional within this first week post-implantation and were localized around the nerve when inspected during CTB injections on day 24 post-lesion, with no apparent damage or displacement. However, connective tissue frequently covered the cuff and prevented removal of the electrode after repeated stimulation without damage to the nerve. Nerves with chronic cuff electrodes were often neovascularized, around and within the cuff, and possible slight compression of the nerve could not be excluded.
ES has no influence on axonal dieback
To determine whether ES also influences axonal dieback, we also quantified the distance of CTB-labeled retraction bulbs from the caudal GFAP/GFP border in animals that underwent a single or repeated session of ES. The border between spinal cord tissue and cell graft was defined in sections double-labeled for GFAP (host tissue) and GFP (graft tissue), and the distance of each retraction bulb to the GFP/GFAP border was measured. Under all experimental conditions, retraction of some axons from the caudal lesion border, on average 250 μm was observed. No influence of CL or ES (single or repeated) on dieback was detected (n=6-7/group, ANOVA p>0.05, data not shown).
Evaluation of hypersensitivity and sensory recovery
To determine whether cuff electrodes or ES are associated with signs of neuropathic pain or result in restoration of function, mechanical thresholds (von Frey stimulation) and thermal sensitivity (Hargreaves’ test) of the hind paws were evaluated in animals with dorsal column lesions (Suppl. Fig.2 A). Plantar heat tests did not show any overall group differences or changes over time (2-way ANOVA p>0.05) (Suppl. Fig.2 B). As expected, thermal sensitivity was not affected by dorsal column lesions. Only animals with CL showed a transient trend towards increased latency at 4 and 7d post-lesion, likely due to the peripheral injury (Suppl. Fig.2 B).
Responses to touch were diminished in all groups immediately after the dorsal column lesion (2-way ANOVA, p<0.00001, Tukey’s posthoc test p<0.05 for all filaments) (Suppl. Fig.2 C) showing a trend towards recovery over time (2-way ANOVA, p<0.00001, Tukey’s posthoc test p<0.05) (Suppl. Fig.2 C).
Fine touch did not recover after a single 1h ES compared to any other group (Suppl. Fig.2) or by repeated stimulation (data not shown). No systematic differences were detected across groups, except in animals that underwent a CL. At 24 days post-lesion, the response rate to 8g and 10g was significantly higher in animals with CL than in animals that received only lesions and cell grafts (Suppl. Fig.2 C), with a similar trend for smaller filaments (2g, 4g, 6g) (Suppl. Fig.2 C). Indeed, responses to smaller filaments (2g and 4g) were significantly increased at 24d post-lesion compared to baseline measures in animals with conditioning lesions suggesting the development of mechanical hyper-responsiveness (2-way ANOVA; p<0.00001, Tukey’s posthoc test p<0.05) (Suppl. Fig.2 C, right panels). Taken together, ES had no adverse effects on thermal hyperalgesia or mechanical allodynia and consistent with histological data, ES-induced short distance sprouting into the graft but did not enhance functional recovery.
DISCUSSION
Neuronal activity and rehabilitative training have profound influences on functional restoration after CNS injury and can modulate sprouting of spared projections (Brus-Ramer et al., 2007; Carmel et al., 2010; Girgis et al., 2007), remyelination (Gautier et al., 2015), synaptic strength (Jiang et al., 2016; Raineteau and Schwab, 2001) and possibly axon guidance (Lim et al., 2016). The influence of activity of injured neurons on axonal regeneration is more controversial with some data suggesting that neuronal silencing rather than activation is beneficial. We therefore first examined the effects of depolarization in adult DRG cultures. Similar to previous studies (Enes et al., 2010), we observed inhibition of neurite extension during prolonged 24h KCl depolarization (Fig.1 A–D). However, brief KCl exposure for 1h, or delayed replating of DRGs after extended KCl exposure (Fig.1 E–L) did increase neurite growth. Thus, duration and timing are crucial parameters and continuous depolarization for 24h or even 72h, while having limited physiological relevance, just require longer intervals for neurons to initiate a growth promoting program.
As KCl-induced depolarization and particularly depolarization for extended time periods only partially simulate action potentials and neuronal activity in vivo, which is normally stimulus evoked and phasic, we investigated in vitro neurite growth after in vivo ES. Indeed, ES of the sciatic nerve stimulated in vitro DRG neurite growth (Fig.2), consistent with previous findings (Udina et al., 2008). However, our data show an effect size approximately half of what was previously reported despite minimal differences in culturing duration, means of stimulation and substrate for neurite growth (Udina et al., 2008). Factors such as surgical manipulation of the sciatic nerve, potentially conditioning DRGs can significantly influence the effect size. To exclude such effects, DRGs from animals that received identical manipulation (electrode implantation without ES) were included as controls in every experiment and cohort in the current study. While cuff electrodes with circumferential contacts and a longitudinal separation recruit nerve fibers more homogenously (Loeb and Peck, 1996), nerve cuffs can result in thickening of epineurium and ingrowth of endometrial connective tissue (Agnew et al., 1989; Loeb and Peck, 1996). Although the cuffs used in this study were designed to avoid compression by leaving sufficient space around the nerve, chronically implanted cuffs were enclosed by connective tissue, and the presence of cuffs over 4 weeks resulted in slightly increased axon growth, acting as a confounding factor (Fig.4 P). Thus, other means of ES such as transcutaneous stimulation might be better suited to investigate repeated in vivo long-term stimulation paradigms.
Inclusion of animals that underwent CL as positive control also demonstrated that the ES-induced increase in neurite growth does not reach the same level as a CL-mediated effects, both in vitro (Fig.2 F) and in vivo (Fig.3 P). Unlike CLs, which increased both neurite elongation and initiation, ES only increased the percentage of neurons with neurites >100μm, without influencing the percentage of neurite bearing neurons (Fig.2 G). The mean increase in neurite length by ES is therefore due to enhanced elongation and not neurite initiation. Importantly, ES did not have any effects on neurite growth when DRGs were isolated 1 day after ES (Fig.3 A), but a delay was necessary for growth-promoting effects to develop. These data further suggest that similar to conditioning lesions, changes in the intrinsic growth potential require time to become effective and might depend on activation of gene expression after ES. Changes in gene expression after in vitro depolarization might also underlie the increased growth of adult DRG neurons upon KCl washout and replating and are consistent with the hypothesis that brief activity induced by ES can stimulate growth at a later time point. Taken together, a similar time dependence, but a smaller effect size and differences in neurite initiation versus extension indicate similarities and differences between ES and CL.
To further enhance DRG neurite growth, repeated stimulation (1 h/day for 7 days) or extended stimulations (7h) were employed in vivo, surprisingly not increasing in vitro neurite growth (Fig.3 B, C). This is consistent with findings after PNS injury, where ES applied continuously for up to 2 weeks did not further enhance motor axon regeneration (Al-Majed et al., 2000b). Similarly, for regeneration of sensory fibers after PNS injuries, ES applied for more than 1 h, such as 3 h, 7 h, 1 day or 14 days did not show significant differences from the unstimulated control femoral nerve (Geremia et al., 2007), and 1h ES daily for 4 weeks, did not further improve sciatic nerve regeneration (Asensio-Pinilla et al., 2009). These studies as well as our results, support the idea that initial activity is the key factor for “priming” neurons, and a brief application of activity is sufficient to trigger the underlying mechanisms. The lack of additional benefits after 7h of stimulation in our study also indicates that unspecific effects on growth from local electrolysis, which is directly proportional to the stimulation time according to Faraday’s Law (Ehl and Ihde, 1954) cannot account for the observed ES-mediated growth.
We also found that a single ES facilitates in vivo growth after a dorsal column lesion, when ES was applied acutely after a SCI (Fig.4). We evaluated in vivo growth with two different methods, either quantifying axonal profiles crossing the tissue-lesion border and extending into the lesion (Fig.4 M, N), or measuring the distance of growth cones/axon tips within the graft from the caudal host/graft interface (Fig.4 O, P). Both methods show a similar increase in the number of fibers extending into the graft after ES. Repeated stimulation in vivo with a first stimulation immediately after spinal cord lesions and cell injection, followed by a stimulation 7 days later, also did not have additive effects on axon extension into a cell graft (Fig.4 N, P) recapitulating in vitro neurite growth data.
Previous studies have shown adverse effects from long term ES of peripheral nerves, such as edema appearing as early as 2 days after ES and early axonal degeneration of large myelinated fibers at 7 days (Agnew et al., 1989; McCreery et al., 1992). However, these studies used considerably higher frequencies (50Hz) and prolonged stimulation (8–16h), and 18h stimulation at 20 Hz did not induce degeneration or edema (Agnew et al., 1989). ES for 8h, with short charge balanced pulses stimulating primarily large axons, has a threshold for damage about 2.2–2.6 times higher than the intensity for full A-fibers recruitment (McCreery et al., 1992). The intensity used here, 2× motor threshold, is considerably below the threshold for damage for 1h stimulation, and likely also for the 7h stimulation. This is also supported by our results from sensory testing. Potential adverse effects such as mechanical allodynia or thermal hyperalgesia due to aberrant spouting of nociceptive fibers were not observed. Neither a decrease in latency to thermal stimuli nor an increase in the response rate to mechanical stimuli was evident comparing animals with ES and sham animals. (Suppl. Fig.2 B, C). However, mechanical sensitivity/fine touch was clearly affected by the spinal dorsal column lesions in all animals as one would expect from this type of lesion (Suppl. Fig.2).
Recovery of sensory function did not differ between animals that underwent ES and sham, a finding that is not surprising without guidance and growth stimulation of dorsal column sensory axons across the lesion to form appropriate synapses with their target (Alto et al., 2009). ES, similar to other approaches addressing only one factor impeding CNS regeneration, has to be combined with other strategies to foster sufficient axonal regeneration for functional reinnervation (Fouad et al., 2005; Houle et al., 2006; Taylor et al., 2006; Tom et al., 2009). Surprisingly however, animals that underwent a CL showed a full recovery of response rates to large von Frey filaments 24d post injury (Suppl. Fig.2 C). In fact, these animals even showed an increased withdrawal response rate to small filaments, a possible sign of mechanical allodynia or overall spinal hyperreflexia. In contrast, thermal sensitivity was not affected by lesions and there was no change in response rates or signs of thermal hyperalgesia in any of the groups (Suppl. Fig.2 B).
Compared to other activity-based strategies such as training, which can be influenced by self-training and compensatory behavior (Caudle et al., 2011; Fouad et al., 2000), ES can be applied immediately post-injury, independent of lesion severity, it is easy to control and can be adjusted to the physiological properties of neural structures. The application and relevance of ES as a therapeutic strategy in SCI is also supported by recent studies that have demonstrated the overall importance of sensory input as well as reduced functional recovery after SCI without appropriate sensory input, revealing complex roles in local plasticity, such as remodeling of CST axons (Jiang et al., 2016), reorganization of circuits and recovery of motor skills after SCI (Takeoka et al., 2014), and modulating spinal circuits underlying semi-automatic movements (Thompson and Wolpaw, 2015).
Taken together, this study provides further support that neuronal activity has time-dependent effects in promoting sensory neuron axon growth without adverse effects on nociception, and that modulating activity through ES can enhance regeneration in the injured CNS. For this promising strategy to become clinically relevant, further optimization of ES parameters, targeted stimulation and a better understanding of mechanisms and potential interactions with other pro-regenerative strategies are needed.
Supplementary Material
Supplementary Figure 1: In vitro neurite branching 7 days after in vivo ES of the sciatic nerve. DRGs (L4-L6) were isolated from animals 7 days after CL, naïve animals, 7 days after sham or after electrical stimulation. Cells were grown on PDL-laminin (5μg/ml) coated plates for 24 hours and labeled for βIII-tubulin. Quantification of branching points of neurites longer than 100 μm shows a reduction in neurite branching after 1h ES comparable to CL (n=7-8/group, Kruskal-Wallis p<0.0001, Dunn’s posthoc test). Mean ± SEM. *p<0.05, **p<0.01.
Supplementary Figure 2: Analysis of thermal and mechanical sensitivity in animals with dorsal column lesions does not indicate ES-mediated adverse effects. (A) Sensory testing was performed one day before lesions (baseline testing) and 4, 7, 15 and 24 days post-surgery. (B) Hargreaves plantar heat test does not show differences across time or groups (2-way ANOVA p>0.05, mean ± SEM). (C) There is no global difference between groups for any of the filaments tested (2, 4, 6, 8 10g). Animals with CL seem to regain sensitivity to thicker filaments (8, 10 g) at 24 days after surgery compared to lesion only. (C, right panel) Time plays a similar role across groups. Responses after von Frey stimulation (2, 4, 6, 8, 10g) indicate a similar decrease in response frequency in all groups after dorsal column lesions, with some recovery over time. Animals with CL develop response rates that are significantly higher than baseline values for small filaments (2g and 4g) at 24 days. Other changes are non-systematic, indicate a tendency for recovery across all groups, but no adverse effects. n=6-7/group, 2-way ANOVA interaction and time p<0.0001, Tukey´s posthoc test, mean ± SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Highlights.
- KCl-induced depolarization can promote or inhibit neurite growth in a time-dependent manner
- increases in neurite growth after electrical stimulation depend on a delay of more than 1 day
- prolonged or repeated electrical stimulation are not superior in enhancing neurite growth
- Electrical stimulation in vivo enhances axon growth into a cellular graft after spinal cord injury
Acknowledgments
Supported by grants from the International Spinal Research Trust (Res: 0011173), the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative, the Indiana Spinal Cord and Brain Injury Research Fund and by a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108. We would like to thank to the Missouri Rat Resource Center for providing GFP-transgenic rats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
none
References
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:630–643. doi: 10.1152/jn.2001.85.2.630. [DOI] [PubMed] [Google Scholar]
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- Agnew WF, McCreery DB, Yuen TG, Bullara LA. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: considerations for the selection of parameters. Ann Biomed Eng. 1989;17:39–60. doi: 10.1007/BF02364272. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000a;12:4381–4390. [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000b;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto LT, Havton LA, Conner JM, Hollis ER, Ii, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J Neurophysiol. 2003;90:3764–3773. doi: 10.1152/jn.00449.2003. [DOI] [PubMed] [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Schwab ME. Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 2003;26:555–563. doi: 10.1016/j.tins.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Blesch A, Lu P, Tsukada S, Taylor L, Roet K, Coppola G, Geschwind D, Tuszynski MH. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to cAMP-mediated effects. Exp Neurol. 2012;235:162–173. doi: 10.1016/j.expneurol.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transection, and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194:221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle KL, Brown EH, Shum-Siu A, Burke DA, Magnuson TS, Voor MJ, Magnuson DS. Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabil Neural Repair. 2011;25:729–739. doi: 10.1177/1545968311407519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. Cochlear Implants: Fundamentals and Applications. Springer; New York: 2006. [Google Scholar]
- Cobianchi S, Casals-Diaz L, Jaramillo J, Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp Neurol. 2013;240:157–167. doi: 10.1016/j.expneurol.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Dobson KR, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411–413. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- Ehl RG, Ihde AJ. Faraday’s electrochemical laws and the determination of equivalent weights. Journal of Chemical Education. 1954;31:226. [Google Scholar]
- Enes J, Langwieser N, Ruschel J, Carballosa-Gonzalez MM, Klug A, Traut MH, Ylera B, Tahirovic S, Hofmann F, Stein V, Moosmang S, Hentall ID, Bradke F. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr Biol. 2010;20:1154–1164. doi: 10.1016/j.cub.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pearson KG. Effects of extensor muscle afferents on the timing of locomotor activity during walking in adult rats. Brain Res. 1997;749:320–328. doi: 10.1016/S0006-8993(96)01328-5. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier HO, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Lao-Peregrin C, Reynolds R, Franklin RJ, Karadottir RT. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat Commun. 2015;6:8518. doi: 10.1038/ncomms9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Gordon T, Brushart TM, Chan KM. Augmenting nerve regeneration with electrical stimulation. Neurol Res. 2008;30:1012–1022. doi: 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- Hoffman PN. Expression of GAP-43, a rapidly transported growth-associated protein, and class II beta tubulin, a slowly transported cytoskeletal protein, are coordinated in regenerating neurons. J Neurosci. 1989;9:893–897. doi: 10.1523/JNEUROSCI.09-03-00893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PN. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp Neurol. 2010;223:11–18. doi: 10.1016/j.expneurol.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma. 2014;31:1088–1106. doi: 10.1089/neu.2013.3096. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YQ, Zaaimi B, Martin JH. Competition with Primary Sensory Afferents Drives Remodeling of Corticospinal Axons in Mature Spinal Motor Circuits. J Neurosci. 2016;36:193–203. doi: 10.1523/JNEUROSCI.3441-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin M, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci. 2016;19:1073–1084. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J Neurosci Methods. 1996;64:95–103. doi: 10.1016/0165-0270(95)00123-9. [DOI] [PubMed] [Google Scholar]
- McCall J, Nicholson L, Weidner N, Blesch A. Optimization of adult sensory neuron electroporation to study mechanisms of neurite growth. Front Mol Neurosci. 2012;5:11. doi: 10.3389/fnmol.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, Yuen TG, Bullara LA. Damage in peripheral nerve from continuous electrical stimulation: comparison of two stimulus waveforms. Med Biol Eng Comput. 1992;30:109–114. doi: 10.1007/BF02446202. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- Neumann S, Skinner K, Basbaum AI. Sustaining intrinsic growth capacity of adult neurons promotes spinal cord regeneration. Proc Natl Acad Sci U S A. 2005;102:16848–16852. doi: 10.1073/pnas.0508538102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Saijilafu Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun. 2013;4:2690. doi: 10.1038/ncomms3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandner B, Ciatipis M, Motsch M, Soljanik I, Weidner N, Blesch A. Limited Functional Effects of Subacute Syngeneic Bone Marrow Stromal Cell Transplantation After Rat Spinal Cord Contusion Injury. Cell Transplant. 2016;25:125–139. doi: 10.3727/096368915X687679. [DOI] [PubMed] [Google Scholar]
- Sleeper AA, Cummins TR, Dib-Hajj SD, Hormuzdiar W, Tyrrell L, Waxman SG, Black JA. Changes in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomy. J Neurosci. 2000;20:7279–7289. doi: 10.1523/JNEUROSCI.20-19-07279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Restoring walking after spinal cord injury: operant conditioning of spinal reflexes can help. Neuroscientist. 2015;21:203–215. doi: 10.1177/1073858414527541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008;210:238–247. doi: 10.1016/j.expneurol.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Wood M, Willits RK. Short-duration, DC electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics. 2006;27:328–331. doi: 10.1002/bem.20214. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, Song XJ, Lamotte RH. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: In vitro neurite branching 7 days after in vivo ES of the sciatic nerve. DRGs (L4-L6) were isolated from animals 7 days after CL, naïve animals, 7 days after sham or after electrical stimulation. Cells were grown on PDL-laminin (5μg/ml) coated plates for 24 hours and labeled for βIII-tubulin. Quantification of branching points of neurites longer than 100 μm shows a reduction in neurite branching after 1h ES comparable to CL (n=7-8/group, Kruskal-Wallis p<0.0001, Dunn’s posthoc test). Mean ± SEM. *p<0.05, **p<0.01.
Supplementary Figure 2: Analysis of thermal and mechanical sensitivity in animals with dorsal column lesions does not indicate ES-mediated adverse effects. (A) Sensory testing was performed one day before lesions (baseline testing) and 4, 7, 15 and 24 days post-surgery. (B) Hargreaves plantar heat test does not show differences across time or groups (2-way ANOVA p>0.05, mean ± SEM). (C) There is no global difference between groups for any of the filaments tested (2, 4, 6, 8 10g). Animals with CL seem to regain sensitivity to thicker filaments (8, 10 g) at 24 days after surgery compared to lesion only. (C, right panel) Time plays a similar role across groups. Responses after von Frey stimulation (2, 4, 6, 8, 10g) indicate a similar decrease in response frequency in all groups after dorsal column lesions, with some recovery over time. Animals with CL develop response rates that are significantly higher than baseline values for small filaments (2g and 4g) at 24 days. Other changes are non-systematic, indicate a tendency for recovery across all groups, but no adverse effects. n=6-7/group, 2-way ANOVA interaction and time p<0.0001, Tukey´s posthoc test, mean ± SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.


