Abstract
Allergy cases are increasing worldwide. Currently allergies are treated after their appearance in patients. However, now there is effort to make a preventive vaccine against allergies. The rationale is to target patient populations that are already sensitized to allergens but have yet to develop severe forms of the allergic disease, or who are susceptible to allergy development but have not yet developed them. Subcutaneous injections and the sublingual route have been used as the primary mode of preventive vaccine delivery. However, injections are painful, especially considering that they have to be given repeatedly to infants or young children. The sublingual route is hard to use since infants can’t be trained to hold the vaccine under their tongue. In the present study, we demonstrate a microneedle (MN)-based cutaneous preventive allergy treatment against ovalbumin (Ova)-induced airway allergy in mice. Insertion of MNs coated with Ova as a model allergen and CpG oligonucleotide as an adjuvant (MNs-CIT) into the skin significantly induced Ova specific systemic immune response. This response was similar to that induced by hypodermic-needle-based delivery of Ova using the clinically-approved subcutaneous immunotherapy (SCIT) route. MNs-CIT upregulated Ova-specific Th2 cytokines (IL-4, IL-5 & IL-13) and anti-inflammatory cytokines (IL-10) in the bronchoalveolar fluid, and IL-2 and IFN-γ cytokines in restimulated splenocyte cultures. Absence of mucus deposition inside the bronchiole wall and low stiffness around the lung bronchioles after Ova-allergen challenge further confirmed the protective role of MNs-CIT. Overall, MNs-CIT represents a novel minimally invasive cutaneous immunotherapy to prevent the progression of Ova induced airway allergy in mice.
Keywords: Airway allergy, Microneedles, Preventive allergy vaccine, Subcutaneous allergy immunotherapy
Graphical abstract
1. Introduction
Appearance of airway allergies is a common public health issue and their prevalence is steadily on the rise worldwide [1, 2]. Airway allergies are largely type 1 hypersensitivity reactions, and they are characterized by elevated levels of systemic allergen-specific IgE antibodies and hyperreactivity of the airway tract [3]. Medications such as anti-IgE therapy, and anti-histamines, which can be administered orally or systemically via injections are available for short term relief [4, 5]. However, allergen specific immunotherapy (ASI) is the only approach to treat allergies permanently [6]. ASI is recommended for allergy patients who have allergen-specific IgE in their serum [6, 7]. In conventional ASI involving subcutaneous allergy immunotherapy (SCIT), multiple injections of increasing doses of a specific allergen are given to the patient until a therapeutic level is reached. The treatment is time consuming because it spans many years, is painful, and is linked to potential systemic reactions, or occasional anaphylaxis [8, 9].
The current form of SCIT is only initiated once a patient has developed allergies. Thus, to halt the allergy pandemic, recently a true mode of vaccination in the form of ‘prophylaxis/preventive allergy treatment’ has come into light [10, 11]. Preventive vaccination can begin in healthy individuals even with a negative skin prick test (i.e., have not yet developed allergen-specific IgE antibodies), but who are genetically susceptible to allergy development. This immunomodulation starts in early childhood to block allergy development in later phases of life, and is thus termed as ‘primary prevention’ [11]. Few case studies have been reported on primary preventive allergy immunomodulation. A dust mite allergy study showed the efficacy of preventive immunomodulation in 111 infants who were less than a year old and received an oral dose of the house dust mite (HDM) extract twice daily for 12 months [12]. As a result, although the risk of allergy development to common allergens was reduced, but allergy development to HDM was not blocked [12]. An important milestone-observation from this study was that the exposure of infants to HDM who were yet not sensitized to the HDM allergen did not cause an increase in their HDM-specific or non-specific allergies, suggesting that such immunomodulatory treatments are potentially safe.
Another form of allergy prevention is ‘secondary prevention’, which has multiple connotations: (i) to block disease development in already sensitized individuals, for example to prevent development of asthma if the patient is already allergic to airway allergens but does not yet have asthma, and, (ii) to prevent development of a second sensitization if a patient is already allergic to one allergen. For instance, allergic rhinoconjunctivitis is a risk factor for asthma development, meaning patients with allergic rhinoconjunctivitis could develop asthma over time. It was recently shown that a three year long subcutaneous ASI in patients with allergic rhinoconjunctivitis to grass and/or birch pollen reduced their risk of asthma development during treatment and two years after discontinuation of treatment [13]. In another study over 800 children between the ages of 5–12 y and with grass-allergen-induced rhinoconjunctivitis but with no signs of asthma were administered grass allergen immunotherapy tablets sublingually [14]. The objective was to test if this could reduce or prevent asthma development during the three years of treatment and two years post-treatment. After immunotherapy a significantly fewer number of children experienced asthma or used asthma medication, and this effect sustained for two years after end of treatment [15]. The trial also demonstrated that allergic rhinoconjunctivitis symptoms were also significantly reduced.
Allergy preventive studies have thus far used the conventional SCIT route, the oral route, or the sublingual route, at preclinical and clinical levels [12, 14, 16–19]. However the problem of holding the formulation under the tongue for few minutes by infants in sublingual immunotherapy is a concern. For example, a pilot study for dust mite allergy prevention in infants was terminated early without any major conclusions due to the inability to train infants to hold the liquid formulation under the tongue for two minutes [20]. The SCIT shots are painful, and the efficacy of the oral route is not as good as the SCIT route [21]. For these reasons, there is a need to develop an efficient preventive immunotherapy treatment approach.
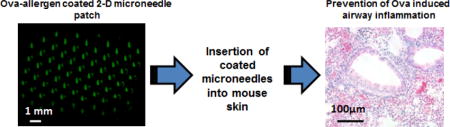
With this perspective, we demonstrate here a novel painless microneedle (MN)-based cutaneous preventive immunotherapy against Ovalbumin (Ova)-induced airway allergy in mice, and compare its efficacy with the conventional SCIT. MNs are micrometer-sized needles, which can deliver the drug or antigen/allergen into the skin by rupturing the stratum corneum (topmost layer of the skin) and they can activate the immune response effectively [15, 22]. They have been widely explored in the development of infectious diseases vaccines [15, 22, 23]. For the very first time, we demonstrate here their efficacy to deliver the allergen in to the skin for primary protection against allergy progression in mice.
2. Materials and methods
2.1 MN fabrication, coating and characterization
MN arrays with 57 individual MNs (Fig. 1A) were fabricated from stainless steel (304) sheets through a wet etch process [24]. MNs were manually bent to make them perpendicular to the base. MNs were coated by using a micro-precision dip coating machine assembled in house [22, 25]. The coating solution consisted of carboxymethyl cellulose (CMC) (1%, w/v) (low viscosity, USP grade, CarboMer, San Diego, CA, USA) as a viscosity enhancer, Lutrol F-68 NF (BASF, Mt. Olive, NJ, USA) as a surfactant, and ovalbumin (Ova) (50 mg/ml) (MP Biomedicals, LLC, Ohio, USA) as a model allergen. To examine deposition of coatings on MNs, Ova was coupled with amine reactive 1-Ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinamide (NHS) activated fluorescein dye (FITC, Thermo Scientific, Pierce Biotechnology, Rockford, IL, USA). This FITC-conjugated Ova was coated on MNs and stereomicrographs were obtained before and after insertion of the MNs into mouse skin using a stereomicroscope (Olympus SZX16, Olympus America Inc.). The delivery efficiency of MNs coated with FITC-conjugated Ova was determined as described previously using calibrated fluorescent spectroscopy [22, 26].
Fig. 1. MNs coating, delivery efficiency and Ova specific immune response.
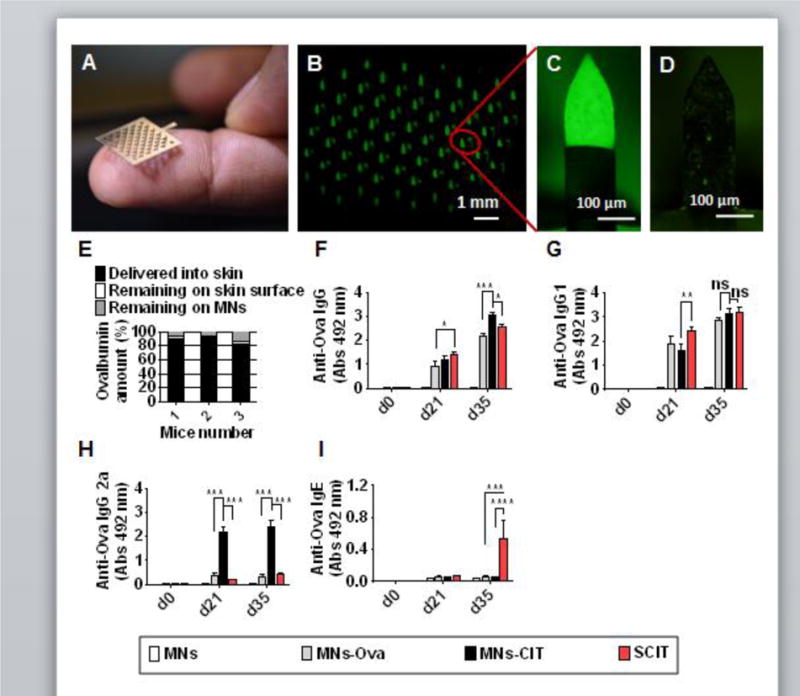
(A) Digital photograph of a MN 2-D patch. (B) Fluorescent micrograph of a MN patch coated with FITC-conjugated Ova. (C, D) Zoom-in fluorescent micrographs of a single MN of the 2-D patch before and after insertion into the mouse skin. (E) In vivo delivery efficiency of Ova-coated MNs after insertion into mouse skin. (F) Anti-Ova IgG response after vaccine administration. (G) Anti-Ova IgG1 response after vaccine administration. (H) Anti-Ova IgG2a response vaccine administration. (I) Anti-Ova IgE response after vaccine administration. Mice were categorized as MNs, MNs-Ova, MNs-CIT and SCIT groups (Table 1). They were immunized at d0, 7 and 14. Sera were collected at d35 to measure anti-Ova response by ELISA. Individual mouse serum was diluted to 1:20 and used in analysis. Error bars denote mean ± SEM. *: p < 0.05; ***:p < 0.0005, and ****: p < 0.0001, ns; not significant.
2.2 Mice
Balb/c female mice, aged 6–8 weeks (Charles River laboratories, USA) were used in the experiments. Mice were housed under climate controlled environment with a 12 h dark and 12h light cycle. Wood shavings, paper and plastic pipes were used as housing enrichment materials in air-ventilated polystyrene cages. Standard food and water were provided throughout the experiments. The diet was confirmed to be egg-free to eliminate oral Ova exposure through the diet. All animal experiments were performed under guidelines of the approved protocol by Institutional Animal Care and Use Committee (IACUC) at Texas Tech University, USA.
2.3 Preventive immunotherapy schedule
Mice were vaccinated on days 0, 7 and 14. Mice were divided in to 6 groups with 5 mice in each group and the different groups were treated as per Table 1: (i) MNs coated with the coating solution but without Ova (MNs), (ii) MNs coated with the coating solution containing Ova (MNs-Ova), (iii) MNs coated with the coating solution containing Ova and CpG (MNs-CIT), (iv) Ova with alum injected subcutaneously (SCIT), (v) naïve mice that received no treatment over the period of the study, and (vi) mice that were not vaccinated but were challenged with Ova. Phosphorothioated CpG-ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was custom synthesized by Integrated DNA Technologies (Coralville, IA).
Table 1.
Treatment groups and dose
| Groups | Vaccination | Sensitization (25 μg Ova + 2 mg alum via IP route)) | Challenge (50 μg Ova in 50 μI PBS via nasal route) | Comment |
|---|---|---|---|---|
| MNs | Uncoated MNs (without Ova) | + | + | Negative control, expected to induce high inflammation |
| MNs+Ova | Ova coated MNs (25 μg Ova) | + | + | Test group to study efficacy of MNs to deliver Ova into skin |
| MNs+Ova+CpG | Ova+CpG coated MNs (25 μg Ova+25 μg CpG) | + | + | Test group to study effect of CpG as adjuvant |
| Ova+alum | Ova (25 μg)+Alum (250 μg) | + | + | Positive control to mimic clinically approved subcutaneous allergy immunotherapy procedure |
| Naive | − | − | − | Negative control (without any treatment) |
| Ova challenge | − | − | + | Negative control to assess effect of Ova challenge through intranasal route |
Sensitization: Two doses of 25 μg Ova+2 mg alum through i.p route at weekly interval
Challenge: 50 μg Ova through the nasal route given on three consecutive days
To apply MNs, a previously described protocol was used [15]. Briefly, mice were first anesthetized under an isoflurane and air mixture, and their back-skin was treated with an electric hair-trimmer. A hair removing lotion was next applied on the trimmed area to fully remove the hair. The treated skin was then gently washed in lukewarm water and dried by gently dabbing with dry cotton. After that, allergen-coated MNs were inserted into the skin and manually held in place for up to 3 minutes to allow the coated allergen to dissolve into the skin. After application, mice were returned to the cage and observed until they were conscious and active again.
Five weeks post-vaccination, mice were sensitized through an intraperitoneal (i.p) injection of 25 μg Ova mixed with 2 mg alum (Fisher Scientific International, USA) on d55 and d62. Ten days later, sensitized mice were challenged with 50 μg Ova dissolved in 50 μl PBS per day, for three consecutive days through the nasal route (Fig. 2A). The very next day after challenge, mice were euthanized through an intraperitoneal (i.p) injection of 10 mg sodium pentobarbital. Blood, lungs, spleen and bronchoalveolar fluid (BAL) fluid were collected for analysis.
Fig. 2. Immunotherapeutic schedule and serum analysis.
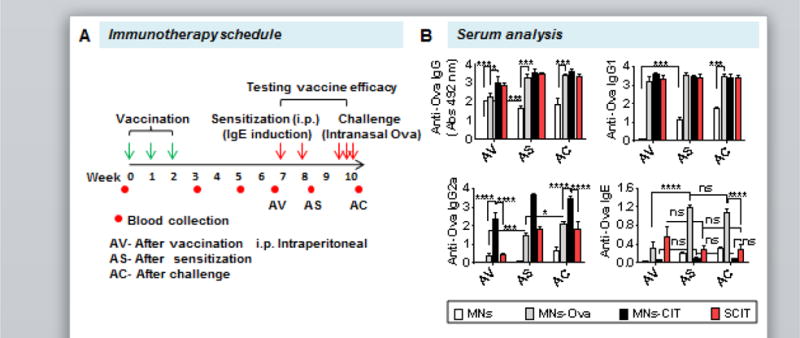
(A) Preventive immunotherapy schedule. In brief mice were vaccinated on day 0, 7 and 14. After a rest period of four weeks, mice were sensitized twice at a weekly interval. Ten days post sensitization, mice were challenged consecutively for three days. (B) Anti-Ova IgG, IgG1, IgG2a and IgE response after vaccination (AV), after sensitization (AS) and after challenge (AC). Individual mouse serum was diluted to 1:500 for IgG, IgG1 and IgG2a analysis, and to 1:100 for IgE dilution. Error bars denote mean ± SEM. *: p < 0.05; ***: p < 0.0005, and ****: p < 0.0001.
2.4 Serum and BAL analysis
Enzyme linked immunosorbent assay (ELISA) was used to measure anti-Ova IgE and IgG subtypes in mouse serum as discussed earlier [22]. Goat anti mouse IgG, IgE, IgG1, and IgG2a antibodies labelled with horseradish peroxidase (Southern Biotech, Birmingham, Al, USA) were used as detection antibodies in ELISA. BAL fluid was collected as described previously [27]. In brief, the trachea region of a mouse was exposed by using a scissor, and then a 21-G catheter was placed into the trachea. One milliliter syringe was fixed into the catheter and lungs were lavaged a total of 10 times with 0.5 ml of Ca2+ and Mg2+ free saline-EDTA solution. All the lavages were centrifuged. The supernatant of first two lavages was frozen at −20°C and stored for analysis. The cell pellets of all lavages were pooled for flow cytometric analysis (Attune NxT, Life Technologies, USA) to determine cell phenotypes in the BAL fluid. Alexa flour 647 anti-mouse Gr-1, FITC-CD11b (M1/70), APC anti-mouse/human CD45R/B220, APC/cy7 anti-mouse CD4, APC/cy7 anti-mouse CD8, and FITC-cKit conjugated monoclonal antibodies purchased from Biolegend (San Diego, CA) were used for cell staining. For staining of cells, they were first incubated with anti-mouse CD16/32 (FcγR blocker) for 40 min at 4°C. After incubation, cells were washed 3 times with 2 % fetal bovine serum (FBS) diluted in 1x PBS, followed by incubation with specific monoclonal antibodies for 30 min. After incubation, cells were again washed and kept in 2 % FBS in 1x PBS at 4°C until counting. Forward and side scatter gating strategy was used for the analysis of single-color-positive cells. In brief, while acquiring data, forward and side scatter plots were created. Dead cells and other debris were excluded from the count by gating the viable cells. Next, single-color-positive cells were sub-gated in the live-cell population on the basis of specific-color signal intensity. Unstained cells were used as a control in the analysis. All colors were compensated before counting the actual experimental samples.
2.5 Splenocyte culture
To assess the type of Th pathway activated during preventive immunotherapy, spleens were harvested at the end of the experiment. Splenocytes were cultured in triplicate at a concentration of one million cells per well of a 96 well plate for 72 h with culture medium alone, or with 200 μg/ml Ova, or with 5μg/ml of Concanavalin A (Sigma Aldrich, USA) in DMEM (Gibco, USA) supplemented with 5% heat-inactivated FBS and penicillin-streptomycin antibiotics. Supernatant of cultured cells were collected after 14 h for IL-2 quantification, and after 72 h for quantification of IFN- γ, IL-4 and IL-13 cytokines through sandwich ELISA. Anti-mouse capture and detection IL-2, IFN-γ, IL-4, IL-13 antibodies and purified proteins were purchased from eBioscience (Thermo Scientific, Waltham, MA).
2.6 Histological analysis
At the end of the experiment, mice were euthanized and lungs were harvested for histology. Lungs were fixed in formalin solution (4% v/v) overnight followed by a 24 h incubation period with ethanol solution (70% v/v). Tissues were sectioned by IDEXX Bioresearch (MO, USA) and also stained for periodic acid-Schiff (PAS) to analyze mucus deposition inside the wall of bronchioles and trichrome blue for collagen deposition around the bronchioles [28]. Bright field images of stained tissues were then taken in our lab by use of a Nikon Eclipse Ti Microscope (Tokyo, Japan).
2.7 Statistical analysis
Statistical analysis was performed by use of Graph pad Prism 6 software. Two-way ANOVA tests were used for statistical calculations between the groups at different time points while one-way ANOVA was used to compare significance between the groups at a given time point. Significance was considered for p < 0.05.
3. Results and discussion
An increasing incidence of allergies calls for a true form of ‘preventive vaccination’ to stop allergy development in healthy individuals. Here, we explored MN-based cutaneous immunization (MNs-CIT) for the development of a prophylactic vaccine to get protection against development of Ova allergy in mice, and compared its efficacy to the existing SCIT.
3.1 Coated MNs successfully delivered Ova into mouse skin and induced allergen-specific immune response comparable to SCIT
Ova as a model allergen was precisely coated on 2D MN arrays, with each array containing 57 MNs (Fig. 1A). This was done with the use of a precision automated dip coater [22, 25]. Coating was uniform and consistent as confirmed by fluorescent micrographs of individual MNs of the patch (Fig. 1B). Coated MNs were sharp, and penetrated into mouse skin. Fluorescent micrographs of MNs before (Fig. 1C) and after insertion (Fig. 1D) show that the coating was removed from the MN surface following skin insertion. Calibrated fluorescent spectroscopy showed that 77% (±3.2) of coated Ova was delivered in to the skin, while 3.2% (±1) Ova was found on the skin, and 19.7% (±4) Ova remained attached to MNs (Fig. 1E). This shows that coated MNs can deliver the coating into the skin with reproducibility. The delivery efficiency observed here is consistent with previous reports of the use of coated MNs for delivery into skin [22, 25, 26, 29].
To confirm the ability of MNs to generate allergen specific immune responses, mice were vaccinated with Ova at d0, 7 and 14, and bled at d0, 21 and 35 to determine Ova-specific Ig responses. IgG levels increased from d21 to d35. At d21, no considerable difference was observed in IgG response between the SCIT and MNs-CIT (p=0.4079) groups. However, at d35, anti-Ova IgG response in the MNs-CIT group was significantly higher than the SCIT group (p=0.0042). As expected, MNs as a negative control group did not show any detectable anti-Ova IgG response since this group did not receive any Ova (Fig. 1F). Unlike total IgG, the anti-Ova IgG1 response at d21 was lower in the MNs-CIT group as compared to the SCIT group (p=0.0068); however at d35, the response in all vaccinated groups increased and no statistically significant (p>0.05) difference was noticed between the different treatment groups (Fig. 1G). Interestingly, anti-Ova IgG2a observed was radically higher in the MNs-CIT group than the MNs (p<0.0001), MNs-Ova (p<0.0001), and SCIT (p<0.0001) groups at both d21 & d35 (Fig. 1H). In allergy immunotherapy, IgG2a response is important since it is considered as a blocking IgG subtype. IgG2a can bind to the allergens and can thus inhibit their attachment to IgE antibodies bound to Fc epsilon RI molecules on mast cells. This in turn prevents allergen-dependent crosslinking of Fc epsilon RI (stimulatory receptors), and avoids mast cell degranulation and state of anaphylaxis. It has also been shown that the IgG2a-allergen complex can enable crosslinking of Fc epsilon RI (stimulatory receptor) and Fc gamma RIIb (inhibitory receptor) to suppress IgE-dependent anaphylaxis [30–32]. Generation of IgG2a suggests activation of Th1-type response by MNs-CIT, which is likely due to the presence of the adjuvant CpG [33]. CpG is a known stimulator of the Th1 cellular pathway and the regulatory response [34]. CpG acts as a toll like receptor 9 agonist [35], and it has been shown in other studies that it can effectively suppresses the atopic inflammatory response by suppressing Th2-type cytokines and allergen specific IgE response in airway allergy [36, 37]. A low level of systemic IgE antibodies in the MNs-CIT group indicates that this approach of vaccination does not induce Ova specific IgE antibodies, and thus poses no risk of allergy-induction or exacerbation from vaccination. In contrast, at d35 the anti-Ova IgE was considerably higher in the SCIT group in comparison to MNs-CIT group (p<0.0001) (Fig. 1I). This state of transient increase of specific IgE in serum during SCIT has also been noted in previous reports [7–9, 38].
Alum is often used as an adjuvant in SCIT allergen formulations [39], especially in Europe. However, there are some concerns over alum-associated granuloma formation when it is given in the subcutaneous region, and other side effects [40, 41]. Therefore, we selected CpG as an adjuvant for MN coatings instead of alum. CpG has been used in clinical trials in more than a thousand human subjects with a relatively benign toxicity profile [42, 43].
3.2 MNs-CIT prevents allergic sensitization of mice against Ova and the protective immune response persists even after allergen challenge
To assess the preventive efficacy of MNs-CIT, vaccinated mice were sensitized to the allergen (Ova) to cause allergy, and then challenged intranasally with a high dose of Ova to simulate allergen exposure (Fig. 2A). In the sensitization phase two injections of Ova+alum (25 μg Ova+2mg alum) were given through the intraperitoneal (i.p) route at weekly intervals. In this phase, naive mice are expected to develop allergen specific IgE antibodies. Ten days later, the sensitized mice received three consecutive doses of Ova intranasally to simulate allergen exposure. In this step, sensitized mice are expected to manifest allergy symptoms. Sensitization through the i.p route and exposure through the intranasal route is a standard protocol to induce airway inflammation in naive mice [18, 44, 45]. Blood was collected after vaccination (AV), after sensitization (AS), and after challenge (AC), to assess if anti-Ova IgG responses generated by vaccination persist or not. At AV (d56), the anti-Ova antibody response was similar to that observed at d35. While the anti-Ova IgG response increased in all groups AS, there was no noteworthy difference between the AV and AS groups except in the MNs group where the antibody response increased AS (p<0.0001) (Fig. 2B). This is because sensitization involves Ova+alum injections i.p., which not only induces IgE, but also generates IgG antibodies [18, 44]. Likewise, the anti-Ova IgG1 response also increased slightly AS, although no significant difference was seen between the different vaccinated groups except the MNs group for which, at AS the anti-Ova IgG1 increased considerably (p<0.0001) in comparison to AV (Fig. 2B). Appearance of systemic allergen-specific IgE is an indication of the development of an allergic response. In this context, it is noteworthy that anti-Ova IgE response did not significantly increase in the MNs-CIT and SCIT groups AV, AS and AC, however, it was significantly elevated in the MNs-Ova control group AS and AC indicating progression of allergic response. Furthermore, AS and AC, the anti-Ova IgE was considerably lower in the MNs-CIT and SCIT groups than the MNs-Ova group, demonstrating the protective efficacy of MNs-CIT and SCIT treatment over the MNs-Ova control group (Fig. 2B). This suggests that Ova alone (without CpG) coated on MNs is unable to suppress IgE development from the sensitization step. Inclusion of CpG adjuvant with Ova in the MNs-CIT group led to the suppression of anti-Ova IgE response (Fig. 2B).
Taken together, this data shows that if mice are vaccinated with Ova and CpG using coated MNs, then a subsequent attempt to cause allergy against Ova is unsuccessful as is evident from poor IgE generation.
At the AC timepoint, anti-Ova IgG, and IgG1 were slightly increased in the MNs group, however these responses were unchanged in the MNs-Ova, MNs-CIT and SCIT groups. However, there was a significant increase observed in the anti-Ova IgG2a response in the MNs-Ova group (Fig. 2B). No considerable changes were observed in anti-Ova IgE response across all treatment groups. Overall, Ova-allergen-specific immune responses persisted even after alum induced sensitization and intranasal allergen exposure. Similar type of persistence of the allergen-specific immune responses have been noted in other pre-clinical studies in mice [18, 44].
3.3 MNs-CIT effectively prevents the development of airway inflammation
To further substantiate the ability of MNs-CIT to prevent airway inflammation, all sensitized mice were challenged with Ova-allergen by intranasal exposure on three consecutive days. After such a challenge, if airway inflammation ensues, it is characterized by infiltration of effector cells like neutrophils, macrophages or mast cells, and up regulation of pro-inflammatory cytokines (IL-4, IL-5 & IL-13) in the lungs [46]. On the other hand, if the allergen challenge does not cause an allergic reaction, it is characterized by down regulation of aforementioned cells and cytokines, and up-regulation of anti-inflammatory cytokines such as IL-10 [18, 44]. In our results, we observed that MNs-CIT suppressed the airway inflammation as revealed by BAL fluid analysis. In BAL fluid, the cell counts of neutrophils, macrophages and mast cells were lower in the MNs-CIT group as compared to the control group of MNs (Fig. 3A). FACS analysis revealed nearly 4 fold reduction in the percentage of neutrophil count (12 ± 1.7% to 3.1 ± 2.8%) and 4 fold reduction in macrophage count (11.8 ± 1.6% to 2.8 ± 2.5%) in the MNs-CIT group in comparison to the MNs group. However, no considerable difference was observed between the MNs-CIT and SCIT groups (Fig. 3A). Additionally, high percentage of mast cells were seen in the MNs group suggesting an ongoing chronic inflammation. The presence of B cells in BAL fluid of all groups might indicate activation of the local immune system after intranasal allergen challenge. Infiltration of lymphocytes in the lungs can also be facilitated by vascular remodeling or leaky vasculature that occurs during asthma pathogenesis [47, 48].
Fig. 3. Bronchoalveolar fluid analysis.
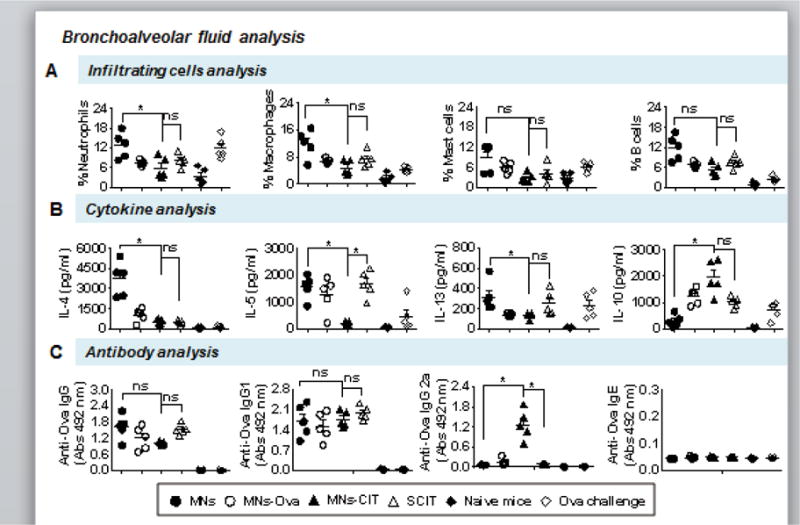
Day after last allergen challenge, mice were euthanized and BAL was harvested for analysis. (A) Percentage cell count of neutrophils, macrophages, mast cells and B cells in BAL. (B) Quantity of pro-inflammatory cytokines IL-4, IL-5, and IL-13, and anti-inflammatory cytokine IL-10 in BAL. (C) Anti-Ova IgG, IgG1, IgG2a, and IgE antibodies in BAL. Individual mouse BAL fluid was used for analysis. Error bars denote mean ± SEM. *: p < 0.05, ns: not significant.
Suppression of pro-inflammatory cytokines, IL-4, IL-5 and IL-13, and activation of anti-inflammatory IL-10 cytokine in BAL fluid of MNs-CIT further confirms the preventive role of MNs-CIT in allergy progression, unlike the MNs group (Fig. 3B). Up-regulation of IL-4 (p=0.0219) in MNs group as compared to MNs-CIT and SCIT groups also points to the establishment of airway inflammation in the MNs group(Fig. 3B). This is because IL-4 is predominantly secreted by T helper cell and it helps in proliferation of allergen reactive Th2 cells [49]. Higher expression of IL-5 and IL-13 cytokines in the MNs group (p=0.0125) than the MNs-CIT group also points towards activation of allergic responses in the MNs group but not the MNs-CIT group (Fig. 3B). IL-5 type 2 cytokines also enhance recruitment, activation and migration of eosinophils [50, 51]. Interestingly, expression of IL-10 was significant higher (p=0.0155) in MNs-CIT group than the MNs group indicating the activation of T regulatory cells (T reg) cells(Fig. 3B), which have been shown to assist in the suppression of allergen specific proliferation of T cells [52]. MNs-CIT and SCIT groups did not significantly differ in IL-10 expression(Fig. 3B).
To assess localized anti-allergen antibody response, anti-Ova antibody response was detected in BAL fluid. There were no considerable differences observed in anti-Ova IgG, IgG1 and IgE responses between the different groups, however the anti-Ova IgG2a response was higher in the MNs-CIT group as compared to the MNs group (p=0.0208) (Fig. 3C). This again can be attributed to the presence of CpG in the MNs-CIT formulation. Appearance of IgG2a in BAL fluid might also contribute towards protection in allergy progression; however, additional experiments are needed to corroborate this hypothesis.
3.4 MNs-CIT mediated protection involves activation of Th1 pathway
From previous studies, it is understood that immunomodulation during allergy treatment involves activation of the Th1 pathway through secretion of IL-2 and IFN-γ cytokines that regulate allergic airway inflammation and improve clinical symptoms of allergy [53, 54]. Therefore, to check if the same type of Th pathway was involved in the ‘prophylactic/preventive’ regime of allergy immunomodulation, at the end of allergen challenge, splenocytes were cultured and stimulated with Ova protein. It was seen that in supernatants the expression of Th1 cytokines (IL-2 and IFN- γ) was significantly higher (p˃0.005) in the MNs-CIT group as compared to MNs control group (Fig. 4), indicating the activation of the Th1 pathway. IL-2 and IFN- γ, which are secreted by Th1 cells, show inhibitory effects on Th2 cell differentiation and synthesis of allergen reactive IgE [55, 56].
Fig. 4. Splenocyte culture supernatant analysis.
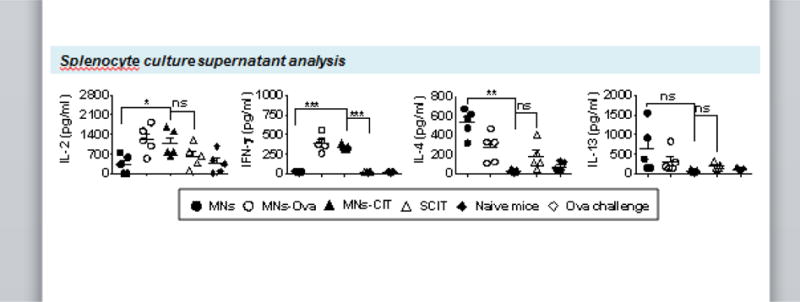
Spleens from mice were harvested at the end of the experiment. Splenocytes were cultured in triplicate at 1×106 cells per well for 72h with medium alone (negative control group) or 200 μg/ml ovalbumin or 5 μg/ml of concanavalin A (positive control group). Supernatant of cultured splenocytes were collected after 14 h for IL-2, and 72 h for IFN- γ, IL-4 and IL-13 analysis. Error bars denote mean ± SEM. *: p < 0.05; **: p < 0.005 and ***: p < 0.0005, ns: not significant.
In contrast, the expression of Th2 cytokines (IL-4 and IL-13) was higher in the control MNs group in comparison to vaccinated groups (Fig. 4) indicating allergy progression in the MNs group. IL-4 and IL-13 cytokines activate the allergen reactive Th2 cells and recruit IgE producing plasma cells, which further activate other effector cells such as basophils, eosinophils, and mast cells to induce allergic inflammation [49]. No significant difference was observed between MNs-CIT and SCIT groups in relation to IL-4 and IL-13 secretion (Fig. 4).
3.5 MNs-CIT effectively suppressed localized inflammation in lungs
Histological analysis of lung tissues further revealed the protective role of MNs-CIT. Absence of mucus on the inner surface of the bronchiole wall (Fig. 5) confirmed prevention of airway allergy progression unlike the MNs and MNs-Ova groups where high deposition of mucus was observed (Fig. 5). Mucus deposition is a condition known as hyper mucusplasia, which is a potent marker for airway allergy pathogenesis [57]. Secretion of mucus in large volumes contributes to airway obstruction, which results in difficulty in breathing and shortening of breath [58]. Moreover, collagen deposition around the air bronchioles of MNs-CIT was no greater than that observed in the naïve mice, indicating normal flexibility of lung bronchioles for breathing (Fig. 5). Overall, MNs-CIT vaccination successfully prevented development of airway allergy in mice.
Fig. 5. Histological analysis of lungs.
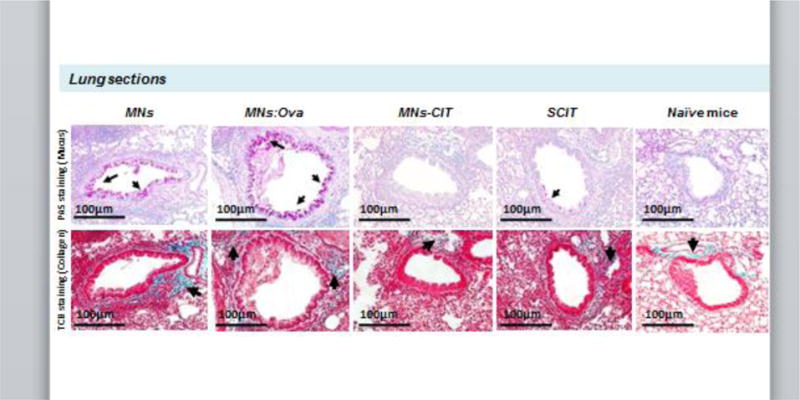
At the end of Ova allergen challenge, lungs were harvested, fixed, cleaned and cut for histology. Tissue sections were stained with either periodic acid-Schiff (PAS) to stain for mucus deposition (top panel), or trichrome blue (TCB) to stain for collagen deposition (bottom panel). Arrows in the top panel point to mucus deposition, and in the bottom panel to collagen deposition.
Conclusion
To halt the allergy pandemic and to develop a preventive vaccine for healthy or allergy-susceptible individuals, we demonstrated that MNs coated with Ova as a model allergen and CpG as an adjuvant can deliver the vaccine into the skin in a minimally invasive manner. The anti-allergy-vaccine successfully showed protection against Ova allergy development in mice, and it was as effective as the conventional SCIT route, which is currently used for treatment of airway allergies. While long term potency and safety concerns of MNs-CIT need to be addressed in the future, this study lays the foundation to study MNs for the development of allergy vaccines.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) [grant number 1R01AI121322-01]. HSG is a co-inventor on a patent related to coated microneedles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
This potential conflict of interest has been disclosed and is managed by Texas Tech University.
References
- 1.Campbell DE, Mehr S. Fifty years of allergy: 1965–2015. J Paediatr Child Health. 2015;51:91–93. doi: 10.1111/jpc.12806. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA. The allergy epidemics: 1870–2010. J Allergy Clin Immunol. 2015;136:3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amato G, Stanziola A, Sanduzzi A, Liccardi G, Salzillo A, Vitale C, Molino A, Vatrella A, D’Amato M. Treating severe allergic asthma with anti-IgE monoclonal antibody (omalizumab): a review. Multidiscip Respir Med. 2014;9:23. doi: 10.1186/2049-6958-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naclerio RM. The effect of antihistamines on the immediate allergic response: a comparative review. Otolaryngol Head Neck Surg. 1993;108:723–730. doi: 10.1177/019459989310800615. [DOI] [PubMed] [Google Scholar]
- 6.Frew AJ. Allergen immunotherapy. J Allergy Clin Immunol. 2010;125:S306–313. doi: 10.1016/j.jaci.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 7.Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy. 2012;2:2. doi: 10.1186/2045-7022-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–157. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukantz SC, Bagg AS, Lockey RF. Adverse effects and fatalities associated with subcutaneous allergen immunotherapy. Clin Allergy Immunol. 2008;21:455–468. [PubMed] [Google Scholar]
- 10.Incorvaia C. Preventive capacity of allergen immunotherapy on the natural history of allergy. J Prev Med Hyg. 2013;54:71–74. [PMC free article] [PubMed] [Google Scholar]
- 11.Larsen JN, Broge L, Jacobi H. Allergy immunotherapy: the future of allergy treatment. Drug Discov Today. 2016;21:26–37. doi: 10.1016/j.drudis.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Zolkipli Z, Roberts G, Cornelius V, Clayton B, Pearson S, Michaelis L, Djukanovic R, Kurukulaaratchy R, Arshad SH. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J Allergy Clin Immunol. 2015;136:1541–1547. e1541–1511. doi: 10.1016/j.jaci.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, Koivikko A, Norberg LA, Valovirta E, Wahn U, Moller C. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 14.Valovirta E, Berstad AK, de Blic J, Bufe A, Eng P, Halken S, Ojeda P, Roberts G, Tommerup L, Varga EM, Winnergard I, G.A.P. investigators Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ-standardized grass allergy immunotherapy tablet in children with grass pollen-induced allergic rhinoconjunctivitis. Clin Ther. 2011;33:1537–1546. doi: 10.1016/j.clinthera.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Wang BZ, Gill HS, He C, Ou C, Wang L, Wang YC, Feng H, Zhang H, Prausnitz MR, Compans RW. Microneedle delivery of an M2e-TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. J Control Release. 2014;178:1–7. doi: 10.1016/j.jconrel.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Zhang Z, Saxon A, Zhang K. Prevention of oral food allergy sensitization via skin application of food allergen in a mouse model. Allergy. 2012;67:622–629. doi: 10.1111/j.1398-9995.2012.02798.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee HJ, Lee NR, Kim BK, Jung M, Kim DH, Moniaga CS, Kabashima K, Choi EH. Acidification of stratum corneum prevents the progression from atopic dermatitis to respiratory allergy. Exp Dermatol. 2016 doi: 10.1111/exd.13144. [DOI] [PubMed] [Google Scholar]
- 18.Hessenberger M, Weiss R, Weinberger EE, Boehler C, Thalhamer J, Scheiblhofer S. Transcutaneous delivery of CpG-adjuvanted allergen via laser-generated micropores. Vaccine. 2013;31:3427–3434. doi: 10.1016/j.vaccine.2012.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Shin JH, Kim SC, Park CK, Kim SW. Preventive effects of oral tolerance on allergic inflammation and airway remodeling in a murine model. Am J Rhinol Allergy. 2013;27:e11–16. doi: 10.2500/ajra.2013.27.3853. [DOI] [PubMed] [Google Scholar]
- 20.Holt PG, Sly PD, Sampson HA, Robinson P, Loh R, Lowenstein H, Calatroni A, Sayre P. Prophylactic use of sublingual allergen immunotherapy in high-risk children: a pilot study. J Allergy Clin Immunol. 2013;132:991–993 e991. doi: 10.1016/j.jaci.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 21.Incorvaia C, Masieri S, Berto P, Scurati S, Frati F. Specific immunotherapy by the sublingual route for respiratory allergy. Allergy Asthma Clin Immunol. 2010;6:29. doi: 10.1186/1710-1492-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm Res. 2014 doi: 10.1007/s11095-014-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly RF, Singh TR Raj, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17:187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakya AK, Gill HS. A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy. Vaccine. 2015;33:4060–4064. doi: 10.1016/j.vaccine.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Jain AK, Lee CH, Gill HS. 5-Aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Control Release. 2016;239:72–81. doi: 10.1016/j.jconrel.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Daubeuf FO, Frossard N. Performing Bronchoalveolar Lavage in the Mouse, Curr. Protoc. Mouse Biol. 2012;2:167–175. doi: 10.1002/9780470942390.mo110201. [DOI] [PubMed] [Google Scholar]
- 28.Polte T, Foell J, Werner C, Hoymann HG, Braun A, Burdach S, Mittler RS, Hansen G. CD137-mediated immunotherapy for allergic asthma. J Clin Invest. 2006;116:1025–1036. doi: 10.1172/JCI23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters EE, Ameri M, Wang X, Maa YF, Daddona PE. Erythropoietin-coated ZP-microneedle transdermal system: preclinical formulation, stability, and delivery. Pharm Res. 2012;29:1618–1626. doi: 10.1007/s11095-012-0674-z. [DOI] [PubMed] [Google Scholar]
- 30.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 31.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochreiter R, Ferreira F, Thalhamer J, Hammerl P. TH1-promoting DNA immunization against allergens modulates the ratio of IgG1/IgG2a but does not affect the anaphylactic potential of IgG1 antibodies: no evidence for the synthesis of nonanaphylactic IgG1. J Allergy Clin Immunol. 2003;112:579–584. doi: 10.1016/s0091-6749(03)01623-3. [DOI] [PubMed] [Google Scholar]
- 33.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. quiz 28-19. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann S, Egeter O, Hausmann S, Lipford GB, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]
- 35.Gnjatic S, Sawhney NB, Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16:382–391. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev. 2009;61:256–262. doi: 10.1016/j.addr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Xu W, Tamura T, Takatsu K. CpG ODN mediated prevention from ovalbumin-induced anaphylaxis in mouse through B cell pathway, Int. Immunopharmacol. 2008;8:351–361. doi: 10.1016/j.intimp.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70:261–271. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 39.Francis JN, Durham SR. Adjuvants for allergen immunotherapy: experimental results and clinical perspectives. Curr Opin Allergy Clin Immunol. 2004;4:543–548. doi: 10.1097/00130832-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Exley C. Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy. Allergy Asthma Clin Immunol. 2014;10:4. doi: 10.1186/1710-1492-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen-Jarolim E. Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ J. 2015;8:7. doi: 10.1186/s40413-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, Pfister T, Maurer P, Bachmann MF, Graf N, Kündig TM. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39:562–570. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 43.Scheiermann J, Klinman DM. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32:6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Moos S, Johansen P, Waeckerle-Men Y, Mohanan D, Senti G, Haffner A, Kundig TM. The contact sensitizer diphenylcyclopropenone has adjuvant properties in mice and potential application in epicutaneous immunotherapy. Allergy. 2012;67:638–646. doi: 10.1111/j.1398-9995.2012.02802.x. [DOI] [PubMed] [Google Scholar]
- 45.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–220. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 47.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 48.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 49.Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology. 1998;3:233–244. doi: 10.1016/s1380-2933(97)10005-7. [DOI] [PubMed] [Google Scholar]
- 50.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 51.Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. 1999;29:1496–1503. doi: 10.1002/(SICI)1521-4141(199905)29:05<1496::AID-IMMU1496>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 53.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon gamma regulate allergic airway inflammation and mucus production. J Exp Med. 1999;190:1309–1318. doi: 10.1084/jem.190.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maggi E. T-cell responses induced by allergen-specific immunotherapy. Clin Exp Immunol. 2010;161:10–18. doi: 10.1111/j.1365-2249.2010.04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm. 2001;10:51–59. doi: 10.1080/09629350120054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulrik CS, von Linstow ML, Nepper-Christensen S, Porsbjerg C, Backer V. Chronic mucus hypersecretion: a marker of asthma in young adults? Respir Med. 2005;99:1576–1582. doi: 10.1016/j.rmed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 58.Rogers DF, Evans TW. Plasma exudation and oedema in asthma. Br Med Bull. 1992;48:120–134. doi: 10.1093/oxfordjournals.bmb.a072529. [DOI] [PubMed] [Google Scholar]


