Abstract
Objective
Longitudinal studies support a prospective relationship between weight suppression (WS) and bulimic syndrome (BN-S) maintenance. Although biobehavioral mechanisms have been proposed to explain this link, such mechanisms have yet to be identified. Given that weight loss would reduce leptin levels which may influence eating, this study examined whether reduced leptin levels mediate the link between greater WS and longer illness duration.
Method
Women (N=53), ages 18–45 years, were recruited from the community if they met criteria for a BN-S, including either DSM-5 bulimia nervosa (BN; n=33) or purging disorder (PD: n=20), and fell within a healthy weight range (18.5–26.5 kg/m2). Participants completed clinical assessments and provided blood samples to measure circulating leptin.
Results
Significant associations were found among greater WS, lower leptin concentrations, and longer duration of illness. Mediation analyses using bootstrapping procedures indicated all paths were significant and that leptin mediated the link between WS and illness duration. An alternative model in which longer illness duration contributed to leptin, via greater WS, was not supported.
Discussion
Longitudinal research is needed to support temporal associations and explore behavioral mechanisms linking leptin to illness trajectory.
Keywords: leptin, weight suppression, mediation, bulimia nervosa, purging disorder
Women with bulimia nervosa (BN) have greater weight suppression (WS) compared to controls (Bodell & Keel, 2015; H. A. Davis, Holland, & Keel, 2014; Van Son, 2013), reflecting a greater difference between their highest prior weight and current weight (Lowe, 1993). WS prospectively predicts illness maintenance in BN (Butryn, Lowe, Safer, & Agras, 2006; Lowe et al., 2011) and bulimic syndromes (BN-S) more broadly (Bodell, Brown, & Keel, 2017; Keel & Heatherton, 2010); however, few studies have examined mediators of the association between WS and illness duration.
Weight loss contributes to reduced leptin concentrations (Rosenbaum et al., 1997; Wolfe, Jimerson, Orlova, & Mantzoros, 2004). Therefore, greater WS in BN could explain reduced leptin concentrations found in BN compared to controls in several studies (Jimerson, Wolfe, Carroll, & Keel, 2010; Monteleone, Di Lieto, Tortorella, Longobardi, & Maj, 2000; Monteleone, Fabrazzo, Tortorella, Fuschino, & Maj, 2002)1. Supporting this hypothesis, we recently found a significant association between greater WS and lower leptin concentrations in women with BN (Bodell & Keel, 2015).
Furthermore, prior research found cross-sectional associations between lower leptin concentrations and longer illness duration in BN (Monteleone, Martiadis, Colurcio, & Maj, 2002). Leptin plays a key role in homeostatic regulation of weight through its modulation of satiation (J. F. Davis et al., 2011). Thus, reduced leptin could maintain binge eating in BN-S. Additionally, circulating leptin influences hedonic regulation of food intake and other rewarding behaviors by crossing the blood-brain barrier where it binds to leptin receptors and inhibits firing of dopamine neurons in the ventral tegmental area (Hommel et al., 2006). Thus, reduced leptin could contribute to BN-S maintenance via increased reinforcement of binge eating, purging, or both (J. F. Davis et al., 2011). Taken together, leptin could mediate associations between WS and BN-S maintenance via homeostatic mechanisms, hedonic mechanisms, or both. Yet, no study has examined whether leptin mediates associations between WS and BN-S maintenance.
The current study sought to examine associations among WS, leptin, and illness duration in BN-S. We predicted that greater WS would be associated with lower leptin and both would be associated with longer illness duration. Further, we hypothesized that the association between greater WS and longer illness duration would be mediated by reduced leptin levels. We also tested a plausible alternative model in which reduced leptin is a consequence of longer illness duration by examining whether WS mediated the association between longer illness duration and leptin.
Methods
Participants
Participants (N=53) included 33 women with DSM-5 BN2, 18 purging and 15 non-purging, and 20 women meeting research criteria for PD (Keel & Striegel-Moore, 2009). Inclusion criteria were body mass index (BMI) between 18.5 and 26.5 kg/m2, and being free of medical and psychiatric conditions (e.g., diabetes, depression) or treatments that might influence weight or appetite. Mean (SD) age was 20.8 (3.5) years, and BMI was 22.9 (2.0) kg/m2. Racial/ethnic diversity was, 71% white, non-Hispanic, 17% African American, 6% Asian, and 6% Hispanic.
Procedures
As part of a larger study, participants completed clinical interviews and self-report questionnaires (visit 1), an ad lib test meal (visit 2), and measurement of gastric emptying and gut peptide responses to a standardized test meal with a placebo and medication (visits 3 and 4), with at least 48 hours between visits. Data for the current report come from participants recruited in Tallahassee, FL, in whom WS and leptin assessments were completed. This study was reviewed and approved by the Human Subjects Committee of the IRB at Florida State University, and participants completed informed consent prior to study participation.
Clinical Assessments
Eating disorder diagnosis and symptom were established with the Eating Disorder Examination (EDE) (Christopher G. Fairburn & Cooper, 1993). Interrater reliability and internal consistency was high (κ=.90 for diagnoses of BN, PD, vs. control, and r>.97 across EDE scales) and α=.96 for EDE total score.
Lifetime eating disorder diagnoses and lifetime and current diagnoses of other Axis I disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-1) (First, 1995). The primary use of the SCID was to assess eligibility inclusion and exclusion criteria. In addition to excluding current disorders influencing weight or appetite in all participants, women with PD could not have a history of DSM-IV anorexia nervosa (AN), BN, binge eating disorder (BED), or recurrent OBEs. Women with DSM-5 BN could not have a history of DSM-IV AN. Interrater reliability across SCID-I diagnoses was high, with κ >.90 for current and lifetime diagnoses.
WS and illness duration were measured using the Oxford Risk Factor Interview 2.3 Obesity Risk Items (RFI) (C. G. Fairburn, Welch, Doll, Davies, & O’Connor, 1997). Participants provided their highest lifetime weight for their current height. Studies have found high correlations (r-values≥0.85) between recalled and objectively measured weight in both community (Tamakoshi et al., 2003) and eating disorder samples (Swenne, Belfrage, Thurfjell, & Engstrom, 2005). The difference between highest prior weight and currently measured weight was used to calculate WS, consistent with its definition in previous studies (Bodell & Keel, 2015; Butryn et al., 2006; Carter, McIntosh, Joyce, & Bulik, 2008; Keel & Heatherton, 2010; Lowe et al., 2011). On the RFI, index age represents onset of full eating disorder criteria. The difference between current age and index age was used to calculate BN-S duration.
Leptin Assessment
Fasting blood samples were drawn on a separate day between 7:45 and 8:15 a.m. With the exception of hormonal contraceptives, participants were free from all medications for a week and instructed not to consume alcohol or recreational drugs 72 hours before these assessments. Blood samples were collected into pre-chilled EDTA vacutainers with 150 μl of ready-to-use aprotinin solution (Sigma #A6279) for 3 ml of blood and gently mixed into solution before being centrifuged at 4° C to separate plasma. Leptin levels in plasma were determined using commercially available ELISA kits (Human Leptin ELISA, EMD Millipore Corporation, St. Charles, MO). Mean intra-assay CV was 5.2%, and leptin values did not differ significantly among assay kits (F[2, 50]=0.41, p=.32).
Symptom levels and BMI were assessed across visits to ensure that participants remained eligible and were weight stable. A paired t-test indicated that participants’ BMIs were stable across visits (t[52]=−.50, p=.62), highly correlated between visits (r[53]=.92, p<.001), and demonstrated a modest mean change of −0.05 kg/m2.
Data Analyses
Following correlations to examine bivariate associations, structural equation models were used to test mediation. Specifically, bootstrapping procedures were used with 1,000 bootstrap resamples to test the indirect effect of WS via leptin on illness duration and the indirect effect of illness duration on leptin via WS while controlling for age (INDIRECT; (Preacher & Hayes, 2008)).
Results
Correlations for BMI, WS, leptin, and symptom severity and illness duration appear in Table 1. As expected, BMI was not significantly correlated with WS but was significantly positively correlated with leptin. Additionally, WS demonstrated a significant negative association with leptin. Neither WS nor leptin was significantly associated with binge or purge frequencies. WS was not significantly associated with total EDE scores; while leptin demonstrated a significant positive association with EDE scores. Finally, greater WS and lower leptin demonstrated significant associations with longer illness duration.
Table 1.
Correlations in Bulimic Syndrome Participants (N=53)
| Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 Body Mass Index† | 22.9 (2.0) | - | |||||
| 2 Weight Suppression (kg) | 6.2 (6.7) | −.20 | - | ||||
| 3 Leptin (ng/mL)† | 11.5 (6.9) | .34* | −.27* | - | |||
| 4 OBE per week | 1.6 (2.1) | .19 | −.03 | −.02 | - | ||
| 5 Purging per week | 4.5 (4.4) | .07 | .13 | −.15 | .03 | - | |
| 6 EDE Total Score | 3. 6 (1.0) | .09 | .02 | .38** | .15 | −.17 | - |
| 7 Illness Duration (mos) | 34.9 (33.4) | −.13 | .43*** | −.32* | .10 | .13 | −.06 |
Note:
p<.05,
p<.01,
p<.001.
mos=months; OBE=Objective Binge Episode.
Body Mass Index (BMI) reflects BMI measured at Study Visit 1 across analyses. Importantly, correlations for leptin and BMI at Study Visit 1 (reported in the table) do not differ from the correlations between leptin and BMI measured at the time of leptin assessment (r(53)=.35, p=.01).
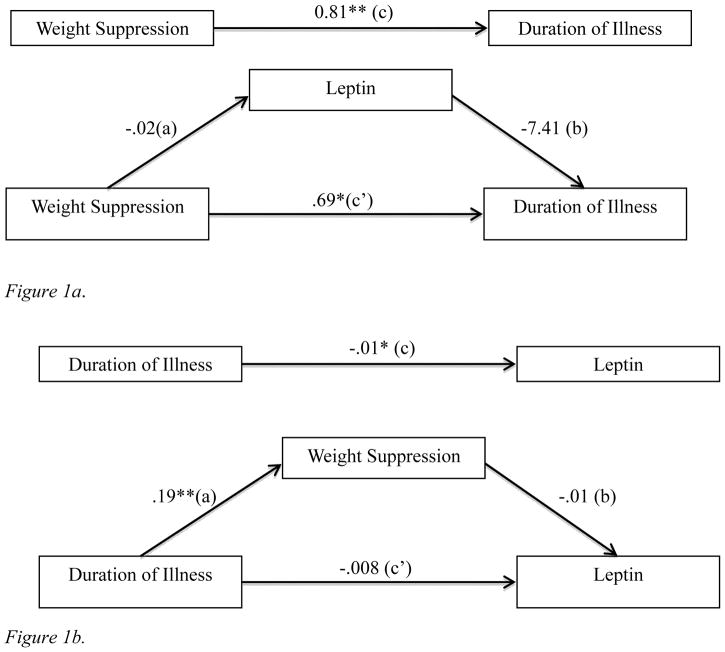
A mediation analysis tested whether the association between WS and illness duration was mediated by leptin. All paths of the model were significant, and the bias corrected 95% confidence interval for the indirect path did not include zero (0.01 to 0.32), indicating that leptin mediated associations between WS and duration of illness (see Figure 1a). Because data were cross-sectional, we ran an alternative model to test the indirect effect of illness duration via WS on leptin. Conceptually, this model posits that longer illness duration contributes to greater WS, which then contributes to lower leptin levels. However, this model was not supported because the 95% confidence interval for the indirect path included zero (−0.007 to 0.001) (see Figure 1b). No other models were considered plausible given research demonstrating that lower leptin contributes to increased food intake and weight (J. F. Davis et al., 2011), which would reduce WS (a direction opposite to that observed).
Figure 1.
Figure 1a. Mediation analysis testing main model (N=53). The top diagram displays the total effect (c) of weight suppression on duration of illness. The bottom diagram displays the direct effect (c′) of weight suppression on duration of illness and the indirect effect (a*b) through the mediator (i.e., leptin). This indirect effect is significant [95% CI: .01–.32]. *p<.05; **p<.01
Figure 1b. Mediation analysis of alternative model (N=53). The top diagram displays the total effect (c) of duration of illness on leptin. The bottom diagram displays the direct effect (c′) of duration of illness on leptin and the indirect effect (a*b) through the mediator (i.e., weight suppression). This indirect effect is not significant [95% CI: −.007–.001]. *p<.05; **p<.01
Discussion
To our knowledge, this is the first study to test whether the effects of WS on illness duration may be mediated by alterations in circulating leptin. Thus, independent replication of our findings is important before firm conclusions may be drawn. Our mediation analyses suggested that the association between WS and illness duration was statistically mediated by reduced leptin levels. An alternative model, in which the association between illness duration and leptin was mediated by WS, was not supported. Future research should examine whether leptin prospectively predicts illness maintenance and accounts for the relationship between WS and future clinical outcomes. If replicated in future research, findings introduce new directions for identifying modifiable factors that may contribute to remission. Identification of such factors is crucial to enhancing treatment efforts, which may focus on biological consequence of WS and their downstream behavioral consequences, such as alterations in homeostatic and hedonic regulation of eating.
Our prior investigation of food reward value in BN supported a significant association between WS and leptin and between WS and greater willingness to work for food as a reward (Bodell & Keel, 2015); however, leptin was not significantly associated with our behavioral measure of reward value. Of note, within that study, there was a small association in the expected direction (r=−.13 and −.11) in both the full sample and in those with BN, respectively. Thus, WS may be linked to greater food reward via leptin, but effects may require a larger sample with a greater range of severity for adequately powered tests of associations.
This study benefited from several methodological strengths. We utilized measures with strong psychometric properties and participants were repeatedly assessed for BMI across visits to ensure weight stability. This represents a particular strength for evaluation of WS as current findings more likely reflect the impact of sustained maintenance of weight below a previously higher weight rather than recent weight fluctuations. This may have greater relevance for understanding factors that contribute to illness maintenance over longer periods of time (Bodell et al., 2017; Keel & Heatherton, 2010). We eliminated known confounds that could have influenced weight or WS. Finally, we employed a large enough sample to detect moderate effect sizes, while controlling for covariates.
Despite strengths, the current study had limitations. Data come from a community-based sample, and findings may not generalize to clinical samples. We did not include all possible covariates for evaluating leptin. For example, we did not measure body composition nor did we control for menstrual cycle phase, and we included participants who were on hormonal contraceptives. The design was cross-sectional, and statistical mediation does not allow us to drawn temporal or causal inferences. Importantly, prior longitudinal studies have established that WS is a prospective predictor of illness maintenance in BN and broadly defined BN-S (Bodell et al., 2017; Butryn et al., 2006; Keel & Heatherton, 2010; Lowe et al., 2011). Further, a model in which we examined whether illness duration contributed to reduced leptin via WS was not supported. Moreover, cross-sectional support is an important first step before undertaking more resource-intensive longitudinal or experimental designs. Our measure of illness duration did not distinguish between individuals who followed an unremitting course and those who remitted and relapsed, and our measure of WS did not include body fat percentage, the period of time over which weight loss occurred, or how long it had been maintained. Finally, some findings may not have achieved statistical significance due to limited power to detect smaller effect sizes.
In summary, findings offer insight into the role leptin may play in the link between WS and illness maintenance for BN-S. Given that complex behavioral symptoms are likely to emerge from an interplay of multiple factors, each exerting a relatively small effect, future research should examine these links longitudinally in larger and more diverse samples. Importantly, even if future studies find that duration of illness drives reduced leptin, these alterations may contribute to a vicious cycle. Identifying new targets for intervention could enhance treatment outcomes in BN-S.
Acknowledgments
This work supported by grants from the National Institute of Mental Health (R01 MH 61836 Keel; R01 MH 111263 Keel; T32 MH 093311 Keel and Eckel).
Footnotes
The influence of weight loss on leptin may reflect reduced body fat in BN compared to control participants at equivalent BMI or a defensive response to recent weight loss in BN even when no difference in body fat exists. Prior studies do not permit conclusions as to the underlying reason for observed differences.
At the time of study initiation, the DSM-5 workgroup had presented proposed revisions to BN criteria for the DSM-5, which were subsequently adopted (APA, 2013), permitting the study to utilize DSM-5 criteria from inception.
The authors have no conflicts of interest to disclose.
Contributor Information
Pamela K. Keel, Florida State University
Lindsay P. Bodell, University of Chicago
Alissa A. Haedt-Matt, Illinois Institute of Technology
Diana L. Williams, Florida State University
Jonathan Appelbaum, Florida State University
References
- Bodell LP, Brown TA, Keel PK. Weight suppression predicts bulimic symptoms at 20-year follow-up: The mediating role of drive for thinness. J Abnorm Psychol. 2017;126(1):32–37. doi: 10.1037/abn00002172016-53088-001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell LP, Keel PK. Weight suppression in bulimia nervosa: Associations with biology and behavior. J Abnorm Psychol. 2015;124(4):994–1002. doi: 10.1037/abn00000772015-31579-001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. Journal of abnormal psychology. 2006;115(1):62–67. doi: 10.1037/0021-843x.115.1.62. [DOI] [PubMed] [Google Scholar]
- Carter FA, McIntosh VVW, Joyce PR, Bulik CM. Weight suppression predicts weight gain over treatment but not treatment completion or outcome in bulimia nervosa. Journal of abnormal psychology. 2008;117(4):936–940. doi: 10.1037/A0013942. [DOI] [PubMed] [Google Scholar]
- Davis HA, Holland LA, Keel PK. A preliminary examination of a nonpurging compensatory eating disorder. Int J Eat Disord. 2014;47(3):239–243. doi: 10.1002/eat.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, … Benoit SC. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69(7):668–674. doi: 10.1016/j.biopsych.2010.08.028. S0006-3223(10)00912-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. 12. New York, NY, US: Guilford Press, New York, NY; 1993. pp. 317–360. [Google Scholar]
- Fairburn CG, Welch SL, Doll HA, Davies BA, O’Connor ME. Risk factors for bulimia nervosa. A community-based case-control study. Arch Gen Psychiatry. 1997;54(6):509–517. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- First MB. In: Structured Clinical Interview for DSM-IV Axis I Disorders - Patient ed. (SCID-I/P) Williams JBW, translator; Spitzer RL, editor. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, … DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–810. doi: 10.1016/j.neuron.2006.08.023. S0896-6273(06)00645-3 [pii] [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Wolfe BE, Carroll DP, Keel PK. Psychobiology of purging disorder: reduction in circulating leptin levels in purging disorder in comparison with controls. Int J Eat Disord. 2010;43(7):584–588. doi: 10.1002/eat.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, Heatherton TF. Weight suppression predicts maintenance and onset of bulimic syndromes at 10-Year follow-up. Journal of abnormal psychology. 2010;119(2):268–275. doi: 10.1037/A0019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, Striegel-Moore RH. The validity and clinical utility of purging disorder. Int J Eat Disord. 2009;42(8):706–719. doi: 10.1002/eat.20718. [DOI] [PubMed] [Google Scholar]
- Lowe MR. The effects of dieting on eating behavior: a three-factor model. Psychol Bull. 1993;114(1):100–121. doi: 10.1037/0033-2909.114.1.100. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Berner LA, Swanson SA, Clark VL, Eddy KT, Franko DL, … Herzog DB. Weight suppression predicts time to remission from bulimia nervosa. J Consult Clin Psychol. 2011;79(6):772–776. doi: 10.1037/a0025714. 2011-23876-001 [pii] [DOI] [PubMed] [Google Scholar]
- Monteleone P, Di Lieto A, Tortorella A, Longobardi N, Maj M. Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiatry Research. 2000;94(2):121–129. doi: 10.1016/S0165-1781(00)00144-X. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Fabrazzo M, Tortorella A, Fuschino A, Maj M. Opposite modifications in circulating leptin and soluble leptin receptor across the eating disorder spectrum. Molecular Psychiatry. 2002;7(6):641–646. doi: 10.1038/sj.mp.4001043. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Martiadis V, Colurcio B, Maj M. Leptin secretion is related to chronicity and severity of the illness in bulimia nervosa. Psychosom Med. 2002;64(6):874–879. doi: 10.1097/01.psy.0000024239.11538.a5. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82(11):3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- Swenne I, Belfrage E, Thurfjell B, Engstrom I. Accuracy of reported weight and menstrual status in teenage girls with eating disorders. Int J Eat Disord. 2005;38(4):375–379. doi: 10.1002/eat.20199. [DOI] [PubMed] [Google Scholar]
- Tamakoshi K, Yatsuya H, Kondo T, Hirano T, Hori Y, Yoshida T, Toyoshima H. The accuracy of long-term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord. 2003;27(2):247–252. doi: 10.1038/sj.ijo.802195802195. [pii] [DOI] [PubMed] [Google Scholar]
- Van Son GE, van der Meer PA, Van Furth EF. Correlates and associations between weight suppression and binge eating symptomatology in a population-based sample. Eating Behaviors. 2013;14(2):102–106. doi: 10.1016/j.eatbeh.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clinical Endocrinology. 2004;61(3):332–338. doi: 10.1111/j.1365-2265.2004.02101.x. [DOI] [PubMed] [Google Scholar]