Abstract
Respiratory infections with Pseudomonas aeruginosa are major health problems, particularly in patients with cystic fibrosis (CF). No vaccine against P. aeruginosa is yet available. A vaccine that controls colonization of the respiratory tract with P. aeruginosa could be useful to prevent chronic infection and exacerbations. Replication-deficient adenoviral (Ad) vectors based on non-human serotypes are attractive vaccine platforms as they can circumvent the problem of pre-existing anti-Ad immunity in humans. The primate-based AdC7 vector AdC7OprF.RGD that expresses the outer membrane protein F (OprF) of P. aeruginosa (AdC7OprF) and that displays an integrin-binding arginine–glycine–aspartic acid (RGD) sequence is a potent inducer of lung mucosal and protective immunity. Here, we investigated the efficacy of immunization with AdC7OprF.RGD to clear an already established P. aeruginosa respiratory infection in mice (wild-type and CF) and rats. Intratracheal administration of the clinical P. aeruginosa strain RP73 embedded in agar beads was used to establish persistent infection. Subsequent intranasal immunization with AdC7OprF.RGD induced robust P. aeruginosa-specific systemic and mucosal, humoral and cellular immune responses. Importantly, the AdC7OprF.RGD immunized mice effectively cleared P. aeruginosa from the lungs. Likewise, immunization with AdC7OprF.RGD to CF mice and Sprague Dawley rats with established P. aeruginosa respiratory infection showed enhanced anti-Pseudomonas immune responses and increased clearance of P. aeruginosa from the lungs. These data suggest that AdC7OprF.RGD can be effective as a post-exposure vaccine and may be useful in clinical settings in particular for patients with CF who frequently harbor the bacteria over prolonged periods.
Introduction
Pseudomonas aeruginosa, a ubiquitous environmental gram-negative microorganism that is found in soil and aquatic environments, is one of the major opportunistic human pathogens. P. aeruginosa can cause chronic infections in the context of damaged or abnormal airway epithelium and compromised local pulmonary clearance mechanisms. Chronic respiratory infections with P. aeruginosa are major problems for patients with chronic pulmonary disorders, such as cystic fibrosis (CF), bronchiectasis and chronic obstructive pulmonary disease (COPD) [1].
P. aeruginosa frequently acquires antibiotic resistance on top of an intrinsic resistance to various antimicrobial agents that often leads to treatment failures [2]. Therefore, alternate prophylactic and therapeutic approaches are needed. Despite some advances in the preclinical development of prophylactic vaccines against P. aeruginosa, a licensed product is not yet available [3]. Several studies have suggested that mucosal immunization can be an effective mode for the management of chronic P. aeruginosa respiratory infection [4–8]. Less effort has been focused on the development of a “therapeutic” vaccine that eliminates or reduces pre-existing P. aeruginosa burden in the respiratory tract [9].
Replication-deficient adenoviral (Ad) vectors are an attractive mucosal vaccine platform to protect against respiratory pathogens [10–13]. The outer membrane protein F (OprF) of P. aeruginosa is a sound vaccine antigen because it has been shown to be immunogenic in various models of acute and chronic lung infections and it is highly conserved [7, 14]. Our previous work identified a human Ad serotype 5 vector expressing OprF or displaying OprF epitopes on the viral capsid as a useful platform for P. aeruginosa vaccine [8, 15, 16]. We have also found that a non-human primate-based AdC7 vector expressing OprF was more potent in inducing lung mucosal and protective immunity compared to a human Ad5-based vector [6]. In addition, genetic modification of the AdC7 fiber to display an integrin-binding arginine–glycine–aspartic acid (RGD) sequence can further enhance mucosal protective immunogenicity of AdC7OprF [5]. Furthermore, the non-human origin of the Ad backbone can circumvent pre-existing anti-human Ad immunity which could limit the effectiveness of an Ad vaccine [17].
In this study we investigated the efficacy of AdC7OprF.RGD as a mucosal vaccine against established P. aeruginosa respiratory infections. We found that immunization with AdC7OprF.RGD induced robust humoral and cellular immunity against P. aeruginosa that resulted in the enhanced clearance of P. aeruginosa from the rodent lungs. These data suggest that the activation of OprF-specific mucosal immune response can be a useful therapeutic vaccine strategy for patients with chronic pulmonary disorders who are frequently colonized with P. aeruginosa.
Materials and methods
Ethics statement
All animal studies were conducted in accordance to the protocols reviewed and approved by the Weill Cornell Institutional Animal Care and Use Committee. All efforts were made to minimize suffering to the animals.
Animals
Female C57BL/6J mice (WT mice), obtained from Jackson Laboratory (Bar Harbor, ME), were housed under specific pathogen-free conditions and were used at 8 weeks of age. CFTR gene knockout mice (Cftrtm1UNC, CF mice) were used at 7–10 weeks of age. Congenic C57BL/6J heterozygous breeding pairs (Cftrtm1UNC) were maintained and continuously bred on regular mouse chow and polyethylene glycol water (GAVIS Pharmaceuticals, NJ) under specific pathogen-free conditions to prevent intestinal obstruction. To maintain congenic status and prevent genetic drift, each new generation of mice was bred to WT C57BL/6J mice and offspring were genotyped at 14 days of age by PCR analysis of tail-clip DNA. Sprague Dawley rats were obtained from Jackson Laboratory and housed under specific pathogen-free conditions and were used at 8 weeks of age.
Adenovirus
The recombinant Ad vectors used in this study are replication-defective E1-, E3- deficient Ad vectors based on the chimpanzee AdC7 genome (kindly provided by JM Wilson, University of Pennsylvania) [5]. AdC7OprF contains the human cytomegalovirus intermediate-early enhancer/promoter, the OprF cDNA, and a simian virus 40 poly(A) stop signal expression cassette in the E1 region as previously described [8]. The RGD peptide was incorporated at the C-terminal of the fiber gene [8]. AdC7Null (kindly provided by JM Wilson), an AdC7 vector that does not lead to transgene expression in mammalian cells, was used as control. The vectors were used on the basis of equal number of particle units (pu) and were propagated, purified and quantified as described previously [18].
Pseudomonas aeruginosa strains
The clinical CF P. aeruginosa strain RP73 (CCBJ/EMBL/GenBank accession number CP006245.1), that can be used to establish persistent respiratory infections in mice [19], was kindly provided by Dr. Bragonzi (San Raffaele Scientific Institute, Italy). P. aeruginosa embedded in agar beads were used to develop murine models of persistent P. aeruginosa lung infection. The embedding of P. aeruginosa within agar beads retains the bacteria within the airways and has been developed to model a CF-like environment of microaeroiosis and bacterial biofilms. Retention of the bacteria presumably avoids physical elimination and leads to a persistent stimulation of host defenses without accounting for initial bacterial colonization [19]. Therefore, the establishment of infection is almost instant, but also wanes over a few weeks. Agar beads embedded with P. aeruginosa were prepared using a modified version of the method described previously [19]. Bacteria from glycerol stocks were streaked for isolation on a tryptic soy agar (TSA) plate and incubated at 37°C overnight. A single colony was used to inoculate 5 ml of tryptic soy broth (TSB). A small aliquot of this overnight culture was used to inoculate 50 ml of TSB. Bacteria were grown to late log phase in a shaking incubator at 37°C, centrifuged at 2,700g for 15 min, and suspended in 1ml of TSB. The bacterial suspension was embedded into agar beads by mixing with 1.5% (for mice) or 2% (for rats) w/v agar (DIFCO™ AGAR granulated, Beckton & Dickenson) in TSB and was then spun into warmed heavy mineral oil. The mineral oil was rapidly stirred for 6 min at room temperature, then cooled for 4°C for 35 min and on ice for 20 min. The beads were centrifuged at 2,700 g for 15 min and then washed six times with phosphate buffered saline (PBS). Only beads sized between 100μm and 200μm were selected using cell strainers (PluriSelect, San Diego, CA) and stored at 4°C. P. aeruginosa embedded agar beads were prepared the day before inoculation. P. aeruginosa concentration was measured in an aliquot of homogenized bead slurry by plating on TSA and calculated as colony forming unit (cfu)/ml. The slurry was diluted with PBS to obtain the desired bacterial concentration before inoculation.
Administration of P. aeruginosa embedded in agar beads to mice and rats
Wild-type mice were intratracheally injected with 1×106 cfu of P. aeruginosa embedded in agar beads in 100μl PBS under isoflurane anesthesia. CF mice and rats were injected with 5×105 or 5×106 cfu of P. aeruginosa embedded in agar beads respectively. Control mice received non-bacterial agar beads prepared similarly with sterile PBS. Aliquots of beads preparations were stained with alcian blue, the numbers of the beads were counted under microscope, and approximately same numbers of beads were used for injection. Body weight was recorded daily.
Post-exposure immunization of mice with adenovirus vector
Mice were immunized by intranasal inoculation of AdC7OprF.RGD (1010 pu diluted in 50μl PBS) 4 days following administration of P. aeruginosa. Control mice received AdC7Null or just PBS intranasally. Rats were immunized with AdC7OprF.RGD or AdC7Null (both at 5×1010 pu) 1 day after the administration of P. aeruginosa embedded in agar beads. The timing of vaccine administration was chosen based on the recovery of mice or rats from the stress of intratracheal administration (minor surgical procedure).
Anti-OprF humoral immune responses
Serum or lung homogenates were collected to measure anti-OprF humoral immune responses. Whole lungs homogenized in 700μl of PBS were centrifuged at 1000 rpm at 4°C for 10 min, and the supernatants were collected to measure anti-OprF IgA titers in lungs. Anti-OprF total IgG, IgG isotypes, IgM and IgA antibody titers were assessed by ELISA using flat-bottomed 96-well EIA/RIA plates (Corning, New York, NY) coated with recombinant OprF (0.5 μg/well in 0.05 M carbonate buffer, pH 7.4) as described [8]. The plates were blocked with 5% dry milk in PBS for 1 h at RT. Serial dilutions of serum or lung homogenate supernatants were added to each well and incubated for 1 h at RT. Following three washes with PBS containing 0.05% Tween20 (PBS-T) a peroxidase-conjugated sheep anti-mouse IgG (Sigma), diluted 1:10,000 in PBS containing 1% dry milk, was added and incubated for 1 h at RT. Anti-OprF IgG isotypes (IgG1, IgG2a, IgG2b and IgG3), IgM and IgA were determined using an isotyping kit (Bio-Rad Laboratories, Hercules, CA). Absorbance at 415 nm was measured with a microplate reader (Bio-Rad Laboratories) and the antibody titers were calculated with a log(OD)-log(dilution) interpolation model and a cutoff value equal to 2-fold the absorbance of the background. Serum from the non-infected and non-immunized mice was used to determine the non-specific reaction levels for the assay. For rats, peroxidase-conjugated sheep anti-rat IgG (Sigma; 1:10,000 dilution) was used as secondary antibody to detect anti-OprF IgG in serum by ELISA.
OprF-specific cellular immune responses
OprF-specific cellular immune responses were assessed 2 weeks after the immunization with AdC7OprF.RGD or AdC7Null. Mice splenocytes were isolated as a single-cell suspension and cultured in RPMI medium supplemented with 2% fetal bovine serum (HyClone, Logan, UT), 10 mM HEPES (pH 7.5; Biosource International, Camarillo, CA), and 10 μM β-mercaptoethanol (Sigma-Aldrich) in 96-well (Millipore) plates. The splenocytes were stimulated with 10 μg/ml OprF for 48 h and culture supernatants were collected. The mouse Interferon-γ (IFN-γ) and IL-4 were measured in the culture supernatant with specific ELISA kits (eBioscience).
Bacterial loads in lungs
Both the lungs from the sacrificed mice and rats were homogenized in 700μl and 3ml of PBS respectively, serially diluted and plated on TSA plates. The concentration of P. aeruginosa were determined after 48 h and bacterial load in lung were calculated as cfu per gram lung.
Statistical analysis
The data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using ANOVA followed by Tukey’s multiple comparisons or unpaired t test. Statistical significance was determined at p<0.05.
Results
Systemic and mucosal humoral immune responses induced by AdC7OprF.RGD in mice with established P. aeruginosa respiratory infection
Mice were infected with P. aeruginosa embedded in agar beads to mimic the disease state of chronic lung infection with P. aeruginosa. The animals were then immunized with AdC7OprF.RGD, AdC7Null or PBS 4 days after the administration of P. aeruginosa. All P. aeruginosa-administrated mice lost about 15% of their body weight by day 3 and regained their starting weight by day 10 (Figure S1). Serum anti-OprF IgG was detected in all groups at 1 and 2 weeks after the immunization. Anti-OprF IgG only increased in the AdC7OprF.RGD-immunized mice at 2 weeks after the immunization, but not in the non-immunized and AdC7Null-immunized mice (p<0.001 all comparisons; Figure 1A). All IgG isotypes were higher in AdC7OprF.RGD immunized mice compared to the mice that had received AdC7Null (p<0.05 all comparisons; Figure 1B).
Figure 1. Anti-P. aeruginosa humoral immune responses after the immunization with AdC7OprF.RGD in mice with established P. aeruginosa respiratory infection.
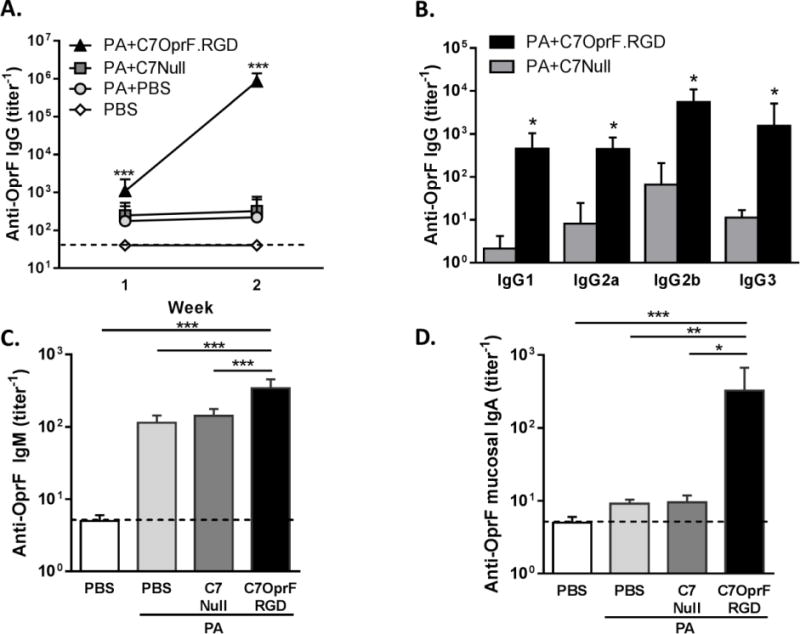
C57BL/6 mice were intratracheally administrated with 1×106 cfu of P. aeruginosa embedded in agar beads and immunized intranasally with either 1×1010 particle units of AdC7OprF.RGD (PA+AdC7OprF.RGD), AdC7Null (PA+AdC7Null) or PBS (PA+PBS) 4 days after the administration of P. aeruginosa. Non-infected and non-immunized mice were used as controls (PBS). Anti-OprF antibody titers were determined in serum at 1 and 2 weeks after the immunization and in lung homogenate supernatants at 2 weeks after the immunization by ELISA. A. Serum anti-OprF IgG (1wk and 2 wk). B. Serum anti-OprF IgG isotypes (2 wk). C. Serum anti-OprF IgM (1 wk). D. Anti-OprF IgA in lung homogenate supernatants (2wk). Data are presented as mean ± SEM of n=7–8 mice per group. *, ** and ***denote significance of p < 0.05, p < 0.01 and p < 0.001 respectively.
Serum anti-OprF IgM was also detected at 1 week after the immunization in all groups but was higher in the mice that had received AdC7OprF.RGD compared to AdC7Null (p<0.001; Figure 1C). Mucosal anti-OprF IgA titers in lungs 2 weeks following immunization were also higher in the mice that had received AdC7OprF.RGD compared to AdC7Null (p<0.05; Figure 1D). This suggests that mucosal administration of AdC7OprF.RGD to the mice with established P. aeruginosa infection enhances the systemic and mucosal humoral immune responses induced by the initial P. aeruginosa infection.
Systemic cellular immune responses induced by AdC7OprF.RGD in mice with established P. aeruginosa respiratory infection
In addition to humoral immune responses, cellular immune responses are known to play an important role to clear the P. aeruginosa infections [20]. Both OprF-specific IFN-γ and IL-4 in splenocytes from the mice with established P. aeruginosa respiratory infection that were immunized with AdC7OprF.RGD were higher compared to AdC7Null or PBS controls (p<0.05, all comparisons; Figure 2). This suggests that both Th1 and Th2 type of cellular immune responses were augmented by immunization with AdC7OprF.RGD in the mice with established P. aeruginosa respiratory infection. Additionally, immunization with AdC7OprF.RGD enhanced the expression of IFN-γ and IL-17A in the lung tissue compared to AdC7Null or PBS controls (p<0.05 all comparisons; Figure S3A–B). No significant change in IL-4 mRNA levels was observed in lungs (Figure S3C).
Figure 2. Cellular immune responses after the immunization with AdC7OprF.RGD in mice with established P. aeruginosa respiratory infection.
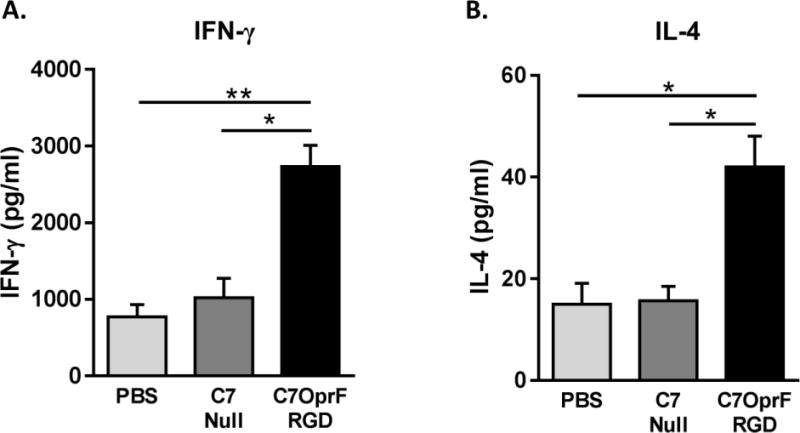
C57BL/6 mice were immunized intranasally with either 1×1010 particle units of AdC7OprF.RGD, AdC7Null or PBS 4 days after the administration of P. aeruginosa embedded in agar beads. Two weeks after the immunization, the splenocytes were collected, cultured and stimulated with OprF protein for 48 h. A. IFN-γ and B. IL-4 levels were measured in the culture supernatants by ELISA. Data are presented as mean ± SEM of n=8 mice per group. * and ** denote significance of p < 0.05 and p < 0.01 respectively.
Enhanced clearance of P. aeruginosa from the lungs after the post-exposure immunization with AdC7OprF.RGD
To evaluate the efficacy of post-exposure immunization with AdC7OprF.RGD to clear the established P. aeruginosa respiratory infection, lung P. aeruginosa bacterial loads were quantified at 1 and 2 weeks after the immunization. Compared to the mice that had received AdC7Null or that were not immunized, the mice that had received AdC7OprF.RGD showed decreased bacterial loads at both time points (p<0.05, both comparisons; Figure 3).
Figure 3. Clearance of P. aeruginosa from lungs after the immunization with AdC7OprF.RGD.
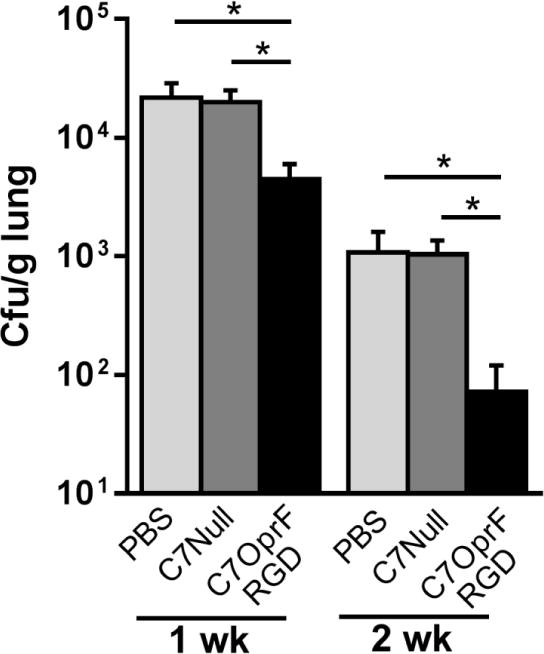
C57BL/6 mice were immunized intranasally with either 1x1010 particle units of AdC7OprF.RGD, AdC7Null or PBS 4 days after the administration of P. aeruginosa embedded in agar beads. P. aeruginosa bacterial loads were determined in lung homogenates 1 and 2 weeks after the immunization. Shown are cfu per gram lung tissue. Data are presented as mean ± SEM of n=7–8 mice per group. * denotes significance of p < 0.05.
Efficacy of post-exposure immunization with AdC7OprF.RGD in CF mice with established P. aeruginosa respiratory infection
We investigated the efficacy of post-exposure immunization with AdC7OprF.RGD in CF mice with established P. aeruginosa respiratory infection. Although it is not an equivalent model for human CF lung disease, CF mice are more susceptible to P. aeruginosa compared to WT mice (Figure S2B). Immunization with AdC7OprF.RGD elicits comparable anti-OprF IgG titers in CF and WT mice (Figure S2A) and protects against a P. aeruginosa challenge in both mice (Figure S2B).
To evaluate the efficacy of immunization on the established P. aeruginosa respiratory infection in CF mice, AdC7OprF.RGD or AdC7Null were intranasally inoculated to CF mice that had been administrated P. aeruginosa embedded in agar beads 4 days prior to the immunization. Ten days after the immunization, the mice that had received AdC7OprF.RGD showed higher levels of serum anti-OprF IgG (Figure 4A) and lower P. aeruginosa bacterial loads in lungs (Figure 4B) compared to the mice that had received AdC7Null (p<0.0001, both comparisons). This suggests that post-exposure immunization with AdC7OprF.RGD is effective to enhance clearance of P. aeruginosa from the lungs in CF mice.
Figure 4. Anti-P. aeruginosa humoral immune responses and enhanced P. aeruginosa clearance from lungs after the immunization with AdC7OprF.RGD in CF mice with established P. aeruginosa respiratory infection.
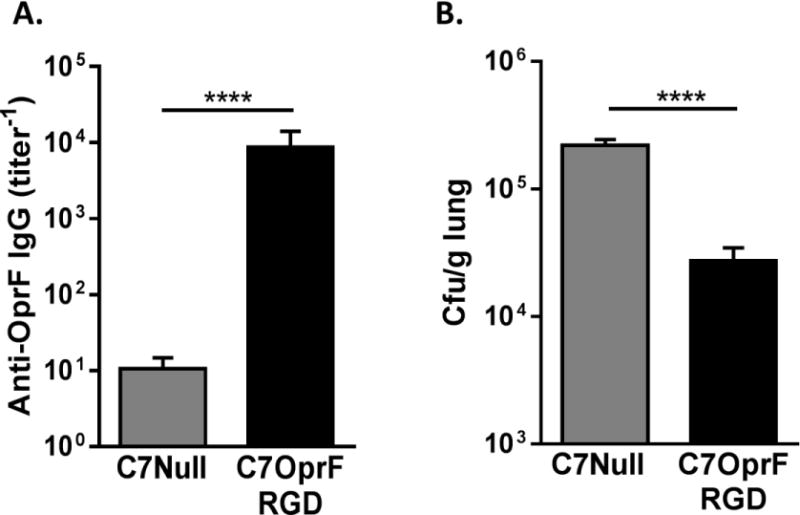
CF mice (Cftrtm1UNC) were immunized intranasally with either 1×1010 particle units of AdC7OprF.RGD or AdC7Null 4 days after the administration of P. aeruginosa embedded in agar beads. Ten days after the immunization, anti-OprF IgG antibody titers were determined in serum and P. aeruginosa bacterial loads were determined in lung homogenates. A. Serum anti-OprF IgG. B. P. aeruginosa bacterial loads in lung homogenates. Data are presented as mean ± SEM of n=8 mice per group. **** denotes significance of p < 0.0001.
Efficacy of immunization with AdC7OprF.RGD in rats with established P. aeruginosa respiratory infection
To evaluate the efficacy of AdC7OprF.RGD in another rodent species, we established persistent P. aeruginosa infections in rat lungs and immunized with AdC7OprF.RGD or AdC7Null 1 day after the administration of P. aeruginosa embedded in agar beads. Two weeks after the immunization, the rats that had received AdC7OprF.RGD had higher levels of serum anti-OprF IgG (p<0.01; Figure 5A) and lower P. aeruginosa bacterial loads in lungs (p<0.05; Figure 5B) compared to the rats that had received AdC7Null. This suggests that post-exposure immunization with AdC7OprF.RGD is also effective to enhance pulmonary clearance of P. aeruginosa in rats.
Figure 5. Anti- P. aeruginosa humoral immune responses and enhanced P. aeruginosa clearance from lungs after the immunization with AdC7OprF.RGD in rats with established P. aeruginosa respiratory infection.
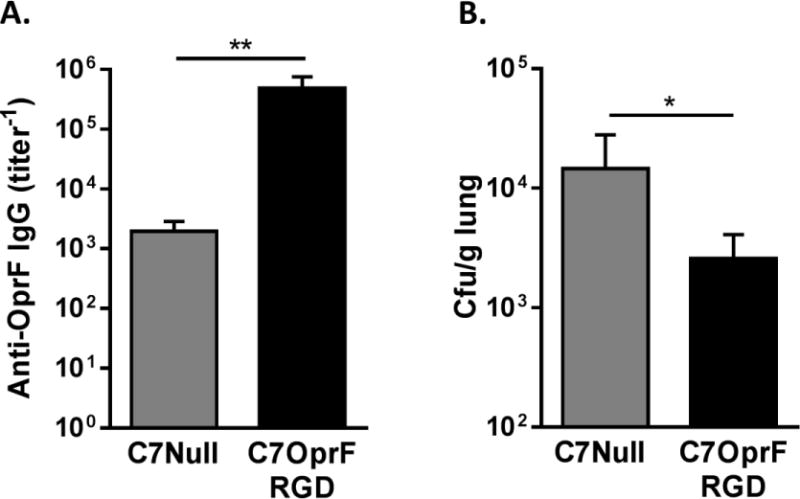
Rats (Sprague Dawley) were immunized intranasally with either 5×1010 particle units of AdC7OprF.RGD or AdC7Null 1 day after the administration of P. aeruginosa embedded in agar beads. Two weeks after the immunization, anti-OprF IgG antibody titers were determined in serum and P. aeruginosa bacterial loads were determined in lung homogenates. A. Serum anti-OprF IgG. B. P. aeruginosa bacterial loads in lung homogenates. Data are presented as mean ± SEM of n=5 mice per group. * and ** denote significance of p < 0.05 and p < 0.01 respectively.
Discussion
This study introduces a novel application for an Ad-based vaccine platform against established P. aeruginosa respiratory infections. Ad-based vaccines against P. aeruginosa such as AdC7OprF.RGD have been mostly developed to function in a classic active immunization approach to prevent infections. With the growing realization of the commonality of bacterial colonization in respiratory tracts that has been a particular problem for patients with chronic pulmonary disorders, a successful vaccine strategy for these conditions is also needed to be able to control pre-existing colonization and to prevent bacterial overgrowth leading to clinical exacerbations. Here we tested, for the first time, such a strategy against respiratory infections with P. aeruginosa and demonstrated that immunization with AdC7OprF.RGD in rodents with already established P. aeruginosa respiratory infection can lead to faster elimination or reduction of the bacteria in the lungs. This can be useful strategy for individuals with CF who are frequently colonized with P. aeruginosa at an early age before their lung function declines.
In CF affected individuals, P. aeruginosa colonizes in respiratory tract early in life with median age at first infection of 5.5 years, and most adults harbor P. aeruginosa in their respiratory tracts [21]. Despite some success of widespread implementation of antibiotic therapy to eradicate initial P. aeruginosa acquisition, the occurrence of multi-drug resistant P. aeruginosa continues to rise [2, 22, 23]. Therefore, a therapeutic vaccine to clear P. aeruginosa colonization would be an attractive strategy. Most therapeutic vaccines have been developed against viruses such as rabies [24], ebola [25, 26], varicella [27], human immunodeficiency virus, hepatitis B and hepatitis C [28]. Bacterial targets have been mainly Mycobacterium spps [29].
We evaluated in the efficacy of post-exposure immunization with AdC7OprF.RGD in wild-type C57BL/6J mice, Cftrtm1UNC mice and Sprague Dawley rats. The exaggerated inflammatory response and increased susceptibility for P. aeruginosa that is characteristic for human CF lung disease is partially mimicked in Cftrtm1UNC mice that are backcrossed on a C57BL/6 background [30–32]. Rats were tested to confirm our findings in another rodent model that is commonly used to study pulmonary P. aeruginosa infections [33, 34]. Based on this and prior data by us and others we assume that humoral, mucosal and systemic opsonizing immunity are effective to prevent and clear colonization against this extracellular pathogen [20]. T-cell responses have also been shown to mediate protective immunity in individuals with P. aeruginosa infections [35–37]. We chose a potent capsid-modified AdC7 vector, which has robust respiratory mucosal immune stimulatory properties, as a vaccine platform to induce strong humoral and cellular immunity to clear established P. aeruginosa respiratory infections. Mucosal immunization with AdC7OprF.RGD induced increased levels of anti-P. aeruginosa humoral and cellular immune responses above those that were induced by the pre-existing P. aeruginosa respiratory infection in mice. The induction of all 4 subclasses of IgG indicates broad activation of transgene-specific humoral responses: IgG2a and IgG1 suggest induction of both Th1 and Th2 type of immune responses; as IgG1, IgG2, and IgG3 play important roles in opsonization, their induction by the vaccine is important as OprF is a major target for opsonizing antibodies to clear the infection [38, 39]. Increased levels of anti-P. aeruginosa humoral immune responses were also observed in CF mice that are characterized by an increased susceptibility to P. aeruginosa [30, 40, 41]. However, CF mice do not mimic the severity and other critical mucus characteristics of human CF lung disease. Other CF animal models such as CF pigs or ferrets could be useful to further evaluate the preclinical feasibility of AdC7OprF.RGD as a therapeutic vaccine [40]. While it would have been interesting to evaluate the bacterial lung titers beyond 2 weeks post-immunization, we anticipate that, based on our prior studies with AdC7OprF.RGD [5, 6], to observe a similar or even higher levels of anti-OprF immunity, the waning P. aeruginosa load over time would have worked against detection of this stronger effect. The choice of this vaccine vector is supported by the promising potential of adenovirus-based vaccines to induce robust anti-transgene immunity as evident in numerous preclinical and clinical studies against a vast variety of infectious agents [42–44]. AdC7, in addition to circumventing the pre-existing immunity against common human Ad serotypes, induces long-term anti-transgene systemic, mucosal and protective immunity in the respiratory tract, particularly when administered via the mucosal route to the respiratory tract [5, 6, 8]. Although only a few mucosal vaccines have been developed for humans, the importance of mucosal immunization against mucosal pathogens is well recognized [45–49]. Addition of RGD to AdC7 vector further enhances the mucosal humoral immunity and protective efficacy of the vaccine [5]. The strategy of RGD incorporation to the fiber of Ad vectors have been previously applied to improve the vaccine and immunomodulatory cancer therapies [50–52]. The RGD peptide facilitates the infections to the cells expressing high levels of αvβ3 or αvβ5 integrins, in particular dendritic cells, which promotes the antigen presentation through both class I and class II pathways. Furthermore, adenovirus-based vaccines are known to favor Th1-biased transgene-specific immune responses [53, 54], which is further enhanced by the addition of the RGD peptide [51]. This could be beneficial in CF where immune responses are often skewed to Th2 [55–57]. Innate immune responses are known to be abnormal in CF [58]. This has also been partially seen in CF mice, which may affect the strength of adaptive immunity induced by vaccines [32, 59]. Interestingly, immunization with AdC7OprF.RGD induced comparable adaptive anti-OprF immunity in the CF mice compared to the WT controls (Figure S2A). Adjuvant properties of Ad vector and the RGD-mediated targeting of dendritic cells might have played a role in induction of these strong adaptive immune responses in the CF mice. In addition to IFN-γ and IL-4, immunization with AdC7OprF.RGD also augmented IL-17A expression, which is a critical component of vaccine-induced protection against pulmonary infection and to control chronic lung infections with P. aeruginosa [60–62]. A more detailed analysis of lung immune cells would be informative and could be subject of future studies.
Overall, our study suggests that AdC7OprF.RGD could be a promising vaccine for patients with chronic pulmonary disorders, especially with colonized P. aeruginosa in their lungs or with chronic P. aeruginosa respiratory infection with frequent clinical exacerbations.
Supplementary Material
Highlights.
A vaccine against established P. aeruginosa respiratory infections would be helpful.
Capsid-modified AdC7 vectors are potent inducers of respiratory mucosal immunity.
Immunization with AdC7OprF.RGD against established P. aeruginosa lung infections was evaluated.
AdC7OprF.RGD immunization induced robust humoral and cellular immunity against P. aeruginosa.
The induced immunity enhanced clearance of P. aeruginosa from rodent lungs.
Acknowledgments
We thank Dr. Alessandra Bragonzi (San Raffaele Scientific Institute, Italy) for providing us the clinical CF P. aeruginosa strain RP73, James M. Wilson for the AdC7 vector, Nancy and Dan Paduano for their enthusiastic support. These studies were supported by RO1 AI103341 to S.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yum HK, Park IN, Shin BM, Choi SJ. Recurrent Pseudomonas aeruginosa Infection in Chronic Lung Diseases: Relapse or Reinfection? Tuberculosis and respiratory diseases. 2014;77:172–7. doi: 10.4046/trd.2014.77.4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Nagrini N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization; 2017. [Google Scholar]
- 3.Priebe GP, Goldberg JB. Vaccines for Pseudomonas aeruginosa: a long and winding road. Expert review of vaccines. 2014;13:507–19. doi: 10.1586/14760584.2014.890053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamei A, Coutinho-Sledge YS, Goldberg JB, Priebe GP, Pier GB. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infection and immunity. 2011;79:1289–99. doi: 10.1128/IAI.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause A, Whu WZ, Qiu J, Wafadari D, Hackett NR, Sharma A, et al. RGD capsid modification enhances mucosal protective immunity of a non-human primate adenovirus vector expressing Pseudomonas aeruginosa OprF. Clinical and experimental immunology. 2013;173:230–41. doi: 10.1111/cei.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause A, Whu WZ, Xu Y, Joh J, Crystal RG, Worgall S. Protective anti-Pseudomonas aeruginosa humoral and cellular mucosal immunity by AdC7-mediated expression of the P. aeruginosa protein OprF. Vaccine. 2011;29:2131–9. doi: 10.1016/j.vaccine.2010.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Human vaccines. 2011;7:999–1011. doi: 10.4161/hv.7.10.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worgall S, Krause A, Qiu J, Joh J, Hackett NR, Crystal RG. Protective immunity to pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P. aeruginosa OprF. Journal of virology. 2007;81:13801–8. doi: 10.1128/JVI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimwood K, Kyd JM, Owen SJ, Massa HM, Cripps AW. Vaccination against respiratory Pseudomonas aeruginosa infection. Human vaccines & immunotherapeutics. 2015;11:14–20. doi: 10.4161/hv.34296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loomis RJ, Johnson PR. Gene-based vaccine approaches for respiratory syncytial virus. Current topics in microbiology and immunology. 2013;372:307–24. doi: 10.1007/978-3-642-38919-1_15. [DOI] [PubMed] [Google Scholar]
- 11.Xiang K, Ying G, Yan Z, Shanshan Y, Lei Z, Hongjun L, et al. Progress on adenovirus-vectored universal influenza vaccines. Human vaccines & immunotherapeutics. 2015;11:1209–22. doi: 10.1080/21645515.2015.1016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Z, Lichty BD. Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization. Tuberculosis (Edinb) 2006;86:211–7. doi: 10.1016/j.tube.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Choi JH, Jonsson-Schmunk K, Qiu X, Shedlock DJ, Strong J, Xu JX, et al. A Single Dose Respiratory Recombinant Adenovirus-Based Vaccine Provides Long-Term Protection for Non-Human Primates from Lethal Ebola Infection. Molecular pharmaceutics. 2015;12:2712–31. doi: 10.1021/mp500646d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock RE, Wong R. Potential of protein OprF of Pseudomonas in bivalent vaccines. Behring Institute Mitteilungen; 1997. pp. 283–90. [PubMed] [Google Scholar]
- 15.Sharma A, Krause A, Xu Y, Sung B, Wu W, Worgall S. Adenovirus-based vaccine with epitopes incorporated in novel fiber sites to induce protective immunity against Pseudomonas aeruginosa. PloS one. 2013;8:e56996. doi: 10.1371/journal.pone.0056996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worgall S, Krause A, Rivara M, Hee KK, Vintayen EV, Hackett NR, et al. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. The Journal of clinical investigation. 2005;115:1281–9. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Current gene therapy. 2011;11:307–20. doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. Journal of virology. 1996;70:7498–509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Facchini M, De Fino I, Riva C, Bragonzi A. Long term chronic Pseudomonas aeruginosa airway infection in mice. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cripps AW, Dunkley ML, Clancy RL, Kyd J. Pulmonary immunity to Pseudomonas aeruginosa. Immunology and cell biology. 1995;73:418–24. doi: 10.1038/icb.1995.65. [DOI] [PubMed] [Google Scholar]
- 21.Cystic Fibrosis Foundation. Patient Registry Annual Data Report. 2015 [Google Scholar]
- 22.Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. International journal of antimicrobial agents. 2015;45:568–85. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T, et al. Cystic Fibrosis Foundation pulmonary guideline. pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Annals of the American Thoracic Society. 2014;11:1640–50. doi: 10.1513/AnnalsATS.201404-166OC. [DOI] [PubMed] [Google Scholar]
- 24.Zhu S, Guo C. Rabies Control and Treatment: From Prophylaxis to Strategies with Curative Potential. Viruses. 2016;8 doi: 10.3390/v8110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzi A, Hanley PW, Haddock E, Martellaro C, Kobinger G, Feldmann H. Efficacy of Vesicular Stomatitis Virus-Ebola Virus Postexposure Treatment in Rhesus Macaques Infected With Ebola Virus Makona. The Journal of infectious diseases. 2016;214:S360–S6. doi: 10.1093/infdis/jiw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong G, Richardson JS, Pillet S, Racine T, Patel A, Soule G, et al. Adenovirus-Vectored Vaccine Provides Postexposure Protection to Ebola Virus-Infected Nonhuman Primates. The Journal of infectious diseases. 2015;212(Suppl 2):S379–83. doi: 10.1093/infdis/jiv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald J. Vaccines for postexposure prophylaxis against varicella (chickenpox) in children and adults. Paediatrics & child health. 2016;21:91–2. doi: 10.1093/pch/21.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edlich RF, Gubler K, Wallis AG, Clark JJ, Dahlstrom JJ, Long WB., 3rd Postexposure prophylaxis for deadly bloodborne viral infections. Journal of environmental pathology, toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer. 2010;29:293–315. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i4.30. [DOI] [PubMed] [Google Scholar]
- 29.Santema W, Rutten V, Segers R, Poot J, Hensen S, Heesterbeek H, et al. Postexposure subunit vaccination against chronic enteric mycobacterial infection in a natural host. Infection and immunity. 2013;81:1990–5. doi: 10.1128/IAI.01121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilbault C, Martin P, Houle D, Boghdady ML, Guiot MC, Marion D, et al. Cystic fibrosis lung disease following infection with Pseudomonas aeruginosa in Cftr knockout mice using novel non-invasive direct pulmonary infection technique. Laboratory animals. 2005;39:336–52. doi: 10.1258/0023677054306944. [DOI] [PubMed] [Google Scholar]
- 31.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. American journal of respiratory cell and molecular biology. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 32.Paroni M, Moalli F, Nebuloni M, Pasqualini F, Bonfield T, Nonis A, et al. Response of CFTR-deficient mice to long-term chronic Pseudomonas aeruginosa infection and PTX3 therapy. The Journal of infectious diseases. 2013;208:130–8. doi: 10.1093/infdis/jis636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cash HA, Woods DE, McCullough B, Johanson WG, Jr, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. The American review of respiratory disease. 1979;119:453–9. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 34.Growcott EJ, Coulthard A, Amison R, Hardaker EL, Saxena V, Malt L, et al. Characterisation of a refined rat model of respiratory infection with Pseudomonas aeruginosa and the effect of ciprofloxacin. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2011;10:166–74. doi: 10.1016/j.jcf.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Dunkley ML, Clancy RL, Cripps AW. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83:362–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Dunkley ML, Cripps AW, Reinbott PW, Clancy RL. Immunity to respiratory Pseudomonas aeruginosa infection: the role of gut-derived T helper cells and immune serum. Advances in experimental medicine and biology. 1995;371B:771–5. [PubMed] [Google Scholar]
- 37.Stevenson MM, Kondratieva TK, Apt AS, Tam MF, Skamene E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clinical and experimental immunology. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin NL, Rawling EG, Wong RS, Rosok M, Hancock RE. Conservation of surface epitopes in Pseudomonas aeruginosa outer membrane porin protein OprF. FEMS microbiology letters. 1993;113:261–6. doi: 10.1111/j.1574-6968.1993.tb06524.x. [DOI] [PubMed] [Google Scholar]
- 39.Staczek J, Gilleland HE, Jr, Gilleland LB, Harty RN, Garcia-Sastre A, Engelhardt OG, et al. A chimeric influenza virus expressing an epitope of outer membrane protein F of Pseudomonas aeruginosa affords protection against challenge with P. aeruginosa in a murine model of chronic pulmonary infection. Infection and immunity. 1998;66:3990–4. doi: 10.1128/iai.66.8.3990-3994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher JT, Zhang Y, Engelhardt JF. Comparative biology of cystic fibrosis animal models. Methods Mol Biol. 2011;742:311–34. doi: 10.1007/978-1-61779-120-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann N, Rasmussen TB, Jensen PO, Stub C, Hentzer M, Molin S, et al. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infection and immunity. 2005;73:2504–14. doi: 10.1128/IAI.73.4.2504-2514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nature reviews Microbiology. 2014;12:765–71. doi: 10.1038/nrmicro3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert SC. Adenovirus-vectored Ebola vaccines. Expert review of vaccines. 2015;14:1347–57. doi: 10.1586/14760584.2015.1077122. [DOI] [PubMed] [Google Scholar]
- 44.Wold WS, Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Current gene therapy. 2013;13:421–33. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afkhami S, Yao Y, Xing Z. Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Molecular therapy Methods & clinical development. 2016;3:16030. doi: 10.1038/mtm.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aldovini A. Mucosal Vaccination for Prevention of HIV Infection and AIDS. Current HIV research. 2016;14:247–59. doi: 10.2174/1570162x14999160224103025. [DOI] [PubMed] [Google Scholar]
- 47.Kim SH, Jang YS. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clinical and experimental vaccine research. 2017;6:15–21. doi: 10.7774/cevr.2017.6.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nature medicine. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 49.Wong-Chew RM, Islas-Romero R, Garcia-Garcia Mde L, Beeler JA, Audet S, Santos-Preciado JI, et al. Immunogenicity of aerosol measles vaccine given as the primary measles immunization to nine-month-old Mexican children. Vaccine. 2006;24:683–90. doi: 10.1016/j.vaccine.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 50.Okada N, Saito T, Masunaga Y, Tsukada Y, Nakagawa S, Mizuguchi H, et al. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer research. 2001;61:7913–9. [PubMed] [Google Scholar]
- 51.Worgall S, Busch A, Rivara M, Bonnyay D, Leopold PL, Merritt R, et al. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. Journal of virology. 2004;78:2572–80. doi: 10.1128/JVI.78.5.2572-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura RE, Hunger A, Fernandes DC, Laurindo FR, Costanzi-Strauss E, Strauss BE. Induction of Oxidants Distinguishes Susceptibility of Prostate Carcinoma Cell Lines to p53 Gene Transfer Mediated by an Improved Adenoviral Vector. Human gene therapy. 2017 doi: 10.1089/hum.2016.139. [DOI] [PubMed] [Google Scholar]
- 53.Lee MB, McMenamin MM, Byrnes AP, Charlton HM, Wood MJ. Th1 cytokines are upregulated by adenoviral vectors in the brains of primed mice. Neuroreport. 2008;19:1187–92. doi: 10.1097/WNR.0b013e3283088e3d. [DOI] [PubMed] [Google Scholar]
- 54.Radosevic K, Rodriguez A, Lemckert AA, van der Meer M, Gillissen G, Warnar C, et al. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clinical and vaccine immunology : CVI. 2010;17:1687–94. doi: 10.1128/CVI.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansen HK, Cryz SJ, Jr, Hougen HP, Moser C, Hoiby N. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia. A new prophylactic principle Behring Institute Mitteilungen. 1997:269–73. [PubMed] [Google Scholar]
- 56.Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. The Journal of allergy and clinical immunology. 2006;117:204–11. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 57.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2000;108:329–35. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 58.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nature medicine. 2012;18:509–19. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y, Krause A, Limberis M, Worgall TS, Worgall S. Low sphingosine-1-phosphate impairs lung dendritic cells in cystic fibrosis. American journal of respiratory cell and molecular biology. 2013;48:250–7. doi: 10.1165/rcmb.2012-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, et al. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181:4965–75. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayes HK, Ritchie ND, Evans TJ. Interleukin-17 Is Required for Control of Chronic Lung Infection Caused by Pseudomonas aeruginosa. Infection and immunity. 2016;84:3507–16. doi: 10.1128/IAI.00717-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lore NI, Cigana C, Riva C, De Fino I, Nonis A, Spagnuolo L, et al. IL-17A impairs host tolerance during airway chronic infection by Pseudomonas aeruginosa. Scientific reports. 2016;6:25937. doi: 10.1038/srep25937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


