Abstract
Formins are a family of regulators of actin and microtubule dynamics that are present in almost all eukaryotes. These proteins are involved in many cellular processes, including cytokinesis, stress fiber formation, and cell polarization. Here we review one sub-family of formins, the inverted formins. Inverted formins as a group break several formin stereotypes, having atypical biochemical properties and domain organization, and they have been linked to kidney disease and neuropathy in humans. In this review, we will explore recent research on members of the inverted formin sub-family in mammals, zebrafish, fruit flies, and worms.
Keywords: INF2, FHDC1, focal segmental glomerulosclerosis, Charcot-Marie-Tooth disease, actin
Introduction
Formins are best known for their interactions with filamentous actin [Breitsprecher and Goode, 2013; Goode and Eck, 2007], but they have also been shown to interact with microtubules [Bartolini and Gundersen, 2010; Chesarone et al., 2010]. Based on homology, there are nine sub-families of formins in animals [Pruyne, 2016]. These sub-families are unified by the typical presence of formin homology 1 (FH1) and formin homology 2 (FH2) domains, which play critical roles in formin-dependent regulation of actin dynamics. Ring-shaped dimers of FH2 domains nucleate actin filaments by bringing together actin monomers and stabilizing a nucleus for filament assembly [Moseley et al., 2004; Pring et al., 2003; Pruyne et al., 2002; Sagot et al., 2002; Xu et al., 2004]. The FH2 dimer stays associated with the growing or barbed end of the actin filament while the FH1 domain brings profilin-bound actin near the barbed end, providing a substrate for elongation [Kovar et al., 2003; Kovar et al., 2006; Moseley et al., 2004, Pring et al., 2003; Pruyne et al., 2002; Romero et al., 2004; Sagot et al., 2002; Zigmond et al., 2003]. This continuous association of the formin with the barbed end during filament elongation is sometimes called “processive capping”.
In many animal formins, an extended region N-terminal to the FH1 domain includes domains that are important for regulation, localization, and dimerization of the formin. Among many formins, this region includes a GTPase binding domain (G), a diaphanous inhibitory domain (DID), and a dimerization domain (DD) (Fig 1) [Li and Higgs, 2005; Otomo et al., 2005; Rose et al., 2005]. In a formin phylogeny study, Higgs and Peterson [Higgs and Peterson, 2005] identified a sub-family of formins with an unusual domain structure in which these N-terminal domains were missing, leaving their FH domains located near the N-terminus (Fig 1 A). Thus, this unusual domain structure appeared inverted compared to other formins, leading the authors to designate this sub-family the “inverted formins”.
Figure 1. Inverted formins of humans and common model systems.
(A) Shown are domains and motifs of inverted formins FH2 Domain-Containing 1 (FHDC1)/Inverted Formin-1 (INF1) and Inverted Formin-2 (INF2) from mouse (Mus musculus, Mm), FORMIN 3 (FORM3) from fruit fly (Drosophila melanogaster, Dm) and Excretory Canal Abnormal-6 (EXC-6) and Inverted Formin/Formin Three Related-2 (INFT-2) from worm (Caenorhabditis elegans, Ce), with M. musculus Diaphanous (DIAPH1) included as a canonical formin for reference. M. musculus INF2 has two known splice forms with alternate final exons, one that encodes a C-terminal CAAX box (red) and one that does not (non-CAAX, blue). Indicated are GTPase-binding domain (G), diaphanous inhibitory domains (DID), dimerization domains (DD), formin homology-1 domains (FH1), formin homology-2 domains (FH2), microtubule binding domain (MTBD), WASp homology-2 motif (WH2), and mixed WH2/diaphanous autoregulatory domain (WH2/DAD). Numbers indicate amino acid position, and the scale bar indicates protein length in amino acids. The INF2 WH2/DAD [Chhabra and Higgs, 2006] and the FHDC1 MTBD [Young et al., 2008] were identified in previous studies, while the positions of other domains and motifs were identified using NCBI CDS [Marchler-Bauer and Bryant, 2004], Eukaryotic Linear Motif Database [Dinkel et al., 2016], and PHYRE2 [Kelley et al., 2015]. (B) Inverted formins of multiple model systems can be separated into those with N-terminal DID and DD regions, resulting in C-terminally positioned FH1 and FH2 domains, and those that lack DID and DD regions, resulting in N-terminally positioned FH1 and FH2 domains.
Naming this group “inverted” was not perfect as of the six original inverted formins, one from puffer fish had a more traditional N-terminal domain organization [Higgs and Peterson, 2005], and further analysis of mammalian Inverted Formin-2 (INF2) has shown that it, too, has N-terminal DID and DD, and C-terminally located FH1 and FH2 domains (Fig 1 A) [Chhabra and Higgs, 2006]. Since then, more inverted formins have been identified, with additional representatives of both categories (Fig 1 B). Among examples of inverted formins with C-terminal FH2 domains are the fly FORMIN 3 (FORM3) and C. elegans Inverted Formin/Formin Three Related-2 (INFT-2), while canonical inverted formins with N-terminal FH2 domains include mammalian FH2 Domain-Containing 1 (FHDC1), also called Inverted Formin-1 (INF1), and C. elegans Excretory Canal Abnormal-6 (EXC-6).
Despite these differences in FH2 domain position, phylogenetic analysis of FH2 domain sequences show that inverted formins fall into a single sub-family distinct from other animal formins [Pruyne, 2016]. Among the nine known sub-families of the animal formins, the inverted formin sub-family is one of only two sub-families that are found in all animal phyla that have been examined. An inverted formin-related protein has also been noted in the choanoflagellate Monosiga brevicollis, a close relative to the animals, but none have been identified in more distantly related organisms [Chalkia et al., 2008]. Many animals encode multiple inverted formins. Interestingly, the presence of an N-terminal FH2-type and a C-terminal FH2-type of inverted formin is a feature conserved among the vertebrates, and is also a feature conserved among nematodes [Pruyne, 2016]. However, there is no obvious increased similarity between the FH2 domain sequences of vertebrate and nematode inverted formins within a given type, suggesting N-terminal FH2-type inverted formins likely arose independently in these different animal lineages [Pruyne, 2016].
Additional regulatory regions are often present C-terminal to the FH2 domain. Many formins have a small, poorly conserved region called the diaphanous autoregulatory domain (DAD), which binds the N-terminal DID in such a manner that actin polymerization by the FH2 domain is blocked [Alberts, 2001; Li and Higgs, 2005; Nezami et al., 2010; Otomo et al., 2010]. However, this property of autoinhibition may not be well conserved among inverted formins. As the N-terminal FH2-type inverted formins lack a DID, such autoinhibition would not be expected. Interestingly, several inverted formins that have a DID lack an obvious DAD. Examples of this include the fly FORM3 and worm INFT-2 (Fig 1 A). Even when DID and DAD are present in an inverted formin, DID/DAD interactions may be only weakly autoinhibitory, and subject to unusual regulation, as will be discussed below for the mammalian formin INF2 [Ramabhadran et al., 2013; Sun et al., 2011].
Additionally, the DAD of many formins shares weak sequence similarity to Wiskott-Aldrich syndrome protein homology-2 (WH2) motifs, which best known for their ability to bind actin [Paunola et al., 2002; Chhabra and Higgs, 2006]. The DADs of several such formins, including such non-inverted formins such as mDia1 and Daam1, have been found to enhance the stimulation of actin assembly by the FH2 domain, presumably through a very weak affinity for monomeric actin [Gould et al., 2011]. For other formins, particularly members of the FMNL sub-family, a bona fide WH2 domain separate from the DAD promotes actin assembly and displays significant affinity for actin [Vaillant et al., 2008; Heimsath and Higgs, 2012]. The inverted formin INF2 represents a third C-terminus organization, having a motif that acts as a DAD (i.e. interacts with the DID), but has stronger sequence similarity to WH2 motifs than other DADs [Chhabra and Higgs, 2006]. Moreover, the INF2 DAD has a much higher affinity (∼ 60 nM) for monomeric actin than what has been observed for other DADs [Chhabra and Higgs, 2006]. As discussed below, the INF2 WH2/DAD motif confers an unusual ability to sever actin filaments [Chhabra and Higgs, 2006]. The worm inverted formin EXC-6 also has a well-conserved WH2 motif in its C-terminues (Fig 1 A), although it remains to be seen whether the motif confers similar filament-severing activities.
Expression Patterns of Inverted Formins
Inverted formin expression has been studied in different tissue and cell types through a variety of methods (Table I). A large qRT-PCR study done by Krainer and colleagues [Krainer et al., 2013] provides some general insight into expression of the two human inverted formin genes, fhdc1 and inf2, in a wide range of human tissues and cell types. They found fhdc1 mRNA expressed in most cell and tissue types analyzed, and gave FHDC1 the designation “homeostatic formin”, suggesting it may have a role in forming or maintaining actin structures common to all cell types [Krainer et al., 2013]. This ubiquitous expression has been found consistently across additional studies (Table I). Probing by qRT-PCR also revealed a ubiquitous presence of mRNA for inf2, but in many tissues the levels were low enough they were considered to exclude inf2 from the homeostatic category [Krainer et al., 2013]. However, the mRNA of inf2 had relatively high expression levels in immune cells, neurons, and many epithelia, suggesting INF2 might be particularly important in those cell types. Protein-based studies have again generally showed consistency with these RNA-based studies (Table I).
Table I. Inverted formin expression in selected mammalian tissues and organs.
Shown are the compiled results from expression studies of FH2 Domain-Containing 1 (FHDC1)/Inverted Formin-1 (INF1) and Inverted Formin-2 (INF2) in human and mouse tissues and organs.
| Organ | FHDC1/INF1a | INF2a | Detection methodsb and references |
|---|---|---|---|
| Brain | + | + | northern blot [Brown et al., 2010] qRT-PCR [Krainer et al., 2013] RT-PCR-ELISA [Nagase et al., 2000] western blot [Young et al., 2008; Chhabra et al., 2009; Ramabhadran et al., 2011] |
| Heart | + | + | qRT-PCR [Krainer et al., 2013; Rosado et al., 2014] RT-PCR-ELISA [Nagase et al., 2000] western blot [Young et al., 2008; Chhabra et al., 2009] |
| Kidney | + | + | northern blot [Brown et al., 2010] RT-PCR-ELISA [Nagase et al., 2000] ISH [Brown et al., 2010] IFM [Brown et al., 2010] western blot [Ramabhadran et al., 2011] |
| Pancreas | + | + | northern blot [Brown et al., 2010] RT-PCR-ELISA [Nagase et al., 2000] |
| Spleen | + | + | northern blot [Brown et al., 2010] RT-PCR-ELISA [Nagase et al., 2000] western blot [Chhabra et al., 2009] |
| Testis | + | n.d. | RT-PCR-ELISA [Nagase et al., 2000] |
| Ovaries | + | n.d. | RT-PCR-ELISA [Nagase et al., 2000] |
| Lungs | + | + | northern blot [Brown et al., 2010] RT-PCR-ELISA [Nagase et al., 2000] western blot [Young et al., 2008]; |
| Liver | + | + | northern blot [Brown et al., 2010] RT-PCR-ELISA [Nagase et al., 2000] western blot [Chhabra et al., 2009] |
| Skeletal Muscle | + | + | northern blot [Brown et al., 2010] qRT-PCR [Krainer et al., 2013] RT-PCR-ELISA [Nagase et al., 2000] |
| Placenta | n.d. | + | northern blot [Brown et al., 2010] |
| Colon | n.d. | + | northern blot [Brown et al., 2010] |
| Skin | + | + | qRT-PCR [Krainer et al., 2013] |
| Blood Cells | + | + | qRT-PCR [Krainer et al., 2013] |
“+ ”indicates expression was detected. “n.d. ” indicates expression was not determined for that organ or tissue.
Abbreviations used are qualitative reverse transcriptase/polymerase chain reaction (qRT-PCR), reverse transcriptase/polymerase chain reaction coupled with enzyme-linked immunosorbent assay (RT-PCR-ELISA), in situ hybridization (ISH), and immunofluorescence microscopy (IFM).
The expression of alternative splice forms for inverted formins has not been exhaustively examined, but mammalian INF2 has been shown to have two prominent but very distinct isoforms (Fig 1 A). One isoform bears a C-terminal CAAX box for prenylation and membrane targeting, while the other lacks this motif [Chhabra et al., 2009; Ramabhadran et al., 2011]. To date, the presence of a CAAX box appears to be unique for INF2 among inverted formins of vertebrates and invertebrates. These INF2 splice forms vary in expression, with INF2 CAAX predominating in the kidney, and being found in the brain and in cultured fibroblasts, while the non-CAAX form predominates in cultured epithelial and immune cells, but is also found in brain and in lower abundance in kidney [Ramabhadran et al., 2011].
Looking in non-mammalian systems, RNA and protein-based studies have found zebrafish inf2 is expressed in the nervous system, eyes, somites, kidney, liver and gut [Sun et al., 2014], corresponding well with its mammalian homolog's expression pattern (Table I). Among invertebrates, RNA for the single fly inverted formin, form3, was found in tracheal cells during embryogenesis by in situ hybridization, where the formin was implicated in lumen formation [Tanaka et al., 2004]. Similarly, the C. elegans inverted formins EXC-6 and INFT-2 are expressed in the excretory canal, the worm osmoregulation device, where they were also implicated in lumen formation [Buechner et al., 1999; Shaye and Greenwald, 2015; Shaye and Greenwald, 2016]. EXC-6 is also expressed in the spermatheca, an epithelial bag-like structure in the worm gonad [Hegsted, et al., 2016]. While a comprehensive analysis of the expression of inverted formins has not been performed in any non-mammalian system, all these studies hint at some conservation of expression, particularly the presence of inverted formins in epithelial tissues, and in kidney-like organs.
Effects of INF2 on Actin Dynamics In Vitro
Formins are best known for their roles in regulating actin dynamics. Analysis of in vitro interactions between actin and an inverted formin have been best characterized for mammalian INF2 (Fig 2 A). In initial work done with mouse INF2, Chhabra and Higgs found that a fragment containing the FH1, FH2 and C-terminus including a WH2/DAD motif (INF2-FFC), or a fragment containing just the FH1 and FH2 (INF2-FF) both nucleate actin and remain associated with the growing barbed end of an actin filament [Chhabra and Higgs, 2006]. As with other formins, mutation of highly conserved isoleucine (I643) and lysine (K792) residues of the INF2 FH2 domain negatively impacted the polymerization activity of INF2-FF constructs. The presence of the C-terminus in INF2-FFC constructs dampened these negative effects, suggesting the WH2/DAD might participate in nucleation (although this might also reflect the formation of new filaments by C-terminus-dependent filament severing producing new filament ends, see below) [Ramabhadran et al., 2012]. Qualitatively similar to other formins, INF2-FFC slows elongation in the absence of profilin, but accelerates elongation in the presence of profilin [Chhabra and Higgs, 2006; Gurel et al., 2015]. One surprising property found for INF2 was that INF2-FFC also severs actin filaments to near completion in a manner dependent on its C-terminal WH2/DAD, and partially dependent on the conserved I643 and K792 residues of the FH2 domain [Chhabra and Higgs, 2006; Ramabhadran et al., 2012].
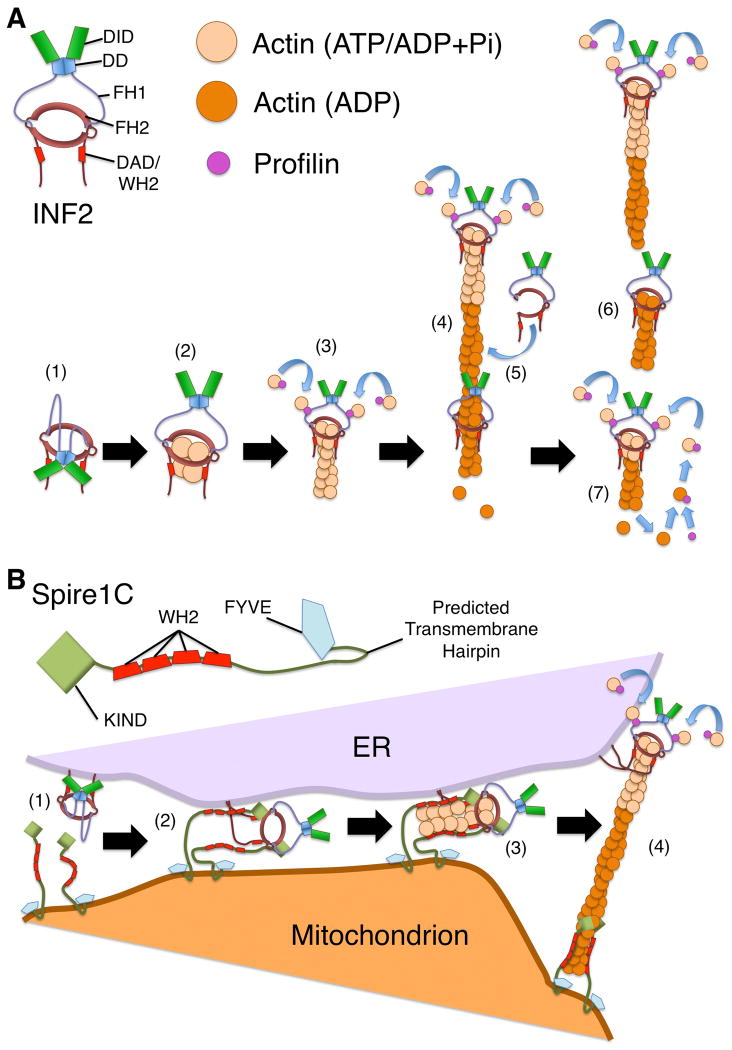
Figure 2. Effects of INF2 on actin filament dynamics.
(A) INF2 stimulates actin polymerization and depolymerization. (1) Interaction between the DID and DAD hold INF2 in an autoinhibited state. (2) With activation of INF2, ATP-actin associates with the FH2 domain and the WH2-like DAD, with a potential ratio of four actin monomers per INF2 dimer [Gurel et al., 2015]. (3) ATP-actin monomers bound to profilin are recruited by INF2 through interaction of profilin with the FH1 domains, followed by transfer of the monomers to the FH2 domain-bound barbed end. (4) As the filament elongates, with INF2 remaining at the growing barbed end, actin hydrolyzes ATP and releases inorganic phosphate, generating ADP-bound F-actin. (5) INF2 associates with ADP-bound actin, possibly through transient opening of the FH2 dimer to encircle the filament. (6) In a WH2/DAD-dependent manner, INF2 promotes filament severing. (7) As ADP-actin depolymerizes, actin monomers bind to profilin, which catalyzes ADP/ATP-nucleotide exchange. This maintains a pool of ATP-bound actin that permits further INF2-stimulated actin polymerization. (B) A model for Spire1C and INF2 cooperation during mitochondrial fission. For mitochondrial fission, INF2 might cooperate with Spire1C to promote actin filament assembly by a “Missile Launcher” type mechanism. (1) The CAAX-form of INF2 associates with the ER membrane through C-terminal prenylation, while Spire1C is anchored to the mitochondrial outer membrane, potentially through a predicted transmembrane hairpin. (2) Analogous to the interaction between Drosophila Spire and CAPU formin, the Spire1C KIND domain binds the INF2 FH2 domain. (3) Actin filaments are nucleated by the Spire1C/INF2 complex in a manner strongly dependent on actin monomer-binding WH2 domains of Spire1C. (4) As the filament elongates, the Spire1C/INF2 interaction is broken, with Spire1C remaining associated near the pointed end at the mitochondrial surface, and INF2 remaining associated with the elongating barbed end at the ER surface.
INF2 dimerizes like other formins, but is distinct from other formins in also being able to oligomerize [Chhabra and Higgs, 2006; Sharma et al., 2014]. INF2 dimers not only bind the barbed ends of actin filaments, but also the sides of actin filaments, with a hypothesized mechanism of partially opening the ring-shaped FH2 dimer and wrapping the FH2 domains around the filament, before re-closing the dimer [Gurel et al., 2014; Sharma et al., 2014]. Alternatively, INF2 FH2 domains of partially opened dimers can also exchange partners to oligomerize, forming what appears to be a daisy chain-like pattern along an actin filament, based on atomic force microscopy data [Sharma et al., 2014]. This side binding is hypothesized to play a prominent role in a model for INF2-mediated severing proposed by Gurel and colleagues [Gurel et al., 2014] (Fig 2 A). Upon side binding, the INF2 WH2/DAD wedges between actin subunits in a filament, weakening their interaction and leading to filament severing, with INF2 usually remaining associated with the new barbed end. INF2 does not have a preference for severing near either the barbed or pointed end of the actin filament, but requires release of phosphate from actin, indicating a specificity for ADP-bound F-actin [Chhabra and Higgs, 2006; Gurel et al., 2014].
Interestingly, in the presence of profilin, which stimulates nucleotide exchange for actin monomers, as well as elongation of INF2-bound filaments, INF2-dependent filament severing does not go to completion [Chhabra and Higgs, 2006]. Rather, the combined effects of profilin, INF2, and an adequate supply of ATP result in a steady-state condition of actin filaments that undergo a continual flux of monomer through addition by filament elongation, and loss by filament severing [Gurel et al., 2015] (Fig 2 A).
Robust biochemical analyses of other inverted formins remain to be done. However, the presence of identifiable WH2-like motifs in at least some other inverted formins (Fig.1 A) leaves open the possibility that some of these distinct properties might be general to members of this sub-family.
INF2 and Cellular Actin Structures
Consistent with the ability of formins to promote actin filament assembly, the expression of exogenous inverted formins in cultured cells has been shown to drive the formation of actin filament-containing structures. For example, expression of the FH1 and FH2 domains of the mammalian inverted formins FHDC1 (FHDC1-FF) and INF2 (INF2-FF) in HeLa cells each caused actin stress fibers to form [Thurston et al., 2012]. In the particular case of INF2, when the C-terminus specific to the CAAX-containing isoform was also included in expression constructs, INF2 associated with the endoplasmic reticulum (ER), and accumulated actin filaments were associated with that organelle [Chhabra et al., 2009; Ramabhadran et al., 2012].
Endogenously expressed INF2 has also been shown to be associated with a number of actin-based structures. Proteomic and immunofluorescence studies have shown INF2 associates with focal adhesions in mouse embryonic fibroblasts, where it promotes the maturation of adhesions into large, elongated structures, and promotes the formation of dorsal stress fibers associated with these adhesions [Skau et al., 2015]. In macrophages, INF2 associates with another adhesive structure, the podosome, which is involved in remodeling the extracellular matrix. Opposite to its effects on focal adhesions, depletion of INF2 from macrophages leads to larger and longer-lived podosomes, and decreased matrix degradation, while expression of INF2 bearing activating mutations promotes smaller, short-lived podosomes [Panzer et al., 2016]. INF2 has also been observed to localize to sarcomere Z-lines in neonatal primary mouse cardiomyocytes, although its importance for sarcomere organization was not reported [Rosado et al., 2014].
One particularly interesting actin-related phenomenon found to involve INF2 is calcium-mediated actin reset (CaAR), a reaction that occurs in a wide variety of cells in response to physical attack, such as prodding with an AFM cantilever or laser ablation of nearby cells. CaAR initiates with a spike of intracellular calcium, which is immediately followed by polymerization of an “actin ridge” around the nucleus, loss of cortical actin but stiffening of the subcortical region, freezing of organelles, and changes in gene expression through myocardin-related transcription factors [Shao et al., 2015; Wales et al., 2016]. Polymerization of the actin ridge is INF2 dependent, and can stimulated by either the CAAX or non-CAAX isoform; for cells in which INF2 has been knocked down, neither polymerization nor other downstream effects occur [Shao et al., 2015; Wales et al., 2016]. While INF2 does not have a known calcium-sensing domain, Wales and colleagues demonstrated that in the presence of calcium, calmodulin binds INF2, pointing to a possible mechanism of activation [Wales et al., 2016].
What remains a challenge is correlating the various effects of INF2 on actin dynamics in vitro with its ability to promote actin organization in vivo. The growth of F-actin-rich structures would be consistent with stimulation of filament nucleation or acceleration of barbed end elongation by INF2. However, it could also be readily explained as the result of new barbed end formation through INF2-mediated filament severing, as has been shown previously for cofilin-mediated severing [Chan et al., 2000].
Invertebrate inverted formins have also been observed to be associated with actin-rich structures. Most of these will be discussed in later sections, but the unusual localization of the inverted formin EXC-6 in the C. elegans spermatheca warrants mention here. The worm's spermatheca is a distensible epithelial sac that holds sperm and stretches to receive oocytes during ovulation. EXC-6 is found in striking ribbon-like patterns that demarcate highly contorted epithelial junctions in the spermatheca [Hegsted et al., 2016]. A minor population of actin filaments is also associated with these junctions. Loss of EXC-6 does not outright abolish these filaments, but it is unclear if their organization or dynamics are affected. The movement of oocytes into the spermatheca is frequently disrupted in exc-6 mutants, suggesting the formin may play a role in allowing spermatheca wall to distend during oocyte entry [Hegsted et al., 2016]. It remains to be determined whether the effect of this formin is through interaction with actin, or by some other mechanism.
INF2-Dependent Mitochondrial Fission
A role for mammalian INF2 in mitochondrial fission was first noted when it was observed that after INF2 was knocked down in human osteocarcinoma cells or mouse fibroblasts, average mitochondrial length increased, whereas expression of activated INF2 caused mitochondria to shorten [Ji et al., 2015; Korobova et al., 2013]. These effects depended on the CAAX-containing isoform of INF2, suggesting INF2 must be associated with the ER to perform this function [Korobova et al., 2013].
Interestingly, further work identified another actin nucleation factor, Spire1, as a protein that cooperates with INF2 in this process. Spire was originally identified in Drosophila as a WH2 domain-containing protein that is able to nucleate actin filaments [Quinlan et al., 2005]. Subsequent work showed that Spire interacts with the Drosophila formin CAPPUCCINO (CAPU) through its Spire kinase noncatalytic C-lobe domain (KIND) [Quinlan et al., 2007; Rosales-Nieves et al., 2006]. The Spire/CAPU complex functions as a unit to promote actin filament assembly, utilizing the highly potent nucleating activity of the Spire WH2 domains, and the processive capping activity of CAPU [Quinlan et al., 2007]. A potential model for this mechanism of actin assembly is the so-called “Rocket Launcher”, as originally described for the formin mDia1 and its nucleating partner, adenomatous polyposis coli (APC) [Breitsprecher et al., 2012]. By this mechanism, the formin/partner complex nucleates an actin filament, and then dissociates as the filament elongates, with the formin acting as a processive cap for the barbed end, while the partner protein remaining near the pointed end. Demonstrating conservation of the Spire/CAPU interaction, two mammalian Spire homologs, Spire1 and Spire2, nucleate actin filaments and interact with the CAPU-related formins FMN1 and FMN2 [Pechlivanis et al., 2009; Pfender et al., 2011; Vizcarra et al., 2011].
A novel splice isoform of Spire1, Spire1C, was implicated in mitochondrial fission when found to localize to mitochondria, and its overexpression was shown to shorten mitochondria, while knockdown or loss-of-function mutations led to elongated mitochondria [Manor et al., 2015]. Although only CAPU-related formins had been previously found to associate with Spire proteins, activated INF2 was shown to interact with the Spire1C KIND domain, and this interaction promotes ER-mediated mitochondrial fission through INF2 [Manor et al., 2015]. Moreover, the Spire1C WH2 domains, which are required for Spire-mediated actin nucleation, were also found to be required to stimulate mitochondrial fission. This supports a model in which INF2 and Spire1C directly cooperate in actin assembly, potentially by a “Rocket Launcher” type of mechanism in which Spire1C provides nucleating activity, while INF2 promotes barbed end elongation (Fig 2 B).
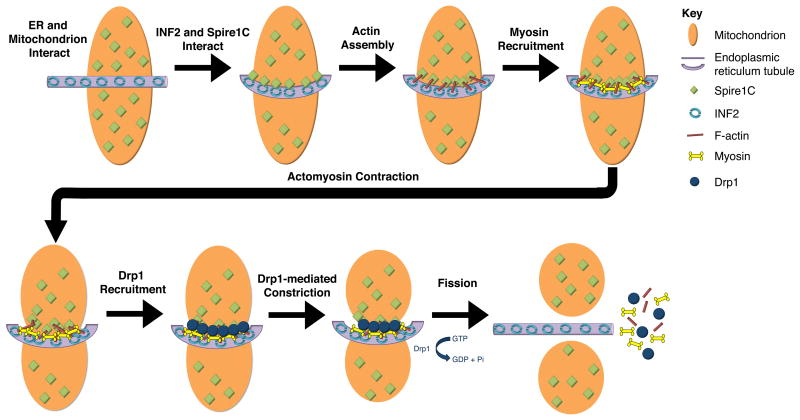
Building off these and additional results, a model for INF2-dependent mitochondrial fission has been proposed by Hatch and colleagues (Fig 3) [Hatch et al., 2014]. Based on its similarity to cytokinesis, they dubbed this model “mitokinesis”. Mitokinesis is initiated in response to an initial interaction between INF2 on the ER, and Spire1C on the outer mitochondrial membrane. F-actin is polymerized at the ER/mitochondrion contact site, and myosin II becomes recruited [Korobova et al., 2014]. Actomyosin contractility is proposed to pre-constrict the fission site, allowing recruitment of the dynamin GTPase Drp1. This is supported by the observation that reduced levels of INF2 or of myosin II result in reduced recruitment of Drp1 to mitochondria [Hatch et al., 2014; Ji et al., 2015; Korobova et al., 2013]. Finally, consistent with previous models for mitochondrial fission [Otera et al., 2013], Drp1 polymerizes into a helical ring that girds the mitochondrion and completes constriction to promote mitochondrial fission.
Figure 3. Mitokinesis — a proposed model of INF2/Spire1C-dependent mitochondrial fission.
Spire1C localizes to the mitochondrial outer membrane and the CAAX-form of INF2 localizes to the endoplasmic reticulum (ER). An ER tubule interacts with a mitochondrion, bringing Spire1C and INF2 together. INF2/Spire1C-dependent actin assembly occurs at the mitochondrion/ER interface. Myosin is then recruited and actomyosin interactions cause “preconstriction,” which cinches the mitochondrion and allows for Drp1 recruitment. Drp1 further constricts the mitochondrion, leading to scission and the completion of mitokinesis. Figure adapted from [Hatch et al., 2014] and [Manor et al., 2015].
An adaptor protein of the CUL3-RBX1 E3 ubiquitin ligase complex, Speckle-type POZ Protein (SPOP), has also been shown to be a player in this process. Specifically, SPOP poly-ubiquitinates INF2, which leads not to degradation, but to translocation of INF2 from the ER to the cytosol [Jin et al., 2017]. Consequently, SPOP overexpression or targeting to the cytosol (by elimination of its nuclear localization signal) leads to mitochondrial lengthening.
Inverted Formins and Microtubule Interactions
While formins are best known for their effects on actin, they also influence the microtubule cytoskeleton, and may serve as bridges between actin filaments and microtubules. This appears particularly true for inverted formins.
Both types of mammalian inverted formins have been shown to directly interact with microtubules. FHDC1 (under its previous name, INF1) was identified as a novel microtubule-associated formin with a microtubule-binding domain (MTBD) near its extreme C-terminus [Young et al., 2008]. No homologous MTBD sequence is apparent in INF2 (or any invertebrate inverted formin), but INF2-FFC was also shown to bind and bundle microtubules in a C-terminal tail-dependent manner in vitro, to cause a decrease in microtubule catastrophe events and a slowing of bundle growth [Gaillard et al., 2011]. For INF2, at least, interaction with actin and microtubules appears to be competitive, as the presence of G-actin inhibits microtubule bundling [Gaillard et al., 2011]. Despite this apparent competition, INF2-FFC can also cause co-bundling of actin filaments and microtubules in vitro [Gaillard et al., 2011].
As with many formins, FHDC1and INF2 have been shown to promote stabilization of microtubules in the cell, based on the increased presence of nocodazole-resistant microtubules, or of acetylated or (for INF2, only) detyrosinated microtubules [Andrés-Delgado et al., 2012; Bartolini et al., 2016; Thurston et al., 2012; Young et al., 2008]. Furthermore, a portion of both formins will colocalize with stabilized microtubules [Bartolini et al., 2016; Young et al., 2008].
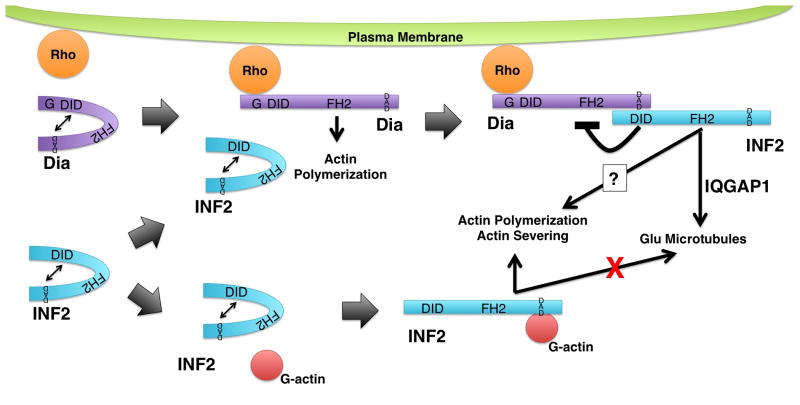
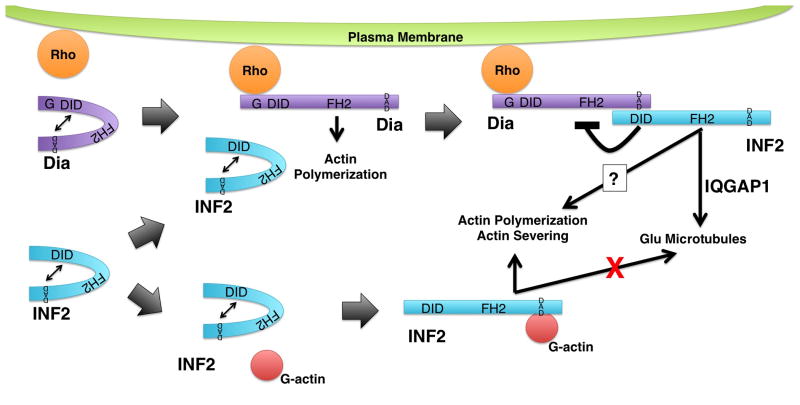
The induction of acetylated microtubules by both inverted formins appears to depend on their FH1 and FH2 domains [Young et al., 2008], but induction of detyrosinated microtubules (also called Glu-microtubules) by INF2 appears to be more complex. That is, INF2 appears to work in a pathway with mDia1, a Diaphanous sub-family formin that was previously shown to induce Glu-microtubules [Bartolini et al., 2016; Palazzo et al., 2001]. In this path, INF2 depends on mDia1 for its activation and ability to associate with microtubules, while mDia1 activation does not depend on INF2 (Fig 4). Thus, loss of mDia1 prevents INF2-induced formation of Glu-microtubules, but over-expression of the INF2 DAD (which is predicted to activate INF2) rescues Glu-microtubules [Bartolini et al., 2016]. IQGAP1 appears to be involved in this, as well. In addition to being known to interact with active mDia1 [Brandt et al., 2007] and with INF2 [Boyer et al., 2011b], IQGAP1 is important to microtubule capture and stability [Fukata et al., 2003; Watanabe et al., 2004; Wickström et al., 2010]. Pointing to a close interaction between these proteins, IQGAP1, INF2 and mDia1 form a complex, and in IQGAP1-depleted cells, INF2 again fails to localize to microtubules [Bartolini et al., 2016].
Figure 4. Regulation of INF2 activity.
INF2 (blue) is autoinhibited by interaction of its DID and DAD. Autoinhibition can be relieved through at least two pathways. (Top) One pathway involves mammalian Diaphanous-related formins (Dia, puple). Dia formins are also autoregulated via interaction between their DID and DAD. This autoregulation can be disrupted by binding of a Rho family GTPase, permitting Dia to stimulate actin polymerization. The INF2 DID has greater affinity for the Dia DAD than for the INF2 DAD leading to INF2 DID/Dia DAD interaction. This breaks the INF2 autoinhibition, allowing INF2 to promote the formation of stabilized Glu microtubules in an IQGAP1-dependent manner. Another consequence of the INF2 DID/Dia DAD interaction is the inhibition of Dia-dependent actin polymerization. (Bottom) A second pathway for breaking INF2 autoinhibition is through binding of G-actin to the INF2 DAD. Interaction with G-actin inhibits INF2 from interacting with microtubules, but permits INF2 to interact with actin to promote polymerization, and perhaps severing. Note, INF2 and Dia are depicted as monomers is for visual clarity, only. Both formins are expected to exist as homodimers.
In addition to affecting microtubule stability, INF2 influences the orientation of the microtubule-organizing center (MTOC). During the formation of the immunological synapse between a T-cell and an antigen-presenting cell, the FH2 domain of INF2 is required for the proper reorientation of the T-cell MTOC toward the synapse [Andrés-Delgado et al., 2012]. This coincides with an INF2-dependent increase in the number of Glu-microtubules in response to T-cell antigen receptor stimulation. Influence of INF2 on MTOC orientation has also been suggested as an explanation for the mechanism by which treatment of gastric cancer cells with mycophenolic acid, which down-regulates of inf2 expression, leads to inhibition of cellular migration [Dun et al., 2013].
Inverted Formins and Membrane Trafficking
In a variety of organisms, inverted formins have been shown to be involved in membrane trafficking. In several cases, this has been shown to involve interactions of the formin with both actin and microtubules. For example, mammalian FHDC1 localizes near the Golgi ribbon in cultured cells, in association with Golgi-derived microtubules [Copeland et al., 2016; Young et al., 2008]. This correlates with the known enrichment of acetylated microtubules in the Golgi-derived microtubule network [Chabin-Brion et al., 2001; Thyberg and Moskalewski, 1993]. Either knockdown or overexpression of FHDC1 results in fragmentation and dispersal of the Golgi [Copeland et al., 2016]. Moreover, the ability of overexpressed FHDC1 to induce Golgi dispersal depends on its MTBD, as well as an intact FH2 domain, but can be blocked by Latrunculin B [Copeland et al., 2016]. These results suggest FHDC1 affects Golgi organization through interactions with both the actin and microtubule cytoskeletons. A sub-population of INF2 is also enriched near the Golgi, but this localization is mostly unaffected by treatment with drugs affecting microtubules [Ramabhadran et al., 2011]. Moreover, knockdown of INF2 also causes Golgi dispersal, but this is blocked with Latrunculin B, suggesting that INF2 maintains Golgi structure by opposing some other actin-dependent event [Ramabhadran et al., 2011].
Two factors that have been identified as cooperating with INF2 in guiding membrane trafficking are myelin and lymphocyte protein (MAL) and myelin and lymphocyte protein-2 (MAL2) [Andrés-Delgado et al., 2010; Madrid et al., 2010]. These two related integral membrane proteins were found to interact with INF2 through yeast two hybrid screens, and have been shown to cooperate with INF2 in distinct cell types [Andrés-Delgado et al., 2010; Madrid et al., 2010]. In T-cells, MAL promotes transport of lymphocyte-specific protein tyrosine kinase (Lck) to the plasma membrane [Antón et al., 2008]. INF2 and MAL normally colocalize at the cell periphery, the pericentriolar region, and along microtubules, while depletion of INF2 from T-cells displaces MAL from these locations, and prevents Lck trafficking to the plasma membrane [Andrés-Delgado et al., 2010]. Interaction between INF2 and MAL2 appears important for lumen formation in several epithelial cell types. A hepatocyte cell line normally establishes lumens between adjacent cells through MAL2-dependent basolateral-to-apical transcytosis [de Marco et al., 2002; Madrid et al., 2010]. However, when INF2 is depleted, these lumens remain small or absent, MAL2 mislocalizes, and MAL2-dependent trafficking is disrupted [Madrid et al., 2010]. Similarly, while Madin-Darby canine kidney cells normally form cysts with large lumens when grown in 3D culture, depletion of INF2 results in the formation of multiple small lumens [Madrid et al., 2010].
Pointing to a role for INF2/actin interactions in this process, normal trafficking correlates with the appearance of MAL2-positive vesicles with actin tails, but these vesicles are scarcer with expression of INF2 bearing FH2 or DAD/WH2 mutations [Madrid et al., 2010]. Despite the absence of a canonical GTPase-binding domain (Fig 1), INF2 can pull down the Rho-family GTPase Cdc42 from extracts of these cells, a relationship that will be explored later in this review. Depletion of Cdc42 similarly results in reduced numbers of lumens and disrupted MAL2 trafficking [Madrid et al., 2010].
An importance for inverted formins during lumen formation has also been observed in invertebrate models. In C. elegans, the inverted formin-coding gene exc-6 was identified in a screen for worms whose excretory canals (a kidney analog) had abnormal lumens [Buechner et al., 1999]. Mutations in exc-6 result in shortened excretory canals with multiple lumens [Buechner et al., 1999; Shaye and Greenwald, 2015]. In particular, point mutations of exc-6 that affect conserved isoleucine and lysine residues of the FH2 domain critical to formin/actin interactions have been shown to result in this phenotype, suggesting an importance for interactions between EXC-6 and actin [Shaye and Greenwald, 2015]. Actin filaments immediately adjacent to the canal lumen are not affected by loss of EXC-6, but a body of actin filaments adjacent to the basal surface during canal cell growth is EXC-6-dependent. Other lines of evidence implicate EXC-6/microtubule interactions as being critical, including colocalization of some EXC-6 with microtubules in the canal cell, a reduction in the number of MTOCs along exc-6 mutant canals, and a randomization of the direction of microtubule growth along exc-6 mutant canals [Shaye and Greenwald, 2015]. Based on these, it was hypothesized that EXC-6 bridges microtubule and F-actin networks in the excretory canal cell. Consistent with this, expression in the canal cell of a synthetic protein with F-actin- and microtubule-binding domains partially rescues lumen defects in exc-6 mutants [Shaye and Greenwald, 2015]. Interestingly, expression of human INF2 bearing activating mutations also partially rescues exc-6 mutants, suggesting similarities in the underlying functions of these inverted formins [Shaye and Greenwald, 2015].
As a final layer of complexity, the second inverted formin of C. elegans, INFT-2, also affects excretory canal development. In this case, INFT-2 is associated with the cortex underlying the lumen, and it promotes the formation of F-actin around the canal lumen in a manner regulated positively by the Rho family GTPase CDC-42, and negatively by the Diaphanous sub-family formin CYK-1 [Shaye and Greenwald, 2016]. A complicated network of genetic interactions supports a model in which EXC-6/microtubule-dependent basal outgrowth is coordinated with INFT-2/F-actin-dependent luminal growth to promote tubulogenesis [Shaye and Greenwald, 2016].
Finally, the sole inverted formin of Drosophila, FORM3, has also been implicated in lumen formation in the fly's respiratory system. Insect respiratory systems are composed of networks of interlinked air-filled tracheal tubes. Mutations affecting form3 result in discontinuities in this network, in which the cells comprising the tracheal tubes connect, but the tube lumens do not fuse [Tanaka et al., 2004]. During normal fusion of two growing tracheal tubes, cells of the fusing tubes make contact. Actin- and E-cadherin-rich projections from the lumens of the fusing tubes grow toward each. After these projections contact each other, lumen continuity is established, whereas in form3 mutants, these projections fail to form [Tanaka et al., 2004].
It is apparent that many inverted formins have an important role in regulating membrane trafficking and lumen formation, and several studies suggest that inverted formins play an important role in linking the actin and microtubule cytoskeletons. Due to the widespread expression of inverted formins in all tissue and cell types examined, it will be interesting to see whether a homeostatic role of inverted formins is to regulate membranes dynamics, possibly through linking these two cytoskeletal systems.
INF2 and Intra- and Intermolecular DID/DAD Interactions
For the most part, the regulation of inverted formin activity is a poorly studied area. Particularly for inverted formins lacking N-terminal DID sequence, such as mammalian FHDC1 or worm EXC-6, it is unclear whether formin activity is subject to any regulation, or if these proteins are constitutively active. For inverted formins with DID and DAD sequences, DID/DAD-mediated autoinhibition is a possibility. Again, mammalian INF2 remains the inverted formin for which this has been analyzed in greatest detail.
INF2 DID and DAD interact [Chhabra et al, 2009], but initial studies were unclear as to whether this interaction resulted in autoinhibition. In vitro, INF2-DID prevents INF2-FFC from promoting depolymerization of actin, but does not block its ability to polymerize actin, which suggested that only the severing activity of INF2 is subject to DID/DAD-mediated autoinhibition [Chhabra et al, 2009]. In vivo, exogenous expression of ER-associated INF2 results in modest accumulation of F-actin around the ER, but this is strongly enhanced if the DID is mutated in a way predicted to disrupt the DID/DAD interaction [Ramabhadran et al., 2013]. This suggests the ability of INF2 to stimulate actin polymerization is subject to autoinhibition in vivo, although this could either be through autoinhibition of nucleation/processive capping, or of severing-dependent formation of new barbed ends.
The absence of a canonical GTPase-binding domain on INF2 would suggest this formin is unlikely to be activated by a Rho family protein in the way many other formins are. Rather, Ramabhadran and colleagues proposed that monomeric actin is a novel regulator of INF2 autoinhibition (Fig 4) [Ramabhadran et al., 2013]. As support for this completely novel mechanism of formin regulation, they demonstrated that while wild-type INF2-DID does not inhibit in vitro actin polymerization by INF2-FFC in the presence of high concentrations of actin monomer, it does become inhibitory in the presence of a low concentration of free actin monomers, similar to what might be expected in vivo [Ramabhadran et al., 2013]. Moreover, INF2-DID bearing a mutation predicted to prevent interaction with the DAD had little inhibitory effect, confirming that the ability of INF2 to promote polymerization in indeed subject to DID/DAD-mediated autoinhibition.
INF2 also physically interacts with a variety of other proteins that might play additional roles in regulating its activity. Profilin-2 and CapZ (xF061)-1 co-purify with INF2 in a manner that increases when the INF2-DID bears activating mutations [Rollason et al., 2016]. And in spite of absence of a canonical formin GBD, the INF2-DID can pull down the Rho-family GTPases Cdc42 and Rac1 from cell extracts, and knockdown of either GTPase phenocopies the effects of INF2 knockdown on MAL- and MAL2-dependent trafficking, as described above [Andrés-Delgado et al., 2010; Madrid et al., 2010]. However, this interaction between INF2 and GTPases has been suggested to be indirect, and in an in vitro system of purified proteins, Cdc42 does not break INF2 autoinhibition [Ramabhadran et al., 2013].
The facts that eliminating GTPases from a cellular system phenocopies INF2 knockdown, yet GTPase/INF2 interactions appear to be indirect, suggests INF2 might be subject to multi-layered regulation. Several studies suggest that formins of the Diaphanous sub-family might participate in such complex regulation. The DADs of the mammalian formins mDia1, mDia2 and mDia3 each interact directly with the INF2-DID, and exogenously expressed full-length mDia formins and INF2 co-localize and co-immunoprecipitate [Sun et al., 2011]. Such inter-formin regulation appears to so far be unique to INF2/mDia proteins. As mentioned earlier, mDia1 and INF2 appear to be in a signaling cascade, in which the activation of INF2 for microtubule stabilization depends on mDia1 [Bartolini et al., 2016]. Binding of mDia1-DAD to INF2-DID might be expected to serve as an activation step (Fig 4). Conversely, in vivo and in vitro assays suggest mDia-dependent actin assembly activity is inhibited when INF2-DID binds mDia-DAD [Sun et al., 2011]. Finally, it might be expected that the ability of INF2 to interact with mDia might depend on previous disruption of the mDia-DID/mDia-DAD interaction. Although this interaction has only been shown to be important for the effect of INF2 on microtubules, a similar interaction might provide an explanation for the dependence of INF2-dependent membrane trafficking on Rho family GTPases, whereby Rho proteins act through an mDia intermediary (Fig 4). Interestingly, mDia only poorly interacts with INF2 bearing disease-causing mutations in its DID (see below), pointing to a potential clinical significance for this unusual interaction [Sun et al., 2011].
The role of INF2 in human disease
Much of the interest in the formin INF2 has been spurred by the identification of inf2 gene mutations associated with two genetic diseases, focal segmental glomerular sclerosis and Charcot Marie Tooth disease.
Focal segmental glomerular sclerosis—a kidney injury
Focal segmental glomerular sclerosis (FSGS) is a lesion of the kidney glomerulus that leads to nephrotic syndrome, typically progressing to end stage renal disease [Chen and Liapis, 2015; Jefferson and Shankland, 2014; Rood et al., 2012]. The key targets of this lesion are podocytes, highly specialized epithelial cells that enwrap the glomerular capillaries with highly elaborate foot processes [Jefferson and Shankland, 2014]. Specialized cell-cell junctions between these foot processes, called slit diaphragms, serve as part of the kidney's filtration barrier. In FSGS, podocyte foot processes become effaced and the slit diaphragms are disrupted, resulting in failure of filtration [Greka and Mundel, 2012; Pavenstädt et al., 2003].
An initial nine mutations were identified in human inf2 that tied this inverted formin gene to late-onset, autosomal dominant FSGS [Brown et al., 2010]. Since then, a growing number of additional FSGS-associated inf2 mutations have been identified (Fig 5). FSGS-causing inf2 mutations have incomplete penetrance, as seen by differing ages of FSGS onset within the same family [Barua et al., 2013; Boyer et al., 2011a; Gbadegesin et al., 2012; Pollack, 2016], but they are prevalent enough that testing for inf2 mutations is recommended in cases of FSGS that are steroid resistant, familial and adolescent/adult onset [Santín et al., 2011]. Additionally, two FSGS-causing inf2 mutations have also been associated with thrombotic microangiopathy (TMA) atypical hemolytic uremic syndrome, a condition that also leads to renal failure [Challis et al., 2017].
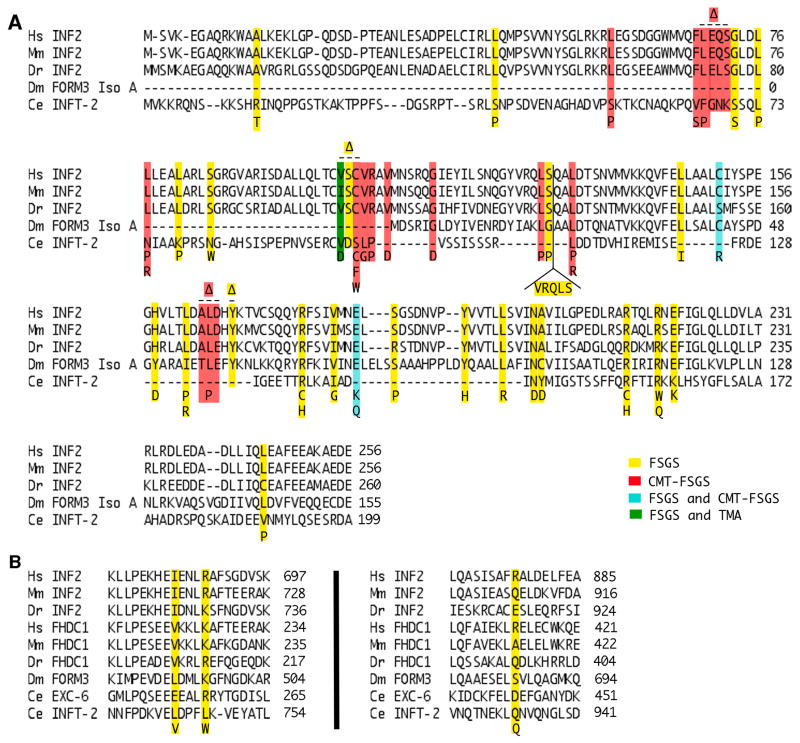
Figure 5. Conservation of disease-linked amino acids of INF2.
Shown are alignments of (A) DID or (B) FH2 domain sequences of inverted formins from human (Homo sapiens, Hs), mouse (Mus musculus, Mm), zebrafish (Danio rerio, Dr), fruit fly (Drosophila melanogaster, Dm), and worm (Caenorhabditis elegans, Ce). Disease-associated amino acid substitutions and insertions of human INF2 are shown below the alignment, while deletions ((x2206)) are shown above the alignment. Mutations are color coded to indicate association with FSGS (yellow), CMT-FSGS (red), both FSGS and CMT-FSGS (cyan), or both FSGS and TMA (green) [Brown et al., 2010; Boyer et al., 2011a; Boyer et al., 2011b; Lee et al., 2011; Gbadegesin et al., 2012; Barua et al., 2013; Lipska et al., 2013; Mademan et al., 2013; Rodriguez et al., 2013; Sanchez-Ares et al., 2013; Toyota et al., 2013; Caridi et al., 2014; Laurin et al., 2014; Park et al., 2014; Quaglia et al., 2014; Roos et al., 2015; Xie et al., 2015; Bullich et al., 2015; Jin et al., 2015; Münch et al., 2016; Rood et al., 2016; Challis et al., 2017]. Numbers indicate amino acid positions.
Most disease-associated inf2 mutations affect residues of the DID that are highly conserved across vertebrate INF2 homologs, and in some cases, even conserved in highly divergent invertebrate homologs, such as C. elegans INFT-2 (Fig 5 A). Pointing to a conserved role for INF2 in podocyte function, introduction into mice of the FSGS-associated inf2 mutation R218Q does not lead to kidney disease, but prevents their glomeruli from recovering from injury [Subramanian et al., 2016]. Similarly, knockdown of inf2 in zebrafish results in several FSGS-like phenotypes that can be rescued by expression of wild-type inf2 mRNA, but not inf2 with the FSGS-associated mutations E184K or R218Q [Sun et al., 2014]. Only three FSGS-linked amino acid changes have been identified that affect the INF2 FH2 domain, with K689 the only affected residue that is well conserved across the inverted formins (Fig 5 B). However, it is not clear that all these mutations are causative of disease, for example, as discussed in [Lipska et al., 2013].
INF2 is expressed in podocytes, where it localizes in the foot processes to the slit diaphragms, and in a diffuse perinuclear pattern [Brown et al., 2010; Sun et al., 2011; Xie et al., 2015]. INF2 colocalizes with other proteins important for slit diaphragm function, including nephrin, podocin, caveolin, mDia2, synaptopodin, and vimentin [Brown et al., 2010; Subramainan et al., 2016; Sun et al., 2011; Sun et al., 2013; Tamura et al., 2016]. In FSGS kidney samples, INF2 staining is reduced or absent [Tamura et al., 2016], and INF2 appears to be shed by podocytes into the urine in exosome-like vesicles in patients with kidney disease [Hogan et al., 2014]. Conversely, INF2 is upregulated in congenital nephrotic syndrome of the Finnish type, caused by a defect in nephrin, and this was suggested to reflect a possible compensatory reaction to establish a functional slit diaphragm [Suvanto et al., 2015].
Based on modeling of the INF2 DID onto the crystal structure of the mDia1 N-terminus, many FSGS-associated DID residues are predicted to be important for interaction with the DAD, and for interacting with IQGAP1 [Gbadegesin et al., 2012]. This suggests FSGS-associated mutations might activate INF2, which would be consistent with the autosomal dominant nature of inf2-dependent FSGS [Brown et al., 2010]. Also consistent with this, several effects of knockdown of INF2 from cultured cells (including cultured podocytes) are opposite of those caused by expression of INF2 bearing FSGS-associated mutations. That is, INF2 knockdown causes an increase in stress fiber abundance, while expression of FSGS-associated mutant INF2 results in a reduction in stress fibers and an increase in diffuse actin [Brown et al., 2010; Sun et al., 2013; Xie et al., 2015].
Several lines of evidence suggest the critical activity of INF2 in podocytes may be to inhibit the mDia formin, mDia2, through the mechanisms discussed in the previous section (Fig 4). In support of this, expression of a constitutively active mDia2 lacking the GBD (mDia2(x2206)GBD) in cultured podocytes results in cytoskeletal and trafficking defects of podocin and nephrin, similar to those caused by knocking down INF2 [Sun et al., 2013]. Moreover, expression of wild-type INF2 will suppress these effects of mDia2(x2206)GBD expression, while expression of INF2 with FSGS-associated mutations will not [Sun et al., 2011; Sun et al., 2013]. Finally, in zebrafish, the FSGS-like phenotypes induced by knockdown of INF2 can be reversed by simultaneous knockdown of Dia2 or the Dia2-activating GTPase, RhoA [Sun et al., 2014].
Charcot-Marie Tooth disease with FSGS—a neurological and kidney disease
Charcot-Marie Tooth disease (CMT) is a group of inherited neuropathies that affect peripheral axons or their myelin sheaths, resulting in weakness and numbness of the limbs. Most commonly, CMT results from duplication of the peripheral myelin protein-22 (pmp22) or mutation of the myelin protein zero (mpz) genes [Liu and Zhang, 2014; Vallat et al., 2013], but a subset of CMT cases also developed FSGS (CMT-FSGS) and were found to be associated with mutations in the DID-coding portion of inf2 [Boyer et al., 2011b]. Some inf2 mutations appear specific for FSGS or for CMT-FSGS, but others can result in either disease (Fig 5), depending on the individual [Caridi et al., 2014; Jin et al., 2015]. To date, inf2 mutations are the only known genetic cause for cases of CMT accompanied by kidney disease, and these cases can also be accompanied by other phenotypes, including cognitive impairment, deafness, and hand deformities [Werheid et al., 2016]. Considering that CMT symptoms arise before FSGS symptoms [Mademan et al., 2013], it has been suggested that CMT patients be screened for proteinuria (a sign of kidney dysfunction) and, potentially, genotyped for inf2, as a guide to the appropriate treatment [De Rechter et al., 2015].
INF2 is present in peripheral axons, and is particularly abundant at the periphery of Schwann cells, colocalizing with MAL in both locations [Boyer et al., 2011b]. When HeLa cells are induced to express INF2 bearing CMT-causing mutations, MAL, Cdc42 and IQGAP1 all mislocalize, and cortical actin and stress fibers are diminished [Boyer et al., 2011b]. Nerve biopsies of patients with inf2-dependent CMT-FSGS reveal lesions in peripheral nerves, with poor or absent axon myelination by Schwann cells. There are also supernumerary filopodia associated with the nonmyelinating Schwann cells, and an overabundance of actin filaments in the Schwann cell cytoplasm [Mathis et al., 2014]. These results suggest that for CMT, the loss of INF2 autoinhibition and a consequent increase in actin polymerization in Schwann cells may be the root cause of the disease.
Conclusions and Perspectives
The inverted formins as a group break numerous stereotypes of the formin family. Rather than being subject to the canonical RhoGTPase-dependent regulation of many formins, these proteins appear to be unregulated, or regulated by unusual factors such as monomeric actin, and at least one member acts in trans as an inhibitor of another formin sub-family. Inverted formins have been tied to cellular functions ranging from promoting lumen formation to regulating mitochondrial fission. The fact that kidney disease and a neuropathy have been tied to defects in a human inverted formin gene only highlight the importance of understanding this group of unusual proteins.
Acknowledgments
The authors would like to thank Arianna Laszlo and Sumana Sundaramurthy for critical reading of the manuscript. We would also like to thank NCBI protein database and WormBase for sequences, as well as NCBI CDART, Eukaryotic Lineage Motif Database and PHYRE2 for structural predictions.
Footnotes
Conflict of Interest: CVY owns stock in Invitae (San Francisco, CA 94103), a company that offers human genetic testing, including of inf2 as part of a Charcot-Marie-Tooth disease panel.
References
- Alberts AS. Identification of a Carboxyl-terminal Diaphanous-related Formin Homology Protein Autoregulatory Domain. J Biol Chem. 2001;276(4):2824–30. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- Andrés-Delgado L, Antón OM, Madrid R, Byrne JA, Alonso MA. Formin INF2 regulates MAL-mediated transport of Lck to the plasma membrane of human T lymphocytes. Blood. 2010;116(26):5919–29. doi: 10.1182/blood-2010-08-300665. [DOI] [PubMed] [Google Scholar]
- Andrés-Delgado L, Antón OM, Bartolini F, Ruiz-Sáenz A, Correas I, Gundersen GG, Alonso MA. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol. 2012;198(6):1025–37. doi: 10.1083/jcb.201202137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón O, Batista A, Millán J, Andrés-Delgado L, Puertollano R, Correas I, Alonso MA. An essentialrole for the MAL protein in targeting Lck to the plasma membrane of human T lymphocytes. J Exp Med. 2008;205(13):3201–3213. doi: 10.1084/jem.20080552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta. 2010;1803(2):164–73. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Andrés-Delgado L, Qu X, Nik S, Ramalingam N, Kremer L, Alonso MA, Gundersen GG. An mDia1-INF2 formin activation cascade facilitated by IQGAP1 regulates stable microtubules in migrating cells. Mol Biol Cell. 2016;27(11):1797–808. doi: 10.1091/mbc.E15-07-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua M, Brown EJ, Charoonratana VT, Genovese G, Sun H, Pollak MR. Mutations in the INF2 gene account for a significant proportion of familial but not sporadic focal and segmental glomerulosclerosis. Kidney Int. 2013;83(2):316–22. doi: 10.1038/ki.2012.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer O, Benoit G, Gribouval O, Nevo F, Tête MJ, Dantal J, Gilbert-Dussardier B, Touchard G, Karras A, Presne C, et al. Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J Am Soc Nephrol. 2011a;22(2):239–45. doi: 10.1681/ASN.2010050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Huynh Cong E, Arrondel C, Tête MJ, Montjean R, et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med. 2011b;365(25):2377–88. doi: 10.1056/NEJMoa1109122. [DOI] [PubMed] [Google Scholar]
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178(2):193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, Goode BL. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science. 2012;336(6085):1164–8. doi: 10.1126/science.1218062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Goode BL. Formins at a Glance. J Cell Sci. 2013;126(Pt 1):1–7. doi: 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010;42(1):72–6. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev Biol. 1999;214(1):227–41. doi: 10.1006/dbio.1999.9398. [DOI] [PubMed] [Google Scholar]
- Bullich G, Trujillano D, Santín S, Ossowski S, Mendizábal S, Fraga G, Madrid Á, Ariceta G, Ballarín J, Torra R, et al. Targeted next-generation sequencing in steroid-resistant nephrotic syndrome: mutations in multiple glomerular genes may influence disease severity. Eur J Hum Genet. 2015;23(9):1192–9. doi: 10.1038/ejhg.2014.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi G, Lugani F, Dagnino M, Gigante M, Iolascon A, Falco M, Graziano C, Benetti E, Dugo M, Del Prete D, et al. Novel INF2 mutations in an Italian cohort of patients with focal segmental glomerulosclerosis, renal failure and Charcot-Marie-Tooth neuropathy. Nephrol Dial Transplant. 2014;29(Suppl 4):iv80–6. doi: 10.1093/ndt/gfu071. [DOI] [PubMed] [Google Scholar]
- Chabin-Brion K, Marceiller J, Perez F, Settegrana C, Drechou A, Durand G, Poüs C. The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell. 2001;12(7):2047–2060. doi: 10.1091/mbc.12.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkia D, Nikolaidis N, Makalowski W, Klein J, Nei M. Origins and evolution of the formin multigene family that is involved in the formation of actin filaments. Mol Biol Evol. 2008;25(12):2717–2733. doi: 10.1093/molbev/msn215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis RC, Ring T, Xu Y, Wong EK, Flossmann O, Roberts IS, Ahmed S, Wetherall M, Salkus G, Brocklebank V, et al. Thrombotic Microangiopathy in Inverted Formin 2-Mediated Renal Disease. J Am Soc Nephrol. 2017;28(4):1084–1091. doi: 10.1681/ASN.2015101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Bailly M, Zebda N, Segall JE, Condeelis JS. Role of focilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J Cell Biol. 2000;148(3):531–42. doi: 10.1083/jcb.148.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YM, Liapis H. Focal segmental glomerulosclerosis: molecular genetics and targeted therapies. BMC Nephrol. 2015;16:101. doi: 10.1186/s12882-015-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol. 2010;11(1):62–74. doi: 10.1038/nrm2816. [DOI] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. INF2 Is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem. 2006;281(36):26754–67. doi: 10.1074/jbc.M604666200. [DOI] [PubMed] [Google Scholar]
- Chhabra ES, Ramabhadran V, Gerber SA, Higgs HN. INF2 is an endoplasmic reticulum-associated formin protein. J Cell Sci. 2009;122(Pt 9):1430–40. doi: 10.1242/jcs.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland SJ, Thurston SF, Copeland JW. Actin- and microtubule-dependent regulation of Golgi morphology by FHDC1. Mol Biol Cell. 2016;27(2):260–76. doi: 10.1091/mbc.E15-02-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rechter S, De Waele L, Levtchenko E, Mekahli D. Charcot-Marie-Tooth: are you testing for proteinuria? Eur J Paediatr Neurol. 2015;19(1):1–5. doi: 10.1016/j.ejpn.2014.08.004. [DOI] [PubMed] [Google Scholar]
- de Marco MC, Martin-Belmonte F, Kremer L, Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA, Alonso MA. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J Cell Biol. 2002;159(1):37–44. doi: 10.1083/jcb.200206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kühn H, Behrendt A, et al. ELM 2016--data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res. 2016;44(D1):D294–300. doi: 10.1093/nar/gkv1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun B, Sharma A, Teng Y, Liu H, Purohit S, Xu H, Zeng L, She JX. Mycophenolic acid inhibits migration and invasion of gastric cancer cells via multiple molecular pathways. PLoS One. 2013;8(11):e81702. doi: 10.1371/journal.pone.0081702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15(5):590–7. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Gaillard J, Ramabhadran V, Neumanne E, Gurel P, Blanchoin L, Vantard M, Higgs HN. Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol Biol Cell. 2011;22(23):4575–87. doi: 10.1091/mbc.E11-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadegesin RA, Lavin PJ, Hall G, Bartkowiak B, Homstad A, Jiang R, Wu G, Byrd A, Lynn K, Wolfish N, et al. Inverted formin 2 mutations with variable expression in patients with sporadic and hereditary focal and segmental glomerulosclerosis. Kidney Int. 2012;81(1):94–9. doi: 10.1038/ki.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Gould CJ, Maiti S, Michelot A, Graziano BR, Blanchoin L, Goode BL. The formin DAD domain plays dual roles in autoinhibition and actin nucleation. Curr Biol. 2011;21(5):384–90. doi: 10.1016/j.cub.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel PS, Ge P, Grintsevich EE, Shu R, Blanchoin L, Zhou ZH, Reisler E, Higgs HN. INF2-mediated severing through actin filament encirclement and disruption. Curr Biol. 2014;24(2):156–64. doi: 10.1016/j.cub.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel PS, A M, Guo B, Shu R, Mierke DF, Higgs HN. Assembly and turnover of short actin filaments by the formin INF2 and profilin. J Biol Chem. 2015;290(37):22494–506. doi: 10.1074/jbc.M115.670166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch AL, Gurel PS, Higgs HN. Novel roles for actin in mitochondrial fission. J Cell Sci. 2014;127(Pt 21):4549–60. doi: 10.1242/jcs.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegsted A, Wright FA, Votra S, Pruyne D. INF2- and FHOD-related formins promote ovulation in the somatic gonad of C. elegans. Cytoskeleton (Hoboken) 2016;73(12):712–28. doi: 10.1002/cm.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimsath EG, Jr, Higgs HN. The C terminus of formin FMNL3 accelerates actin polymerization and contains a WH2 domain-like sequence that binds both monomers and filament barbed ends. J Biol Chem. 2012;287(5):3087–98. doi: 10.1074/jbc.M111.312207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16(1):1–13. doi: 10.1091/mbc.E04-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Johnson KL, Zenka RM, Charlesworth MC, Madden BJ, Mahoney DW, Oberg AL, Huang BQ, Leontovich AA, Nesbitt LL, et al. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 2014;85(5):1225–37. doi: 10.1038/ki.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson JA, Shankland SJ. The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21(5):408–16. doi: 10.1053/j.ackd.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji WK, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S, Wang W, Wang R, Lv H, Zhang W, Wang Z, Jiao J, Yuan Y. INF2 mutations associated with dominant inherited intermediate Charcot-Marie-Tooth neuropathy with focal segmental glomerulosclerosis in two Chinese patients. Clin Neuropathol. 2015;34(5):275–81. doi: 10.5414/NP300835. [DOI] [PubMed] [Google Scholar]
- Jin X, Wang J, Gao K, Zhang P, Yao L, Tang Y, Tang L, Ma J, Xiao J, Zhang E, et al. Dysregulation of INF2-mediated mitochondrial fission in SPOP-mutated prostate cancer. PLoS Genet. 2017;13(4):e1006748. doi: 10.1371/journal.pgen.1006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M, Katoh M. Identification and characterization of human FHDC1, mouse Fhdc1 and zebrafish fhdc1 genes in silico. Int J Mol Med. 2004;13(6):929–34. [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–58. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339(6118):464–7. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Gauvin TJ, Higgs HN. A role for myosin II in mammalian mitochondrial fission. Curr Biol. 2014;24(4):409–14. doi: 10.1016/j.cub.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Kuhn JR, Tichy AL, Pollard TD. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol. 2003;161(5):875–87. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124(2):423–35. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Krainer EC, Ouderkirk JL, Miller EW, Miller MR, Mersich AT, Blystone SD. The multiplicity of human formins: Expression patterns in cells and tissues. Cytoskeleton (Hoboken) 2013;70(8):424–38. doi: 10.1002/cm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin LP, Lu M, Mottl AK, Blyth ER, Poulton CJ, Weck KE. Podocyte-associated gene mutation screening in a heterogeneous cohort of patients with sporadic focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2014;29(11):2062–9. doi: 10.1093/ndt/gft532. [DOI] [PubMed] [Google Scholar]
- Lee HK, Han KH, Jung YH, Kang HG, Moon KC, Ha IS, Choi Y, Cheong HI. Variable renal phenotype in a family with an INF2 mutation. Pediatr Nephrol. 2011;26(1):73–6. doi: 10.1007/s00467-010-1644-5. [DOI] [PubMed] [Google Scholar]
- Li F, Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem. 2005;280(8):6986–92. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- Lipska BS, Iatropoulos P, Maranta R, Caridi G, Ozaltin F, Anarat A, Balat A, Gellermann J, Trautmann A, Erdogan O, et al. Genetic screening in adolescents with steroid-resistant nephrotic syndrome. Kidney Int. 2013;84(1):206–13. doi: 10.1038/ki.2013.93. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang R. Intermediate Charcot-Marie-Tooth disease. Neurosci Bull. 2014;30(6):999–1009. doi: 10.1007/s12264-014-1475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mademan I, Deconinck T, Dinopoulos A, Voit T, Schara U, Devriendt K, Meijers B, Lerut E, De Jonghe P, Baets J. De novo INF2 mutations expand the genetic spectrum of hereditary neuropathy with glomerulopathy. Neurology. 2013;81(22):1953–8. doi: 10.1212/01.wnl.0000436615.58705.c9. [DOI] [PubMed] [Google Scholar]
- Madrid R, Aranda JF, Rodríguez-Fraticelli AE, Ventimiglia L, Andrés-Delgado L, Shehata M, Fanayan S, Shahheydari H, Gómez S, Jiménez A, et al. The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev Cell. 2010;18(5):814–27. doi: 10.1016/j.devcel.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Manor U, Bartholomew S, Golani G, Christenson E, Kozlov M, Higgs H, Spudich J, Lippincott-Schwartz J. A mitochondria-anchored isoform of the actin-nucleating spire protein regulates mitochondrial division. Elife. 2015;4 doi: 10.7554/eLife.08828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bryant SH. CD-search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:W327–31. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis S, Funalot B, Boyer O, Lacroix C, Marcorelles P, Magy L, Richard L, Antignac C, Vallat JM. Neuropathologic characterization of INF2-related Charcot-Marie-Tooth disease: evidence for a Schwann cell actinopathy. J Neurophathol Exp Neurol. 2014;73(3):223–33. doi: 10.1097/NEN.0000000000000047. [DOI] [PubMed] [Google Scholar]
- Mi-Mi L, Votra S, Kemphues K, Bretscher A, Pruyne D. Z-line formins promote contractile lattice growth and maintenance in striated muscles of C. elegans. J Cell Biol. 2012;198(1):87–102. doi: 10.1083/jcb.201202053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;15(2):896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch J, Grohmann M, Lindner TH, Bergmann C, Halbritter J. Diagnosing FSGS without kidney biopsy - a novel INF2-mutation in a family with ESRD of unknown origin. BMC Med Genet. 2016;17(1):73. doi: 10.1186/s12881-016-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Kikuno R, Hattori A, Kondo Y, Okumura K, Ohara O. Prediction of the coding sequences of unidentified human genes. XIX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000;7(6):347–55. doi: 10.1093/dnares/7.6.347. [DOI] [PubMed] [Google Scholar]
- Nezami A, Poy F, Toms A, Zheng W, Eck MJ. Crystal structure of a complex between amino and carboxy terminal fragments of mDia1: insights into autoinhibition of diaphanous-related formins. PLoS One. 2010;5(9):e12992. doi: 10.1371/journal.pone.0012992. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833(5):1256–68. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18(3):273–81. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Otomo T, Tomchick DR, Otomo C, Machius M, Rosen MK. Crystal structure of the Formin mDia1 in autoinhibited conformation. PLoS One. 2010;5(9):e12896. doi: 10.1371/journal.pone.0012896. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3(8):723–9. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- Panzer L, Trübe L, Klose M, Joosten B, Slotman J, Cambi A, Linder S. The formins FHOD1 and INF2 regulate inter- and intra-structural contractility of podosomes. J Cell Sci. 2016;129(2):298–313. doi: 10.1242/jcs.177691. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim HJ, Hong YB, Nam SH, Chung KW, Choi BO. A novel INF2 mutation in a Korean family with autosomal dominant intermediate Charcot-Marie-Tooth disease and focal segmental glomerulosclerosis. J Peripher Nerv Syst. 2014;19(2):175–9. doi: 10.1111/jns5.12062. [DOI] [PubMed] [Google Scholar]
- Paunola E, Mattila PK, Lappalainen P. WH2 domain: a small, versatile adapter for actin monomers. FEBS Lett. 2002;513(1):92–7. doi: 10.1016/s0014-5793(01)03242-2. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Pechlivanis M, Samol A, Kerkhoff E. Identification of a short Spir interaction sequence at the C-terminal end of formin subgroup proteins. J Biol Chem. 2009;284(37):25324–33. doi: 10.1074/jbc.M109.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E, Schuh M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol. 2011;21(11):955–60. doi: 10.1016/j.cub.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Genetics of Familial FSGS. Semin Nephrol. 2016;36(6):467–472. doi: 10.1016/j.semnephrol.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Pring M, Evangelista M, Boone C, Yang C, Zigmond SH. Mechanism of formin-induced nucleation of actin filaments. Biochemistry. 2003;42(2):486–96. doi: 10.1021/bi026520j. [DOI] [PubMed] [Google Scholar]
- Pruyne D. Revisiting the Phylogeny of the Animal Formins: Two New Subtypes, Relationships with Multiple Wing Hairs Proteins, and a Lost Human Formin. PLoS One. 2016;11(10):e0164067. doi: 10.1371/journal.pone.0164067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297(5581):612–5. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- Quaglia M, Musetti C, Ghiggeri GM, Fogazzi GB, Settanni F, Boldorini RL, Lazzarich E, Airoldi A, Izzo C, Giordano M, et al. Unexpectedly high prevalence of rare genetic disorders in kidney transplant recipients with an unknown causal nephropathy. Clin Transplant. 2014;28(9):995–1003. doi: 10.1111/ctr.12408. [DOI] [PubMed] [Google Scholar]
- Quinlan ME, Heuser JE, Kerkhoff E, Mullins RD. Drosophila Spire is an actin nucleation factor. Nature. 2005;433(7024):382–8. doi: 10.1038/nature03241. [DOI] [PubMed] [Google Scholar]
- Quinlan ME, Hilgert S, Bedrossian A, Mullins RD, Kerkhoff E. Regulatory interactions between two actin nucleators, Spire and Cappuccino. J Cell Biol. 2007;179(1):117–28. doi: 10.1083/jcb.200706196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramabhadran V, Korobova F, Rahme GJ, Higgs HN. Splice variant-specific cellular function of the formin INF2 in maintenance of Golgi architecture. Mol Biol Cell. 2011;22(24):4822–33. doi: 10.1091/mbc.E11-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramabhadran V, Gurel PS, Higgs HN. Mutations to the formin homology 2 domain of INF2 protein have unexpected effects on actin polymerization and severing. J Biol Chem. 2012;287(41):34234–45. doi: 10.1074/jbc.M112.365122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramabhadran V, Hatch AL, Higgs HN. Actin monomers activate inverted formin 2 by competing with its autoinhibitory interaction. J Biol Chem. 2013;288(37):26847–55. doi: 10.1074/jbc.M113.472415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PQ, Lohkamp B, Celsi G, Mache CJ, Auer-Grumbach M, Wernerson A, Hamajima N, Tryggvason K, Patrakka J. Novel INF2 mutation p. L77P in a family with glomerulopathy and Charcot-Marie-Tooth neuropathy. Pediatr Nephrol. 2013;28(2):339–43. doi: 10.1007/s00467-012-2299-1. [DOI] [PubMed] [Google Scholar]
- Rollason R, Wherlock M, Heath JA, Heesom KJ, Saleem MA, Welsh GI. Disease causing mutations in inverted formin 2 regulate its binding to G-actin, F-actin capping protein (CapZ (xF061)-1) and profilin 2. Biosci Rep. 2016;36(1):e00302. doi: 10.1042/BSR20150252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119(3):419–29. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Rood IM, Deegens JK, Wetzels JF. Genetic causes of focal segmental glomerulosclerosis: implications for clinical practice. Nephrol Dial Transplant. 2012;27(3):882–90. doi: 10.1093/ndt/gfr771. [DOI] [PubMed] [Google Scholar]
- Rood IM, Bongers EM, Lugtenberg D, Klein IH, Steenbergen EJ, Wetzels JF, Deegens JK. Familial focal segmental glomerulosclerosis: mutation in inverted formin 2 mimicking Alport syndrome. Neth J Med. 2016;74(2):82–5. [PubMed] [Google Scholar]
- Roos A, Weis J, Korinthenberg R, Fehrenbach H, Häusler M, Züchner S, Mache C, Hubmann H, Auer-Grumbach M, Senderek J. Inverted formin 2-related Charcot-Marie-Tooth disease: extension of the mutational spectrum and pathological findings in Schwann cells and axons. J Peripher Nerv Syst. 2015;20(1):52–9. doi: 10.1111/jns.12106. [DOI] [PubMed] [Google Scholar]
- Rosado M, Barber CF, Berciu C, Feldman S, Birren SJ, Nicastro D, Goode BL. Critical roles for multiple formins during cardiac myofibril development and repair. Mol Biol Cell. 2014;25(6):811–27. doi: 10.1091/mbc.E13-08-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales-Nieves AE, Johndrow JE, Keller LC, Magie CR, Pinto-Santini DM, Parkhurst SM. Coordination of microtubule and microfilament dynamics by Drosophila Rho1, Spire and Cappuccino. Nat Cell Biol. 2006;8(4):367–76. doi: 10.1038/ncb1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435(7041):513–8. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat Cell Biol. 2002;4(8):626–31. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ares M, Garcia-Vidal M, Antucho EE, Julio P, Eduardo VM, Lens XM, Garcia-Gonzalez MA. A novel mutation, outside of the candidate region for diagnosis, in the inverted formin 2 gene can cause focal segmental glomerulosclerosis. Kidney Int. 2013;83(1):153–9. doi: 10.1038/ki.2012.325. [DOI] [PubMed] [Google Scholar]
- Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6(5):1139–48. doi: 10.2215/CJN.05260610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Li Q, Mogilner A, Bershadsky AD, Shivashankar GV. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. Proc Natl Acad Sci U S A. 2015;112(20):E2595–601. doi: 10.1073/pnas.1504837112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Grintsevich EE, Woo J, Gurel PS, Higgs HN, Reisler E, Gimzewski JK. Nanostructured self-assembly of inverted formin 2 (INF2) and F-actin-INF2 complexes revealed by atomic force microscopy. Langmuir. 2014;30(25):7533–9. doi: 10.1021/la501748x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I. The disease-associated formin INF2/EXC-6 organizes lumen and cell outgrowth during tubulogenesis by regulating F-actin and microtubule cytoskeletons. Dev Cell. 2015;32(6):743–55. doi: 10.1016/j.devcel.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I. A network of conserved formins, regulated by the guanine exchange factor EXC-5 and the GTPase Cdc42, modulates tubulogenesis in vivo. Development. 2016;143(22):4173–4181. doi: 10.1242/dev.141861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau CT, Plotnikov SV, Doyle AD, Waterman CM. Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly. Proc Natl Acad Sci U S A. 2015;112(19):E2447–56. doi: 10.1073/pnas.1505035112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Subramanian B, Sun H, Yan P, Charoonratana VT, Higgs HN, Wang F, Lai KM, Valenzuela DM, Brown EJ, Schlöndorff JS, et al. Mice with mutant Inf2 show impaired podocyte and slit diaphragm integrity in response to protamine-induced kidney injury. Kidney Int. 2016;90(2):363–72. doi: 10.1016/j.kint.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Schlöndorff JS, Brown EJ, Higgs HN, Pollak MR. Rho activation of mDia formins is modulated by an interaction with inverted formin 2 (INF2) Proc Natl Acad Sci U S A. 2011;108(7):2933–8. doi: 10.1073/pnas.1017010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Schlöndorff J, Higgs HN, Pollak MR. Inverted formin 2 regulates actin dynamics by antagonizing Rho/diaphanous-related formin signaling. J Am Soc Nephrol. 2013;24(6):917–29. doi: 10.1681/ASN.2012080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Al-Romaih KI, MacRae CA, Pollak MR. Human Kidney Disease-causing INF2 Mutations Perturb Rho/Dia Signaling in the Glomerulus. EBioMedicine. 2014;1(2-3):107–15. doi: 10.1016/j.ebiom.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvanto M, Jahnukainen T, Kestilä M, Jalanko H. Podocyte proteins in congenital and minimal change nephrotic syndrome. Clin Exp Nephrol. 2015;19(3):481–8. doi: 10.1007/s10157-014-1020-z. [DOI] [PubMed] [Google Scholar]
- Tamura H, Nakazato H, Kuraoka S, Yoneda K, Takahashi W, Endo F. Reduced INF2 expression in nephrotic syndrome is possibly related to clinical severity of steroid resistance in children. Nephrology (Carlton) 2016;21(6):467–75. doi: 10.1111/nep.12627. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Takasu E, Aigaki T, Kato K, Hayashi S, Nose A. Formin3 is required for assembly of the F-actin structure that mediates tracheal fusion in Drosophila. Dev Biol. 2004;274(2):413–25. doi: 10.1016/j.ydbio.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Thurston SF, Kulacz WA, Shaikh S, Lee JM, Copeland JW. The ability to induce microtubule acetylation is a general feature of formin proteins. PLoS One. 2012;7(10):e48041. doi: 10.1371/journal.pone.0048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J, Moskalewski S. Relationship between the Golgi complex and microtubules enriched in detyrosinated or acetylated alpha-tubulin: studies on cells recovering from nocodazole and cells in the terminal phase of cytokinesis. Cell Tissue Res. 1993;273(3):457–66. doi: 10.1007/BF00333700. [DOI] [PubMed] [Google Scholar]
- Toyota K, Ogino D, Hayashi M, Taki M, Saito K, Abe A, Hashimoto T, Umetsu K, Tsukaguchi H, Hayasaka K. INF2 mutations in Charcot-Marie-Tooth disease complicated with focal segmental glomerulosclerosis. J Peripher Nerv Syst. 2013;18(1):97–8. doi: 10.1111/jns5.12014. [DOI] [PubMed] [Google Scholar]
- Vaillant DC, Copeland SJ, Davis C, Thurston SF, Abdennur N, Copeland JW. Interaction of the N- and C-terminal autoregulatory domains of FRL2 does not inhibit FRL2 activity. J Biol Chem. 2008;283(48):33750–62. doi: 10.1074/jbc.M803156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat JM, Mathis S, Funalot B. The various Charcot-Marie-Tooth diseases. Curr Opin Neurol. 2013;26(5):473–80. doi: 10.1097/WCO.0b013e328364c04b. [DOI] [PubMed] [Google Scholar]
- Vizcarra CL, Kreutz B, Rodal AA, Toms AV, Lu J, Zheng W, Quinlan ME, Eck MJ. Structure and function of the interacting domains of Spire and Fmn-family formins. Proc Natl Acad Sci U S A. 2011;108(29):11884–9. doi: 10.1073/pnas.1105703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales P, Schuberth CE, Aufschnaiter R, Fels J, García-Aguilar I, Janning A, Dlugos CP, Schäfer-Herte M, Klingner C, Wälte M, et al. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. ELife. 2016;5:e19850. doi: 10.7554/eLife.19850. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7(6):871–83. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Werheid F, Azzedine H, Zwerenz E, Bozkurt A, Moeller MJ, Lin L, Mull M, Häusler M, Schulz JB, Weis J, et al. Underestimated associated features in CMT neuropathies: clinical indicators for the causative gene? Brain Behav. 2016;6(4):e00451. doi: 10.1002/brb3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström SA, Lange A, Hess MW, Polleux J, Spatz JP, Krüger M, Pfaller K, Lambacher A, Bloch W, Mann M, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell. 2010;19(4):574–88. doi: 10.1016/j.devcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Hao X, Azeloglu EU, Ren H, Wang Z, Ma J, Liu J, Ma X, Wang W, Pan X, et al. Novel mutations in the inverted formin 2 gene of Chinese families contribute to focal segmental glomerulosclerosis. Kidney Int. 2015;88(3):593–604. doi: 10.1038/ki.2015.106. [DOI] [PubMed] [Google Scholar]
- Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–23. doi: 10.1016/s0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- Young KG, Thurston SF, Copeland S, Smallwood C, Copeland JW. INF1 is a novel microtubule-associated formin. Mol Biol Cell. 2008;19(12):5168–80. doi: 10.1091/mbc.E08-05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, Evangelista M, Boone C, Yang C, Dar AC, Sicheri F, Forkey J, Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr Biol. 2003;13(2):1820–3. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]