Abstract
In organ transplantation, the function and longevity of the graft critically rely on the success of controlling immunological rejection reactivity against human leukocyte antigens (HLA). Histocompatibility guidelines are based on laboratory tests of anti-HLA immunity, which presents either as pre-existing or de novo generated HLA antibodies that constitute a major transplantation barrier. Current tests are built on a single-antigen beads (SAB) platform using a fixed set of ~100 preselected recombinant HLA antigens to probe transplant sera. However, in humans there exist a far greater variety of HLA types, with no two individuals other than identical twins who can share the same combination of HLA sequences. While advanced technologies for HLA typing and direct sequencing can precisely capture any mismatches in DNA sequence between a donor's and recipient's HLA, the SAB assay, due to its limited variety in sequence representation, is unable to precisely detect alloantibodies specifically against the donor HLA mismatches. We sought to develop a complementary method using a different technology to detect and characterize anti-donor HLA antibodies on a personalized basis. The screening tool is a custom peptide array of donor HLA-derived sequences for probing post-transplant sera of the organ recipient to assess the risk for antibody-mediated rejection. On a single array for one donor-recipient pair, up to 600 unique peptides are made based on the donor's HLA protein sequences, each peptide carrying at least one mismatched residue in a 15-amino acid sequence. In our pilot experiments to compare antigen patterns for pre- and post-transplant sera on these arrays, we were able to detect anti-HLA signals with the resolution that also allowed us to pinpoint the immune epitopes involved. These personalized antigen arrays allow high-resolution detection of donor-specific HLA epitopes in organ transplantation.
Keywords: Biochemistry, Issue 127, Organ transplantation, Antibody mediated transplant rejection (AMR), Human leukocyte antigen (HLA), HLA mismatch, Alloantibody, Antigen array, SPOT synthesis
Introduction
Organ replacement therapy that is routinely conducted across the world has saved millions of lives. Solid organ transplantation occurs in approximately 100 patients per million people in the USA annually, while a greater number still are on waitlists to receive donor organs due to a severe shortage of supply (according to information provided by the Organ Procurement and Transplantation Network - OPTN/UNOS: optn.transplant.hrsa.gov). Organ transplant is highly regulated in order to reduce organ waste and save lives, but the scientific tools used to inform these regulations are limited in effectiveness. For instance, the scientific community fully recognizes the highly polymorphic states of HLA molecules and accurate genetic tests of DNA using high-resolution typing and sequence-based typing (SBT) have been developed in recent years1,2. However, alloantibody testing methods have not yet been able to produce the vast variety of individual HLA sequences as antigen probes. The standard test nowadays uses an invariable panel of ~100 allelic antigens that are comprised of common variants of HLA, A, B, C, DQ, DP and DR sequences in human populations3,4,5,6. Frequently, the actual donor's HLA sequences are not included in the test panel, forcing transplant physicians and surgeons to infer donor-specific reactivity based on shared similarities between donor's actual sequences and corresponding "standards" in the test set7,8. Consequently, it is sometimes challenging to make a reliable estimation of rejection risk based on antibody test results9,10,11,12. Therefore, new personally customizable tests for alloantibodies are urgently needed13,14.
The HLA genes encode the major histocompatibility complex (MHC) receptors that have a key function in immune responses6. HLA genes are known to be the most polymorphic genes of the human genome6. Due to the rapid advancements in DNA sequencing strategies for the HLA genes, new allelic variants (or simply referred to as alleles) are being discovered at an explosive rate15,16. By March 2017, 16,755 validated alleles had been deposited to the IMGT/HLA Database (http://www.ebi.ac.uk/ipd/index.html), of which 12,351 were of class I and 4,404 were of class II groups. In stark contrast, only a little over 100 distinct alleles are represented in the standard single-antigen beads (SAB) assay, which is routinely used to detect alloantibodies in organ transplantation. The SAB method is built on a Luminex platform using flow cytometry. Since the assay utilizes an invariable set of antigens, apart from minor batch to batch variabilities in production, the antiserum test can be robustly standardized across individuals and across laboratories5. However, this test is unable to capture all alloantibodies developed specifically against the donor alleles, particularly when the donor sequences are absent from the SAB set. Although custom production of donors’ antigens based on true sequences are desirable, there remain technical challenges in streamlining the necessary production and testing procedures.
We recently described an alternative methodology in a feasibility study of renal transplant subjects17. The method used peptide antigens in an array format for probing pre- and post-transplant sera of individual subjects. Each array was custom built using the SPOT synthesis method18,19,20,21,22,23 that produces peptide antigens, each 15 amino acids in length, entirely based on the respective organ donor's HLA alleles of A, B, C, DQA1, DQB1 and DRB1. SPOT synthesis is operated on a cellulose membrane using standard Fmoc-chemistry22 and can produce hundreds of custom peptides in parallel with a fully automated robotic system19,21. The membrane array can withstand multiple rounds of stripping and reprobing cycles. In our retrospective study17, we detected changes in antigen patterns with stored transplant antisera collected in a time series (i.e., before and after transplantation). Herein we describe the technical protocol for the workflow including array design, manufacturing, antiserum probing and result analysis. The method is intended for detecting alloantibodies against specific linear epitopes on transplant donors' HLA molecules.
Protocol
All methods described here have been approved by the Northwestern University Institutional Review Board (IRB protocol#: STU00104680). An overall workflow of the protocol is illustrated in Figure 1.
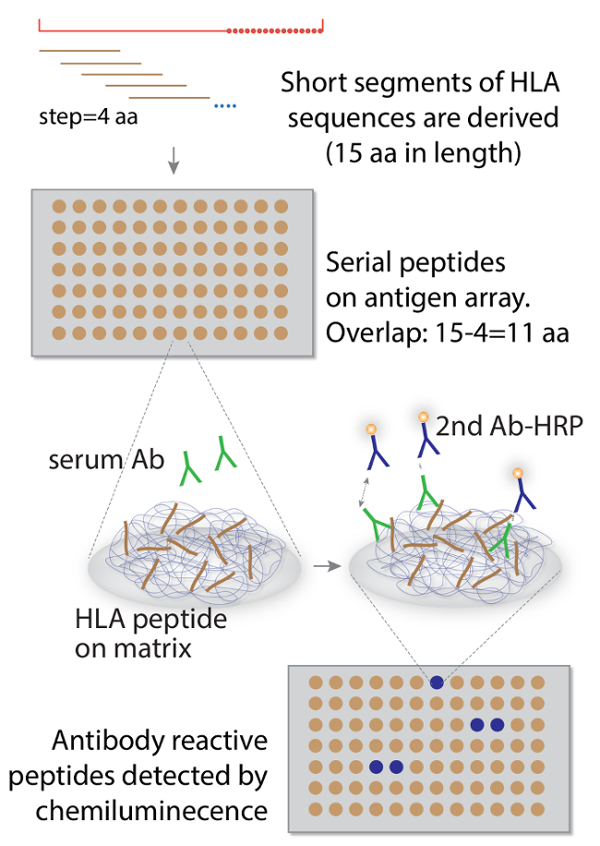
1. Bioinformatic Analysis of Donor and Recipient HLA Sequences
- Retrieve sequences from IMGT/HLA database15.
- Obtain HLA typing reports of both the organ donor and his/her recipient. NOTE: Ensure proper procedures to protect confidential medical records are utilized. Typically, an Institutional Review Board (IRB) approval of the study protocol or the experimental test is required per institutional IRB guidelines. If HLA reports were from PCR-based typing (of either high or low resolution) or sequencing-based typing (SBT), go directly to step 1.1.3. If sequences were obtained from genomic/HLA sequencing, and if they are complete, use these sequences directly instead. Occasionally, when only incomplete typing or sequencing results are available, follow the additional step 1.1.2. to assign the "missing" alleles via the web tool described in step 1.1.2.
- Open the Ambiguous Allele Combinations following web link - http://www.ebi.ac.uk/ipd/imgt/hla/ambig.html, and search using the “Ambiguous Allele Combinations Search Tool” function to retrieve the hypothetical alleles. Then, proceed to step 1.1.3. to enter the allele name.
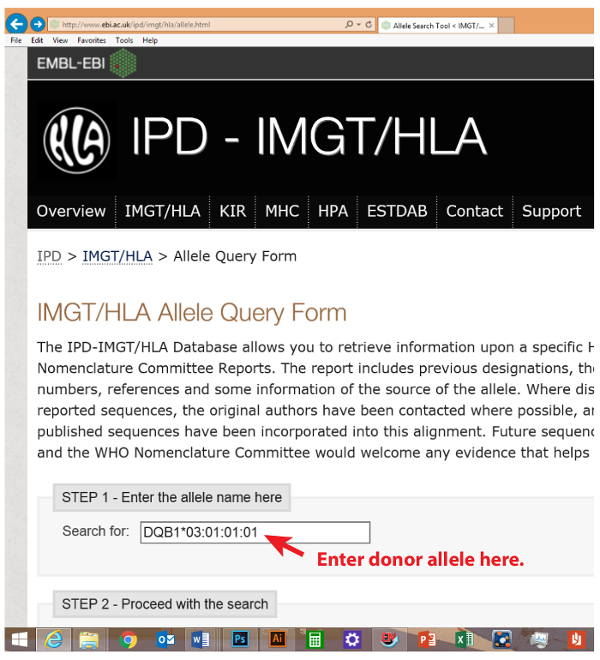
- Open the IMGT/HLA Allele Query Form at the following the web link - http://www.ebi.ac.uk/ipd/imgt/hla/allele.html, and input subject’s allele names (each individually, as shown in Figure 2) into the “Search for” box. Click on the “Search for alleles now” button. HLA allele names should use standard naming systems (i.e., A*, A*01, A*01:01:01:01, and any previous designations like A*01010101).
- Find the matching allele name displayed on the screen. Click on the allele button.
- Copy the "Protein sequence", and paste it into a text document underneath a header line of ">allele name". Effectively, the text document is in a FASTA (FAST-All) format of the allele name and sequence.
- Perform gene-based multiple alignments of donor and recipient/patient alleles.
- One gene (i.e., DQB1*03:01:01:01) at a time, copy and paste all four alleles of the donor and patient FASTA sequences (two from the donor and two from the patient) into a combined text document. At this point, it is important to differentially denote donor allele names from patient allele names (Options include differential use of upper vs. lower cases; or create a distinguishing prefix or suffix extension to each allele name to separately mark donor vs. patient alleles). The input order of the sequences is not important because following alignment the order will be shuffled according to the levels of similarity between any pair of sequences.
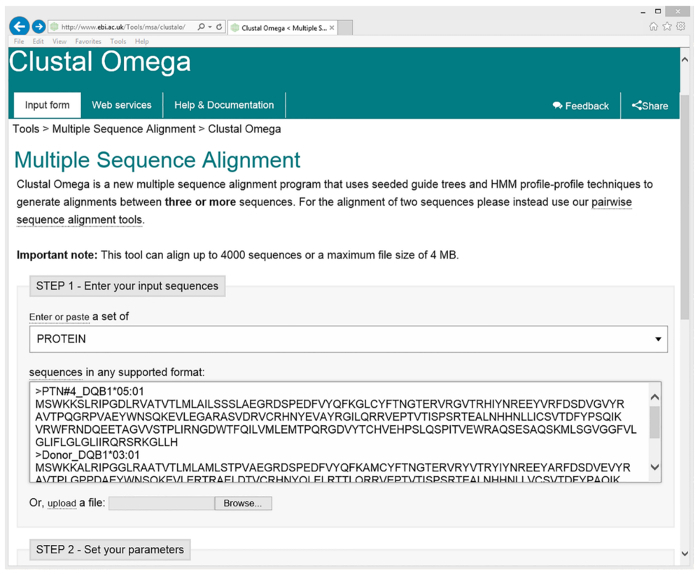
- Copy and paste the sequences in FASTA format (as seen in Figure 3) from above into the “Enter your input sequences” box on the Cluster Omega website: http://www.ebi.ac.uk/Tools/msa/clustalo/.
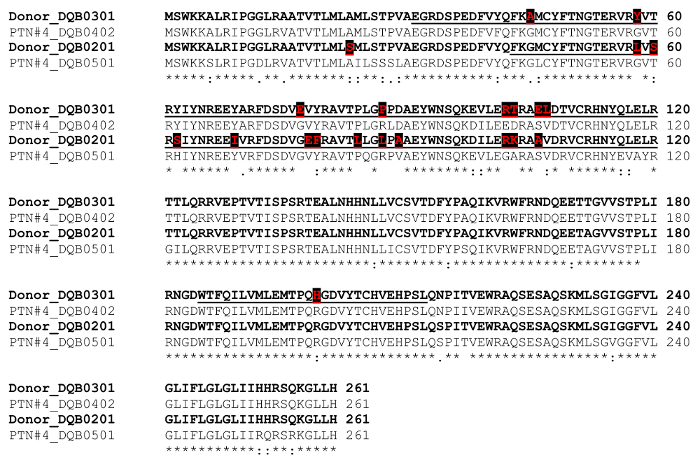
- Click on "Submit your job" using standard settings of parameters: such as "PROTEIN"; and "Clustal w/ numbers". Perform alignment by clicking on "Submit". An alignment of the four sequences is displayed on the screen (Figure 4).
- Mark donor-specific mismatches. NOTE: It should be specially noted that the allelic sequences given here were based on the results of HLA typing, not sequencing. Therefore, there is a risk that relevant sequence variation in the particular donor-recipient pair may be missed. This potential problem will be mitigated as more and more transplant centers adopt accurate high-resolution typing and direct DNA sequencing.
- Copy and paste the alignment text into a text document and create a new document. If the format of the aligned file is disturbed, fix the format by changing font style and size: Always use "Courier New" font in small type size to fit the rows. Save the document after the formatting issues are solved.
- Manually inspect the aligned sequences to identify all mismatched residues that belong uniquely to the donor. Mark each of these donor-specific residues using a distinguishing font color.
- Underline all letters in the donor's sequences that extend 14 residues both up- and downstream of the marked donor-specific mismatches (calculated as 15 amino acid peptide minus 1 residue of the mismatch).
- Derive 15-mer peptide sequences in an overlapping series. NOTE: The reason for choosing 15-mer peptides was based on the general knowledge of typical epitopes being 4-9 amino acids in length. Therefore, when we applied a "moving window" procedure with a step size of 4 amino acids ( Figure 1), our selection of a 15 amino acid length left a 15-4=11 amino acid overlap between any neighboring peptides in a series. In theory, this 11 amino acid overlap is sufficient to cover all immune epitopes of up to 9 amino acids in length, so that no epitope will be inadvertently "split" in half with only partial sequences on the array.
- Use the underlined sequences as templates to sequentially derive short 15 amino acid sequences in a series that overlap by 4 residues between any two immediately adjacent sequences in the series.
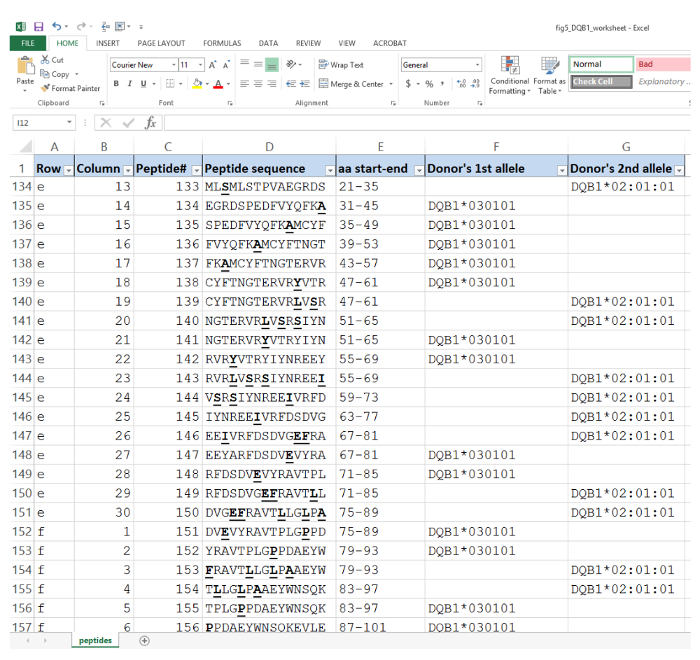
- Copy and paste these 15 amino acid sequences into a spreadsheet( Figure 5) in a column format accompanied by notation columns that include the corresponding names of the donor alleles. An additional column to include aa residue positions may be useful.
Repeat the steps from 1.1.3. to 1.3. for each HLA allele (i.e., A, B, C, DQA1, DQB1, DRB1 and DP) of the donor and the recipient pair to derive the 15 aa short sequences of the donor.
Copy and paste all columns into a master spreadsheet to create a long continuous column of all the 15 aa sequences from all the alleles of the donor. Write down the total number of rows (for the peptide sequences) in the document.
2. Design of Custom Array Layout and Production
- Generate a spreadsheet of peptide sequences with corresponding allele callings.The array has 20 rows and 30 columns and can hold up to 20x30=600 spots for peptides. Depending on the total number of peptides recorded in step 1.5 from one particular donor, and based our past experience with two examples that we had tried in Liu et al.17, the 600 spot array can hold all the sequences generated from ~2 separate transplant cases.
- Rationally plan the most efficient way to fit the sets of peptides within the 600-spot format of the array.
- If more than one subject can fit into one whole array, insert empty rows after the first donor's sequences to match the total number of rows that can be divided by 30 (the number of spots in a row on the array).
- Immediately following the last empty row for the first set of sequences, paste in the entire column of sequence contents from the second transplant case.
- Repeat these steps until the array is filled and no more cases can be added without exceeding the 600 spots. If entire empty rows are left to fill at the bottom, "move" the empty row to be positioned between the two donor sets. This wide space left between cases makes cutting of the membrane after completion of the synthesis easier - following step 3.2. below.
Program the peptide sequences in the context of array layout. The program of the SPOT synthesizer takes a text format of the sequences (one row one peptide sequence), which can be directly obtained by saving the resulting spreadsheet from step 2.1.4 as a simple text document (without the extra column(s) for allele names, aa positions etc.).
Run automated peptide array synthesis via the SPOT synthesizer. The entire operation of the synthesis is detailed in Kudithipudi et al.21. Note that Fmoc synthesis of two arrays takes ~4-5 days.
3. Probe and Reprobe Antisera from a Time Series of an Individual Transplant Recipient.
NOTE: The 600-spot membrane array has the dimensions ~7 cm x 13 cm. After synthesis, the arrays can be stored at room temperature as dry membranes for at least two years when shielded from direct light. Avoid excessive folding of the membrane to preserve its longevity for repeated use.
- Rehydrate the membrane array with ethanol and visualize peptide spots stained with Ponceau S.
- Rehydrate the membrane array carrying the peptides following a stepwise procedure optimized in Li et al.24.
- Immerse the array membrane in 20 mL of 100% ethanol.
- Add 20 mL distilled water to dilute the solution to 50% ethanol and incubate at room temperature for 15 min.
- Change the immersion solution to 40 mL of 100% water three times and incubate 15 min each time.
- Wash in a proper working buffer, e.g., 20 mL of TBST (Tris-buffered saline with 0.1%), three times for 5 min each.
- Ponceau S staining of the array to visualize synthetic peptides
- Add 20 mL of pre-formulated Ponceau S solution directly to the hydrated array and incubate for ~30 s with shaking.
- Run distilled water continuously over the membrane to de-stain background Ponceau S color. During the process, peptide spots of red color may become visible.
- Separate the portions of the array for each donor by carefully cutting between the sections of the array for individual cases. Mark the orientation of each array.
- Pre-block array.
- Block the membrane in 20 mL of 5% non-fat milk dissolved in TBST buffer. This milk-based solution will further de-stain Ponceau S color. Replace the milk buffer several times in order to achieve the clearest peptide images.
- For record-keeping purposes, take a photo of the Ponceau S image of the array using a hand-held camera (example in Figure 6).
- Continue blocking of the membrane in 5% non-fat milk at 4 °C overnight or at room temperature for 2 h with rocking.
- Incubate array with transplant antiserum. NOTE: It should be noted that a phenomenon known as prozone or the Hook effect may result from complement-dependent interference in serum antibody analysis. To circumvent this problem, an optional serum pretreatment step can be considered with either EDTA or heat inactivation of serum samples.
- Remove blocking buffer and wash the membrane with 20 mL of TBST three times the next day for 5 min each time.
- Add 20 µL of the crude serum of the recipient to 20 mL of 2.5% milk in TBST buffer and incubate with the membrane for 2-3 h at room temperature. Note that in this initial round of probing, it is recommended that the last post-transplant serum (or likely most sensitized serum) in the time series is firstly used. This is based on the assumption that earlier specimens in the series, particularly from pre-transplant time points, have less variety of alloantibodies that tend to develop over time. This way, any possibility of signal interference from "carry-over" between probing rounds can be clearly distinguished from truly developed alloantibody reactivity due to immune responses against the graft.
- Wash the array using 20 mL of TBST and incubate with secondary antibody.
- Wash the membrane three times for 10 min each time.
- Incubate with goat anti-human IgG-HRP (Horseradish peroxidase) secondary antibody at 1:10,000 dilution in TBST buffer supplemented with 1% milk for another 2 h.
- Wash and develop the blot.
- Wash the membrane three times with TBST for 10 min each time.
- Perform enhanced chemiluminescence (ECL) using 5 mL of luminol solution freshly mixed with 5 mL of peroxide solution to develop the membrane (for 1 min).
- Visualize ECL signals using a suitable imager (i.e., ChemiDoc Imaging Systems or Azure C600).
- Scan and quantify the blot.
- Save the developed images (Figure 7, lower image) and perform quantification of spot intensity.
4. Compare Antiserum Reactivity across a Clinical Time Series.
- Strip the array.
- At this point, keep the membrane wet at all times.
- Strip the membrane by incubating with 20 mL of commercial stripping buffer at 37 °C for 20 min, and then wash the membrane with TBST three times for 10 min each. Then repeat the blocking (step 4.2) and probing (step 4.3) steps using a different serum of the patient taken at a different time point.
- Block stripped membrane. Note: Following stripping, the membrane can be reused for another round of probing of a different serum from the same patient in a time series.
- Block the membrane using 5% milk buffer as before (see step 3.2).
Reprobe a different antiserum from the same time series (repeat steps from 3.3-3.6; example in Figure 7, upper image). NOTE: The stripped array can then be used to reprobe another serum specimen. Since the peptides are covalently conjugated to the supporting matrix of the membrane, we showed that the array can be reused for up to 20 rounds of stripping and reprobing cycles without losing its performance.
- Long-term storage of arrays.
- Store the membrane in TBS buffer supplemented with 0.02% w/w sodium azide as preservative. Use a sealed plastic bag for long-term storage. At 4 °C under protection from direct light, the wet membrane can be stored for at least 2 years.
5. Data Acquisition and Analysis
- Manually annotate positive antigen peptides.
- Locate spots showing positive antibody signals and determine each of their grid positions.
- Highlight the corresponding rows in the master worksheet for the positive peptides.
- Retrieve peptide sequences listed in the master worksheet ( Figure 7, bottom list). According to the design principle outlined in step 1.4.1., neighboring serial spots share significantly overlapping sequences (15-4=11), therefore reactive epitopes may be shared by peptides in a consecutive series.
- Highlight any positive spots occurring in succession.
- Determine minimum epitope length for each peptide in a series.
- Highlight any potentially shared epitope sequences based on overlapping segments of reactive peptides.
- Structurally model antigen epitopes.
- Obtain prototype HLA crystal structures from the Protein Data Bank found at the following the web link: http://www.rcsb.org/pdb/home/home.do.
- Display the prototype HLA structure in Pymol (download from www.pymol.org).
- Map" or model discovered anti-donor epitopes to the 3D structures of the prototype HLA molecules by highlighting the reactive peptide sequences ( Figure 8). Click on "Display", then "Background", and select "White". To save the image, go to "File" and "Save Image As" and select the file format "png".
Report results and assign reactive epitopes to the corresponding alleles.
- Compare array results to the single-antigen beads (SAB) results, if available. NOTE: The SAB test using single allelic antigens measures antibody reactivity towards a fixed panel of HLA proteins. Individual peptides that show antibody reactivity belong to certain HLA alleles that, if also included in the SAB panel, allow direct comparison between the array and the SAB results. Our previous study17 showed a high level of correlation between the results, suggesting that the peptide epitopes significantly contribute to the overall reactivity of SAB. In addition, the array results also reveal amino acid-levels of specificity.
- Obtain SAB results from an HLA lab which provides information about whether and which donor alleles are reactive to post-transplant sera of the patient. Results from step 5.5. based on the array analysis also provide information about corresponding donor alleles positive for alloantibody reactivity.
- Compare SAB and array results.
Representative Results
In the original study using the array screening method17, we enrolled a total of 5 kidney transplant subjects. We obtained the HLA typing results of our cohort and of their respective donors. Their medical history and allelic antibody titers from SAB tests were also available to us. In our pilot study of these 5 patients, we devised two different methodologies: a standard array comprised of a fixed panel of peptides and personalized arrays that were custom made for each donor and recipient pair. While the first method had allowed us to technically validate the performance of the array platform in achieving high levels of specificity in individual transplants, the personalized protocol for this latter method is described in this article and video intended for comprehensive screening of donor-specific antibodies (DSA), which often correlate with poor graft outcomes5,25,26.
The personalized arrays were applied to two of the five cases17, and for illustrating the overall workflow of the method, here we focus on one of these two patients (namely PTN#4). The key technology for making custom peptide array involves the SPOT synthesizer. It is a fully automated robotic system that makes peptides using Fmoc synthesis directly onto a specially derived cellular matrix on a membrane (Figure 1). We exploited the flexibility of the synthesizer to assemble large sets of peptides derived from individual organ donors' HLA sequences. In order to have sufficient coverage to avoid a situation when a continuous epitope is being "split" and loses antibody reactivity, we followed a "walking" design of serial peptides to have 15-4=11 residues of overlap between any immediately adjacent peptides, so that the series is always sufficient to cover the typical length of antibody epitopes estimated to be 4-9 aa in length (Figure 1).
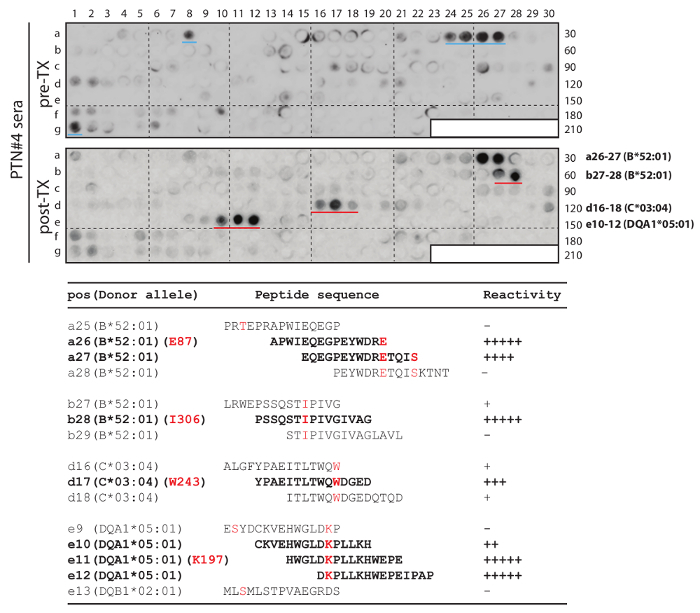
The template HLA sequences were directly downloaded from the repository database maintained at EMBL-EBI (the European Bioinformatics Institute). As an example, PTN#4's donor's HLA-DQB1*03:01:01:01 allele was searched for (webpage illustration in Figure 2). In conjunction, we separately downloaded sequences for the donor's second HLA-DQB1 allele of *02:01:01, as well as those of PTN#4 himself, HLA-DQB1*04:02 and *05:01. Multiple sequence alignment was then performed using Clustal Omega's web functions (Figure 3). The resulting alignment file was obtained (Figure 4), from which all mismatched amino acid residues were identified (donor's residues are highlighted). Next, we marked template sequences (underlined) of the donor that were sufficiently long to cover all his mismatched residues. Based on these template sequences and following a "walking" design as described above, three series of peptides each 15 aa in length were derived for HLA-DQB1. This process was repeated to the rest of the donor's alleles of HLA-A, B, C, DQA1, and DRB1 (DRA1 and DP were excluded due to unavailability of the pertinent clinical record) in comparison to the corresponding alleles of his recipient. At the end, a total of 202 peptides were enlisted for making the array (Peptide sequences in a worksheet in Figure 5 and an array illustration in Figure 6) specifically for probing PTN#4's post-transplant serum, and compared the results with those obtained from a subsequent reprobing of his pre-transplant serum (Figure 7). Out of the 202 peptides, a total of 10 showed antibody signals associated with the post-transplant specimen of which donor-specific residues (mismatched from the recipient) E87, I306, W243 and K197 of HLA-B*52:01, -B*52:01, -C*03:04 and -DQA1*05:01 seemed to be involved in inciting antibody reactivity (residues denoted in red letters in Figure 7).
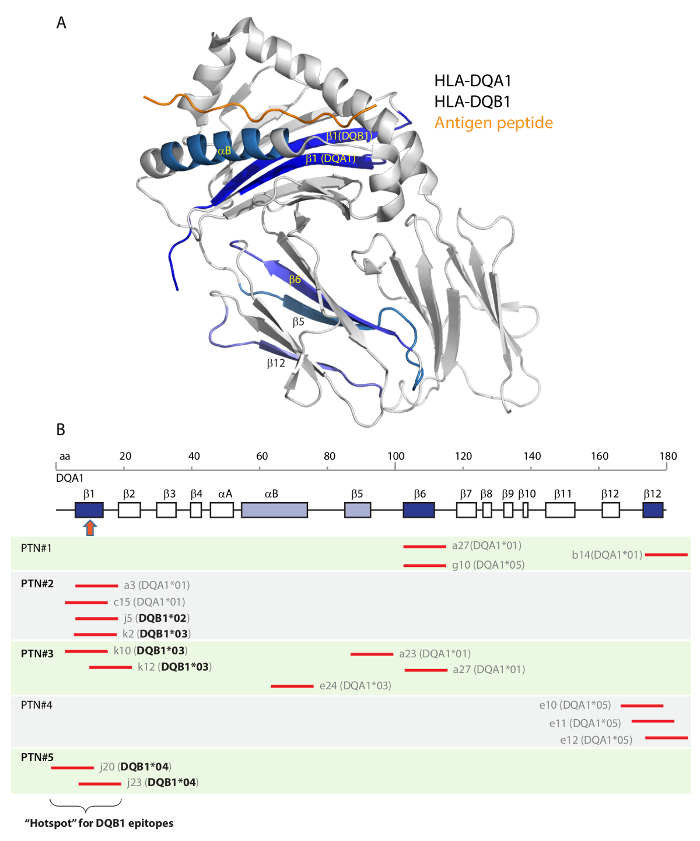
Additionally, one of the key advantages of the array method as compared to the traditional SAB test is that epitope information can be readily obtained, as shown in Figure 7. We compared the antibody-reactive epitopes on HLA-DQA1 and -DQB1 from all five patients (from separate array results in Liu et al.17) by having them all modeled to a co-crystal structure of DQA1 and DQB1 heterodimer (Figure 8). Encouragingly, a prominent segment of the structure known as the β1 strand is a "hotspot" that had a much higher chance (3/5 in patients 2,3,5) to be targeted by alloantibodies among the transplant subjects in the 5 case cohort.
Figure 1. The overall scheme of HLA-based antigen array method for detecting anti-donor HLA alloantibodies in organ recipients. (Adapted and modified from Liu et al.17) Please click here to view a larger version of this figure.
Figure 2. Data repository website To retrieve HLA sequences, visit the following website: http://www.ebi.ac.uk/ipd/imgt/hla/allele.html Please click here to view a larger version of this figure.
Figure 3. Multiple protein sequences alignment of donor vs. recipient HLA-DQB1 alleles using Clustal Omega web-based tool. Copy and paste sequences of patient #4(PTN#4)’s DQB1*0201 and 03:01, and of donor’s DQB1*04:02 and 05:01 in FASTA format into http://www.ebi.ac.uk/Tools/msa/clustalo/ (For illustration, DQB1*05:01 of PTN#4 and DQB1*03:01 of the donor are shown in the input box). Select the “Clustal w/ numbers” option in “STEP2” for output format and run alignment. Please click here to view a larger version of this figure.
Figure 4. Comparison of donor vs. patient/recipient HLA DQB1 and selection of template sequences for peptide synthesis. The donor's sequences are in bold, with donor-specific mismatches in red font and also highlighted with black boxes. Template sequences (underlined) for deriving peptides contain these donor-specific residues. (This figure was adapted and modified from Liuet al.17) Please click here to view a larger version of this figure.
Figure 5. An illustrative example of the master worksheet in a spreadsheet of donor HLA peptides. Peptide position on the array is indicated by row vs. column coordinates. Peptide sequences are used to program synthesis using robotic SPOT synthesizer. Donor-specific residues (mismatched from the recipient's corresponding alleles) are highlighted in bold and underlined. The start and end positions of the peptide corresponding to full length HLA-DQB1 sequences are also indicated. Please click here to view a larger version of this figure.
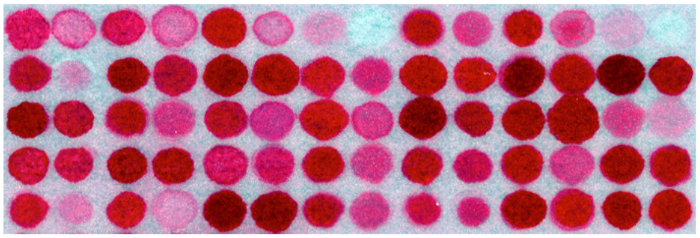
Figure 6. Image of an array section stained with Ponceau S Note disparity in color intensity due to difference in amino acid composition among peptides, while peptide concentrations among all spot areas are the same. (The image is an illustrative example, not the one from this actual study.) Please click here to view a larger version of this figure.
Figure 7.Donor-specific HLA-A, B, C, DQ, DR array study of mismatched epitopes in PTN#4. Serial peptides were derived from the donor's sequences to cover mismatched residues (An example of DQB1 in Figure 4). The array was used to probe the post-transplant serum (lower blot: post-TX) and subsequently the pre-transplant serum (upper blot: pre-TX) from the same patient. The four sets of strong spots from post-transplant probing are marked by red lines (in the lower blot), while two medium intensity spots that were only associated with pre-transplant serum are marked by blue lines (in the upper blot). The bottom table shows the corresponding peptide sequences and their reactivity to the post-transplant serum. Donor-specific (mismatched) residues E87, I306, W243 and K197 of their respective alleles are in red letters and peptides showing strong antibody reactivity are in bold fonts. This figure was adapted and modified from Liu et al.17. Please click here to view a larger version of this figure.
Figure 8. Structural locations of HLA-DQ epitopes. Co-crystal structure of HLA-DQ8 was used as a template. The structure was comprised of a DQA1 and a DQB1 subunits together with an antigen peptide (A). Protein secondary structures of the α-helices and β-strands are shown in panel (B). DQA1 and DQB1 peptides that reacted with either one of the five serum were located on the co-crystal structure (in A and in B: shaded in blue). Three β-strands, β1, β6 and β12 (dark blue in B), each represented by multiple peptides (short red lines corresponding to the linear aa positions of DQA1), reacted with multiple patients' samples (original results in Liu et al.17). All six DQB1 peptides (in bold font) reactive to antibodies in patients #2, #3, #5 are located to the β1 "hotspot" segment (in B: pointed by the red arrow). This figure was adapted from Liu et al.17. Please click here to view a larger version of this figure.
Discussion
The design of the SPOT array described here is for experimental study of alloantibody specificity in transplantation against an organ donor's HLA antigens. In contrast to the existing SAB assay broadly used in clinic, the antigen array method has a major advantage in its flexible design that can accommodate the true HLA sequences of the individual donor. The new platform exploits the potentials of the rapidly advancing DNA sequencing technology that will soon be able to produce accurate HLA allelic sequence readings without ambiguity16,27 and the potential of designated databases for repository HLA sequences15 in the global population. Our current array-screening protocol was developed based on the success of several pilot studies of transplant sera. Here we should emphasize that at this stage, our prototype arrays are constructed only for research use, not for diagnostic applications in clinical practice.
Another notable distinction between SAB and our antigen array is that the latter has a higher resolution to distinguish potential antigen epitopes within the 15 aa segments of HLA sequences, which could provide valuable information about alloantigenicity18. However, unlike SAB that presents whole protein antigens to adapt their folded structures, the antigen array only incorporates short peptides, which excludes information about conformational folding. Therefore, the arrays cannot detect antibodies that only recognize conformational epitopes, which some believe constitute a majority of all functional epitopes relevant to antibody mediated rejection28,29. Still, in other contexts, antibody actions against linear epitopes, such as those in short peptides, are being exploited in viral antigen responses and in vaccine design30,31. We should also note that, in the limited number of patient samples tested, the detection sensitivity performance of our array exceeded that of SAB and allowed the detection of donor-specific antibodies sooner17 during the clinical progression of antibody-mediated rejection. This is due to the high molar concentration of local antigen peptides on the array as compared to SAB. This feature was particularly helpful in cases when SAB detected no reactivity while clinical and pathological evidence of antibody-mediated rejection was apparent, as we showed previously17. However, it is important to point out that this personalized test is more time-consuming than the standard SAB test. The array test requires obtaining the organ donor's and (proposed) recipient's HLA sequences before starting the design of antigen peptides. It then takes several days to produce the array and another 7-8 hours to obtain antibody results. Therefore, the lengthy procedure is not suitable for deceased donor transplant, unless the main purpose of the antibody test is for determining the emergence of donor-specific antibodies following transplantation, rather than for the pre-transplant evaluation of existing antibodies.
The array method had a major improvement from the existing SAB test in detecting antigen epitopes. Furthermore, we made an attempt to map reactive epitopes in five transplant patients to a template structure of HLA-DQ, in which we noted at least one prominent "hotspot" epitope in its β1 strand that occurred in three out of the five patients17. Intriguingly, the β1 strands of HLA-DQA1 and -DQB1 are located at the groove of the antigen-presentation concave (Figure 8), a location known to be antigenic in the context of transplant rejection32. Therefore, we anticipate that future exploration of our personalized antigen array method in extended transplant cohort studies will yield valuable insights on antigenic hotspots in HLA molecules. A catalogue of these identified hotspots, when considered in conjunction with highly accurate DNA sequencing typing of HLA genes between prospective donors and recipients, will ultimately assist pre-transplant decisions through acceptable mismatch programs33, intended to more effectively avoid certain mismatches in vicinity to catalogued hotspots.
In summary, our original study17 in exploring the personalized design of an antigen array for detection of specific anti-HLA alloantibodies was the first of its kind. The study's focus was on the feasibility of array design and implementation, which served as a prototype for potential future technology. Our pilot study not only generated encouraging results from clinical samples, but also allowed us to estimate the overall cost and speed of the test. The production cost of one personalized array in our current laboratory setting is under $1,000 USD, which is a one-time cost since the array can then be used over time in up to 20 rounds of reprobing cycles without a loss of performance. It is possible that additional testing of the array protocol with a broader cohort of transplant subjects will further streamline the method that could one day be used in clinical practice.
Disclosures
No conflicts of interests declared.
Acknowledgments
We thank Drs. Shawn Li and Xing Li of Western University in Canada for their kind assistance with SPOT array production. We are grateful to the staff members at the Histocompatibility Core and at the Comprehensive Transplant Center of Northwestern University for providing sample services. This work has been partly supported by the Auxiliary Board of Northwestern Memorial Hospital, and by a faculty startup fund provided by Northwestern University to J.J..
References
- Bradshaw RA, Dunn PP. Unambiguous high resolution genotyping of human leukocyte antigens. J Immunol Methods. 2017. [DOI] [PubMed]
- Robinson J, Halliwell JA, McWilliam H, Lopez R, Marsh SG. IPD--the Immuno Polymorphism Database. Nucleic Acids Res. 2013;41:D1234–D1240. doi: 10.1093/nar/gks1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait BD. Solid phase assays for HLA antibody detection in clinical transplantation. Curr Opin Immunol. 2009;21(5):573–577. doi: 10.1016/j.coi.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Tait BD. Detection of HLA Antibodies in Organ Transplant Recipients - Triumphs and Challenges of the Solid Phase Bead Assay. Front Immunol. 2016;7:570. doi: 10.3389/fimmu.2016.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebel HM, Bray RA. HLA antibody detection with solid phase assays: great expectations or expectations too great? Am J Transplant. 2014;14(9):1964–1975. doi: 10.1111/ajt.12807. [DOI] [PubMed] [Google Scholar]
- Horton R, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5(12):889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- Duquesnoy RJ. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination I. Description of the algorithm. Hum Immunol. 2002;63(5):339–352. doi: 10.1016/s0198-8859(02)00382-8. [DOI] [PubMed] [Google Scholar]
- Duquesnoy RJ, Marrari M. HLAMatchmaker-based definition of structural human leukocyte antigen epitopes detected by alloantibodies. Curr Opin Organ Transplant. 2009;14(4):403–409. doi: 10.1097/MOT.0b013e32832ca2b8. [DOI] [PubMed] [Google Scholar]
- Wedel J, Bruneau S, Kochupurakkal N, Boneschansker L, Briscoe DM. Chronic allograft rejection: a fresh look. Curr Opin Organ Transplant. 2015;20(1):13–20. doi: 10.1097/MOT.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostaing LP, Malvezzi P. HLA-Incompatible Kidney Transplantation--Worth the Risk? N Engl J Med. 2016;374(10):982–984. doi: 10.1056/NEJMe1601379. [DOI] [PubMed] [Google Scholar]
- Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003;3(12):1488–1500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- Haas M. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2014;19(3):315–322. doi: 10.1097/MOT.0000000000000072. [DOI] [PubMed] [Google Scholar]
- Cecka JM, Reed EF, Zachary AA. HLA high-resolution typing for sensitized patients: a solution in search of a problem? Am J Transplant. 2015;15(4):855–856. doi: 10.1111/ajt.13169. [DOI] [PubMed] [Google Scholar]
- Tambur AR, Claas FH. HLA epitopes as viewed by antibodies: what is it all about? Am J Transplant. 2015;15(5):1148–1154. doi: 10.1111/ajt.13192. [DOI] [PubMed] [Google Scholar]
- Robinson J, et al. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423–D431. doi: 10.1093/nar/gku1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel C, et al. HLA typing by next-generation sequencing - getting closer to reality. Tissue Antigens. 2014;83(2):65–75. doi: 10.1111/tan.12298. [DOI] [PubMed] [Google Scholar]
- Liu P, et al. A Novel Method for Anti-HLA Antibody Detection Using Personalized Peptide Arrays. Transplant Direct. 2016;2(11) doi: 10.1097/TXD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank R, Overwin H. SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes. Methods Mol Biol. 1996;66:149–169. doi: 10.1385/0-89603-375-9:149. [DOI] [PubMed] [Google Scholar]
- Frank R. The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports--principles and applications. J Immunol Methods. 2002;267(1):13–26. doi: 10.1016/s0022-1759(02)00137-0. [DOI] [PubMed] [Google Scholar]
- Amartely H, Iosub-Amir A, Friedler A. Identifying protein-protein interaction sites using peptide arrays. J Vis Exp. 2014. [DOI] [PMC free article] [PubMed]
- Kudithipudi S, Kusevic D, Weirich S, Jeltsch A. Specificity analysis of protein lysine methyltransferases using SPOT peptide arrays. J Vis Exp. 2014. p. e52203. [DOI] [PMC free article] [PubMed]
- Hilpert K, Winkler DF, Hancock RE. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat Protoc. 2007;2(6):1333–1349. doi: 10.1038/nprot.2007.160. [DOI] [PubMed] [Google Scholar]
- McBride R, Head SR, Ordoukhanian P, Law M. Low-Cost Peptide Microarrays for Mapping Continuous Antibody Epitopes. Methods Mol Biol. 2016;1352:67–83. doi: 10.1007/978-1-4939-3037-1_6. [DOI] [PubMed] [Google Scholar]
- Li SS, Wu C. Using peptide array to identify binding motifs and interaction networks for modular domains. Methods Mol Biol. 2009;570:67–76. doi: 10.1007/978-1-60327-394-7_3. [DOI] [PubMed] [Google Scholar]
- Kaczmarek I, et al. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6(3):229–235. [PubMed] [Google Scholar]
- Claas FH. Clinical relevance of circulating donor-specific HLA antibodies. Curr Opin Organ Transplant. 2010;15(4):462–466. doi: 10.1097/MOT.0b013e32833b9c38. [DOI] [PubMed] [Google Scholar]
- Brown NK, Kheradmand T, Wang J, Marino SR. Identification and characterization of novel HLA alleles: Utility of next-generation sequencing methods. Hum Immunol. 2016;77(4):313–316. doi: 10.1016/j.humimm.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Cino EA, Choy WY, Karttunen M. Conformational Biases of Linear Motifs. Journal of Physical Chemistry B. 2013;117(50):15943–15957. doi: 10.1021/jp407536p. [DOI] [PubMed] [Google Scholar]
- Duquesnoy RJ. Human leukocyte antigen epitope antigenicity and immunogenicity. Curr Opin Organ Transplant. 2014;19(4):428–435. doi: 10.1097/MOT.0000000000000100. [DOI] [PubMed] [Google Scholar]
- Lavinder JJ, et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci U S A. 2014;111(6):2259–2264. doi: 10.1073/pnas.1317793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2(3):179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- Filippone EJ, Farber JL. The Humoral Theory of Transplantation: Epitope Analysis and the Pathogenicity of HLA Antibodies. J Immunol Res. 2016;2016:5197396. doi: 10.1155/2016/5197396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas FH, Witvliet MD, Duquesnoy RJ, Persijn GG, Doxiadis II. The acceptable mismatch program as a fast tool for highly sensitized patients awaiting a cadaveric kidney transplantation: short waiting time and excellent graft outcome. Transplantation. 2004;78(2):190–193. doi: 10.1097/01.tp.0000129260.86766.67. [DOI] [PubMed] [Google Scholar]


