Abstract
Electroacupuncture (EA) has been widely used to treat cognitive impairment following cerebral ischemia. However, the functional mechanisms of EA have not been fully elucidated. The aim of the present study was to investigate whether EA at the GV 20 and DU 24 acupoints can improve the learning and memory ability via alteration of the neurochemical metabolism in the hippocampus (HPC) and prefrontal cortex (PFC) of rats with ischemia and reperfusion (I/R) injury. Sprague-Dawley male rats were randomly divided into three groups, namely the sham group (n=12), the middle cerebral artery occlusion (MCAO) group (n=12) and the EA treatment (MCAO + EA) group (n=12). MCAO was performed to establish the left focal cerebral I/R injury model, and the GV 20 and DU 24 acupoints were then stimulated with EA for 30 min per time, once daily, for 7 consecutive days. The Morris water maze (MWM) test was used to assess learning and memory ability. T2-weighted imaging was used to assess the cerebral infarct volume. Magnetic resonance spectroscopy was used to assess neurochemical metabolism of HPC and PFC. The neurological scores of the MCAO + EA group were significantly reduced compared with those of the MCAO group 7 days after EA treatment (P<0.01). The escape latency of the MWM test in the MCAO + EA group was found to be shorter compared with that in the MCAO group (P<0.01). The number of rats crossing through the platform area was significantly higher in the MCAO + EA group compared with that in the MCAO group (P<0.01). The cerebral infarct volume was also decreased in the MCAO + EA group compared with the MCAO group (P<0.05). The ratios of N-acetylaspartate (NAA)/creatine (Cr) and choline (Cho)/Cr of left-to-right HPC were increased in the MCAO + EA group compared with the MCAO group; however, the ratio of glutamate (Glu)/Cr did not change significantly (P>0.05). The ratios of NAA/Cr, Cho/Cr and Glu/Cr of left-to-right PFC were elevated (P<0.05). In conclusion, EA at the GV 20 and DU 24 acupoints may ameliorate learning and memory ability, possibly through increasing the levels of NAA and Cho in the HPC and PFC of rats with I/R injury.
Keywords: electroacupuncture, ischemia and reperfusion, learning and memory impairment, magnetic resonance spectroscopy
Introduction
Cognitive impairment and decline, particularly the impairment of learning and memory, in patients with stroke during the acute and chronic phases, markedly affect the rehabilitation programs of physical ability and the activities of daily living (1,2). Electroacupuncture (EA) treatment, originating from acupuncture in ancient China, delivers electrical stimulation to acupoints through acupuncture needles, which is a simple, convenient and cost-effective treatment that has been widely used for treating cognitive impairment following cerebral ischemia (3,4). However, the functional mechanisms of EA have not been fully elucidated.
Functional imaging studies have been used to identify the brain regions that are associated with cognitive behavioral alterations. Some of these regions, such as the hippocampus (HPC) and prefrontal cortex (PFC), are crucial for regulating learning and memory behaviors, such as spatial exploration and motor learning (5,6). Moreover, it is becoming increasingly clear that certain neurochemical and metabolic changes occur in the brain after learning and training, which are correlated with the relative specificity of brain regions, biochemical substances and behaviors (7). However, it has been previously demonstrated that focal brain ischemia caused neurochemical and metabolic changes in the brain, including creatine (Cr), 1actic acid, N-acetylaspartate (NAA), γ-aminobutyric acid, glutamate (Glu), glutamine and myoinositol alterations (8). During early acute cerebral ischemia, the excitatory neurotoxicity induced by excessive secretion of certain neurochemicals may lead to neuronal damage; by contrast, during the chronic phase, these neurochemicals act as a neurotransmitters or neuromodulators that may improve nervous system activity (9,10). It has been reported that EA may improve cognitive behavior via regulation of neuromodulator signaling in HPC following focal cerebral ischemia (11). Our previous reports have demonstrated that EA at the GV 20 and DU 24 acupoints may improve learning and memory ability and alleviate the histopathological lesions of HPC in a rat model of ischemic stroke (12). However, the association between the improved cognitive function by EA at the GV 20 and DU 24 acupoints and the neurochemical changes in HPC and FPC following an ischemic stroke remains unknown.
Proton magnetic resonance spectroscopy (1H-MRS) is a novel approach to non-invasive detection of metabolites through recording the chemical biology wave frequency in the brain, with generation of visual images (13). The aim of the present study was to elucidate whether EA at the GV 20 and DU 24 acupoints improved the learning and memory impairment via neurochemical biomarker detection with 1H-MRS in the HPC and PFC of rats with ischemia and reperfusion (I/R) injury.
Materials and methods
Ethics statement
A total of 36 male Sprague-Dawley rats (2 months old; weighing 260±20 g) was obtained from the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All experiments were performed strictly in accordance with the International Ethical Guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Ethics Committee of Fujian University of Traditional Chinese Medicine (protocol no. FUTCM-2014019). For euthanasia, 3% sodium pentobarbital (40 mg/kg body weight, i.p.) was used. The middle cerebral artery occlusion (MCAO) surgery was peformed under general anesthesia (1.5% isoflurane in 68.5% N2O and 30% O2). All efforts were made to minimize animal suffering.
Grouping and model of ischemic stroke
The animals were randomly divided into three groups (n=12 per group) as follows: i) The sham operation group (sham), ii) the MCAO and reperfusion group (MCAO) and iii) the MCAO and EA treatment group (MCAO + EA). The MCAO model of ischemic stroke was established as previously reported (14,15). Briefly, a 18–22-mm nylon monofilament (Jialing-Bio, Guangzhou, China) with a rounded tip was inserted into the left common external carotid artery, and was advanced through the internal carotid artery until the origin of the middle cerebral artery (MCA) was blocked. Ischemia was monitored using transcranial temporal laser Doppler (BIOPAC Systems, Inc., Goleta, CA, USA) and an 80% decrease in blood flow after the occlusion was noted. After 2 h of occlusion, reperfusion was achieved by extracting the filament to restore blood flow. The sham-operated rats underwent the same procedure, but arterial occlusion was not performed.
EA treatment
The rats in the MCAO + EA group received EA treatment at the Baihui (GV 20, located in the center of the parietal bone) and Shenting (DU 24, located in the anterior median line) acupoints using an EA apparatus (model G6805; SMIF, Shanghai, China). The stimulation parameters were as follows: Dilatational wave of 1–20 Hz (adjusted to the muscle twitch threshold), peak voltage of 6 V, 1 mA intensity for 30 min/day for 7 consecutive days.
Assessment of neurological deficit scores
Neurological deficit scores were assessed to confirm successful MCAO and the therapeutic efficacy of EA. The neurological deficit scores were assessed in each animal at 2 h, 24 h, 3, 5 and 7 days following I/R in a blinded manner, according to a well-established four-point neurological scale (16): Score 0, no apparent deficits; 1, failure to fully extend the right forepaw; 2, circling to the right; 3, falling or leaning over to the right; 4, no spontaneous walking and depressed level of consciousness. Rats subjected to MCAO with neurological deficit scores of 1–3 were used in subsequent experiments.
Morris water maze (MWM) test
Cognitive function was tested with the MWM test (17,18), which was conducted in a circular pool with a diameter of 150 cm and a height of 60 cm. The pool was filled to a depth of 30 cm with water (22±1°C) and divided into four equal quadrants. A circular escape platform (10 cm in diameter) was placed at the midpoint of the target quadrant and submerged ~1.5 cm below the surface of the water. For the place navigation trials, the rats were trained for 4 days. Each trial was started by placing the rats in one of the four quadrants. The animals were allowed to swim in the pool for 90 sec to find the hidden platform. If an animal did not find the platform within this period, it was manually guided to the platform by the researchers. The rats rested for 10 sec between two consecutive trials. Post-training probe trial tests were conducted 1 day after the final training session. The hidden platform was removed, and rats were allowed to swim freely for 90 sec. The occupancy and crossing of animals in the proximity of the target quadrant (the quadrant including the hidden platform during training trials), were then recorded.
Magnetic resonance spectroscopy (MRS) scans
After EA treatment for 7 days and the MWM test, single-voxel 1H-MRS experiments were performed on a 7.0 T MRI scanner (BioSpec 70/20USR; Bruker BioSpin MRI GmbH, Ettlingen, Germany) using a 38-mm birdcage rat brain quadrature resonator for radiofrequency transmission and reception. The animals were anesthetized with isoflurane/O2 (with 3% induction for 5 min and 1.2–1.5% to maintain the rats in a state of deep anesthesia) and kept body temperature with a hot-water circuit. After anesthesia, the rats were placed in a prone position on a custom-made holder to minimize head movements, with a set head position and real-time monitoring of the breathing rate at 40 breaths/min. The rats' temperature was maintained at 37±2°C during scanning in the holder to minimize head movements while respiration was maintained.
T2-weighted imaging (T2WI) in three planes with a fast spin echo pulse sequence was first acquired to control rat head positioning. T2WI scans were acquired using rapid acquisition with relaxation enhancement pulse sequence with the following parameters: Field of view, 32 × 32 mm; matrix size, 256×256; repetition time (TR), 4200 msec; echo time (TE), 55 msec; slice thickness, 1.0 mm; slice gap, 0 mm; and acquisition time, 4 min and 51 sec.
For single-voxel 1H-MRS, the volume of interest (20×20×20 mm3) was placed at the HPC and PFC regions in the coronal T2W image centre (bregma value −2.40 and +3.24 mm of the standard rat brain atlas). Point resolved selective spectroscopy sequence was used for signal acquisition, with TR, 1,500 msec; TE, 16.168 msec, and scan duration for each side, 9 min. In addition, the order of acquisition of the right and left HPC and PFC spectra alternated between scanned animals, to minimize the introduction of artifactual hemispheric differences.
Measurement of cerebral infarct volume
ImageJ analysis and processing was applied for T2W images. The volume of each brain cerebral infarct was calculated as follows: Percentage of cerebral infarct volume = (cerebral infarct volume/whole brain volume) ×100.
1H-MRS spectral processing
Spectral image analysis and data processing were performed using automatic analysis software to analyze spectrum signal with MR (19). The metabolite area under the peak was quantified using a quantum estimation method with a subtraction approach for background modeling. A simulated basis set was used to estimate peak areas. To reduce systematic variations between animals, a relative quantification method using the Cr peak as the internal spectral reference was applied. NAA/Cr, Cho/Cr and Glu/Cr were statistically evaluated. Each MRS metabolite was identified by its part per million (ppm) position of the nuclear spectrum (20), including NAA 2.02 ppm, Cr 3.05 ppm, choline compounds (Cho) 3.20 ppm and Glu 2.2 ppm.
Statistical analysis
All data were analyzed using SPSS 18.0 software (IBM SPSS, Armonk, NY, USA). Statistical data analysis was performed with the unpaired Student's t-test or analysis of variance (ANOVA). Considering that the acquisition trials of neurological deficit scores and MWM test were carried out on 4 time points, repeated-measures ANOVA was performed. All data were presented as mean ± standard deviation (SD). P<0.05 was considered to indicate a statistically significant difference.
Results
EA attenuates neurological deficits and infarct volume in rats with I/R injury
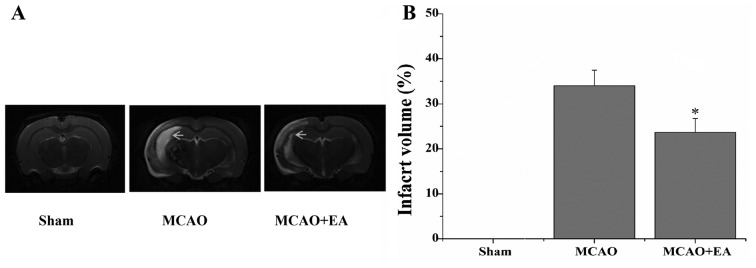
In the present study, the neurological scores were used to evaluate whether EA at the GV 20 and DU 24 acupoints can improve neurological function at 24 h, 3, 5 and 7 days after I/R injury. The rats in the sham group did not display any signs of neurological deficits. However, the rats in the MCAO and the MCAO + EA groups exhibited different degrees of neurological deficits (Fig. 1). At 24 h and 3 days following I/R injury, the difference in the neurological scores between the MCAO and the MCAO + EA groups was not statistically significant (P>0.05). However, at 7 days after I/R injury, the neurological score of the MCAO + EA group was significantly reduced compared with the MCAO group (P<0.01). The infarct volume was measured using T2WI, and the infarct region displayed high signal intensity. The infarct volume in the MCAO + EA group was significantly lower compared with the MCAO group (P<0.05; Fig. 2).
Figure 1.
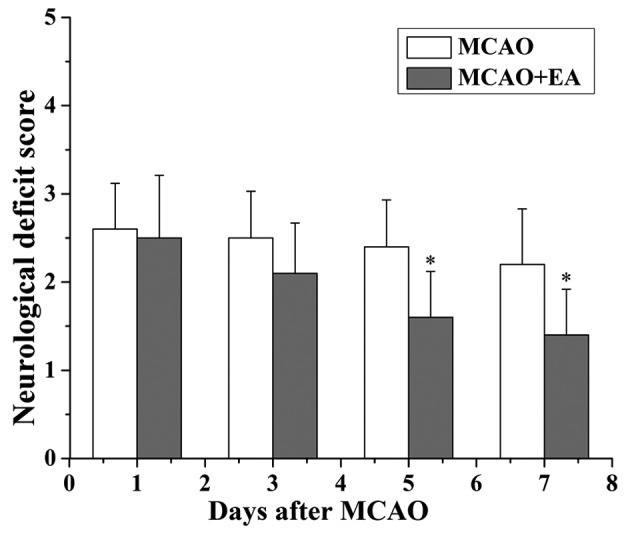
Effect of EA at the DU 20 and DU 24 acupoints on the neurological deficits in rats with cerebral I/R injury. The neurological deficit score was evaluated, and data are shown as the mean ± standard deviation from 12 individual rats. *P<0.05 vs. the MCAO group. I/R, ischemia and reperfusion; MCAO, middle cerebral artery occlusion; EA, electroacupuncture.
Figure 2.
Effect of EA at the DU 20 and DU 24 acupoints on infarct volume in rats with cerebral I/R injury. (A) The cerebral infarct volume was measured by T2-weighted magnetic resonance imaging in the sham, MCAO and MCAO + EA groups. (B) Infarct volume is represented as a percentage of the total brain volume and data are presented as the mean ± standard deviation from 12 individual rats in each group. *P<0.05 vs. the MCAO group. EA, electroacupuncture; I/R, ischemia and reperfusion; MCAO, middle cerebral artery occlusion.
EA improves learning and memory in rats with I/R injury
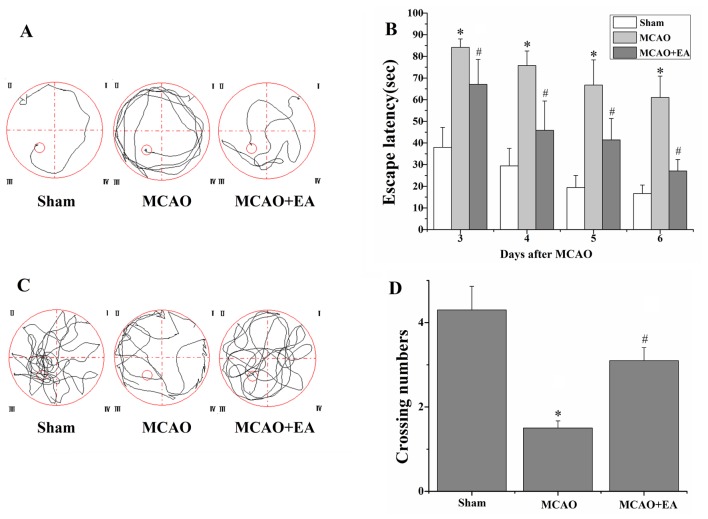
The MWM experiment was performed to evaluate the effects of EA at the GV 20 and DU 24 acupoints on learning and memory in rats with I/R injury. In the place navigation test, the escape latency in rats of the MCAO group was longer compared with the sham group (P<0.01), and that of rats of the MCAO + EA group was shorter compared with the MCAO group (P<0.01; Fig. 3). In addition, in the spatial probe test, the swim time of the rats of the MCAO group in the platform quadrant was significantly lower compared with the sham group (P<0.01). However, the swim time of the rats in the MCAO + EA group was significantly longer compared with the MCAO group (P<0.01; Fig. 3).
Figure 3.
Effect of EA on learning and memory in rats with cerebral I/R injury. The MWM test was applied to evaluate learning and memory ability on days 3-7 following I/R injury. (A) Tracing images from the MWM test in the sham, MCAO and MCAO + EA groups (n=12) on days 3-6. (B) Escape latency for the rats to locate the platform (within 90 sec). (C) Tracing images from the MWM test on day 7. (D) Numbers of rats crossing through the area in which the platform was located. *P<0.05 vs. the sham group and #P<0.05 vs. the MCAO group. EA, electroacupuncture; I/R, ischemia and reperfusion; MWM, Morris water maze; MCAO, middle cerebral artery occlusion.
Effect of EA on NAA/Cr, Cho/Cr and Glu/Cr in HPC and PFC of rats with I/R injury
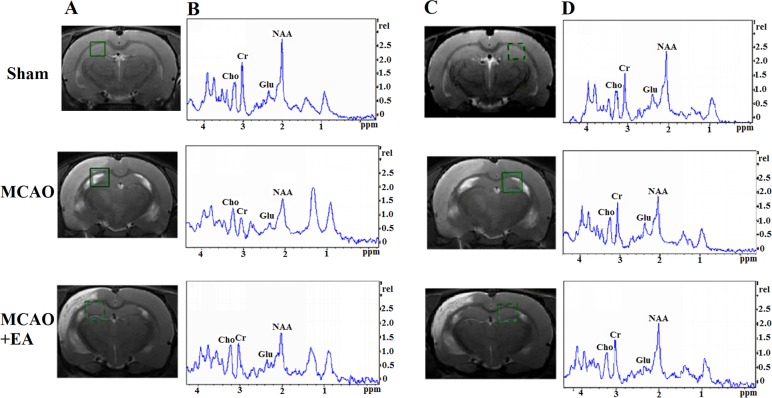
The analysis of neurochemicals in the brain at 7 days after EA treatment: As can be seen in the green box area (Fig. 4, left), T2-weight imaging was measured with shimming and detected for MRS. Compared with the sham group, the ratio of NAA/Cr in the left HPC of the MCAO group was decreased (P<0.05), whereas the Cho/Cr and Glu/Cr ratios did not change significantly (P>0.05). In addition, there was no statistically significant difference in the NAA/Cr, Cho/Cr and Glu/Cr ratios in the right HPC between the sham and the MCAO groups (P>0.05). However, a significant decrease in NAA/Cr and Cho/Cr ratios was observed in the left/right HPC of the MCAO group compared with the sham group (P<0.05); the left/right HPC Glu/Cr ratio did not differ significantly between the MCAO and sham groups (P>0.05; Fig. 4 and Table I).
Figure 4.
Changes in brain metabolites in HPC by MRS in rats with cerebral I/R injury. (A and C) Localization of the left and right HPC VOI on the T2-weighted scan, which is an MRS shimming region. (B and D) 1H-MRS exhibited the NAA peak at 2.02 ppm, the Glu peak at 2.2 ppm, the Cho peak at 3.20 ppm, and the Cr peak at 3.05 ppm. HPC, hippocampus; MRS, magnetic resonance spectroscopy; I/R, ischemia and reperfusion; VOI, volume of interest; NAA, N-acetylaspartate; Glu, glutamate; Cho, choline; Cr, creatine.
Table I.
Metabolite ratios in the right and left HPC regions.
| Laterality | Metabolites | Sham | MCAO | MCAO + EA |
|---|---|---|---|---|
| Left | NAA/Cr | 1.44±0.29 | 1.08±0.25a | 1.31±0.28b |
| Glu/Cr | 0.56±0.07 | 0.56±0.16 | 0.55±0.10 | |
| Cho/Cr | 0.65±0.12 | 0.62±0.10 | 0.67±0.15 | |
| Right | NAA/Cr | 1.39±0.11 | 1.37±0.13 | 1.37±0.20 |
| Glu/Cr | 0.57±0.09 | 0.55±0.17 | 0.58±0.11 | |
| Cho/Cr | 0.70±0.13 | 0.63±0.14 | 0.67±0.09 | |
| Left/right | NAA/Cr | 0.95±0.10 | 0.76±0.12a | 0.84±0.20b |
| Glu/Cr | 0.85±0.13 | 0.87±0.16 | 0.83±0.23 | |
| Cho/Cr | 0.93±0.25 | 0.81±0.10a | 0.90±0.25 |
Metabolite ratios in the right and left HPC regions of the sham, MCAO and MCAO + EA groups, and left/right ratios in each group. Data are presented as mean ± standard deviation (n=12 per group).
P<0.05, MCAO vs. sham;
P<0.05, MCAO vs. MCAO + EA. HPC, hippocampus; NAA, N-acetylaspartate; Cr, creatine; Glu, glutamate; Cho, choline-containing compounds; MCAO, middle cerebral artery occlusion; EA, electroacupuncture.
Compared with the MCAO group, EA reduced the NAA/Cr ratio in the left HPC (P<0.05), whereas the Cho/Cr and Glu/Cr ratios exhibited no significant changes (P>0.05). In the right HPC, the NAA/Cr, Cho/Cr and Glu/Cr ratios exhibited no differences following EA treatment compared with the MCAO group (P>0.05). As regards the ratios of left̸right neurochemicals in HPC after EA treatment, the NAA/Cr ratio in the MCAO + EA group was improved compared with the MCAO group (P<0.05). Although there was no difference in the Cho/Cr ratio between the MCAO and the MCAO + EA groups, Cho/Cr exhibited an increasing tendency following EA treatment. However, the difference in the Glu/Cr ratio between the two groups was not statistically significant (P>0.05; Fig. 4 and Table I).
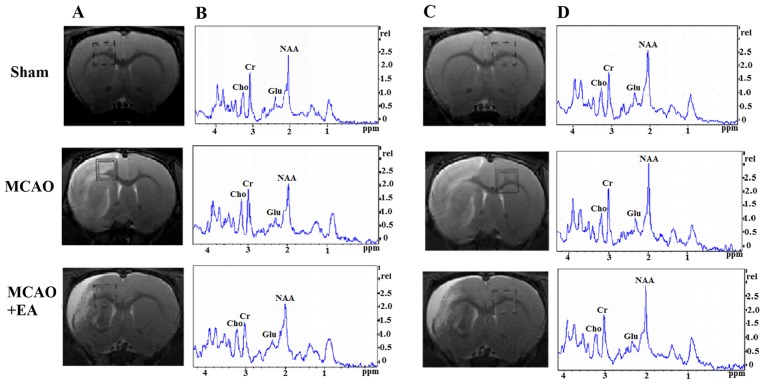
In the MCAO group, the NAA/Cr and Glu/Cr ratios in the left PFC were decreased (P<0.05), whereas the Cho/Cr ratio exhibited no significant changes compared with the sham group (P>0.05). The differences in the NAA/Cr, Cho/Cr and Glu/Cr ratios of the right PFC between the MCAO and the MCAO + EA groups were not statistically significant (P>0.05). However, the NAA/Cr, Cho/Cr and Glu/Cr ratios in left/right PFC were decreased in the MCAO group compared with the sham group (P<0.05; Fig. 5 and Table II).
Figure 5.
Changes in brain metabolites in TPC by MRS detection in rats rats with cerebral I/R injury. (A and C) Localization of the left and right TPC VOI on the T2-weighted scans, which is an MRS shimming region. (B and D) 1H-MRS exhibited the NAA peak at 2.02 ppm, the Glu peak at 2.2 ppm, the Cho peak at 3.20 ppm, and the Cr peak at 3.05 ppm. MRS, magnetic resonance spectroscopy; I/R, ischemia and reperfusion; VOI, volume of interest; NAA, N-acetylaspartate; Glu, glutamate; Cho, choline; Cr, creatine.
Table II.
Metabolite ratios in the right and left TPC regions.
| Laterality | Metabolites | Sham | MCAO | MCAO + EA |
|---|---|---|---|---|
| Left | NAA/Cr | 1.47±0.10 | 0.92±0.15a | 1.27±0.20b |
| Glu/Cr | 0.69±0.10 | 0.37±0.08a | 0.57±0.11b | |
| Cho/Cr | 0.74±0.17 | 0.68±0.18 | 0.70±0.15 | |
| Right | NAA/Cr | 1.45±0.20 | 1.34±0.18 | 1.38±0.19 |
| Glu/Cr | 0.63±0.06 | 0.60±0.11 | 0.57±0.07 | |
| Cho/Cr | 0.77±0.13 | 0.72±0.11 | 0.73±0.20 | |
| Left/right | NAA/Cr | 0.97±0.24 | 0.68±0.17a | 0.84±0.16b |
| Glu/Cr | 0.83±0.28 | 0.69±0.24a | 0.79±0.12b | |
| Cho/Cr | 0.98±0.16 | 0.81±0.11a | 0.89±0.11b |
Metabolite ratios in the right and left TPC regions of the sham, MCAO and MCAO + EA groups, and left/right ratios in each group. Data are presented as mean ± standard deviation (n=12 per group).
P<0.05, MCAO vs. sham;
P<0.05, MCAO vs. MCAO + EA. NAA, N-acetylaspartate; Cr, creatine; Glu, glutamate; Cho, choline-containing compounds; MCAO, middle cerebral artery occlusion; EA, electroacupuncture.
Compared with the MCAO group, the NAA/Cr and Glu/Cr ratios in the PFC of the EA+MCAO group were increased (P<0.05), whereas Cho/Cr exhibited no obvious changes (P>0.05). The NAA/Cr, Cho/Cr and Glu/Cr ratios in the right PFC did not exhibit statistically significant differences (P>0.05). However, the NAA/Cr, Cho/Cr and Glu/Cr ratios in the left/right PFC increased following EA treatment (P<0.05; Fig. 5 and Table II).
Discussion
MRS is a novel technique for detecting brain metabolites in vivo non-invasively and non-radioactively, according to different nuclei and compounds forming different magnetic resonance phenomena and chemical shifts. It was previously confirmed that the HPC and PFC regions play key roles in learning and memory through their specific structure, location and interconnection with other brain regions (21). Thus, in the present study, 1H MRS was used to monitor the neurochemical alterations in the HPC and PFC regions associated with learning and memory changes following cerebral I/R injury.
NAA is a type of specific amino acid, which is mainly present in neurons and axons. NAA also is a neuronal marker and its concentration may be sensitive to the density of neurons. When NAA declines in the brain, nervous functional impairment may develop (22,23). Previous studies demonstrated that the NAA level is positively correlated with learning and memory in different diseases (24,25), whereas a higher ratio of NAA/Cr is accompanied with better Mini-Mental State Examination scores in Alzheimer's disease (26). Moreover, Cho is an important neurotransmitter precursor compound of acetylcholine, which is related to memory, recognition and emotional behavior (27). It has been reported that it is crucial for learning and memory to maintain a stable level of acetylcholine in the frontal cortex and HPC (28).
The results of the present study revealed that the left/right NAA/Cr and Cho/Cr ratios were obviously decreased in HPC as well as PFC at 7 days following cerebral I/R injury accompanied with learning and memory impairment as indicated by the MWM test. These results are consistent with those of other studies reporting that HPC neurochemicals and spatial learning and memory are closely correlated (29). It has been reported that NAA rapidly decreased in the ischemic core within 6 h, and declined to 0 at 7 days after acute cerebral infarction; however, there was no obvious change in the ischemic penumbra within 48 h, after which time it gradually declined to 20–40% (30,31). In the present study, we observed that the NAA ratio of left/right HPC and PFC was decreased to 20–40% at the 7 days following cerebral I/R injury.
EA is an effective novel treatment based on the combination of traditional Chinese acupuncture with modern electrotherapy, exhibiting confirmed clinical efficacy in the treatment of stroke and cognitive impairment (32). In the present study, an MWM test was performed to assess the effect of EA on learning and memory following cerebral I/R injury. The results demonstrated that EA at the GV 20 and DU 24 acupoints improved learning and memory ability, along with ameliorated neurological deficits and reduced cerebral infarct volume, which were consistent with our previous findings (33).
In the HPC and PFC regions, we observed that EA at GV 20 and DU 24 increased the metabolism of left/right NAA and Cho at 7 days after EA treatment. These findings are similar to those of a previous study, which demonstrated that EA at the bilateral Hegu (LI 4) and Taichong (LR 3) acupoints may increase the levels of NAA in the ischemic penumbra and improve the memory scale score (34). In addition, it has been reported that the dynamic changes of Glu in the cerebral cortex are associated with cognitive function. The excessive secretion of extracellular Glu following cerebral ischemia induced excitatory toxic effects or oxidative stress, which may damage neurons and cause cognitive impairment (35). In the present study, it was demonstrated that EA at the GV 20 and DU 24 acupoints increased the left/right Glu level in PFC at 7 days after EA treatment. A possible explanation for this finding is that EA at the GV 20 and DU 24 acupoints may promote Glu-mediated synaptic transmission; this was observed found in PFC but not in HPC, which requires further investigation.
In conclusion, the results of the present study demonstrated that EA at the GV 20 and DU 24 acupoints may alleviate neurological deficits, reduce infarct volume and improve the learning and memory ability following cerebral I/R injury, possibly via enhancing the neurochemical metabolism of NAA and Cho in the HPC and FPC regions.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (grant no. 81403450).
References
- 1.Renjen PN, Gauba C, Chaudhari D. Cognitive impairment after stroke. Cureus. 2015;7:e335. doi: 10.7759/cureus.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JD, Jacova C, Sepehry AA, Pratt B, Benavente OR. A quantitative systematic review of domain-specific cognitive impairment in lacunar stroke. Neurology. 2013;80:315–322. doi: 10.1212/WNL.0b013e31827deb85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Wang X, Zheng Y, Shang G, Huang J, Tao J, Chen L. Electroacupuncture inhibits inflammatory injury by targeting the miR-9-mediated NF-κB signaling pathway following ischemic stroke. Mol Med Rep. 2016;13:1618–1626. doi: 10.3892/mmr.2015.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Guo F, Zhang Q, Huo T, Liu L, Wei H, Xiong L, Wang Q. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/S1 transgenic mice. BMC Complement Altern Med. 2014;14:37. doi: 10.1186/1472-6882-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding X, Li CY, Wang QS, Du FZ, Ke ZW, Peng F, Wang J, Chen L. Patterns in default-mode network connectivity for determining outcomes in cognitive function in acute stroke patients. Neuroscience. 2014;277:637–646. doi: 10.1016/j.neuroscience.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg T, Gal-Ben-Ari S, Dieterich DC, Kreutz MR, Ziv NE, Gundelfinger ED, Rosenblum K. The roles of protein expression in synaptic plasticity and memory consolidation. Front Mol Neurosci. 2014;7:86. doi: 10.3389/fnmol.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Wang S, Hao F, Li Y, Tang H, Shi X. NMR analysis of the rat neurochemical changes induced by middle cerebral artery occlusion. Talanta. 2012;88:136–144. doi: 10.1016/j.talanta.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Mattfeld AT, Stark CEL. Functional contributions and interactions between the human hippocampus and subregions of the striatum during arbitrary associative learning and memory. Hippocampus. 2015;25:900–911. doi: 10.1002/hipo.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi L, Pu J, Xu L, Malaguit J, Zhang J, Chen S. The efficacy and safety of cilostazol for the secondary prevention of ischemic stroke in acute and chronic phases in Asian population - an updated meta-analysis. BMC Neurol. 2014;14:251. doi: 10.1186/s12883-014-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu ZY, Guo H, Zhang XL, Liu J, Qu HY, Peng W, Bao YM, Yin LL, Song YX. Impacts of electroacupuncture on left hippocampus NAA/Cr for patients of Uygur and Han nationality with mild cognitive impairment. Zhongguo Zhen Jiu. 2011;31:773–777. In Chinese. [PubMed] [Google Scholar]
- 12.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 13.Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30:353–363. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Tao J, Xue XH, Chen LD, Yang SL, Jiang SM, Gao YL, Wang XB. Electroacupuncture improves neurological deficits and enhances proliferation and differentiation of endogenous nerve stem cells in rats with focal cerebral ischemia. Neurol Res. 2010;32:198–204. doi: 10.1179/174313209X414506. [DOI] [PubMed] [Google Scholar]
- 16.Lan L, Tao J, Chen A, Xie G, Huang J, Lin J, Peng J, Chen L. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med. 2013;31:75–80. doi: 10.3892/ijmm.2012.1184. [DOI] [PubMed] [Google Scholar]
- 17.Pouzet B, Zhang WN, Feldon J, Rawlins JN. Hippocampal lesioned rats are able to learn a spatial position using non-spatial strategies. Behav Brain Res. 2002;133:279–291. doi: 10.1016/S0166-4328(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 18.Veng LM, Granholm AC, Rose GM. Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats. Physiol Behav. 2003;80:27–36. doi: 10.1016/S0031-9384(03)00219-1. [DOI] [PubMed] [Google Scholar]
- 19.Hui Xi G, Zhang J, Liu Z, Zhang S, Teng X, Chan G, Wu KC, Nie EX, Shan BB, et al. Learning and memory alterations are associated with hippocampal N-acetylaspartate in a rat model of depression as measured by 1H-MRS. PLoS One. 2011;6:e28686. doi: 10.1371/journal.pone.0028686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou IY, Chan RW, Ho LC, Wu EX. Longitudinal metabolic changes in the hippocampus and thalamus of the maternal brain revealed by proton magnetic resonance spectroscopy. Neurosci Lett. 2013;553:170–175. doi: 10.1016/j.neulet.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 21.Milner B, Klein D. Loss of recent memory after bilateral hippocampal lesions: Memory and memories-looking back and looking forward. J Neurol Neurosurg Psychiatry. 2016;87:230. doi: 10.1136/jnnp-2015-311092. [DOI] [PubMed] [Google Scholar]
- 22.Jones RS, Waldman AD. 1H-MRS evaluation of metabolism in Alzheimer's disease and vascular dementia. Neurol Res. 2004;26:488–495. doi: 10.1179/016164104225017640. [DOI] [PubMed] [Google Scholar]
- 23.Bertolino A, Frye M, Callicott JH, Mattay VS, Rakow R, Shelton-Repella J, Post R, Weinberger DR. Neuronal pathology in the hippocampal area of patients with bipolar disorder: A study with proton magnetic resonance spectroscopic imaging. Biol Psychiatry. 2003;53:906–913. doi: 10.1016/S0006-3223(02)01911-X. [DOI] [PubMed] [Google Scholar]
- 24.Jayaweera HK, Lagopoulos J, Duffy SL, Lewis SJ, Hermens DF, Norrie L, Hickie IB, Naismith SL. Spectroscopic markers of memory impairment, symptom severity and age of onset in older people with lifetime depression: Discrete roles of N-acetylaspartate and glutamate. J Affect Disord. 2015;183:31–38. doi: 10.1016/j.jad.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhou IY, Ding AY, Li Q, McAlonan GM, Wu EX. Magnetic resonance spectroscopy reveals N-acetylaspartate reduction in hippocampus and cingulate cortex after fear conditioning. Psychiatry Res. 2012;204:178–183. doi: 10.1016/j.pscychresns.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Penner J, Wells JL, Borrie MJ, Woolmore-Goodwin SM, Bartha R. Reduced N-acetylaspartate to creatine ratio in the posterior cingulate correlates with cognition in Alzheimer's disease following four months of rivastigmine treatment. Dement Geriatr Cogn Disord. 2015;39:68–80. doi: 10.1159/000367685. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Wang X. Correlation of iron deposition and change of gliocyte metabolism in the basal ganglia region evaluated using magnetic resonance imaging techniques: An in vivo study. Arch Med Sci. 2016;12:163–171. doi: 10.5114/aoms.2016.57593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoiljkovic M, Leventhal L, Chen A, Chen T, Driscoll R, Flood D, Hodgdon H, Hurst R, Nagy D, Piser T, et al. Concentration-response relationship of the α7 nicotinic acetyl-choline receptor agonist FRM-17874 across multiple in vitro and in vivo assays. Biochem Pharmacol. 2015;97:576–589. doi: 10.1016/j.bcp.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell KK, Goggin SL, Tyler CR, Allan AM. Prenatal alcohol exposure is associated with altered subcellular distribution of glucocorticoid and mineralocorticoid receptors in the adolescent mouse hippocampal formation. Alcohol Clin Exp Res. 2014;38:392–400. doi: 10.1111/acer.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: A literature review of animal research on brain ischaemia. J Neurochem. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- 31.Aoki Y, Inokuchi R, Suwa H. Reduced N-acetylaspartate in the hippocampus in patients with fibromyalgia: A meta-analysis. Psychiatry Res. 2013;213:242–248. doi: 10.1016/j.pscychresns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Lin R, Wu Y, Tao J, Chen B, Chen J, Zhao C, Yu K, Li X, Chen LD. Electroacupuncture improves cognitive function through Rho GTPases and enhances dendritic spine plasticity in rats with cerebral ischemia-reperfusion. Mol Med Rep. 2016;13:2655–2660. doi: 10.3892/mmr.2016.4870. [DOI] [PubMed] [Google Scholar]
- 33.Lin R, Lin Y, Tao J, Chen B, Yu K, Chen J, Li X, Chen LD. Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Mol Med Rep. 2015;12:6807–6814. doi: 10.3892/mmr.2015.4321. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Shen Y. Magnetic resonance spectroscopic study of memory impairment after cerebral infarction treated with electroacupuncture. Zhongguo Zhen Jiu. 2015;35:657–660. In Chinese. [PubMed] [Google Scholar]
- 35.Zhao L, Zhang H, Zheng Z, Huang J. Electroacupuncture on the head points for improving gnosia in patients with vascular dementia. J Tradit Chin Med. 2009;29:29–34. doi: 10.1016/S0254-6272(09)60027-3. [DOI] [PubMed] [Google Scholar]