Abstract
Pulmonary fibrosis (PF) is a chronic lung disease. The transforming growth factor-β1 (TGF-β1)/Smad3 signaling pathway plays an important role in the pathogenesis of pulmonary fibrosis. Bone marrow-derived mesenchymal stem cells (BMSCs) have been shown to be a modulator of the molecular aspects of the fibrosis pathway. However, it is still unknown as to whether the conditioned medium from BMSCs (BMSCs-CM) inhibits the epithelial-mesenchymal transition (EMT) process. This study confirmed the hypothesis that BMSCs-CM exerts an anti-fibrotic effect on human type II alveolar epithelial cells (A549) by suppressing the phosphorylation of Smad3. We used the A549 cells in vitro to detect morphological evidence of EMT by phase-contrast microscopy. These cells were randomly divided into 4 groups as follows: the control group, the TGF-β1 group, the SIS3 (specific inhibitor of Smad3) group and the BMSCs-CM group. The immunofluorescence method was used to determined the location of E-cadherin (E-calcium mucins; E-cad), α-smooth muscle actin (α-SMA) and p-Smad3. The expression levels of E-cad, CK8, α-SMA, vimentin, p-Smad3, Snail1, collagen I (COLI) and collagen III (COLIII) were detected by western blot analysis. Following exposure to TGF-β1, the A549 cells displayed a spindle-shaped fibroblast-like morphology. In accordance with these morphological changes, the expression levels of E-cad and CK8 were downregulated, while the expression levels of α-SMA and vimentin were upregulated. Along with this process, the expression levels of p-Smad3, Snail1, COLI and COLIII were increased. However, the cells in the BMSCs-CM group and SIS3 group exhibited a decrease in the levels of α-SMA and vimentin (which had been upregulated by TGF-β1), and an increase in the levels of E-cad and CK8 expression (which had been downregulated by TGF-β1). On the whole, these results indicated that BMSCs-CM suppressed the EMT which might be associated with TGF-β1/Smad3. This study provides the theoretical basis for the research of the mechanisms responsible for pulmonary disease.
Keywords: bone marrow-derived mesenchymal stem cells, conditioned medium, epithelial-mesenchymal transition, type II alveolar epithelial cells, A549
Introduction
Pulmonary fibrosis (PF) is a chronic, progressive, irreversible and lethal pulmonary disease, and currently, no effective treatments are available (1,2). There is evidence to indicate that epithelial-mesenchymal transition (EMT) is closely associated with the process of fibrosis (3). EMT involves profound phenotypic changes, including the loss of cell-cell adhesion and cell polarity, and endows cells with a migratory ability and involves the synthesis of the extracellular matrix (4).
Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine that regulates a wide range of biological phenomena, including tissue morphogenesis, differentiation and extracellular matrix remodeling (5). TGF-β1 can effectively induce the conversion of alveolar type II epithelial cells into mesenchymal cells. TGF-β1-mediated EMT in several cell types is primarily dependent on the canonical TGF-β1/Smad signaling pathway (6,7). Smad proteins are the key molecules in the process of TGF-β1 signal transduction, and among these, Smad3 is a main substrate of TGF-β1 type I receptor kinase and mediates signal transduction in the cell biology (8,9).
Bone marrow-derived mesenchymal stem cells (BMSCs) are pluripotent stem cells derived from bone marrow, with the potential of self-renewal and differentiation into various cell types, such as adipocytes, cardiomyocytes (10), endotheliocytes and neurons (11). BMSCs can perceive damage signals and migrate into the damaged area. BMSCs also have a stronger immune regulatory function, and can avoid immune rejection when in allografts (12). Moreover, it has been demonstrated that BMSCs have anti-inflammatory and regenerative properties (13). In addition, certain studies have indicated that BMSCs can modify the tissue microenvironment and repair the damaged cells via secreting soluable factors. Such soluble factors secreted from BMSCs play a considerable role in terms of the regulation of the immune system, and are associated with anti-inflammatory and anti-fibrotic effects (14–16). Since the transplantation of BMSCs is associated with poor differentiation and survival rates, the utilization of conditioned medium (CM) including trophic factors from BMSCs has emerged as an alternative method (17,18). In a previous study, we demonstrated that BMSCs exhibit anti-fibrotic properties by alleviating the pathological state of silicosis, reducing the inflammatory reaction and suppressing the deposition of collagen. In addition, we also confirmed that BMSCs in lung tissues of rats with silicosis inhibited the expression of tumor necrosis factor (TNF)-α and TGF-β, and promoted the restoration of lung injury via interleukin-1 receptor antagonist (IL-1RA) (19). However, whether BMSCs-CM can mediate the EMT process, as well as the mechanism involved in this process remain unknown.
In this study, to explore the effect of BMSCs-CM on the development of EMT, we used A549 cells to confirm whether BMSCs-CM can suppress the EMT process. Additionally, to elucidate the possible molecular mechanisms responsible for the protective effect of BMSCs-CM, we examined the levels of E-cadherin (E-calcium mucins; E-cad), CK8, α-smooth muscle actin (α-SMA), vimentin, p-Smad3, Snail1 and collagen I (COLI) and collagen III (COLIII). This study provides some major theoretical and experimental fundamental principles for BMSCs-CM as a novel therapeutic agent.
Materials and methods
Culture of BMSCs
All experiments using rats were performed in compliance with the guidelines of the national Institutes of Health Guide for Care and use of Laboratory Animals. The experimental procedures were approved by the north China university of Science and Technology Experimental Ethics Committee. BMSCs were generated from male Sprague-Dawley (SD) rats (n=15; 3–6 weeks old; weighing 100–120 g; obtained from Beijing Weitong Lihua, Beijing, China). Rats were anesthetized with an intraperitoneal injection of chloral hydrate (350–400 mg/kg) and were then sacrificed by cervical dislocation. Fresh bone marrow cells were collected by flushing the medullary cavity of rat femurs with Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Carlsbad, CA, USA). After filtering, the cells were centrifuged at 1,000 rpm for 5 min. Purified cells were dispersed into cell culture flasks (Corning Life Sciences, Tewksbury, MA, USA), grown in DMEM supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and then cultured at 37°C with 5% CO2. After 48 h, non-adherent cells were removed and fresh medium was added. The medium was replaced every 3 days. The adhered cells were allowed to grow to approximately 90% confluency, and then trypsinized and reseeded. Passage 3 (P3) BMSCs were used for this experiment.
Preparation of BMSCs-CM
After the 3rd generation of BMSCs had adhered to the wall of the flasks for 24 h, they were washed with phosphate-buffered saline (PBS) twice. The cells were then cultured with serum-free DMEM, and the supernatant was collected after 48 h (cells had reached a confluence of 80%). The collected supernatant was then centrifuged at 1,000 rpm for 10 min. After the supernatants were re-centrifuged at 3,000 rpm for 5 min, the CM was collected with a clean filter to filter to eliminate any bacteria, and then used in the later experiments.
Culture of A549 cells
The A549 cells were obtained from the Chinese Academy of Science Cell Library (TCHu 150, Shanghai, China), and were cultured in DMEM supplemented with 10% FBS (both from Gibco-BRL) and 1% antibiotics (penicillin-streptomycin; Sigma-Aldrich) in a humidified 5% CO2 at 37°C. The A549 cells were serum-starved for 24 h, and then stimulated with 5 ng/ml TGF-β1 (Peprotech, Rocky Hill, NJ, USA) for 48 h.
Experiment groups
There were 4 experimental groups as follows: i) the control group, in which cells were cultured in serum-free DMEM; ii) the TGF-β1 stimulation group (TGF-β1 group), in which the cells were cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; iii) the SIS3 inhibitor group (SIS3 group), in which the cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure; iv) the BMSCs-CM group (BMSCs-CM group), in which the BMSCs-CM was added prior to 5 ng/ml TGF-β1 exposure. Following incubation for 48 h, the morphology of the cells undergoing EMT was observed under phase-contrast microscope (CKX41; Olympus, Tokyo, Japan).
Observation of cell internal structure by transmission electron microscopy (TEM)
The cells were digested with pancreatin and centrifuged at 1,500 rpm for 5 min at 4°C. The cells were washed with pre-cooled and sterile PBS, centrifuged under the same conditions and the supernatant was discarded. The sediment was fixed with 2.5% glutaraldehyde, and then subjected to electron microscopy by first fixin gin 1% osmic acid, resin embedding and sample preparation, and images were then acquired using a transmission electron microscope (H-7650; Hitachi, Tokyo, Japan).
Transwell migration assay
The migration ability of the cells was evaluated by a Transwell migration chamber assay. The polycarbonate filters (8 μm pore size) were purchased from Corning Life Sciences. The cells (1×104/insert) that were suspended in 100 μl serum-free DMEM were added to the upper chamber, while 600 μl of complete medium were added to the lower chamber at 37°C in a humidified atmosphere with 5% CO2. Following incubation for 24 h, the cells that had passed through the polycarbonate filter were stained with crystal violet and photographed using an inverted phase contrast microscope. Each migration assay was carried out in triplicate and repeated in 3 independent experiments.
Immunofluorescence analysis
The slides of the cells were fixed in 4% paraformaldehyde at room temperature for 48 h, and washed 3 times in PBS. The cell samples were permeabilized with 0.4% Triton X-100 for 30 min at room temperature, then washed with PBS. Following incubation with blocking solution (5% normal goat serum) for 1 h, the cells were incubated at 4°C overnight with 150 μl diluted primary antibody mixture [E-cad (dilution, 1:50; 610181; BD Transduction Laboratories, Franklin Lakes, NJ, USA), α-SMA (dilution, 1:1,000; ab32575), p-Smad3 (dilution, 1:100; ab52903; both from Abcam Ltd., Shanghai, China)]. The following day, after being washed with PBS, secondary antibodies labeled with tetramethyl rhodamine isothiocyanate (TRITC; Abcam, Cambridge, MA, USA) and fluorescein isothiocyanate (FITC; BD Transduction Laboratories) were added to each slide and the slides were incubated at room temperature in a wet dark box for 2 h. Then slides were then incubated with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 10 min. Fluorescent images were obtained using a super resolution laser confocal microscope (Leica TCS SP8 STED 3X; serial no. 8100001247; Wetzlar, Germany), and 5 fields from each slide were randomly selected for observation and imaging.
Western blot analysis
The cells were washed with PBS 3 times. RIPA lysis buffer (Aidlab Biotechnologies Co., Ltd., Beijing, China) was added to cover the cell surface, on ice for 30 min. The cells were scraped from the 6-well plates, and collected in an EP tube. The cell suspension was centrifuged at 12,000 x g for 15 min at 4°C and the supernatant was collected and stored at −80°C. The protein concentration of the samples was determined using the BCA reagent (Solarbio, Beijing, China). Proteins from each sample were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then electrotransferred onto polyvinylidene fluoride (PVDF) nitrocellulose membranes (Roche Diagnostics, Mannheim, Germany). The membranes were then incubated with primary antibodies [CK8 (dilution, 1:200; ab59400; Abcam), E-cad (dilution, 1:3,000; 610181; BD Transduction Laboratories), vimentin (dilution, 1:200; ab92547; Epitomics, Burlingame, CA, USA), α-SMA (dilution, 1:2,000; ab32575), p-Smad3 (dilution, 1:1,000; ab52903), Snail1 (dilution, 1:200; ab53519; Anbobio, Shanghai, China), COLI (dilution, 1:500; sc-59772) and COLIII (dilution, 1:500; sc-271249), β-actin (dilution, 1:500; sc-47778; all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)], overnight at 4°C. The following day, the membranes were incubated with secondary antibodies [goat anti-rabbit IgG (dilution, 1:3,000; SA1021) and goat anti-mouse IgG (dilution, 1:3,000; SA1022); both from Wuhan Boster Biotechnology Ltd., Wuhan, China] for 2 h at 37°C. The immunoreactive bands were visualized by enhanced chemiluminescence reagent (ECL; Bio-rad, Hercules, CA, USA). The blots were scanned by densitometry, and the integrated density of pixels was quantified using Image Quant 5.2 software (Molecular Dynamics, Sunnyvale, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. Data are presented as the means ± standard deviation (SD). Each result represents data from at least 3 independent experiments. One-way ANOVA was used to compare overall difference in various groups. A P-value ≤0.05 was considered to indicate a statistically significant difference.
Results
Morphological observation of BMSCs
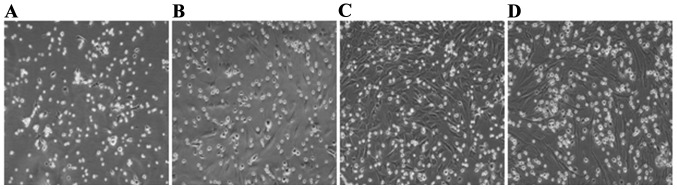
The original generation of BMSCs cells gradually adhered following inoculation for 24 h, and had completely adhered within 48 h. The morphological characteristics of the primary cells were circles and polygons, nuclear-centered and occasional polygonal-shaped cells. Cell proliferated mainly in a clone mode. BMSCs were purified by changing the medium several times and during this process, hematopoietic stem cells and other non-adherent growth cells were removed. After 10 days, primary cells which had reached 95% confluence were passaged. Passaged cells overcame the growth inhibition period and underwent accelerated growth. Following passaging, cell morphology was more uniform and consistent, forming mainly fibroblast-like flattened cells (Fig. 1).
Figure 1.
Morphology of BMSCs as observed under an inverted phase contrast microscope (magnification, ×200) (A) Primary cells. (B) The first passage BMSCs. (C) The second passage BMSCs. (D) The third passage BMSCs. BMSCs, bone marrow-derived mesenchymal stem cells.
Changes in the morphological characteristics of A549 cells
EMT was significantly promoted in the A549 cells following exposure to 5 ng/ml TGF-β1 for 48 h. When the cells were pretreated with the BMSCs-CM, EMT was attenuated, as shown in Fig. 2. First, we investigated the morphological changes in A549 cells following their exposure to TGF-β1 for 48 h. Inverted phase contrast microscopy revealed that the A549 cells has an epithelial cobblestone-like morphology, a round or polygonal shape and exhibited very close cell-cell proximity reminiscent of cellular tight-junctions in the control group, while following exposure to TGF-β1, the cells displayed a spindle-shaped elongated fibroblast-like morphology. These changes became clearer with the passing of time. These morphological changes became noticeably suppressed in the SIS3 group. Furthermore, the cells in the BMSCs-CM group exhibited similar morphological characteristics as those in the SIS3 grou (Fig. 2).
Figure 2.
Cell morphology as observed under an inverted phase contrast microscope (magnification, ×200). The control group included A549 cells with an epithelial cobblestone-like morphology, a round or polygonal shape. The TGF-β1 group included cells that displayed a spindle-shaped elongated fibroblast-like morphology. The cells in the SIS3 group and BMSCs-CM group exhibited a similar morphology; the morphological changes induced by TGF-β1 were suppressed. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived mesenchymal stem cells; CM, conditioned medium; TGF-β1, transforming growth factor-β1.
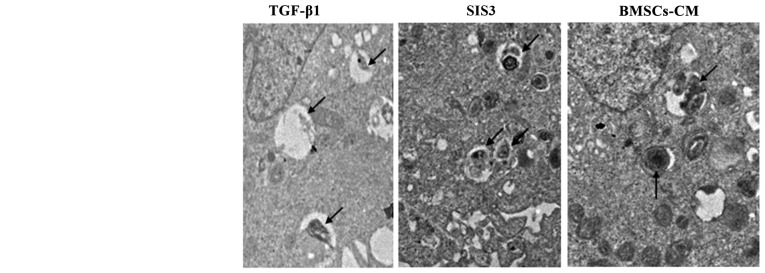
Changes in the lamellar corpuscle in A549 cells
TEM assay indicated that the characteristics of A549 cell osmiophilic multilamellar body had changed, as evidenced by degeneration, swelling and the formatoin of vacuoles following TGF-β1 exposure. However, these phenomena were attenuated in the the SIS3 group and BMSCs-CM group (Fig. 3).
Figure 3.
Ultrastructural evidence of EMT. Normal A549 cells can be identified by the presence of LBs in the cytoplasm. In the TGF-β1 group, LBs were degeneration, swollen and with vacuoles. Arrows indicate LBs; magnification, ×7,000. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specifc inhibitor of Smad3), which was added 4 h prior to 5 ng/ml TGF-β1 exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ml TGF-β1 exposure. Nu, cell nucleus; LB, lamellar body; EMT, epithelial-mesenchymal transition; TGF-β1, transforming growth factor-β1.
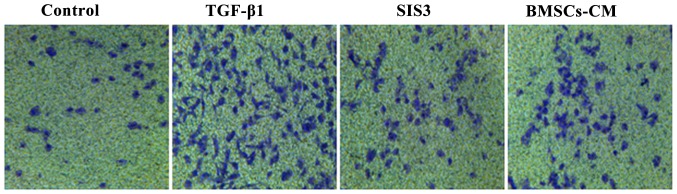
Effects of BMSCs-CM on the migratory ability of A549 cells
As TGF-β1 has been reported to induce EMT (20–22), we used it as an experimental system to evaluate the anti-metastatic potential of BMSCs-CM in A549 cells. The migration assay revealed that the migratory ability of the cells exposed to TGF-β1 was significantly enhanced compared with that of the cells in the control group. However, the TGF-β1-induced enhancement of the cell migratory ability was substantially attenuated in the cells pre-treated with SIS3 or BMSCs-CM compared with the TGF-β1 group. Moreover, the migratory ability of the A549 cells in the BMSCs-CM group exhibited no difference when compared with that of the cells in the SIS3 group (Fig. 4).
Figure 4.
The cell migratory ability observed under an inverted phase contrast microscope during the process of EMT. A549 cells (1×104) were seeded on 8 μm pore size Transwell chambers and then stimulated with 5 ng/ml of TGF-β1 for 48 h, with or without SIS3 and BMSCs-CM. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived mesenchymal stem cells; CM, conditioned medium; EMT, epithelial-mesenchymal transition; TGF-β1, transforming growth factor-β1.
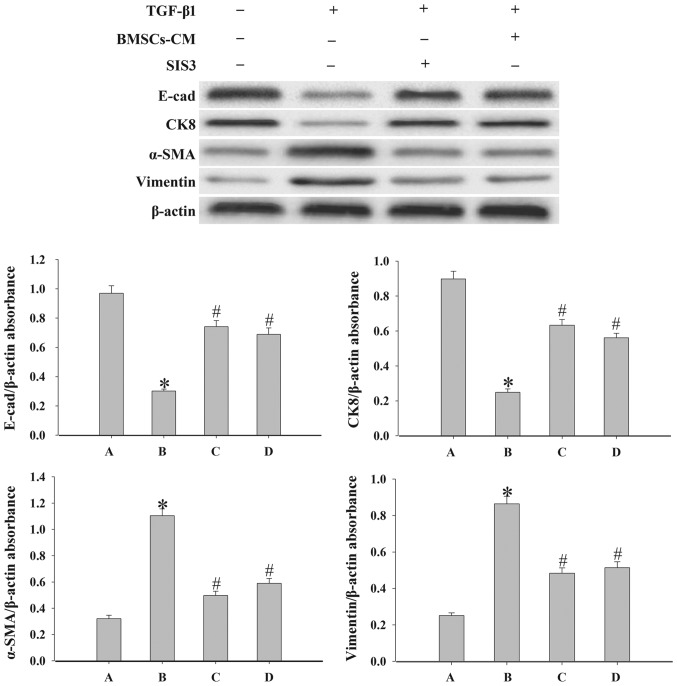
Effects of BMSCs-CM on the expression of epithelial cell markers and mesenchymal cell markers
To demonstrate the effect of BMSCs-CM on the EMT process in A549 cells, we examined the expression levels of the epithelial cell markers, E-cad and CK8, and the mesenchymal cell markers, vimentin and α-SMA, by western blot analysis. As shown in Fig. 5, the results revealed that the expression levels of E-cad and CK8 were significantly decreased, while the expression levels of α-SMA and vimentin were increased following exposure to 5 ng/ml TGF-β1 compared with the control group (P<0.05). By contrast, in the cells in the SIS3 group and the BMSCs-CM group the levels of vimentin and α-SMA were decreased, while those of E-cad and CK8 were increased (P<0.05).
Figure 5.
Evaluation of the epithelial cell markers, E-cad and CK8, and the mesenchymal cell markers, α-SMA and vimentin, expression in A549 cells. Western blot analysis was used to examine the levels of E-cad, CK8, α-SMA and vimentin in the cells. Densitometric analysis of protein expression with β-actin as the control. The results demonstrated that treatment with BMSCs-CM or SIS3 significantly increased the expression of E-cad and CK8, and decreased α-SMA and vimentin protein expression following exposure to TGF-β1 (*P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group). The bars are labeled as follows: A, control group; B, TGF-β1 group; C, SIS3 group; and D, BMSCs-CM group. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived mesenchymal stem cells; CM, conditioned medium; TGF-β1, transforming growth factor-β1; E-cad, E-calcium mucins; α-SMA, α-smooth muscle actin.
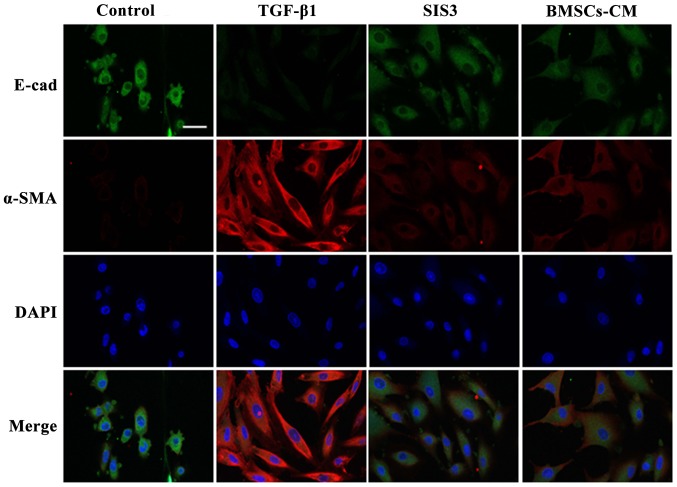
The results from immunofluorescence assay revealed that E-cad fluorescence expression was decreased in the cell membrane in the TGF-β1-exposed cells. By contrast, the expression of E-cad in the cells in the BMSCs-CM group was inhibited to a lesser extent compared with the cells in the TGF-β1 group. On the other hand, α-SMA fluorescence expression was increased and uniformly distributed in the cytoplasm in the TGF-β1-exposed cells. However, the BMSCs-CM reduced the intensity of α-SMA fluorescence expression and attenuated the spindle-like morphological changes. The cells in the SIS3 group exhibited similar expression levels of these markers as those in the BMSCs-CM group. These results strongly suggest that BMSCs-CM promote the expression of E-cad and suppress the expression of α-SMA (Fig. 6).
Figure 6.
Co-localization of E-cad and α-SMA occurs in A549 cells as determined by immunofluorescent staining. E-cad (green) was present in the cytoplasm, FITC-stained; α-SMA (red) was present in the cytoplasm, TRITC-stained. Cell nuclei were counterstained with DAPI (blue). Scale bar, 40 μm. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ ml TGF-β1 exposure. E-cad, E-cadherin (E-calcium mucins); α-SMA, α-smooth muscle actin; TRITC, tetramethyl rhodamine isothiocyanate; FITC, fluorescein isothiocyanate; DAPI, 4′,6-diamidino-2-phenylindole.
BMSCs-CM regulate the EMT process by inhibiting the TGF-β1/Smad3 signaling pathway
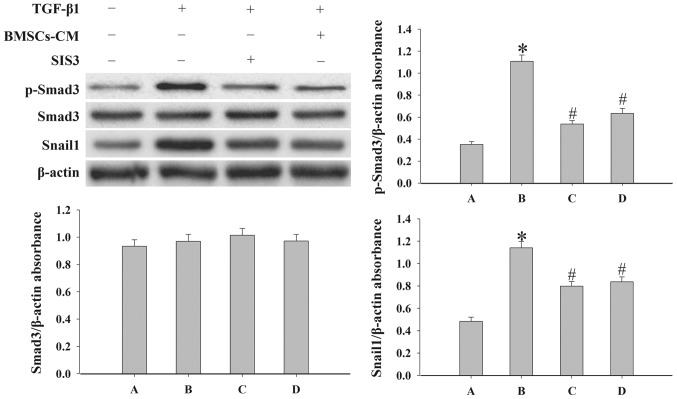
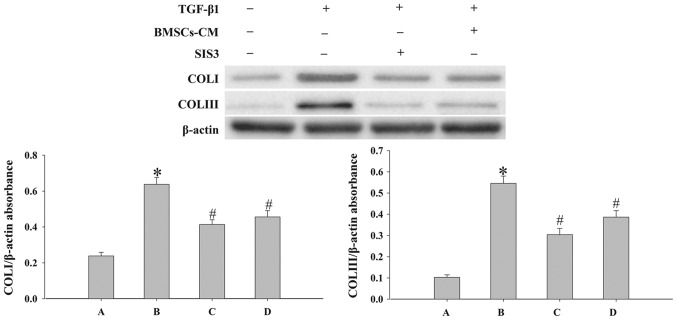
In order to elucidate the mechanisms responsible for the effects of BMSCs-CM on EMT, we examined the expression levels of p-Smad3, Snail1, COLI and COLIII by western blot analysis. SIS3 markedly decreased the expression of p-Smad3 that was induced by TGF-β1 (P<0.05; Fig. 7). However, SIS3 did not affect total Smad3 expression. In addition, the results revealed that BMSCs-CM decreased the expression levels of p-Smad3 and Snail1 (P<0.05; Fig. 7). In addition, the process of EMT induced by TGF-β1 was accompanied by an increased expression of COLI and COLIII (P<0.05; Fig. 8). However, when the cells were pre-treated with SIS3 or BMSCs-CM, the expression levels of COLI, COLIII were decreased (P<0.05).
Figure 7.
Evaluation of the expression levels of p-Smad3, Smad3 and Snail1 in A549 cells. Western blot analysis was used to examined the levels of p-Smad3, Smad3 and Snail1 in the cells. Densitometric analysis of protein expression with β-actin as the control. The results demonstrated that treatment with BMSCs-CM or SIS3 significantly decreased the expression of p-Smad3 and Snail1 following exposure to TGF-β1 (*P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group). The bars are labeled as follows: A, control group; B, TGF-β1 group; C, SIS3 group; and D, BMSCs-CM group. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure. BMSCs-CM, BMSCs-CM was added prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived mesenchymal stem cells; CM, conditioned medium; TGF-β1, transforming growth factor-β1.
Figure 8.
Evaluation of the expression levels of COLI and COLIII in cells. Western blot analysis was used to examine the levels of COLI and COLIII in the cells. Densitometric analysis of protein expression with β-actin as the control. The results demonstrated that treatment with BMSCs-CM or SIS3 significantly decreased the expression of COLI and COLIII following exposure to TGF-β1 (*P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group). The bars are labeled as follows: A, control group; B, TGF-β1 group; C, SIS3 group; and D, BMSCs-CM group. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived mesenchymal stem cells; CM, conditioned medium; TGF-β1, transforming growth factor-beta 1; COLI, collagen I.
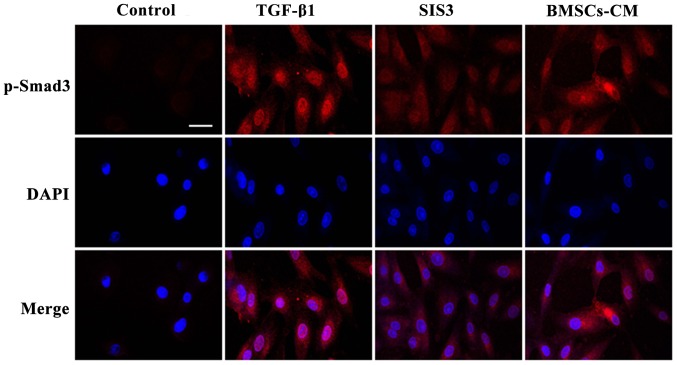
The results from immunofluorescence assay also indicated that the A549 cells had an obvious nuclear expression of p-Smad3 accompanied by a spindle-shaped-like morphology following TGF-β1 exposure. At the same time, the fluorescent expression of p-Smad3 was observed in the cytoplasm. Following pre-treatment with SIS3, the TGF-β1-induced morphological changes were not evident and the fluorescence intensity of p-Smad3 was weakened mainly in the nucleus. Furthermore, no significant difference in fluorescence expressions was observed between the BMSCs-CM group and the SIS3 group (Fig. 9).
Figure 9.
Localization of p-Smad3 occurs in the cells as shown by immunofluorescence. p-Smad3 is shown in red, TRITC-stained; counterstaining of nuclei with DAPI is shown in blue. Scale bar, 50 μm. The cell groups were as follows: control, cells cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1 exposure. TRITC, tetramethylrhodamine isothiocyanate; DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
PF is a chronic, progressive, irreversible and lethal pulmonary disease, with a poor prognosis and complex pathogenesis. At present, the main aim of PF treatment is to relieve symptoms as much as possible. EMT is a biological process through which epithelial cells undergo a phenotypic transition into mesenchymal cells. This process was initially identified in normal tissue development, such as during embryonic development and organogenesis (23). Previous studies have suggested that EMT plays an important role in the formation and development of organ fibrosis (24,25). Following TGF-β1 exposure in vitro, the morphology of pleural mesothelial cells (MET-5A) has been shown to change from a flat paved stone-like appearance into a spindle-shaped appearance, and this is accompanied by a high α-SMA expression and decreased E-cad expression (26). Individual cell motility has been shown to be enhanced in this process, which is a feature of TGF-β1-induced EMT (27). In this study, we demonstrated that polygonal A549 cells acquired a spindle-shaped morphology following exposure to TGF-β1 for 48 h. Immunofluorescence analysis revealed that the expression of α-SMA-positive filaments was increased and the levels of E-cad protein in the cytoplasm were decreased following exposure to TGF-β1. Similar results were observed by western blot analysis. Taken together, all these results suggest that TGF-β1 successfully induced A549 cell transformation into myofibroblasts.
TGF-β1 can be secreted by macrophages, lung epithelial cells and fibroblasts, and is one of the most potent EMT mediators (28–30). The present study demonstrated that TGF-β1-mediated EMT was primarily dependent on canonical TGF-β1/Smad signaling; and canonical TGF-β1 signaling has been demonstrated in several cell types (31,32). The signaling of Smad3 is modulated via phosphorylation and cytosol-nucleus translocation (33). Smad3 regulates the lung phenotype in pulmonary fibrosis, and the inhibition of p-Smad3 can reduce TGF-β1-induced EMT and fibrosis (34,35). A number of studies have reported that Smad2/3 are involved in the EMT process (36–39). For example, in hepatic fibrosis, a number of fibrogenic genes (collagens) and markers (α-SMA and E-cad) are Smad3-dependent, and the deletion of Smad3 inhibits COLI expression and blocks EMT (40). In addition, increased p-Smad2/3 expression was detected during EMT in retinal pigment epithelium cells, which indicated an association between increased p-Smad2/3 expression and the EMT process (41). Our experimental results revealed that the expression of p-Smad3 was elevated following exposure to TGF-β1. This phenomenon was accompanied by a reduction in the levels of epithelial cell markers, while the levels of mesenchymal cell markers and COLI, COLIII were increased. This further supports a role for p-Smad3 in the regulation of EMT.
Snail has been known as an essential regulator in TGF-β1-induced EMT (42). TGF-β1 has been shown to induce Snai1 expression either through a Smad3-mediated mechanism or through interaction with other signaling pathways, including Ras, Wnt and Notch (43,44). In this study, the resutls of western blot analysis indicated that the expression levels of E-cad were increased, while those of Snail1, α-SMA were decreased significantly in the SIS3 group and the BMSCs-CM group. This further confirmed that p-Smad3 and Snail1 play an important role in the EMT process.
Studies in vivo have demonstrated the anti-fibrotic properties of BMSCs in the liver (45), kidneys (46) and heart (47), and the application of BMSCs in the treatment of pulmonary fibrosis has gained increasing attention. In vitro, BMSCs can inhibit collagen synthesis and suppress the transdifferentiation of hepatocytes into myofibroblasts (48). Our group has also shown that BMSCs can inhibit silica-induced pulmonary fibrosis and collagen synthesis in vivo (49). However, whether BMSCs-CM is capable of regulating EMT by regulating the TGF-β1/p-Smad3 signaling pathways has not yet been reported, at least to the best of our knowledge. Our present results revealed that treatment of the cells with BMSCs-CM prior to TGF-β1 exposure, downregulated the expression of p-Smad3, Snail1, α-SMA and vimentin, and upregulated the expression levels of E-cad and CK8. This confirms that BMSCs-CM suppresses TGF- β1-induced EMT via the modulation of p-Smad3, Snail1, COLI and COLIII expression. On the other hand, the migratory ability of the A549 cells was weakened. Similar findings were observed in cells pre-treated with SIS3. Taken together, our results suggest that BMSCs-CM inhibits EMT by modulating p-Smad3 and Snail1 expression, thus suppressing pulmonary fibrosis.
In conclusion, the results of this study demonstrate that TGF-β1/Smad3 signaling is a key pathway in TGF-β1-induced EMT. Furthermore, BMSCs-CM can prevent the EMT process, and ameliorate pulmonary fibrosis, which may provided a novel multitarget treatment agent for pulmonary fibrosis.
Acknowledgments
This study was supported by grants from the Science and Technology Support Key Funding Project of Hebei Province (no. 09276191D); Hebei Province Occupational Disease Prevention and Control research (no. 13277709D); the Natural Science Foundation of Hebei Province (grant no. H2015209309).
Abbreviations
- PF
pulmonary fibrosis
- TGF-β1
transforming growth factor-β1
- BMSCs
bone marrow-derived mesenchymal stem cells
- EMT
epithelial-mesenchymal transition
- E-cad
E-cadherin (E-calcium mucin)
- α-SMA
α-smooth muscle actin
- CM
conditioned medium
- COLI
collagen I, COLIII, collagen III
- IL-1RA
interleukin-1 receptor antagonist
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- TEM
transmission electron microscopy
- PBS
phosphate-buffered saline
- TRITC
tetramethyl rhodamine isothiocyanate
- FITC
fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
- PVDF
polyvinylidene fluoride
- ECL
chemiluminescence
References
- 1.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 2.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. doi: 10.1016/S0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 5.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: The master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 6.Pirozzi G, Tirino V, Camerlingo R, Franco R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N, Rocco G. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS On. 2011;6:e21548. doi: 10.1371/journal.pone.0021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang G, Liang Y, Zheng T, Song R, Wang J, Shi H, Sun B, Xie C, Li Y, Han J, et al. FCN2 inhibits epithelial-mesenchymal transition-induced metastasis of hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett. 2016;378:80–86. doi: 10.1016/j.canlet.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Wang C, Huang Q, Wu D, Cao J, Xu X, Yang C, Li X. Caveolin-1 plays an important role in the differentiation of bone marrow-derived mesenchymal stem cells into cardiomyocytes. Cardiology. 2017;136:40–48. doi: 10.1159/000446869. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 12.Mezey E. The therapeutic potential of bone marrow-derived stromal cells. J Cell Biochem. 2011;112:2683–2687. doi: 10.1002/jcb.23216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 14.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G, Traggiai E, et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci USA. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suganuma S, Tada K, Hayashi K, Takeuchi A, Sugimoto N, Ikeda K, Tsuchiya H. Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration. J Orthop Sci. 2013;18:145–151. doi: 10.1007/s00776-012-0306-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS On. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Luo Q, Sun J, Song G. Conditioned medium from mesenchymal stem cells enhances the migration of hepatoma cells through CXCR4 upregulation and F-actin remodeling. Biotechnol Lett. 2015;37:511–521. doi: 10.1007/s10529-014-1710-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhao MM, Cui JZ, Cui Y, Li R, Tian YX, Song SX, Zhang J, Gao JL. Therapeutic effect of exogenous bone marrow derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Mol Med Rep. 2013;8:741–746. doi: 10.3892/mmr.2013.1580. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe-Takano H, Takano K, Hatano M, Tokuhisa T, Endo T. DA-Raf-mediated suppression of the Ras-ERK pathway is essential for TGF-β1-induced epithelial-mesenchymal transition in alveolar epithelial type 2 cells. PloS On. 2015;10:e0127888. doi: 10.1371/journal.pone.0127888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM, Tang MJ. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP. Epithelial-mesenchymal transitions in cancer onset and progression. Bull Acad Natl Med. 2009;193:1969–1979. In French. [PubMed] [Google Scholar]
- 24.Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347:103–116. doi: 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 25.Hosper NA, van den Berg PP, de Rond S, Popa ER, Wilmer MJ, Masereeuw R, Bank RA. Epithelial-to-mesenchymal transition in fibrosis: Collagen type I expression is highly upregulated after EMT, but does not contribute to collagen deposition. Exp Cell Res. 2013;319:3000–3009. doi: 10.1016/j.yexcr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Wettstein G, Bellaye PS, Kolb M, Hammann A, Crestani B, Soler P, Marchal-Somme J, Hazoume A, Gauldie J, Gunther A, et al. Inhibition of HSP27 blocks fibrosis development and EMT features by promoting Snail degradation. FASEB J. 2013;27:1549–1560. doi: 10.1096/fj.12-220053. [DOI] [PubMed] [Google Scholar]
- 27.Perez RE, Navarro A, Rezaiekhaligh MH, Mabry SM, Ekekezie II. TRIP-1 regulates TGF-β1-induced epithelialmesenchymal transition of human lung epithelial cell line A549. Am J Physiol Lung Cell Mol Physiol. 2011;300:L799–L807. doi: 10.1152/ajplung.00350.2010. [DOI] [PubMed] [Google Scholar]
- 28.He X, Young SH, Schwegler-Berry D, Chisholm WP, Fernback JE, Ma Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem Res Toxicol. 2011;24:2237–2248. doi: 10.1021/tx200351d. [DOI] [PubMed] [Google Scholar]
- 29.Aschner Y, Downey GP. Transforming growth factor-β: Master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54:647–655. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamminen JA, Myllärniemi M, Hyytiäinen M, Keski-Oja J, Koli K. Asbestos exposure induces alveolar epithelial cell plasticity through MAPK/Erk signaling. J Cell Biochem. 2012;113:2234–2247. doi: 10.1002/jcb.24094. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes-Reyes EM, Ramos IN, Tavera-Garcia MA, Ramos KS. The aryl hydrocarbon receptor agonist benzo(a)pyrene reactivates LINE-1 in HepG2 cells through canonical TGF-β1 signaling: Implications in hepatocellular carcinogenesis. Am J Cancer Res. 2016;6:1066–1077. [PMC free article] [PubMed] [Google Scholar]
- 33.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Song X, Li Y, Han F, Gao S, Wang X, Xie S, Lv C. Low-dose paclitaxel ameliorates pulmonary fibrosis by suppressing TGF-β1/Smad3 pathway via miR-140 upregulation. PLoS One. 2013;8:e70725. doi: 10.1371/journal.pone.0070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L, Zhou D, Xiong J, You J, Zeng Y, Peng L. Paraquat induce pulmonary epithelial-mesenchymal transition through transforming growth factor-β1-dependent mechanism. Exp Toxicol Pathol. 2016;68:69–76. doi: 10.1016/j.etp.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Wang Y, Yan R, Li S, Shi M, Xiao Y, Guo B. Oxymatrine inhibits renal tubular EMT induced by high glucose via upregulation of SnoN and inhibition of TGF-β1/Smad signaling pathway. PLoS On. 2016;11:e0151986. doi: 10.1371/journal.pone.0151986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Z, Tai W, Lei W, Wang Y, Li Z, Zhang T. IL-27 inhibits the TGF-β1-induced epithelial-mesenchymal transition in alveolar epithelial cells. BMC Cell Biol. 2016;17:7. doi: 10.1186/s12860-016-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Cheng X, Gao Y, Zhang C, Bao J, Guan H, Yu H, Lu R, Xu Q, Sun Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via downregulation of the TGF-β/Smad2/3 signaling pathway. Exp Cell Res. 2016;341:157–165. doi: 10.1016/j.yexcr.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Kong D, Zhang F, Shao J, Wu L, Zhang X, Chen L, Lu Y, Zheng S. Curcumin inhibits cobalt chloride-induced epithelial- to-mesenchymal transition associated with interference with TGF-β/Smad signaling in hepatocytes. Lab Invest. 2015;95:1234–1245. doi: 10.1038/labinvest.2015.107. [DOI] [PubMed] [Google Scholar]
- 40.Masszi A, Kapus A. Smaddening complexity: The role of Smad3 in epithelial-myofibroblast transition. Cells Tissues Organs. 2011;193:41–52. doi: 10.1159/000320180. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Mei Y, Lei H, Tian R, Ni N, Han F, Gan S, Sun S. LYTAK1, a TAK1 inhibitor, suppresses proliferation and epithelial mesenchymal transition in retinal pigment epithelium cells. Mol Med Rep. 2016;14:145–150. doi: 10.3892/mmr.2016.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith KA, Zhou B, Avdulov S, Benyumov A, Peterson M, Liu Y, Okon A, Hergert P, Braziunas J, Wagner CR, et al. Transforming growth factor-1 induced epithelial mesenchymal transition is blocked by a chemical antagonist of translation factor eIF4E. Sci Rep. 2015;5:18233. doi: 10.1038/srep18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zavadil J, Cermak L, Soto-Nieves N, Böttinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZC, Yang S, Huang JJ, Chen SL, Li QQ, Li Y. Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats. Asian Pac J Trop Med. 2014;7:321–324. doi: 10.1016/S1995-7645(14)60048-1. [DOI] [PubMed] [Google Scholar]
- 46.Lang H, Dai C. Effects of bone marrow mesenchymal stem cells on plasminogen activator inhibitor-1 and renal fibrosis in rats with diabetic nephropathy. Arch Med Res. 2016;47:71–77. doi: 10.1016/j.arcmed.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Cai B, Tan X, Zhang Y, Li X, Wang X, Zhu J, Wang Y, Yang F, Wang B, Liu Y, et al. Mesenchymal stem cells and cardiomyocytes interplay to prevent myocardial hypertrophy. Stem Cells Transl Med. 2015;4:1425–1435. doi: 10.5966/sctm.2015-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang YO, Kim MY, Cho MY, Baik SK, Cho YZ, Kwon SO. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol. 2014;14:198. doi: 10.1186/s12876-014-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao MM, Cui JZ, Li R, Du LJ, Tian YX, Kan Q, Zhang J, Gao JL. Effects of bone mesenchymal stem cell transplantation on silicosis fibrosis in rats. Chin J Pathophysiol. 2012;28:2205–2210. [Google Scholar]