Abstract
The superior colliculus is one of the most well-studied structures in the brain, and with each new report, its proposed role in behavior seems to increase in complexity. Forty years of evidence show that the colliculus is critical for reorienting an organism toward objects of interest. In monkeys, this involves saccadic eye movements. Recent work in the monkey colliculus and in the homologous optic tectum of the bird extends our understanding of the role of the colliculus in higher mental functions, such as attention and decision making. In this review, we highlight some of these recent results, as well as those capitalizing on circuit-based methodologies using transgenic mice models, to understand the contribution of the colliculus to attention and decision making. The wealth of information we have about the colliculus, together with new tools, provides a unique opportunity to obtain a detailed accounting of the neurons, circuits, and computations that underlie complex behavior.
Keywords: attention, decision making, saccades, orienting, population coding, normalization, movement, vision
1. INTRODUCTION AND OVERVIEW
The superior colliculus (colliculus) is one of the most well-studied structures in the brain, and with each new experimental report, its proposed role in behavior seems to increase in complexity. The original electrophysiological recordings from single neurons in the colliculus of alert monkeys in the early 1970s showed that collicular neurons discharged in relationship to the generation of saccadic eye movements, in addition to responding to visual stimuli. This groundbreaking result ushered in a generation of studies focused on understanding the role of the colliculus in the initiation and dynamics of saccadic eye movements, as well as the place of the colliculus in systems engineering–based models of saccadic eye movement control. Forty years of evidence leaves no doubt that the colliculus is a critical node in the network responsible for reorienting an organism toward objects of interest. One could easily ask, what more can be learned by studying the colliculus? This high level of understanding, however, is precisely the reason for continuing to study it. The colliculus is one of the few brain areas for which we have a detailed accounting of inputs and outputs, as well as of neuronal cell types based on morphology and neurotransmitter profiles and of neuronal response properties and their association with behavior. We now know that the colliculus also plays some role in higher processes, such as target selection, attention, and decision making. As the sophistication of behavioral and statistical methods increases, we are well poised to solve key questions about the relationship between the activity of single neurons and higher mental function, and given the knowledge we have about the colliculus, it is an ideal place to make these links. With technical advances in electrophysiological methods, particularly those allowing for recording of multiple neurons simultaneously and optical imaging approaches, we can begin to answer questions regarding how populations of neurons work together to give rise to higher mental function, such as choosing from among multiple target options. Finally, the development of transgenic mouse models and molecular genetic techniques will allow us to dissect neuronal cell-specific circuits underlying these behaviors. The knowledge gained from these approaches will link with the knowledge gained from traditional techniques to provide a circuit-based understanding of collicular mechanisms underlying higher mental function, such as attention and decision making.
In what follows, we briefly review the organization of the superior colliculus and discuss the recent results showing its role in behavior that extends beyond simple orienting to include attention and decision making. We then discuss findings from experiments in which the microcircuits of the colliculus have been explored in an effort to reveal how this structure mediates behaviors such as orienting and attention. We conclude by highlighting some of the remaining key questions.
2. BASIC ANATOMY OF THE SUPERIOR COLLICULUS, A LAYERED STRUCTURE
The colliculus is a layered structure found at the roof (tectum) of the midbrain. The layers of the colliculus are commonly organized into two divisions, a dorsally located visuosensory division and a ventrally located motor division. This dichotomy holds for all vertebrates explored to date, with the dorsal division receiving retinal input and the ventral division controlling orienting movements. In monkeys, the most well-studied orienting movement is the quick movement of the eyes to an object of interest—a saccade. Species with less-sophisticated oculomotor behaviors still make orienting movements and have well-developed superior colliculi (mammals) or optic tecti (nonmammalian vertebrates) that direct these movements. The motor layers of the colliculus also receive multisensory information, including auditory and somatosensory signals.
The pattern of collicular layering appears similar in all mammals that have been examined. Figure 1 shows a schematic of the location of the colliculus in the brain of a monkey and of a mouse along with a schematic of an axial section revealing the layers. Immediately under the pia mater is the stratum zonale (SZ), a thin, neuronal cell-sparse layer, and below the SZ is the stratum griseum superficiale (SGS). Below the SGS is the stratum opticum (SO), which contains scattered neurons but is mostly made up of retinal afferent fibers. Below the SO is the cellular stratum griseum intermediale (SGI). The next deepest layers are termed stratum album intermediale (SAI), stratum griseum profundum (SGP), and stratum album profundum (SAP). The fibers of SAP demarcate the colliculus from the underlying periaqueductal gray matter. SGS and SGI are commonly subdivided into sublaminae, and the size and complexity of these layers show species-specific differences (May 2006). This schema is commonly used in anatomical studies, with some species differences beyond the scope of this review (Huerta & Harting 1984, May 2006). However, neurophysiologists generally divide the layers into two: the upper layers, including the SO, are referred to as the superficial or visuosensory layers, whereas SGI together with the SGP are lumped together as motor layers. For simplicity, we use the terms visuosensory and motor layers, unless otherwise specified.
Figure 1.
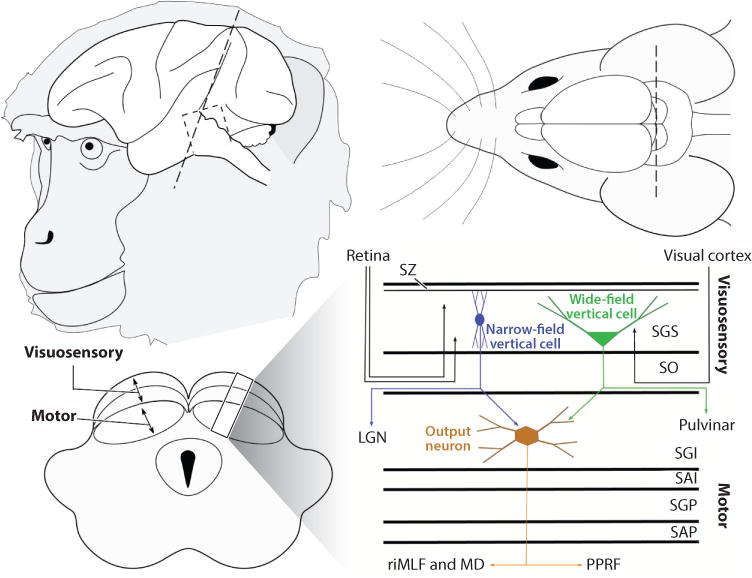
Schematic representation of the brain, highlighting the location of the superior colliculus in two mammalian species, a monkey and a mouse (not drawn to scale). The dashed line indicates an axial cut through the colliculus to reveal the layers of the colliculus. Stratum griseum superficiale (SGS) and stratum opticum (SO) together comprise the visuosensory layers, and the stratum griseum intermediale (SGI) together with the deeper layers comprise the motor layers. The schematic on the right shows the known neuronal types within the colliculus and their projection patterns. Narrow-field vertical cells (blue) project to the lateral geniculate nucleus (LGN), and the wide-field vertical cells (green) project to the pulvinar. Output neurons of the motor layers (brown) project to the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) to control vertical eye movements and to the paramedian pontine reticular formation (PPRF) to control horizontal eye movements. The motor layers also project upstream to the medial dorsal nucleus of the thalamus (MD). Abbreviations: SAI, stratum album intermediale; SAP, stratum album profundum; SGP, stratum griseum profundum; SZ, stratum zonale.
2.1. Overview of Superficial Layer Inputs and Outputs
The primary inputs to the visuosensory layers are the retina, striate, and extrastriate cortex (Schiller 1984) (Figure 1). Similarly, the avian optic tectum receives input from the retina and the Wulst, the avian homolog of the primary visual cortex (Karten & Dubbeldam 1973). Other inputs arise from the ventral lateral geniculate nucleus, parabigeminal nucleus, pretectum, and locus coeruleus. Visuosensory colliculus receives inputs from both M- and K-type retinal ganglion cells. Visual cortical afferents to the colliculus terminate more ventrally in the visuosensory layers, compared to retinal afferents (Harting et al. 1992, Huerta & Harting 1984). The outputs of the visuosensory layers of the colliculus also follow a segregated pattern: More dorsally located neurons tend to project to the lateral geniculate nucleus, whereas the more ventrally located (lower SGS and SO) neurons tend to project to the pulvinar (Albano et al. 1979, Harting et al. 1991). Consistent with this, retinal afferents more densely target colliculo-geniculate neurons, and cortical afferents more densely target tectopulvinar neurons (Graham et al. 1979). The precise roles of these different channels in visual behavior remain unknown but are an active area of research. Molecular genetic studies suggest that wide-field vertical cells express a transcription factor called Ntsrl-GN209. These neurons respond best to slowly moving stimuli and project to the pulvinar, whereas the horizontal neurons express GABA and project to the lateral geniculate nucleus, as well as the parabigeminal nucleus (Gale & Murphy 2014). Recent optogenetic studies show that there are also excitatory projections to the dorsal lateral geniculate nucleus (Bickford et al. 2015). These new results, together with the known properties of retinal afferents and their recent molecular identification (Bowling & Michael 1980, Huberman et al. 2009, Sachs & Schneider 1984, Tamamaki et al. 1995), suggest the visuosensory layers process and relay visual information related to motion and orienting (Hall & Colby 2016, White et al. 2009). Consistent with this, transneuronal retrograde labeling of collicular neurons after injections of rabies virus into extrastriate cortical areas shows that areas V3 and MT of the dorsal stream, but not areas V2 and V4 of the ventral stream, are targets of collicular outputs (Clower et al. 2001, Lyon et al. 2010). This general organization led to the view that the colliculus is involved in rudimentary visual processing designed to guide orienting movements, rather than for detailed visual processing.
2.2. Overview of Intermediate and Deep-Layer Inputs and Outputs
Unlike the limited inputs and outputs of the visuosensory layers, the motor layers receive inputs from virtually the entire brain and parts of the spinal cord (Edwards et al. 1979), and their outputs target nuclei throughout the neuraxis, descending to the pons and spinal cord and ascending to multiple thalamic nuclei. These anatomical connections of the colliculus have been discussed in many detailed reviews over the years (Borra et al. 2014, Butler et al. 2016, Harting 1977, Harting et al. 1992, Huerta & Harting 1984, May 2006, Sparks & Hartwich-Young 1989). Worth noting, and discussed more fully below (see Figure 5), is that the ascending outputs of the motor layers of the colliculus appear denser than the descending outputs. The bulk of the work in the primate colliculus focuses on the role of the descending pathways in control of orienting. But the ascending pathways likely play an equal or even more important role and point toward a collicular contribution to higher aspects of visual function, such as attention and decision making. Another organizing principle is that inputs to the motor layers of the colliculus form patches or puffs. A series of patches between 300 and 600 µm in diameter densely labeled for acetylcholinesterase (AChE) were the first indication of this organization (Illing & Graybiel 1985, Mana & Chevalier 2001). Subsequently, other molecular markers, such as the calcium binding proteins calbindin and parvalbumin, were identified as having a patchy or modular arrangement, particularly within the motor layers. Furthermore, a number of inputs to the intermediate layers terminate in patches, which sometimes correlate with AChE patches (Harting et al. 1997; Illing & Graybiel 1985, 1986; Illing et al. 1990), suggesting that understanding the architecture of the patches could provide key information about collicular function. However, the relationship between the patchy architecture and the functional organization of the colliculus remains to be determined. In fact, considering the large number of inputs to the colliculus and the large number of outputs, there are few studies of the precise input/output relationships of these populations.
Figure 5.
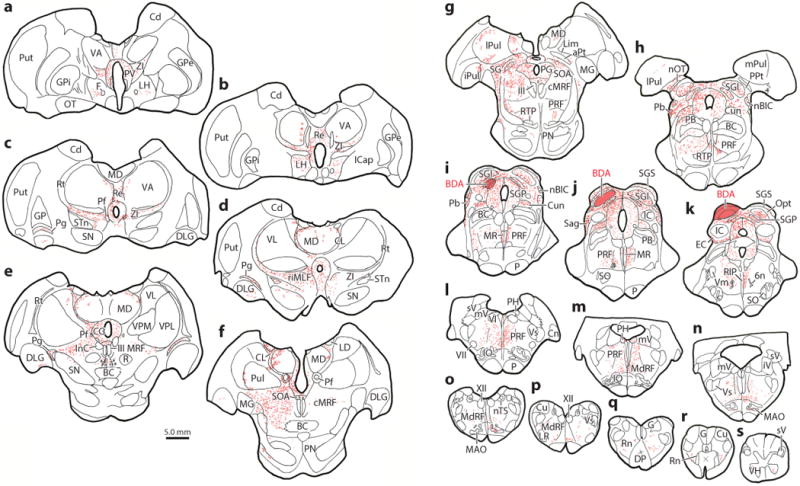
Distribution of terminals following an injection of biotinylated dextran amine (BDA) into the superior colliculus. Injection site shown in panels i, j,and k. Note denser terminal labeling in panels a–h rostral to the injection site, compared to panels i–s caudal to the injection site.
3. SUPERIOR COLLICULUS MAPS
By the late nineteenth century, it was known that the colliculus played a role in moving the eyes (Adamuk 1870), but it was the work of Apter in 1945 that laid the groundwork for understanding that a prominent collicular feature is an organized map, or, more precisely, two maps: one of visual space, superficially, and one of saccadic eye movement space, ventrally. Using recording techniques, Apter found that the retina projects to the feline superior colliculus in an orderly fashion, with the right hemifield mapped onto the left colliculus and the left hemifield mapped to the right colliculus (Apter 1945, Hess et al. 1946). By using strychnine in combination with light stimulation, she showed that “each point on the surface of the colliculus is responsible for movement of the eyes toward the particular part of the visual field which projects to that point on the colliculus” (Apter 1946, p. 74). Subsequent work in monkeys revealed that neurons in the visuosensory layers discharge in relationship to visual stimuli appearing in particular regions of the visual field—they have visual receptive fields (Goldberg & Wurtz 1972a). Furthermore, neurons in the motor layers discharge in close temporal relationship to the generation of eye movements, with movement field locations similar to the overlying visual receptive fields (Wurtz & Goldberg 1972). Indeed, the term movement field was introduced at this time in analogy to the receptive field to describe the activity co-occurring with movement onsets rather than stimulus onsets. Around the same time, Schiller & Stryker (1972) showed alignment of the maps of visual and saccadic eye movement space in that electrical stimulation evoked eye movements to regions of the visual field for which neurons in the overlying superficial layers discharged maximally when stimulated with light. This led to the foveation hypothesis, the idea that the role of the neurons in the motor layers was to point the eyes toward the region of visual space encoded by the overlying visual areas.
At around the same time as the recording experiments, Robinson discovered an orderly map of eye movement space using electrical stimulation (Figure 2) (Fuchs & Robinson 1966, Robinson 1972). A number of experiments since have demonstrated that the amplitude and direction of the eye movements produced by the colliculus depend only on the site of the electrical stimulation within the motor layers, because the same saccade vector occurs with stimulation regardless of the position of the eye in the orbit. This led to the notion referred to as the dual coding hypothesis; the location of the maximal discharge of movement neurons on the collicular map determines the vector of saccades, whereas the frequency of their discharge determines their speed (Edelman & Goldberg 2001, 2003; Gnadt et al. 1991; Hikosaka & Wurtz 1985; Klier et al. 2001; Sparks & Mays 1990; Stanford et al. 1996; Van Opstal et al. 1990).
Figure 2.
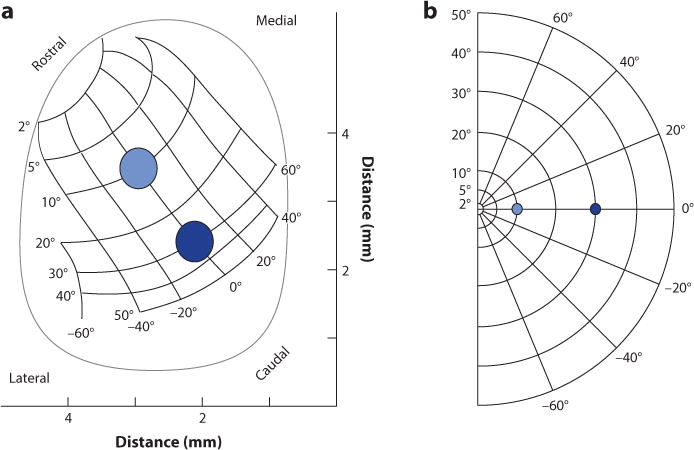
(a) The map of saccadic eye movement space across the movement layers of the left superior colliculus as discovered by Robinson. Rostral is at the top and caudal is at the bottom. (b) The visual field representation corresponding to the left colliculus. Blue dots show locations of visual stimuli in visual space and correspondingly on the collicular map. Adapted from Robinson (1972).
Although saccadic eye movements in monkeys are the most well studied, activation of the colliculus evokes other species-specific orienting movements as well. These include pinnae movements, whisker movements, whole-body movements, and even head and limb movements (Corneil et al. 2002, Courjon et al. 2015, Dean et al. 1989, Hemelt & Keller 2008, Hess et al. 1946, McHaffie & Stein 1982, Pélisson et al. 1991, Stein & Clamann 1981, Syka & Radil-Weiss 1971, Tehovnik & Yeomans 1986, Vidal et al. 1988). Furthermore, the colliculus also contains auditory and somatosensory maps that are aligned with the visual and movement maps (Chalupa & Rhoades 1977, Drager & Hubel 1975, Ghose et al. 2014, Knudsen 1982, Middlebrooks & Knudsen 1984, Palmer & King 1982, Stein & Meredith 1993, Wickelgren 1971, Wise & Irvine 1983). The study of the maps in the colliculus has moved in two main directions over the years. The first is directed at understanding how neurons integrate information from multiple sensory sources (Rowland & Stein 2014, Stein & Meredith 1993, Wallace et al. 1993). The second stems from the pioneering work of Knudsen and colleagues in the barn owl (Knudsen & Brainard 1991) demonstrating the importance of visual information for the development and formation of accurate sensory maps (Wang et al. 2015). Less attention has been paid to determining the basis of the coordination between the maps. Schiller proposed a direct link between the visual and movement maps with the foveation hypothesis, reasoning that a saccade would be directed to the retinotopic location activated by the overlying visual neurons. Drager & Hubel (1975) proposed that somatosensory maps were organized relative to how body parts would be seen from the eye, implying that information from other modalities was converted to retinotopic coordinates. Sparks (1988) proposed that all the maps were organized according to motor error—the position of the eyes relative to the target position. A number of experiments support the motor error hypothesis (Groh & Sparks 1996, Jay & Sparks 1984, Krauzlis et al. 2000, Mays & Sparks 1980). An important implication of this idea is that target activity within the colliculus is not stationary but changes as the location of the target of interest relative to current eye position changes. However, how the information arising from the visual, auditory, and somatosensory systems maps is translated into collicular motor error signals for eye, head, or limb movements remains to be determined. With the advent of new molecular genetic tools, we are poised to begin dissecting these circuits to define neurons within the colliculus that perform the computations leading to the conversion of sensory information into movement commands.
4. HIGHER COGNITIVE FUNCTION: TARGET SELECTION, DECISION MAKING, AND VISUAL ATTENTION
4.1. Population Coding and Saccade Choice
McIlwain (1986, 1991) was among the first to propose that the colliculus used a population code to produce saccades. He noted that individual collicular neurons encoded saccade vectors very coarsely, so that they could not produce appropriately precise saccades (Robinson 1972; Schiller & Stryker 1972; Sparks 1975, 1978; Wurtz & Goldberg 1972). Thus, a weighted sum (vector average) of the activity of collicular neurons across the entire map was thought to determine the saccade vector. However, the data leading to this conclusion are largely from computational efforts or from experiments performed using only a single visual target, a condition not usually found naturally. More recently, investigators began exploring collicular activity when multiple possible targets are available (Basso & Wurtz 1997, 1998; Li & Basso 2005; McPeek & Keller 2004; Port & Wurtz 2003). These experiments point to the possibility that the colliculus operates using a winner-takes-all code, in which, ultimately, the population of neurons discharging at the greatest levels determines the saccade. Thus, for single targets, the colliculus appears to use a population vector average scheme to determine the saccade vector, and for multiple possible targets, a winner-takes-all scheme. This two-model conundrum is evident in behavioral studies too. When two visual stimuli appear in close proximity, saccades land at a location between the two stimuli, a phenomenon called the global effect or averaging saccades (Edelman & Keller 1998, Findlay 1982, Glimcher & Sparks 1993, Kowler & Blaser 1995, McGowan et al. 1998, Melcher & Kowler 1999). However, if the targets appear further apart or more time is provided, a saccade can be made to one or the other stimulus (Ottes et al. 1984).
Probabilistic population coding strategies offer a solution to this conundrum (Ma et al. 2006). So, studies explored whether saccadic eye movements could be encoded using a probabilistic framework (Kim & Basso 2008, 2010). Recording from multiple neurons simultaneously from the colliculus while monkeys performed a simple task in which they chose one differently colored stimulus from an array of four stimuli revealed that the relative level of activity across both colliculi predicted whether monkeys would perform well or poorly, regardless of the saccade vector. This result indicates two things: First, the activity of neurons encoding saccades that are not made also contributes to the resulting saccadic eye movement, and second, the relative level of activity across the collicular map encodes the saccade choice, not just the saccade vector. Figure 3 shows this result schematically (Kim & Basso 2008). A further analysis comparing different models—vector averaging, winner-takes-all, and one using a Bayesian estimator, to understand how the colliculus encodes saccades—revealed that the Bayesian estimator provided the most accurate prediction of the choices made by the monkeys compared to the winner-takes-all and the vector average models. A probabilistic approach is superior to winner-takes-all or population vector averaging in part because all the information contained in individual neuronal turning curves is used to determine the choice, and when all the information is combined across multiple neurons, the population activity encodes multiple options simultaneously. The distribution of choices encoded by the population provides a conditional probability for each of the alternatives, eliminating the need for a switch between population coding schemes. Both the vector averaging code and the winner-takes-all code disregard information from distractor neurons. Consequently, variations in behavior, uncertainty, or even attentional modulation cannot be resolved using these other encoding methods (McAdams & Maunsell 1999, Pouget et al. 1999, Spitzer et al. 1988). Furthermore, a Bayesian framework also allows prior information to influence saccade choices, an area yet to be explored. How Bayesian models are implemented in neuronal circuits remains an enigma in the field (Ma et al. 2006, Pouget et al. 2003), but exploring the circuits of the colliculus, with its known relationship to behavior, may provide a useful model system in which to do this.
Figure 3.
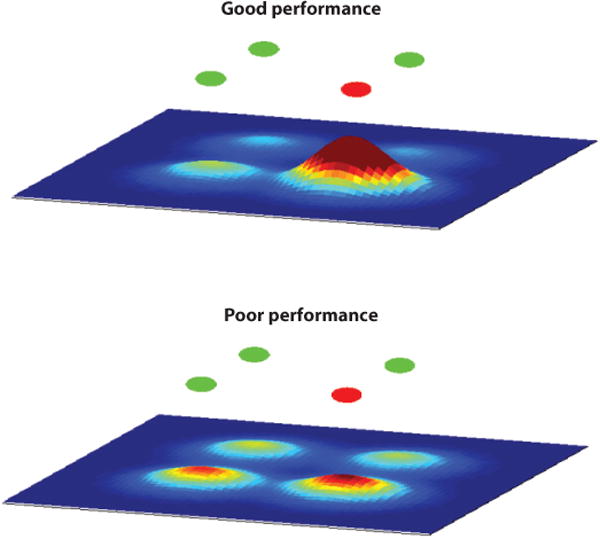
Schematic representation of activity across the movement map of the superior colliculus during a simple task in which one differently colored stimulus is chosen as a target for a saccade. The circles on the top are the possible targets (red or green), and the maps below are heat maps in which warmer colors represent higher levels of neuronal activity. One could think of these hills of activity as likelihoods; a linear sum across all activity will naturally lead to a probability distribution of all possible saccades. The peak would be the most likely saccade, whereas the width of population distribution provides an implicit representation of the certainty of the saccade choice. Adapted from Kim & Basso (2008, 2010).
The physiological work described above reported another interesting observation. When comparing the relative level of activity between the neurons encoding saccade targets and neurons encoding distractors using signal-detection-theory analytical methods, receiver operating characteristic (ROC) and d′, the investigators found that the neuronal activity occurring as early as 20 ms before eye movement initiation correlated best with saccade target choices, and not the parameters of the saccades such as amplitude and velocity (Figure 4) (Kim & Basso 2008). This work together with that of others (Carello & Krauzlis 2004, Krauzlis & Dill 2002, McPeek & Keller 2004) provides strong support for the idea that motor-layer neurons of the colliculus encode something more than just the saccade vector (Moschovakis & Highstein 1994; Moschovakis et al. 1988a,b; Rodgers et al. 2006).
Figure 4.
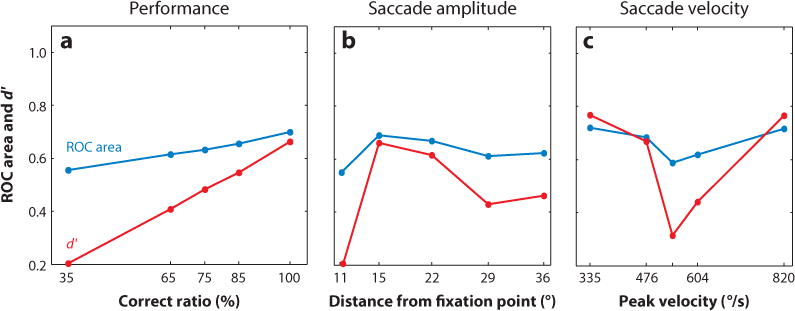
The relative level of activity across the collicular map encodes saccadic eye movement choice. (a) Receiver operating characteristic (ROC) area and d′, two measures of discriminability, are plotted against the performance accuracy in a simple target selection task. The relative activity scales linearly with performance accuracy. (b) The same data are plotted as a function of saccade amplitude or distance to the saccade from the fixation point. (c) The same data are plotted as a function of saccade velocity. Discriminability correlates best with target choice and least with saccade parameters. From Kim & Basso (2008).
4.2. Intrinsic Properties of Superior Colliculus Neurons—Delay-Period Activity
The experiments described above focused on the neurons in the motor layers of the colliculus with delay-period activity. In monkeys, there are at least two types of neurons found in the motor layers of the colliculus that have activity associated with the onset of saccadic eye movements. These neurons are generally referred to as burst and as buildup, or prelude, neurons because of their characteristic response profiles. Burst neurons discharge action potentials robustly at the time of saccade generation, whereas buildup, or prelude, neurons have a low rate of discharge while a monkey waits for a cue to make a saccade that culminates in a saccade-related burst of action potentials, known as delay-period activity (Glimcher & Sparks 1992, Munoz & Wurtz 1995). Both of these types of neuron project out of the colliculus (Moschovakis et al. 1988a,b; Rodgers et al. 2006). This arrangement leaves open the possibility that neurons with delay-period activity process information related to cognition, which is then transmitted to the burst neurons that are responsible for sending out the movement command. The superior colliculus of the rodent is an excellent model in which we can capitalize on novel molecular tools to unravel the neuronal circuits responsible for incorporating cognitive signals into movement commands. As a first step toward this, rodent motor layer output neurons have been characterized as regular, burst, and late-spiking types, and they have multipolar morphology. Similar neurons are found in monkeys and have been classified as X and T neurons. X neurons have larger somata and larger, more complex dendritic fields, whereas T neurons have smaller somata and smaller, less complex dendritic fields. Both have axons that cross beneath the oculomotor nucleus and descend in the brainstem, but T neurons also have a commissural projection to the other colliculus. Both also display recurrent local collaterals (Moschovakis et al. 1988a). These recurrent collaterals form a local excitatory circuit that in rodents has been shown to modulate the robust bursting in neurons associated with the generation of orienting movements (Isa et al. 1998, Saito & Isa 2003). Further work, using the GAD67 knock-in mouse expressing GFP in GABAergic neurons, shows that the regulation of bursting activity in the motor layers of the colliculus depends in part upon activation of GABAergic neurons in the visuosensory layers (Kaneda et al. 2008). Thus, local inhibitory circuits in both layers may modulate the bursting of motor layer neurons to drive a movement. A recent study reported that collicular neurons show a persistent Na++ current that is intrinsic to the neurons (Ghitani et al. 2016). This finding suggests that the delay-period activity of collicular neurons may not be a simple, passive reflection of synaptic inputs but an intrinsic property of the neurons themselves, providing cellular evidence for a collicular role in mechanisms that intervene between vision and action.
4.3. Role of Superior Colliculus in Attention—Old and New
A careful look at the outputs of the superior colliculus reveals a surprising point: The terminations from ascending projections are much denser and more extensive than are the descending projections to the pons (Figure 5). The ascending projections target regions of the thalamus, including the pulvinar, underscoring the idea that the superior colliculus plays an important role in cognitive aspects of brain function by influencing circuits upstream. Indeed, the first evidence that the colliculus might play a role in spatial attention was that the visual responses of superficial layer neurons showed enhancement when monkeys made saccades to targets in their receptive fields, compared to when they remained fixating in the presence of the targets (Goldberg & Wurtz 1972b). This enhancement of visual responses was interpreted as indicating an overt shift in the focus of attention to the target location. This effect also appears in more complicated attentional tasks and even when a saccade is cued before it is made, suggesting a role in covert shifts of attention as well (Gattass & Desimone 1996, Li & Basso 2008).
Spatial attention enhances sensory signals, presumably to facilitate the processing of sensory information (Colby & Goldberg 1999, Egeth & Yantis 1997, Kastner & Ungerleider 2000, Maunsell 2015, Reynolds & Chelazzi 2004, Wolfe & Horowitz 2004). Because volitional saccades reorient the line of sight, it is reasonable to propose that getting ready to move the eyes to a new location and shifting attention to that new location are served by similar neuronal mechanisms and processes (Deubel & Schneider 1996; Kowler et al. 1995; Rizzolatti et al. 1987; Sheliga et al. 1994, 1995). Consistent with this, low-intensity electrical stimulation of the colliculus that does not evoke eye movements enhances motion direction discrimination (Müller et al. 2005) and detection (Cavanaugh & Wurtz 2004, Cavanaugh et al. 2006).
Krauzlis and colleagues followed up on these findings with crucial experiments (Lovejoy & Krauzlis 2010, Zenon & Krauzlis 2012). They presented monkeys with four patches of motion stimuli (Figure 6). While fixating centrally, one of the patches changed its direction of motion. Monkeys reported this change by looking at a target placed elsewhere, dissociating the location of attention from the location of the required eye movement. One of the four locations was cued, indicating the location where the change would occur. Importantly, they also provided a foil patch that contained a motion direction change that differed from that occurring in the cued patch. When they inactivated a small region of the colliculus corresponding to the cued location, they found that monkeys ignored the cued motion change and reported the motion change occurring at the foil location. Conversely, when the foil signal appeared in the inactivated region of the colliculus, the monkeys ignored this and reported the motion change from the cued location. This result was obtained whether monkeys reported the motion change with a saccade (Figure 6b,c) or a button press (not shown), providing compelling evidence that the colliculus plays a critical role in determining the location of covert spatial attention. However, when they recorded from neurons in cerebral cortical areas MT and MST during this task, they found that although the monkeys were impaired at performing the task, all the signatures of attention in the cerebral cortex remained after collicular inactivation (Zenon & Krauzlis 2012). This suggests that the colliculus can modulate spatial attention through circuits operating independently of these cortical regions.
Figure 6.
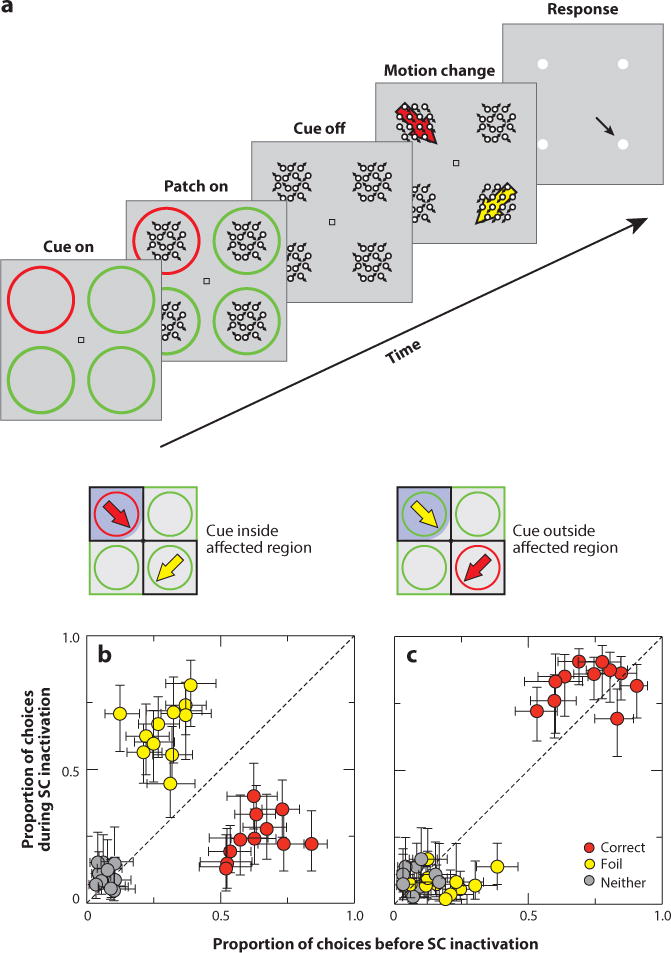
A role for the superior colliculus (SC) in attention. (a) Monkeys attended to one of four motion patches determined by a colored ring cue. One patch then changed its motion direction, and monkeys reported this change by making a saccade to a choice target (or pressing a button) corresponding to the change in motion direction. (b) Inactivation of the colliculus in the region of the colliculus representing the cue led to impaired detection at that location and increased responding to the foil. The inset shows the trial condition. Points falling along the dashed line indicate no change with activation. (c) Same as in panel b for the condition in which the foil appeared in the inactivated region. In this case, monkeys ignored it. Adapted with permission from Krauzlis et al. (2013).
4.4. Multiple Pathways, Possible Circuits, and a More Parsimonious Interpretation—The Superior Colliculus Determines Decision Criteria
How can the superior colliculus influence attention, while bypassing cortical circuits? If the projections from the colliculus to thalamic nuclei, including the medial dorsal nucleus and the pulvinar that project in turn to the cerebral cortex, are not necessary for attention, what targets are involved? Subcortical circuits through the basal ganglia have been proposed for visual selection and attention (Krauzlis et al. 2014, McHaffie et al. 2005). The motor layers project to the intralaminar nuclei of the thalamus, including the centromedian nucleus and the parafascicular nucleus, and these project to the striatum, the key input nucleus of the basal ganglia. The striatum then projects to the substantia nigra pars reticulata, which projects directly back to the superior colliculus (McHaffie et al. 2005). It is possible that the activity in this subcortical loop could be responsible for changes in attention seen with inactivation (Krauzlis et al. 2013). A second, perhaps more parsimonious, hypothesis is based on a well-known fact from signal detection theory—attention is composed of at least two components: a change in perceptual sensitivity and a change in response bias or decision criteria (Green & Swets 1966). The results of Krauzlis and colleagues can be explained simply if the superior colliculus is responsible for only one component, the decision criterion. In this model, cortical activity is responsible for attentional mechanisms related to perception, and collicular activity is responsible for setting the decision criterion that determines how perceptual information is reported. Results from two recent experiments are consistent with this idea. In one, Luo & Maunsell (2015) used different reward contingencies to entice monkeys to perform an attention task using two different strategies. On some trials, monkeys adjusted their sensitivity to improve performance, and on other trials, they adjusted their criterion or response bias to improve performance. The investigators found that V4 activity correlated with changes in sensitivity and not changes in criterion. Because both processes are a part of attention, they reasoned that the experiments performed by Krauzlis and colleagues interfered with only one part of the attentional process. In a second experiment, trained monkeys performed a simple Yes/No perceptual discrimination under conditions in which they changed only their decision criteria, without changing their perceptual sensitivity (Crapse & Basso 2014). Recording in the motor layers of the colliculus showed that the neuronal activity correlated with the changes in the monkeys’ decision criterion. Furthermore, performing the same experiment but substituting electrical stimulation of the superior colliculus mimicked the behavioral results (Figure 7). These results are consonant with a series of previous studies in the superior colliculus in monkeys and rodents finding choice biases following collicular manipulations (Carello & Krauzlis 2004, Felsen & Mainen 2008, Lovejoy & Krauzlis 2010, McPeek & Keller 2004, Müller et al. 2005, Nummela & Krauzlis 2010, Thevarajah et al. 2009). Thus, neurons in the motor layers of the superior colliculus may not be shifting the focus of attention per se, but rather, their activity may signal the decision criterion and, therefore, how attention is expressed.
Figure 7.
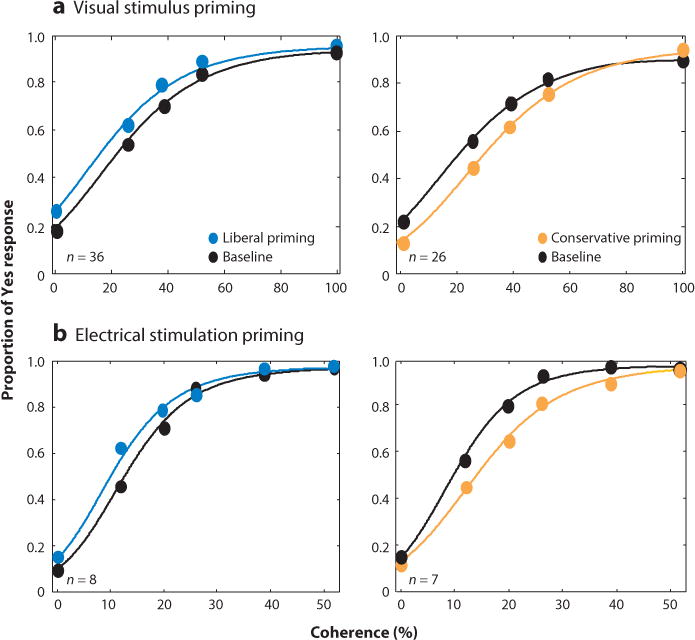
Electrical stimulation of the colliculus changes decision criteria in predictable ways. Proportion of a Yes response is plotted as a function of decision difficulty (coherence). Panel a shows the results of priming with a visual stimulus, and panel b shows the results of priming with electrical stimulation of the motor layers of the colliculus. Blue shows the results with liberal priming, and orange shows the results with conservative priming. Black is baseline data with no priming or stimulation. n indicates the number of sessions averaged. From Crapse & Basso (2014).
This line of work extends the domain of the superior colliculus into decision making (Redgrave et al. 1999). Indeed, the notion that the colliculus of the alert monkey plays a role in decision making beyond its role in saccade generation was suggested by the observation that superior colliculus neurons in the motor layers contained signals correlating with target uncertainty (Basso & Wurtz 1997, 1998) and perceptual decision-making performance (Horwitz & Newsome 1999, Horwitz et al. 2004). A recent computational effort informed by data from monkeys performing a motion discrimination task (Roitman & Shadlen 2002) suggests the colliculus detects crossings of decision thresholds (Lo & Wang 2006). In this work, the colliculus operates in partnership with corticobasal ganglia circuits, and the decision threshold is set by the weight of corticostriatal synapses. In this model, the colliculus plays a passive role by simply detecting threshold crossings and initiating the choice response, similar to the traditional role that the basal ganglia–superior colliculus circuit plays in saccade initiation (Hikosaka et al. 2000). However, the results described above, together with other recent results, suggest that the role of the basal ganglia and its relationship to the colliculus is more complicated and may include processing events occurring before saccade generation, such as selecting and remembering the locations of targets and even determining decision criteria (Basso & Sommer 2011, Basso & Wurtz 2002, Crapse & Basso 2014); therefore, consideration of an active role of the colliculus in decision-making processes is warranted.
5. MICROCIRCUITS OF THE SUPERIOR COLLICULUS
5.1. Pathways Linking Superficial and Deep Layers of the Superior Colliculus
Considerable experimental focus is placed on the role of the monkey colliculus in cognitive processing, but far less experimental attention is placed on the circuits in the colliculus that can mediate these behaviors. Early on, the role of the colliculus in orienting was predicated on the idea that the visuosensory and motor layers of the colliculus were linked directly, but this was controversial (Casagrande & Diamond 1974, Edwards 1980, Mays & Sparks 1980, Schiller & Stryker 1972). Recent experiments using coronal slices of the superior colliculus, in vitro, confirmed this direct link. In these experiments, superficial layer stimulation evoked excitatory postsynaptic currents (EPSCs) and potentials (EPSPs) in motor layer neurons (Helms et al. 2004, Isa et al. 1998, Lee et al. 1997, Özen et al. 2004). Application of biccuculine, a GABAA receptor antagonist, enhanced these responses, resulting in robust bursts of action potentials, similar to those observed in vivo during saccades. These results, together with anatomical experiments (Behan & Appell 1992, Kardamakis et al. 2015, Lee & Hall 1995, Mooney et al. 1988a, Rhoades et al. 1989, Tardif et al. 2005), now provide solid evidence for the existence of a disynaptic pathway from the retina to the motor layer neurons. This pathway may underlie the generation of ultrafast, express saccades. Recent slice work also shows that photoactivation of superficial layer neurons up to 1,000 µm from motor layer neurons in the horizontal direction can evoke EPSCs, suggesting that the linkage between the visuosensory and motor layers is not strictly columnar (Casagrande et al. 1972, Lee & Hall 2006). This phenomenon corresponds to the known geometry of the visuosensory neurons providing inputs to motor layer neurons (Isa & Hall 2009). For example, wide-field vertical cells (Figure 1) have dendritic fields on the order of ~2,000 µm (Endo & Isa 2001, Langer & Lund 1974, Lee & Hall 1995, Mooney et al. 1988b, Saito & Isa 1999).
In addition to a pathway linking the visuosensory layers to the motor layers, recent slice work provides evidence for at least two pathways from the motor layers back to the visuosensory layers. One is inhibitory and may contribute to saccadic suppression—the phenomenon that explains why we do not perceive the high-velocity motion that occurs every time we move our eyes. Recent results show that motor output neurons have recurrent collaterals that terminate on local GABAergic interneurons. These interneurons inhibit visuosensory layer neurons that project up to the dorsal lateral geniculate nucleus and pulvinar (Lee et al. 2007, Phongphanphanee et al. 2011). A second pathway arises from the motor layers and is excitatory (Ghitani et al. 2014). It appears sparser than the inhibitory pathway, but it may underlie the enhancement of visuosensory neuronal activity prior to saccades or, possibly, visual stabilization (Dunn & Colby 2010, Dunn et al. 2010, Goldberg & Wurtz 1972b, Li & Basso 2008).
5.2. Microcircuits of Orienting Behavior
Because the visuosensory neurons have projections to neurons in the motor layers of the colliculus, it is tempting to suggest that this circuit is responsible for the alignment of the sensory and motor maps and, thus, the generation of visually guided orienting movements. This is likely for species with small telencephalons but more complicated for mammals with a developed cerebral cortex. First, when two visual stimuli appear in rapid succession and monkeys look at each flash sequentially, neurons in the superficial layers discharge topographically in response to the two flashes, but the underlying motor activity correlates with the first target location and not the second. The location of activity related to the second saccade is defined by the saccade vector, indicating a dissociation between the visual and motor layer activities (Mays & Sparks 1980). Second, reversible inactivation of the primary visual cortex in monkeys eliminates the visual responsiveness of neurons in the motor layers of the colliculus (Schiller et al. 1974). Therefore, it is likely that interlaminar flow plays little role in conventional volitional saccades in primates. Instead, this pathway is likely to play a role in express saccades, the ultrashort latency saccades in which the target location is predefined and target onset is just a cue to move (Baro et al. 1995, Boch et al. 1984, Fischer & Ramsperger 1984, Sommer 1994). Under express-saccade conditions, when there is a higher level of activity in the motor layer neurons, a visual stimulus excites the intralaminar input, which pushes motor layer neurons over their threshold, producing a very short latency saccade (Isa 2002, Isa & Hall 2009, Isa et al. 1998). The direct visuosensory-to-motor pathway is enhanced by the bath application of a GABA antagonist, indicating that the efficacy of this pathway is controlled by GABAergic inputs. Whether this inhibitory control arises from local inhibitory circuits or external sources, like the substantia nigra, is unknown (Bickford & Hall 1992, Chevalier et al. 1981, Deniau & Chevalier 1992, Westby et al. 1994). However, patients with Parkinson’s disease have altered basal ganglia output signals (Prescott et al. 2008), yet they show normal express-saccade distributions (Roll et al. 1996).
5.3. Microcircuits of Attention
The delay-period activity seen in collicular neurons is thought to underlie the processes that intervene between vision and action, such as target selection, shifts of attention, and decision making. How these neurons relate to the circuits that control attention in mammals is unknown. Circuits in the bird may hold the key. The nucleus isthmus pars parvocellularis (Ipc) and the nucleus isthmus pars magnocellularis (Ipm) in the brainstem project to the optic tectum and receive reciprocal, topographic inputs from the motor layers of the optic tectum (Knudsen 2011, Sereno & Ulinski 1987, Wang 2003, Wang et al. 2006). The cholinergic inputs from the Ipc are focal and target the motor and visuosensory layers of the avian optic tectum (layers 1–9; Figure 8). Because of this anatomical arrangement, it has been proposed that this brainstem network creates focal excitation and global surround inhibition within the tectum, contributing to the establishment of a priority map in the colliculus (Mysore & Knudsen 2014). With aid from forebrain input, the priority map (Itti & Koch 2000, Koch & Ullman 1985) identifies the spatial location of interest that ultimately drives an orienting movement toward a target. Visual stimuli produce bursts in tectal neurons and in axon terminals of Ipc neurons. However, after inactivation of Ipc, the bursting disappears. Because the projections from Ipc and the optic tectum are reciprocal and focal, this activity may represent a reentrant excitatory drive that enhances the saliency of a visual stimulus at its topographic tectal location (Marín et al. 2005). Furthermore, Ipc neurons exhibit competitive interactions and thus can signal the strength of the sensory signal to the optic tectum (Asadollahi et al. 2010, Marín et al. 2007). Nicotinic cholinergic receptors are also found on mammalian retinal axonal terminals, and the release of acetylcholine enhances visual signal transmission in the mammalian colliculus (Baginskas et al. 2011, 2012; King 1990; Titmus et al. 1999), suggesting that the mammalian colliculus may operate similarly.
Figure 8.
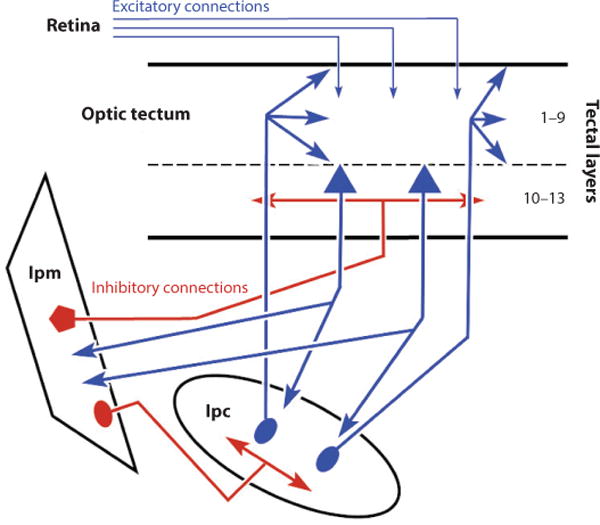
Schematic illustration of the circuits in the avian tectum thought to control attention. Red shows inhibitory connections and blue shows excitatory connections. Numbers indicate tectal layers in the bird. Arrowheads show terminals, whereas ovals, pentagons, and triangles show neuronal cell bodies. Abbreviations: Ipc, nucleus isthmus pars parvocellularis; Ipm, nucleus isthmus pars magnocellularis.
Neurons in the avian optic tectum also project to the GABAergic nucleus, Ipm. The Ipm GABAergic neurons project back to the optic tectum broadly in layers 10–13 (Figure 8). Simultaneous recordings from Ipm and Ipc neurons and experiments inactivating Ipm neurons in pigeons support the idea that this inhibitory input underlies the suppression of activity in unattended regions of the tectal map (Goddard et al. 2014). Moreover, neurons in the Ipc show reductions in visually evoked activity in the presence of a second visual stimulus, and this inhibition is eliminated after reversible inactivation of Ipm. Thus, the cholinergic drive serves to highlight a focused region of the map, the attended locations, while the GABA input serves to dampen activity in adjacent locations, creating a winner-takes-all attentional system.
Recently, the ability of this brainstem network to generate gamma frequency oscillations like those recorded in the cerebral cortex associated with attention was reported in a slice preparation of the avian brain (Goddard et al. 2012). The results suggest that the oscillations arise in the motor layers of the tectum and the frequency is determined by local GABAergic circuits, which in turn, through their cholinergic inputs to the visuosensory layers, are responsible for generating the oscillations in activity of the neurons in the visuosensory layer (Goddard et al. 2012, King 1990).
The homologous cholinergic nuclei to the Ipm and Ipc in the mammal are the parabigeminal nucleus and the parabrachial nuclei, including the lateral tegmental nucleus (Appell & Behan 1990, Graybiel 1978, Hall et al. 1989). Acetylcholine, through the activation of nicotinic receptors on retinal ganglion terminals (King 1990), enhances the transmission of signals in visuosensory layer neurons, and through the activation of both nicotinic and muscarinic receptors, it also activates GABAergic interneurons in the visuosensory layers. This latter activation may enhance lateral inhibition (Binns & Salt 1997, Endo et al. 2005, Lee et al. 2001). Nicotine enhances the generation of express saccades when injected in monkey colliculus (Watanabe et al. 2005), and activation of these cholinergic nuclei in smaller mammals produces orienting (Wolf et al. 2015). It is unknown whether oscillations seen in the avian circuit appear in the mammalian colliculus, although there is some evidence that the relative timing of activity across the colliculus may signal information about orienting movements (Brecht et al. 2004). Ultimately though, it is the number, not the timing, of action potentials generated by motoneurons that determines a movement, so efforts toward understanding how a temporal code is converted to a rate code will be required to understand the operation of these circuits fully. Also, whether these oscillations are a mechanism of attention requires experimental investigation (Ray & Maunsell 2015).
5.4. Normalization Circuits
In the mammalian colliculus, experiments using whole-cell patch recordings and voltage-sensitive dye imaging in slices suggest that local circuits may be capable of performing computations known to be performed by cortical circuits and to underlie sensory and attentional processes. For example, normalization is a computational process that explains the well-known observation that presenting two stimuli in a sensory cortical neuron’s response field results in neuronal activity that is approximately the average of the responses of the neuron to the presentation of each stimulus alone, rather than the sum. Normalization provides a description of how neurons integrate their multiple sources of excitatory and inhibitory inputs and is a key computation thought to underlie attention (Boynton 2009, Carandini & Heeger 2012, Lee & Maunsell 2009, Maunsell 2015, Ni et al. 2012, Reynolds & Heeger 2009). In vivo, collicular neurons show properties of normalization similar to those seen in the visual cortex. Specifically, visually responsive neurons in the colliculus show saturating contrast response functions (Li & Basso 2008). Similarly, when multiple stimuli appear and one is in a collicular neuron’s response field and one is outside of the response field, even in the opposite hemifield, the discharge rate is reduced, compared to when a stimulus is presented in the preferred location alone (Basso & Wurtz 1997, 1998; Rizzolatti et al. 1973, 1974). Collicular neurons also show suppressed activity with increases in the size of a visual stimulus (Gale & Murphy 2016, Goldberg & Wurtz 1972a) and show averaged rather than summed levels of activity when two stimuli appear in individual response fields (Li & Basso 2005). The results from these studies indicate that signals generated by visual input to the colliculus may be integrated within the colliculus through a process of normalization.
Interestingly, brain slice experiments indicate that local circuits within the colliculus are capable of performing normalization (Vokoun et al. 2014) (Figure 9). Applying electrical stimulation to one region of the motor layers of the colliculus evokes a circumscribed level of activation measured with voltage-sensitive dye. The activation of the motor layers propagates synaptically to the visuosensory layers (Figure 9b) (Ghitani et al. 2014, Vokoun et al. 2010). Presenting dual-site stimulation results in two distinct population responses in the motor layers, but the population responses in the visuosensory layers merge when the sites are close (Figure 9d) or remain distinct when the sites are farther away. Note that the overall level of the population response is not the sum of the population responses measured from the individual sites but rather is the approximate average of the responses evoked at the individual sites (Figure 9d–f).
Figure 9.
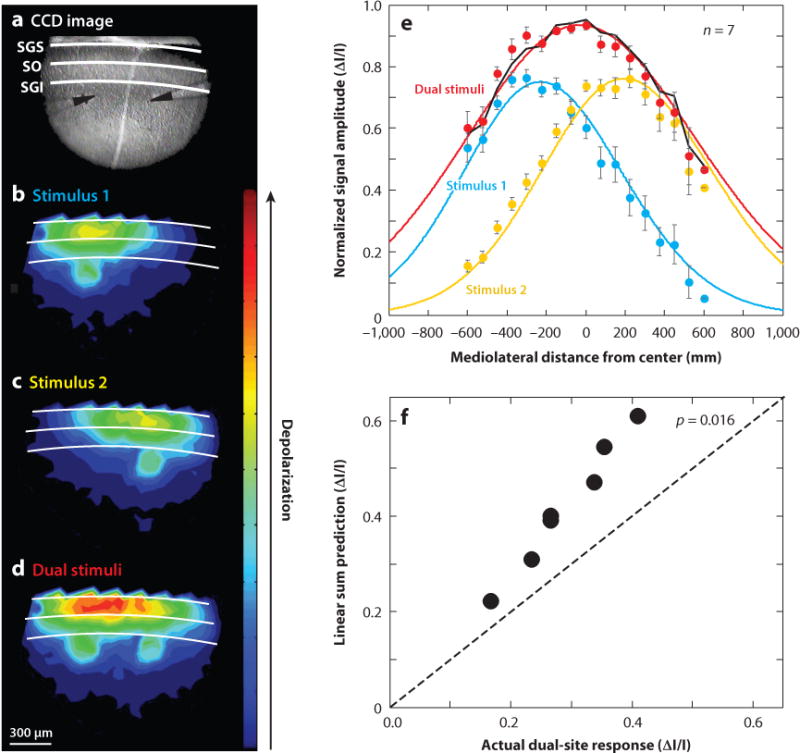
Normalization in a collicular slice. (a) Image of the colliculus through the microscope during the experiment showing the two electrodes. (b) Activation of one site results in a population membrane depolarization in the motor layers that extends to the visuosensory layers. The magnitude of the depolarization is indicated by the heat map with warmer colors indicating more depolarization. (c) Same as in panel b for site 2. (d) Population response to dual-site stimulation. (e) Population responses fitted with Gaussians to extract the center and amplitude of the responses. Circles show data, and lines show fits. (f) Linear sum model prediction (ΔI/I) is plotted against the actual response evoked with two sites of stimulation (ΔI/I). Points falling above the line indicate that the linear sum does not predict the dual-site response. Adapted with permission from Vokoun et al. (2014). Abbreviations: I/I, delta intensity/intensity; CCD, charge-coupled device; SGI, stratum griseum intermediale; SGS, stratum griseum superficiale; SO, stratum opticum.
Local inhibitory circuits in the mammalian colliculus also may form a hardwired priority map. The maps of locations in cortical areas and the colliculus are thought to signal locations within the visual world containing behaviorally relevant stimuli, thus forming a map of prioritized locations. In parasagittal slices of the colliculus, electrical stimulation evokes responses in the visuosensory layers that have a stronger and more caudally directed spread than rostrally directed spread. The asymmetry in the spread of activation is mediated by GABAA receptors within the visuosensory layers, suggesting that the colliculus is hardwired to give preferential processing to stimuli that appear more peripherally, consistent with its role in guiding orienting movements. Indeed, a recent study in the monkey also revealed that the motor map in the colliculus contains an inhomogeneous representation of saccadic eye movement space that favors upward locations (Hafed & Chen 2016).
5.5. New Insights from Molecular Genetics
In the cerebral cortex, mouse transgenic models expressing reporter genes have revealed that there are at least three subclasses of inhibitory neurons identified by their coexpression of GABA and either the Ca++ binding protein, parvalbumin (PV), or vasointestinal peptide (VIP), or somatostatin (SOM) (Gupta et al. 2000, Kawaguchi & Kubota 1997, Markram et al. 2004, Taniguchi 2014, Taniguchi et al. 2011). These inhibitory neurons play different roles in behavior (Pi et al. 2013). PV/GABA neurons in the cortex have also been implicated in controlling the frequency of oscillations in cortex that may mediate attention (Sohal 2012, Sohal et al. 2009), and because of the high levels of expression of PV in the chicken tectum, it was suggested that these neurons may play a similar role in the colliculus (Goddard et al. 2012). Recent work in the colliculus suggests that the principles of inhibitory circuitry discovered in the cortex may not apply to the superior colliculus. For example, activating PV neurons expressing channel rhodopsin in the visuosensory layers of the colliculus in mice results in the activation of a circuit involving the parabigeminal nucleus and the amygdala to produce avoidance and freezing behavior (Shang et al. 2015). Furthermore, some PV neurons in the colliculus may use glutamate and not GABA (Hormigo et al. 2016). Finally, recent work expressing the Ca++ indicator GCaMP6 in the superior colliculus to obtain two-photon imaging of the signals in the visuosensory layers in response to visual stimulus presentation reveals that the mouse colliculus contains orientation columns that seem more like those of the primate visual cortex (Feinberg & Meister 2015). Combining experiments such as these with new molecular genetic tools and behavioral measures is an exciting direction for future work and could lead to great gains in our understanding of how circuits perform computations that give rise to behavior.
6. SOME REMAINING QUESTIONS
The superior colliculus plays a role in visual processing, the generation of orienting behaviors, and the events that intervene between seeing and acting, such as selecting targets, making decisions, and paying attention. Over the past 40+ years, we have gone from thinking of the colliculus as a simple structure controlling eye movements to what we now think is a structure that is a key node in the network of brain areas responsible for controlling the location of attention and even the decisions we make. With the advent of new transgenic mouse models and new molecular and genetic tools to identify and selectively manipulate specific neuronal cell types, we can begin to relate the different collicular neuronal cell types to specific behavioral processes and we can determine how the different neuronal cell types are wired up to generate complex behavior. In spite of, or perhaps because of, the 40+ years of scientific effort devoted to the superior colliculus, there remain many open questions. The visuosensory layers of the colliculus contain some of the highest levels of GABA measured in the brain. What is it doing there? There is considerably less GABA in the motor layers. Why? Are there different neurons that contribute to attention, decision making, and the generation of orienting movements, or are the same neurons involved in each of these behaviors? Do the neurons that express PV, SOM, and VIP in the colliculus form canonical circuit motifs with output neurons of the colliculus? How are inputs to the motor layers organized vis-à-vis the output populations? What is the specific role of the cholinergic input from the parabrachial region? What is the role of the serotonergic input from the raphe nuclei? The list of open questions is long, but given the wealth of information that we have about the relationship of the neurons in the colliculus with behavior in monkeys, we believe that this structure provides an opportunity to obtain a detailed accounting of the neuronal cells, circuits, and computations that underlie complex behavior.
SUMMARY POINTS.
The distinctive layering pattern of the superior colliculus allows a detailed understanding of the organization of inputs and outputs, the topography of the physiological characteristics of neurons, and the connectivity within and between the layers.
Precise maps of visual space and saccadic eye movement space in the colliculus provide a model system for understanding basic processes of vision and action.
Neurons in the motor layers of the superior colliculus play a role in action generation and events that precede action, such as decision making and attention.
The colliculus encodes action choice using a probabilistic population code.
Intrinsic circuits of the avian colliculus support a winner-takes-all mechanism of attention.
Intrinsic circuits of the mammalian colliculus are capable of performing a simple computation of normalization.
FUTURE ISSUES.
We need cellular-level detail, rather than layer-level detail, about the inputs and outputs of the colliculus.
We need to know the molecular signatures of neurons in the colliculus and how they relate to the neuronal morphology and physiological phenotype. Does the canonical circuit architecture of the cerebral cortex apply to the colliculus?
A striking feature of the colliculus is the complexity of extrinsic and intrinsic inhibitory connections. What are the roles of these distinct inhibitory elements?
If there is a relationship between specific behavioral processes and specific neuronal cell types, what is it?
The colliculus receives inputs from a variety of brainstem regions with different neurotransmitters (e.g., acetylcholine, serotonin). What is the role of acetylcholine in the colliculus? What is the role of serotonin in the colliculus?
Acknowledgments
We are grateful to our collaborators in the laboratory, Drs. Trinity Crapse, Alessandra Perugini, and Claudio Villalobos, and to our colleague Dr. Martha Bickford, who provided thoughtful comments on our manuscript. We thank Donna Crandall for assistance with some of the images. The work in our laboratories is supported by NIH NEI EY19692 (M.A.B.); EY019663 (M.A.B. and P.J.M.); EY024153 (M.A.B.); EY024516 (M.A.B.).
Footnotes
The Annual Review of Vision Science is online at vision.annualreviews.org
LITERATURE CITED
- Adamuk E. Über die Innervation der Augenbewegungen. Zent Med Wiss. 1870;8:65. [Google Scholar]
- Albano JE, Norton TT, Hall WC. Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Res. 1979;173:1–11. doi: 10.1016/0006-8993(79)91090-4. [DOI] [PubMed] [Google Scholar]
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus of the cat. J Comp Neurol. 1990;302:143–58. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- Apter JT. Projection of the retina on superior colliculus of cats. J Neurophysiol. 1945;8:123–34. [Google Scholar]
- Apter JT. Eye movements following strychninization of the superior colliculus of cats. J Neurophysiol. 1946;9:73–86. doi: 10.1152/jn.1946.9.2.73. [DOI] [PubMed] [Google Scholar]
- Asadollahi A, Mysore SP, Knudsen EI. Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci. 2010;13:889–95. doi: 10.1038/nn.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginskas A, Kuraite V, Kuras A. Presynaptic nicotinic potentiation of a frog retinotectal transmission evoked by discharge of a single retina ganglion cell. Neurosci Res. 2011;70:391–400. doi: 10.1016/j.neures.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Baginskas A, Kuraite V, Kuras A. Phasic nicotinic potentiation of frog retinotectal transmission enhances intrinsic activity of tectum column. Neurosci Res. 2012;74:42–47. doi: 10.1016/j.neures.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Baro JA, Hughes HC, Peck CK. Express saccades in cat: effects of task and target modality. Exp Brain Res. 1995;103:209–17. doi: 10.1007/BF00231707. [DOI] [PubMed] [Google Scholar]
- Basso MA, Sommer MA. Exploring the role of the substantia nigra pars reticulata in eye movements. Neuroscience. 2011;198:205–12. doi: 10.1016/j.neuroscience.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature. 1997;389:66–69. doi: 10.1038/37975. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–34. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci. 2002;22:1883–94. doi: 10.1523/JNEUROSCI.22-05-01883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Appell PP. Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J Comp Neurol. 1992;315:230–43. doi: 10.1002/cne.903150209. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. The nigral projection to predorsal bundle cells in the superior colliculus of the rat. J Comp Neurol. 1992;319:11–33. doi: 10.1002/cne.903190105. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Zhau N, Krahe TE, Govindaiah G, Guido W. Retinal and tectal “driver-like” inputs converge in the shell of the mouse lateral geniculate nucleus. J Neurosci. 2015;35:10523–34. doi: 10.1523/JNEUROSCI.3375-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol. 1997;504:629–39. doi: 10.1111/j.1469-7793.1997.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch R, Fischer B, Ramsperger E. Express-saccades of the monkey: reaction times versus intensity, size, duration and eccentricity of their targets. Exp Brain Res. 1984;55:223–31. doi: 10.1007/BF00237273. [DOI] [PubMed] [Google Scholar]
- Borra E, Gerbella M, Rozzi S, Tonelli S, Luppino G. Projections to the superior colliculus from inferior parietal, ventral premotor, and ventrolateral prefrontal areas involved in controlling goal-directed hand actions in the macaque. Cereb Cortex. 2014;24:1054–65. doi: 10.1093/cercor/bhs392. [DOI] [PubMed] [Google Scholar]
- Bowling DB, Michael CR. Projection patterns of single physiologically characterized optic tract fibres in cat. Nature. 1980;286:899–902. doi: 10.1038/286899a0. [DOI] [PubMed] [Google Scholar]
- Boynton GM. A framework for describing the effects of attention on visual responses. Vis Res. 2009;49:1129–43. doi: 10.1016/j.visres.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Singer W, Engel AK. Amplitude and direction of saccadic eye movements depend on the synchronicity of collicular population activity. J Neurophysiol. 2004;92:424–32. doi: 10.1152/jn.00639.2003. [DOI] [PubMed] [Google Scholar]
- Butler BE, Chabot N, Lomber SG. A quantitative comparison of the hemispheric, areal, and laminar origins of sensory and motor cortical projections to the superior colliculus of the cat. J Comp Neurol. 2016;524:2623–42. doi: 10.1002/cne.23980. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–83. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Diamond IT. Ablation study of the superior colliculus in the tree shrew (Tupaia glis) J Comp Neurol. 1974;156:207–37. doi: 10.1002/cne.901560206. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF. Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science. 1972;177:444–47. doi: 10.1126/science.177.4047.444. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Alvarez BD, Wurtz RH. Enhanced performance with brain stimulation: attentional shift or visual cue? J Neurosci. 2006;26:11347–58. doi: 10.1523/JNEUROSCI.2376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–43. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Rhoades RW. Responses of visual, somatosensory, and auditory neurones in the golden hamster’s superior colliculus. J Physiol. 1977;270:595–626. doi: 10.1113/jphysiol.1977.sp011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM, Thierry AM, Feger J. The nigro-tectal pathway. An electrophysiological reinvestigation in the rat. Brain Res. 1981;213:253–63. doi: 10.1016/0006-8993(81)90232-8. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–91. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;23:319–49. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol. 2002;88:2000–18. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- Courjon J-H, Zénon A, Clément G, Urquizar C, Olivier E, Pélisson D. Electrical stimulation of the superior colliculus induces non-topographically organized perturbation of reaching movements in cats. Front Syst Neurosci. 2015;9:109. doi: 10.3389/fnsys.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Basso MA. Presented at Annu Meet Soc for Neurosci. Washington, DC: 2014. Nov, Sensory priming influences form-based perceptual decision-making and superior colliculus population activity; pp. 15–19. [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–47. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience. 1992;46:361. doi: 10.1016/0306-4522(92)90058-a. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis Res. 1996;36:1827–37. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975;38:690–713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Dunn CA, Colby CL. Representation of the ipsilateral visual field by neurons in the macaque lateral intraparietal cortex depends on the forebrain commissures. J Neurophysiol. 2010;104:2624–33. doi: 10.1152/jn.00752.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CA, Hall NJ, Colby CL. Spatial updating in monkey superior colliculus in the absence of the fore-brain commissures: dissociation between superficial and intermediate layers. J Neurophysiol. 2010;104:1267–85. doi: 10.1152/jn.00675.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JA, Goldberg ME. Dependence of saccade-related activity in the primate superior colliculus on visual target presence. J Neurophysiol. 2001;86:676–91. doi: 10.1152/jn.2001.86.2.676. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Goldberg ME. Saccade-related activity in the primate superior colliculus depends on the presence of local landmarks at the saccade endpoint. J Neurophysiol. 2003;90:1728–36. doi: 10.1152/jn.00016.2003. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Keller EL. Dependence on target configuration of express saccade-related activity in the primate superior colliculus. J Neurophysiol. 1998;80:1407–26. doi: 10.1152/jn.1998.80.3.1407. [DOI] [PubMed] [Google Scholar]
- Edwards S. The deep cell layers of the superior colliculus: their reticular characteristics and structural organization. In: Hobson JA, Brazier MAB, editors. The Reticular Formation Revisited. New York: Raven Press; 1980. pp. 193–209. [Google Scholar]
- Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol. 1979;184:309–30. doi: 10.1002/cne.901840207. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–97. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Endo T, Isa T. Functionally different AMPA-type glutamate receptors in morphologically identified neurons in rat superficial superior colliculus. Neuroscience. 2001;108:129–41. doi: 10.1016/s0306-4522(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Endo T, Yanagawa Y, Obata K, Isa T. Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus. J Neurophysiol. 2005;94:3893–902. doi: 10.1152/jn.00211.2005. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Meister M. Orientation columns in the mouse superior colliculus. Nature. 2015;519:229–32. doi: 10.1038/nature14103. [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron. 2008;60:137–48. doi: 10.1016/j.neuron.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JM. Global visual processing for saccadic eye movements. Vis Res. 1982;22:1033–45. doi: 10.1016/0042-6989(82)90040-2. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express-saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res. 1984;57:191–95. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–70. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gale SD, Murphy GJ. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J Neurosci. 2014;34:13458–71. doi: 10.1523/JNEUROSCI.2768-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Murphy GJ. Active dendritic properties and local inhibitory input enable selectivity for object motion in mouse superior colliculus neurons. J Neurosci. 2016;36:9111–23. doi: 10.1523/JNEUROSCI.0645-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Desimone R. Responses of cells in the superior colliculus during performance of a spatial attention task in the macaque. Rev Bras Biol. 1996;56:257–79. [PubMed] [Google Scholar]
- Ghitani N, Bayguinov PO, Basso MA, Jackson MB. A sodium afterdepolarization in rat superior colliculus neurons and its contribution to population activity. J Neurophysiol. 2016;116:191–200. doi: 10.1152/jn.01138.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, Basso MA. Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. J Neurosci. 2014;34:6822–33. doi: 10.1523/JNEUROSCI.3137-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose D, Maier A, Nidiffer A, Wallace MT. Multisensory response modulation in the superficial layers of the superior colliculus. J Neurosci. 2014;34:4332–44. doi: 10.1523/JNEUROSCI.3004-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–45. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Representation of averaging saccades in the superior colliculus of the monkey. Exp Brain Res. 1993;95:429–35. doi: 10.1007/BF00227135. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Bracewell RM, Andersen RA. Sensorimotor transformation during eye movements to remembered visual targets. Vis Res. 1991;31:693–715. doi: 10.1016/0042-6989(91)90010-3. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Mysore SP, Bryant AS, Huguenard JR, Knudsen EI. Spatially reciprocal inhibition of inhibition within a stimulus selection network in the avian midbrain. PLOS ONE. 2014;9:e85865. doi: 10.1371/journal.pone.0085865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard CA, Sridharan D, Huguenard JR, Knudsen EI. Gamma oscillations are generated locally in an attention-related midbrain network. Neuron. 2012;73:567–80. doi: 10.1016/j.neuron.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J Neurophysiol. 1972a;35:542–59. doi: 10.1152/jn.1972.35.4.542. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972b;35:560–74. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- Graham J, Lin C-S, Kaas JH. Subcortical projections of six visual cortical areas in the owl monkey, Aotus trivirgatus. J Comp Neurol. 1979;187:557–80. doi: 10.1002/cne.901870307. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res. 1978;145:365–74. doi: 10.1016/0006-8993(78)90870-3. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- Groh JM, Sparks DL. Saccades to somatosensory targets. II. Motor convergence in primate superior colliculus. J Neurophysiol. 1996;75:428–38. doi: 10.1152/jn.1996.75.1.428. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–78. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Chen C-Y. Sharper, stronger, faster upper visual field representation in primate superior colliculus. Curr Biol. 2016;26:1647–58. doi: 10.1016/j.cub.2016.04.059. [DOI] [PubMed] [Google Scholar]
- Hall NJ, Colby CL. Express saccades and superior colliculus responses are sensitive to short-wavelength cone contrast. PNAS. 2016;113:6743–48. doi: 10.1073/pnas.1600095113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol. 1989;287:495–514. doi: 10.1002/cne.902870408. [DOI] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1977;173:583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Harting JK, Feig S, Van Lieshout DP. Cortical somatosensory and trigeminal inputs to the cat superior colliculus: light and electron microscopic analyses. J Comp Neurol. 1997;38:313–26. doi: 10.1002/(sici)1096-9861(19971117)388:2<313::aid-cne9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Hashikawa T, van Lieshout DP. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J Comp Neurol. 1991;304:275–306. doi: 10.1002/cne.903040210. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, Van Lieshout DP. Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol. 1992;324:379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- Helms MC, Ozen G, Hall WC. Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. J Neurophysiol. 2004;91:1706–15. doi: 10.1152/jn.00705.2003. [DOI] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior colliculus control of vibrissa movements. J Neurophysiol. 2008;100:1245–54. doi: 10.1152/jn.90478.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess WR, Burgi S, Bucher V. Motorisch Funktion des Tektal und Temgentalgebietes. Monatsschr Psychiatr Neurol. 1946;112:1–52. [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–78. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–91. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hormigo S, Vega-Flores G, Castro-Alamancos MA. Basal ganglia output controls active avoidance behavior. J Neurosci. 2016;36:10274–84. doi: 10.1523/JNEUROSCI.1842-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol. 2004;91:2281. doi: 10.1152/jn.00872.2003. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–61. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an on-off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H, editor. Comparative Neurology of the Optic Tectum. New York: Plenum Publ; 1984. pp. 687–773. [Google Scholar]
- Illing R-B, Graybiel AM. Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cat’s superior colliculus. Neuroscience. 1985;14:455–82. doi: 10.1016/0306-4522(85)90303-3. [DOI] [PubMed] [Google Scholar]
- Illing R-B, Graybiel AM. Complementary and non-matching afferent compartments in the cat’s superior colliculus: innervation of the acetylcholinesterase-poor domain of the intermediate gray layer. Neuroscience. 1986;18:373. doi: 10.1016/0306-4522(86)90160-0. [DOI] [PubMed] [Google Scholar]
- Illing R-B, Vogt DM, Spatz WB. Parvalbumin in rat superior colliculus. Neurosci Lett. 1990;120:197. doi: 10.1016/0304-3940(90)90037-a. [DOI] [PubMed] [Google Scholar]
- Isa T. Intrinsic processing in the mammalian superior colliculus. Curr Opin Neurobiol. 2002;12:668. doi: 10.1016/s0959-4388(02)00387-2. [DOI] [PubMed] [Google Scholar]
- Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci. 1998;18:8496–504. doi: 10.1523/JNEUROSCI.18-20-08496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102:2581–93. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vis Res. 2000;40:1489–506. doi: 10.1016/s0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Auditory receptive fields in primate superior colliculus shift with changes in eye position. Nature. 1984;309:345–47. doi: 10.1038/309345a0. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Phongphanphanee P, Katoh T, Isa K, Yanagawa Y, et al. Regulation of burst activity through presynaptic and postsynaptic GABAB receptors in mouse superior colliculus. J Neurosci. 2008;28:816–27. doi: 10.1523/JNEUROSCI.4666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardamakis AA, Saitoh K, Grillner S. Tectal microcircuit generating visual selection commands on gaze-controlling neurons. PNAS. 2015;112:E1956–65. doi: 10.1073/pnas.1504866112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Dubbeldam JL. The organization and projections of the paleostriatal complex in the pigeon (columba livia) J Comp Neurol. 1973;148:61–89. doi: 10.1002/cne.901480105. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–55. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WM. Nicotinic depolarization of optic nerve terminals augments synaptic transmission. Brain Res. 1990;527:150–54. doi: 10.1016/0006-8993(90)91074-q. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4:627–32. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Auditory and visual maps of space in the optic tectum of the owl. J Neurosci. 1982;2:1177–94. doi: 10.1523/JNEUROSCI.02-09-01177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci. 2011;33:1961–72. doi: 10.1111/j.1460-9568.2011.07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Brainard MS. Visual instruction of the neural map of auditory space in the developing optic tectum. Science. 1991;253:86–87. doi: 10.1126/science.2063209. [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol. 1985;4:219–27. [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vis Res. 1995;35:1897–916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kowler E, Blaser E. The accuracy and precision of saccades to small and large targets. Vis Res. 1995;35:1741–54. doi: 10.1016/0042-6989(94)00255-k. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol. 2000;84:876–91. doi: 10.1152/jn.2000.84.2.876. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Bollimunta A, Arcizet F, Wang L. Attention as an effect not a cause. Trends Cogn Sci. 2014;18:457–64. doi: 10.1016/j.tics.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron. 2002;35:355–63. doi: 10.1016/s0896-6273(02)00756-0. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zénon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–82. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer TP, Lund RD. The upper layers of the superior colliculus of the rat: a Golgi study. J Comp Neurol. 1974;158:405–35. doi: 10.1002/cne.901580404. [DOI] [PubMed] [Google Scholar]
- Lee J, Maunsell JH. A normalization model of attentional modulation of single unit responses. PLOS ONE. 2009;4:e4651. doi: 10.1371/journal.pone.0004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Hall WC. Interlaminar connections of the superior colliculus in the tree shrew. II: projections from the superficial gray to the optic layer. Vis Neurosci. 1995;12:573–88. doi: 10.1017/s0952523800008464. [DOI] [PubMed] [Google Scholar]
- Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. J Neurosci. 2006;26:4763–68. doi: 10.1523/JNEUROSCI.0724-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. PNAS. 1997;94:13299–304. doi: 10.1073/pnas.94.24.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Schmidt M, Hall WC. Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J Neurosci. 2001;21:8145–53. doi: 10.1523/JNEUROSCI.21-20-08145.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, Hall WC. Identity of a pathway for saccadic suppression. PNAS. 2007;104:6824–27. doi: 10.1073/pnas.0701934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci. 2005;25:11357–73. doi: 10.1523/JNEUROSCI.3825-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci. 2008;28:4561–77. doi: 10.1523/JNEUROSCI.5683-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C-C, Wang X-J. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci. 2006;9:956. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–66. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo TZ, Maunsell JHR. Neuronal modulations in visual cortex are associated with only one of multiple components of attention. Neuron. 2015;86:1182–88. doi: 10.1016/j.neuron.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010;65:270–79. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Beck JM, Latham PE, Pouget A. Bayesian inference with probabilistic population codes. Nat Neurosci. 2006;9:1432. doi: 10.1038/nn1790. [DOI] [PubMed] [Google Scholar]
- Mana S, Chevalier G. The fine organization of nigro-collicular channels with additional observations of their relationships with acetylcholinesterase in the rat. Neuroscience. 2001;106:357. doi: 10.1016/s0306-4522(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Marín G, Mpdozis J, Sentis E, Ossandón T, Letelier JC. Oscillatory bursts in the optic tectum of birds represent re-entrant signals from the nucleus isthmi pars parvocellularis. J Neurosci. 2005;25:7081–89. doi: 10.1523/JNEUROSCI.1379-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J Neurosci. 2007;27:8112–21. doi: 10.1523/JNEUROSCI.1420-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR. Neuronal mechanisms of visual attention. Annu Rev Vis Sci. 2015;1:373–91. doi: 10.1146/annurev-vision-082114-035431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–78. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Mays LE, Sparks DL. Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol. 1980;43:207–32. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–41. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JW, Kowler E, Sharma A, Chubb C. Saccadic localization of random dot targets. Vis Res. 1998;38:895. doi: 10.1016/s0042-6989(97)00232-0. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–7. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res. 1982;247:243–53. doi: 10.1016/0006-8993(82)91249-5. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Point images in the visual system: new interest in an old idea. Trends Neurosci. 1986;9:354–58. [Google Scholar]
- McIlwain JT. Distributed spatial coding in the superior colliculus: a review. Vis Neurosci. 1991;6:3–13. doi: 10.1017/s0952523800000857. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–63. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Melcher D, Kowler E. Shapes, surfaces and saccades. Vis Res. 1999;39:2929. doi: 10.1016/s0042-6989(99)00029-2. [DOI] [PubMed] [Google Scholar]
- Middlebrooks J, Knudsen E. A neural code for auditory space in the cat’s superior colliculus. J Neurosci. 1984;4:2621–34. doi: 10.1523/JNEUROSCI.04-10-02621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RD, Nikoletseas MM, Hess PR, Allen Z, Lewin AC, Rhoades RW. The projection from the superficial to the deep layers of the superior colliculus: an intracellular horseradish peroxidase injection study in the hamster. J Neurosci. 1988a;8:1384–99. doi: 10.1523/JNEUROSCI.08-04-01384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RD, Nikoletseas MM, Ruiz SA, Rhoades RW. Receptive-field properties and morphological characteristics of the superior collicular neurons that project to the lateral posterior and dorsal lateral geniculate nuclei in the hamster. J Neurophysiol. 1988b;59:1333–51. doi: 10.1152/jn.1988.59.5.1333. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Highstein SM. The anatomy and physiology of primate neurons that control rapid eye movements. Annu Rev Neurosci. 1994;17:465–88. doi: 10.1146/annurev.ne.17.030194.002341. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. I. Morphological classification of efferent neurons. J Neurophysiol. 1988a;60:232–62. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus. II. Morphological identification of presaccadic neurons. J Neurophysiol. 1988b;60:263–302. doi: 10.1152/jn.1988.60.1.263. [DOI] [PubMed] [Google Scholar]
- Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. PNAS. 2005;102:524–29. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol. 1995;73:2313–33. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- Mysore SP, Knudsen EI. Descending control of neural bias and selectivity in a spatial attention network: rules and mechanisms. Neuron. 2014;84:214–26. doi: 10.1016/j.neuron.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni AM, Ray S, Maunsell JHR. Tuned normalization explains the size of attention modulations. Neuron. 2012;73:803–13. doi: 10.1016/j.neuron.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela SU, Krauzlis RJ. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. J Neurophysiol. 2010;104:1538–48. doi: 10.1152/jn.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JA, Eggermont JJ. Metrics of saccade responses to visual double stimuli: two different modes. Vis Res. 1984;24:1169–79. doi: 10.1016/0042-6989(84)90172-x. [DOI] [PubMed] [Google Scholar]
- Özen G, Helms MC, Hall WC. The intracollicular neuronal network. In: Hall WC, Moschovakis A, editors. The Superior Colliculus: New Approaches for Studying Sensorimotor Integration. Boca Raton, FL: CRC Press; 2004. pp. 147–58. [Google Scholar]
- Palmer AR, King AJ. The representation of auditory space in the mammalian superior colliculus. Nature. 1982;299:248–49. doi: 10.1038/299248a0. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Guitton D, Munoz DP. Superior colliculus and feedback control of gaze shift in the head-free cat. In: Schmid R, Zambarbieri D, editors. Oculomotor Control and Cognitive Processes. Amsterdam: Elsevier Sci. Publ BV; 1991. pp. 213–28. [Google Scholar]
- Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, Hall WC. A circuit model for saccadic suppression in the superior colliculus. J Neurosci. 2011;31:1949–54. doi: 10.1523/JNEUROSCI.2305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–24. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J Neurophysiol. 2003;90:1887–903. doi: 10.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- Pouget A, Dayan P, Zemel RS. Inference and computation with population codes. Annu Rev Neurosci. 2003;26:381–410. doi: 10.1146/annurev.neuro.26.041002.131112. [DOI] [PubMed] [Google Scholar]
- Pouget A, Deneve S, Ducom J-C, Latham P. Narrow versus wide tuning curves: What’s best for a population code? Neural Comp. 1999;11:85–90. doi: 10.1162/089976699300016818. [DOI] [PubMed] [Google Scholar]
- Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain. 2008;132:309–18. doi: 10.1093/brain/awn322. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR. Do gamma oscillations play a role in cerebral cortex? Trends Cogn Sci. 2015;19:78–85. doi: 10.1016/j.tics.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: the vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–23. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–47. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–85. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Rohrer WH, Nikoletseas MM, Fish SE. Organization of the projection from the superficial to the deep layers of the hamster’s superior colliculus as demonstrated by the anterograde transport of Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;283:54–70. doi: 10.1002/cne.902830106. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Grupp LA, Pisa M. Inhibition of visual responses of single units in the cat superior colliculus by the introduction of a second visual stimulus. Brain Res. 1973;61:390–94. doi: 10.1016/0006-8993(73)90544-1. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Grupp LA, Pisa M. Inhibitory effect of remote visual stimuli on visual response of cat superior colliculus: spatial and temporal factors. J Neurophysiol. 1974;37:1262–75. doi: 10.1152/jn.1974.37.6.1262. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vis Res. 1972;12:1795–808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Rodgers CK, Munoz DP, Scott SH, Pare M. Discharge properties of monkey tectoreticular neurons. J Neurophysiol. 2006;95:3502–11. doi: 10.1152/jn.00908.2005. [DOI] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–89. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll A, Wierzbicka MM, Wolf W. The “gap paradigm” leads to express-like saccadic reaction times in Parkinson’s disease. Exp Brain Res. 1996;111:131–38. doi: 10.1007/BF00229562. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Stein BE. A model of the temporal dynamics of multisensory enhancement. Neurosci Biobehav Rev. 2014;41:78–84. doi: 10.1016/j.neubiorev.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GM, Schneider GE. The morphology of optic tract axons arborizing in the superior colliculus of the hamster. J Comp Neurol. 1984;230:155–67. doi: 10.1002/cne.902300202. [DOI] [PubMed] [Google Scholar]
- Saito Y, Isa T. Electrophysiological and morphological properties of neurons in the rat superior colliculus. I. Neurons in the intermediate layers. J Neurophysiol. 1999;82:754–67. doi: 10.1152/jn.1999.82.2.754. [DOI] [PubMed] [Google Scholar]
- Saito Y, Isa T. Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus. J Neurosci. 2003;23:5854–64. doi: 10.1523/JNEUROSCI.23-13-05854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH. The superior colliculus and visual function. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology. Philadelphia: Lippincott Williams and Wilkins; 1984. pp. 457–505. [Google Scholar]
- Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1972;35:915–24. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker M, Cynader M, Berman N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J Neurophysiol. 1974;37:181–94. doi: 10.1152/jn.1974.37.1.181. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Ulinski PS. Caudal topographic nucleus isthmi and the rostral nontopographic nucleus isthmi in the turtle, Pseudemys scripta. J Comp Neurol. 1987;261:319–46. doi: 10.1002/cne.902610302. [DOI] [PubMed] [Google Scholar]
- Shang C, Liu Z, Chen Z, Shi Y, Wang Q, et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science. 2015;348:1472–77. doi: 10.1126/science.aaa8694. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Exp Brain Res. 1994;98:507–22. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Spatial attention and eye movements. Exp Brain Res. 1995;105:261–75. doi: 10.1007/BF00240962. [DOI] [PubMed] [Google Scholar]
- Sohal VS. Insights into cortical oscillations arising from optogenetic studies. Biol Psychiatry. 2012;71:1039–45. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA. Express saccades elicited during visual scan in the monkey. Vis Res. 1994;34:2023–38. doi: 10.1016/0042-6989(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Response properties of eye movement-related neurons in the monkey superior colliculus. Brain Res. 1975;90:147–52. doi: 10.1016/0006-8993(75)90690-3. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Functional properties of neurons in the monkey superior colliculus: coupling of neuronal activity and saccade onset. Brain Res. 1978;156:1–16. doi: 10.1016/0006-8993(78)90075-6. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Neural cartography: sensory and motor maps in the superior colliculus. Brain Behav Evol. 1988;31:49–56. doi: 10.1159/000116575. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev Oculomot Res. 1989;3:213–55. [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Signal transformations required for the generation of saccadic eye movements. Annu Rev Neurosci. 1990;13:309–36. doi: 10.1146/annurev.ne.13.030190.001521. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–40. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol. 1996;76:3360–81. doi: 10.1152/jn.1996.76.5.3360. [DOI] [PubMed] [Google Scholar]
- Stein BE, Clamann HP. Control of pinna movements and sensorimotor register in cat superior colliculus. Brain Behav Evol. 1981;19:180–92. doi: 10.1159/000121641. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Syka J, Radil-Weiss T. Electrical stimulation of the tectum in freely moving cats. Brain Res. 1971;28:567–72. doi: 10.1016/0006-8993(71)90068-0. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Uhlrich DJ, Sherman SM. Morphology of physiologically identified retinal X and Y axons in the cat’s thalamus and midbrain as revealed by intraaxonal injection of biocytin. J Comp Neurol. 1995;354:583–607. doi: 10.1002/cne.903540408. [DOI] [PubMed] [Google Scholar]
- Taniguchi H. Genetic dissection of GABAergic neural circuits in mouse neocortex. Front Cell Neurosci. 2014;8:8. doi: 10.3389/fncel.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif E, Delacuisine B, Probst A, Clarke S. Intrinsic connectivity of human superior colliculus. Exp Brain Res. 2005;166:316–24. doi: 10.1007/s00221-005-2373-z. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS. Two converging brainstem pathways mediating circling behavior. Brain Res. 1986;385:329–42. doi: 10.1016/0006-8993(86)91080-2. [DOI] [PubMed] [Google Scholar]
- Thevarajah D, Mikulic A, Dorris MC. Role of the superior colliculus in choosing mixed-strategy saccades. J Neurosci. 2009;29:1998–2008. doi: 10.1523/JNEUROSCI.4764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titmus MJ, Tsai HJ, Lima R, Udin SB. Effects of choline and other nicotinic agonists on the tectum of juvenile and adult Xenopus frogs: a patch-clamp study. Neuroscience. 1999;91:753–69. doi: 10.1016/s0306-4522(98)00625-3. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JAM, Smit AC. Comparison of saccades evoked by visual stimulation and collicular electrical stimulation in the alert monkey. Exp Brain Res. 1990;79:299–312. doi: 10.1007/BF00608239. [DOI] [PubMed] [Google Scholar]
- Vidal PP, May PJ, Baker R. Synaptic organization of the tectal-facial pathways in the cat. I. Synaptic potentials following collicular stimulation. J Neurophysiol. 1988;60:769–97. doi: 10.1152/jn.1988.60.2.769. [DOI] [PubMed] [Google Scholar]
- Vokoun CR, Huang X, Jackson MB, Basso MA. Response normalization in the superficial layers of the superior colliculus as a possible mechanism for saccadic averaging. J Neurosci. 2014;34:7976–87. doi: 10.1523/JNEUROSCI.3022-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokoun CR, Jackson MB, Basso MA. Intralaminar and interlaminar activity within the rodent superior colliculus visualized with voltage imaging. J Neurosci. 2010;30:10667–82. doi: 10.1523/JNEUROSCI.1387-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69:1797–809. doi: 10.1152/jn.1993.69.6.1797. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu M, Segraves MA, Cang J. Visual experience is required for the development of eye movement maps in the mouse superior colliculus. J Neurosci. 2015;35:12281–86. doi: 10.1523/JNEUROSCI.0117-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-R. The nucleus isthmi and dual modulation of the receptive field of tectal neurons in non-mammals. Brain Res Rev. 2003;41:13–25. doi: 10.1016/s0165-0173(02)00217-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol. 2006;494:7–35. doi: 10.1002/cne.20821. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol. 2005;93:519–34. doi: 10.1152/jn.00558.2004. [DOI] [PubMed] [Google Scholar]
- Westby GW, Collinson C, Redgrave P, Dean P. Opposing excitatory and inhibitory influences from the cerebellum and basal ganglia converge on the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci. 1994;6:1335–42. doi: 10.1111/j.1460-9568.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- White BJ, Boehnke SE, Marino RA, Itti L, Munoz DP. Color-related signals in the primate superior colliculus. J Neurosci. 2009;29:12159–66. doi: 10.1523/JNEUROSCI.1986-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren BG. Superior colliculus: some receptive field properties of bimodally responsive cells. Science. 1971;173:69–72. doi: 10.1126/science.173.3991.69. [DOI] [PubMed] [Google Scholar]
- Wise LZ, Irvine DR. Auditory response properties of neurons in deep layers of cat superior colliculus. J Neurophysiol. 1983;49:674–85. doi: 10.1152/jn.1983.49.3.674. [DOI] [PubMed] [Google Scholar]
- Wolf AB, Lintz MJ, Costabile JD, Thompson JA, Stubblefield EA, Felsen G. An integrative role for the superior colliculus in selecting targets for movements. J Neurophysiol. 2015;114:2118–31. doi: 10.1152/jn.00262.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS. What attributes guide the deployment of visual attention and how do they do it? Nat Rev Neurosci. 2004;5:495–501. doi: 10.1038/nrn1411. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey: III. Cells discharging before eye movements. J Neurophysiol. 1972;35:575–86. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–37. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]


