Abstract
Transcranial Alternating Current Stimulation (tACS) is a neuromodulatory technique able to act through sinusoidal electrical waveforms in a specific frequency and in turn modulate ongoing cortical oscillatory activity. This neurotool allows the establishment of a causal link between endogenous oscillatory activity and behavior. Most of the tACS studies have shown online effects of tACS. However, little is known about the underlying action mechanisms of this technique because of the AC-induced artifacts on Electroencephalography (EEG) signals. Here we show a unique approach to investigate online physiological frequency-specific effects of tACS of the primary motor cortex (M1) by using single pulse Transcranial Magnetic Stimulation (TMS) to probe cortical excitability changes. In our setup, the TMS coil is placed over the tACS electrode while Motor Evoked Potentials (MEPs) are collected to test the effects of the ongoing M1-tACS. So far, this approach has mainly been used to study the visual and motor systems. However, the current tACS-TMS setup can pave the way for future investigations of cognitive functions. Therefore, we provide a step-by-step manual and video guidelines for the procedure.
Keywords: Neuroscience, Issue 127, tACS, TMS, primary motor cortex, oscillatory activity, MEPs, tACS-TMS, tES, neuromodulation, beta frequency, 20 Hz
Introduction
Transcranial Electrical Stimulation (tES) is a neuromodulatory technique which allows the modification of neuronal states through different current waveforms1. Among different types of tES, transcranial Alternating Current Stimulation (tACS) enables the delivery of sinusoidal external oscillatory potentials in a specific frequency range and the modulation of physiological neural activity underlying perceptual, motor and cognitive processes2. Using tACS, it is possible to investigate potential causal links between endogenous oscillatory activity and brain processes.
In vivo, it has been shown that spiking neural activity is synchronized at different driving frequencies, suggesting that neuronal firing can be entrained by electrically applied fields3. In animal models, weak sinusoidal tACS entrains the discharged frequency of the widespread cortical neuronal pool4. In humans, tACS combined with online Electroencephalography (EEG) allows the induction of the so-called "Entrainment" effect on endogenous oscillatory activity by interacting with brain oscillations in a frequency-specific manner5. However, combining tACS with neuroimaging methods for a better understanding of the online mechanisms is still questionable because of AC-induced artifacts6. In addition, it is not possible to directly record the EEG signal over the stimulated target area without using a ring-like electrode which is a questionable solution7. Thus, there is a lack of systematic studies on this topic.
So far, there is no clear evidence about the lasting effects of tACS after stimulation cessation. Only a few studies have shown weak and unclear after-effects of tACS on the motor system8. Moreover, EEG evidence is still not clear about the after-effects of tACS9. On the other hand, most tACS studies showed prominent online effects10,11,12,13,14,15,16,17,18, which are difficult to measure at a physiological level because of technical constraints. Thus, the overall goal of our method is to provide an alternative approach to test online and frequency-dependent effects of tACS on the motor cortex (M1) by delivering single pulse Transcranial Magnetic Stimulation (TMS). TMS allows researchers to "probe" the physiological state of the human motor cortex19. Moreover, by recording Motor Evoked Potentials (MEP) on the subject's contralateral hand, we can investigate the effects of the ongoing tACS11. This approach lets us accurately monitor changes in corticospinal excitability by measuring MEP amplitude during online electrical stimulation delivered at different frequencies in an artifact-free fashion. In addition, this approach can also test online effects of any other waveform of tES.
To demonstrate the combined tACS-TMS effects, we will show the protocol by applying 20 Hz AC stimulation over the primary motor cortex (M1) while online neuronavigated single pulse TMS is delivered interspersed by random intervals from 3 to 5 s in order to test M1 cortical excitability.
Protocol
All procedures were approved by the local research ethics committee of the Higher School of Economics (HSE), Moscow, with consent from all participants.
NOTE: Participants must report no history of implanted metal devices, neurological or psychiatric disease, drug abuse or alcoholism. TMS is used according to the most recent safety guidelines20. Subjects must be fully informed of the nature of the research and sign an informed consent form before starting the experiment. We show an entire set of equipment needed to run the online-combined tACS-TMS protocol by stimulation of the dominant M1 ( Figure 1; Table of Materials).
1. Place Electromyography (EMG) Electrodes in a Bipolar Belly-tendon Montage
Clean the skin using a cleaning scrub under all the electrodes to achieve low skin impedance (below 10 kOhm).
Place the active EMG electrode on the first dorsal interosseous (FDI) muscle, reference electrode on the bone 2 cm distally and the ground electrode more proximally on the arm.
2. Identifying the Target for the Stimulation Protocol
NOTE: Here, we use the frameless TMS navigation system to achieve a proper positioning of the TMS coil.
Place the tracking sensors over the glabella between the eyebrows and above the nose of the participant.
Open the navigation system software. Use individual participants' structural T1 Magnetic Resonance Imaging (MRI) data and perform a co-registration of the participant's head and a 3D MRI head via the navigation system.
Accurately, place the coil over the primary motor hand-area, the so called "motor knob" region (Figure 2).
Start applying single pulse TMS and test MEPs; TMS is delivered by a stimulator (see Table of Materials) connected to a standard figure-of-eight 75-mm coil. To localize the "hotspot" of the left M1, hold the coil tangential to the scalp, with the handle pointing backward and laterally, angled at 45° from the midline sagittal axis of the participant's head.
Once the hotspot (i.e., the scalp point eliciting MEPs at threshold from the contralateral examined hand muscles) is found, mark it with a pencil to facilitate the application of the tACS target electrode.
3. tACS Electrodes Preparation
Connect 2 surface saline-soaked sponge electrodes (size: 5 cm x 7 cm) to the stimulation device, which can generate electrical alternating current (e.g., Brainstim).
In order to minimize skin sensation, constantly saturate the electrodes with a saline solution to keep impedances below 10 kOhm throughout the whole stimulation session.
4. tACS Protocol Set Up
To set up the tACS protocol using the stimulator device, first check the battery status.
- Using the software, open a new session and manage a new stimulation protocol.
- Name the protocol (e.g., "Beta").
- Set the frequency of the stimulation (e.g., 20 Hz).
- Choose the waveform (e.g., sinusoidal).
- Set the total duration of the stimulation protocol (e.g., 600 s).
- Finally, set the intensity of stimulation (e.g., 1 mA), set offset, fade in, fade out, and phase at "0". NOTE: A little timing to fade in and out the stimulation (around 30 s) can be suggested, in order to avoid any adverse or uncomfortable neurosensory effects for the subject.
- Activate the device's "Bluetooth" function and upload the protocol from the software to the stimulator.
5. tACS Electrodes Montage
Place the "target" electrode over the scalp corresponding to the marked point. Place the "reference" electrode over the ipsilateral shoulder by using specific sticky tape, in a "monopolar montage"21.
Carefully adjust the first elastic strap on the head with respect to the neuro-navigation head-sensors position. Then, by using the second strap, fix the target electrode position.
Once tACS electrodes are placed both on the scalp and on the ipsilateral shoulder, connect them to the stimulator.
Before the start of the stimulation session, ensure by visual inspection that position of the target electrode is centered over the marked hotspot.
6. Identifying the Resting Motor Threshold (RMT)
Place the TMS coil over the target tACS electrode and carefully adjust the coil position over the hotspot (Figure 3) by using the neuro-navigation system.
- Measure the RMT accordingly to the combined tACS-TMS setup (i.e., TMS coil over the electrode). Specifically, adjust the TMS intensity with respect to the thickness of the tACS electrode in order to check for a reliable RMT.
- Measure the RMT individually, it is defined as the minimum intensity required to induce an MEP in the FDI muscle with an amplitude of 50 mV (peak-to-peak) in 5 out of 10 trials22.
Set the intensity of the TMS stimulation at 110% of the RMT in order to start the experimental session.

7. Experimental Procedure
Open the EMG software and start EMG recording.
Start the tACS stimulation.
During the stimulation, deliver TMS single pulses interspersed by random intervals from 3 to 5 seconds.
Ensure that each session of the stimulation (e.g., 20 Hz tACS stimulation followed by a sham/another control frequency) lasts no more than 90 seconds with an inter-session interval around 3 minutes, in order to avoid possible carryover effect of the preceding stimulation frequency/condition11,13.
Representative Results
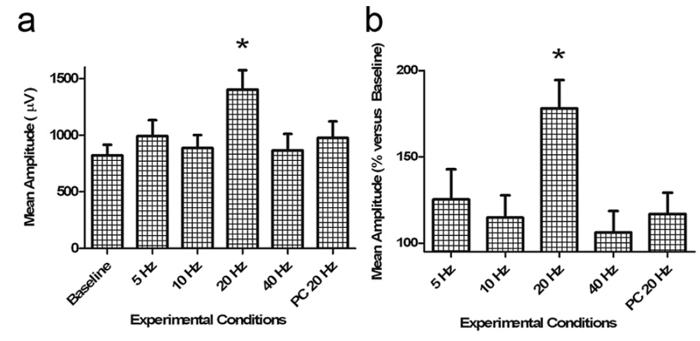
The first evidence of a tACS/TMS combined approach was shown by Kanai et al. in 2010. In that study, the authors applied tACS over the primary visual cortex (V1) and demonstrated a frequency-specific modulation of the visual cortical excitability measured by online TMS-induced phosphene perception15. A more refined version of the protocol was adopted to investigate a physiological modulation of the motor cortex excitability by Feurra et al. in 2011. To do so, these authors recorded MEPs during single pulse TMS while ongoing tACS was delivered (Figure 4). Authors reported the first causal evidence of possible entrainment of 20 Hz stimulation of the endogenous idling Beta rhythm of M1 by enhancing the corticospinal output with respect to other control frequencies, control site (parietal stimulation) and control experiment (peripheral ulnar nerve experiment)11 (Figure 5).
In a following study, Feurra and collaborators showed that effects of tACS are not only frequency but also state-dependent13. By using the same combined montage, tACS was applied over the M1 under two different conditions: rest and motor imagery (subjects were asked to imagine pinch-to grip movements). In line with the previous findings11, only Beta stimulation (20 Hz) enhanced primary motor cortex excitability at rest, while during the motor imagery task the enhancement effect was prominent during theta (5 Hz) and alpha (10 Hz) stimulation. This represented the first physiological evidence of a state-dependent effect by tACS.
To date, this combined approached has been used to further study the functioning of the motor cortex (Table 1). Guerra and collaborators applied tACS-TMS, by using a similar approach, to show how specific interneuronal circuits react to the stimulation delivered at motor frequency (20 Hz) and non-motor resonance frequency (7 Hz). They showed that 20 Hz stimulation abolished the effect of cholinergic short-latency afferent inhibition (SAI), regardless of the phase of the stimulation. Interestingly, changes in glutamatergic intracortical facilitation (ICF) and GABAAergic short-interval intracortical inhibition (SICI) were phase-specific23.
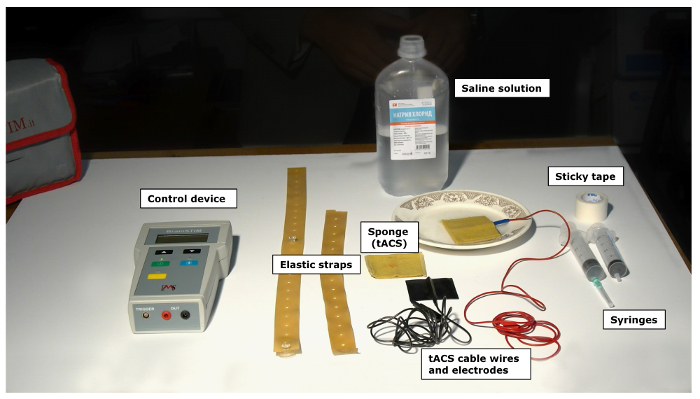
Figure 1: List of the required materials for simultaneous tACS stimulation. Saline solution, control device, elastic straps, sponges (tACS), tACS cable wires and electrodes, syringes, sticky tape. Please click here to view a larger version of this figure.
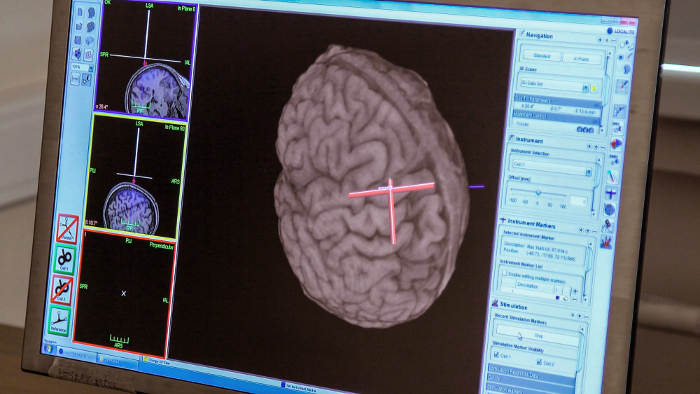
Figure 2: Neuronavigation during the tACS-TMS protocol. The red cross indicates a memorized TMS hot-spot on the primary motor cortex. The overlapping white cross indicates an online positioning of the TMS coil during the protocol, as a sign of correct orientation. Please click here to view a larger version of this figure.
Figure 3: tACS-TMS on the subject's scalp. The TMS coil must be placed over the target tACS electrode. The investigator should maintain the position of the coil in accordance with the neuronavigation coordinates of the hotspot. Please click here to view a larger version of this figure.
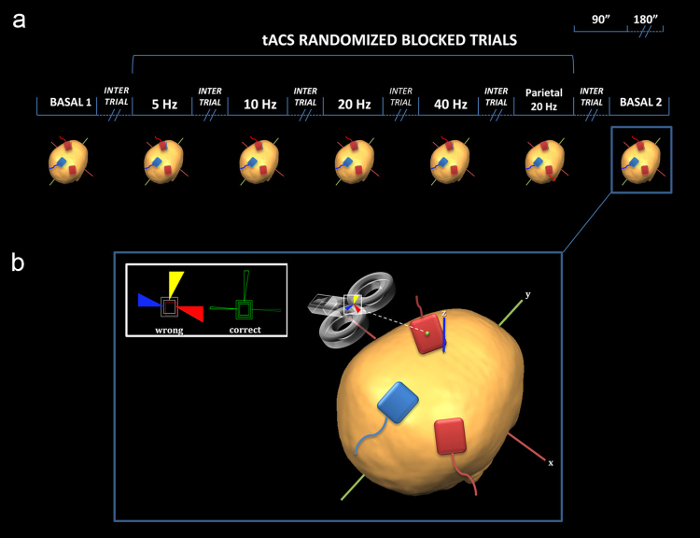
Figure 4: Schematic representation of the experimental design by using the online tACS-TMS approach. (a) Red ("target") electrodes are placed on the scalp overlying the left motor cortex and the right parietal cortex (P4 position of the 10-20 International EEG System). The blue ("reference") electrode is placed on the midline corresponding to the PZ (10 -20 International EEG System) position (bipolar/cephalic montage). Of note, the reference electrode of the current proposal is placed on the ipsilateral shoulder (monopolar montage), while P4 is used as a control site. (b) Neuronavigated TMS: The coil is held on the sponge electrode placed over the left M1. The colored triangles indicate the online feedback of the coil displacement from the exact target, with a tolerance of 2 mm (This figure has been modified from Feurra et al., 2011)11. Please click here to view a larger version of this figure.
Figure 5: Representative results. (a) Average log-transformed MEP amplitude (error bars denote Standard Error) values (raw data) obtained through different experimental conditions. Only tACS delivered at beta range (20 Hz) on the motor cortex increases the corticospinal output versus all the other conditions (baseline, 5 Hz, 10 Hz, 40 Hz, and 20 Hz on the parietal cortex). An asterisk (*) indicates a significant difference of 20 Hz stimulation with respect to all the other conditions. (b) Percentage changes versus baseline of raw MEP amplitude values (This figure has been modified from Feurra et al., 2011)11. Please click here to view a larger version of this figure.
| Authors | Task | Frequency | Intensity | Electrodes position | Results |
| Feurra et al., 2011 | measurements of corticospinal excitability at rest | 5 Hz, 10 Hz, 20 Hz, 40 Hz | 1 mA | Left M1, parietal cortex, ulnar nerve | 20 Hz increased MEPs size at rest |
| Feurra et al., 2013 | measurements of corticospinal excitability at rest and during motor imagery | 5 Hz, 10 Hz, 20 Hz, 40 Hz | 1 mA | Left M1 | 20 Hz increased MEPs size at rest while 5 and 10 Hz increased MEPs size during motor imagery |
| Cancelli et al., 2015 | measurements of corticospinal excitability at rest | 20Hz | 2.2 mA | Bilateral M1 | Differences in cortical excitability enhancement with respect to personalized and non-personalized electrodes |
| Guerra et al., 2016 | measurements of corticospinal excitability at rest | 7 Hz, 20 Hz | 1 mA | Left M1 | 20 Hz tACS modulated SICI, ICF and SAI |
Table 1: tACS effects on the primary motor cortex through different conditions. Frequency, intensity, cortical site of stimulation and results.
Discussion
This approach represents a unique opportunity to directly test online effects of tACS of the primary motor cortex by measuring corticospinal output through MEPs recording. However, the placement of the TMS coil over the tACS electrode represents a critical step that should be accurately performed. Therefore, we would firstly suggest experimenters find a target point by single pulse TMS, then mark it on the scalp and, only after that, place the tACS electrode over the hotspot. Moreover, the availability of a neuronavigation system crucially supports localization of an optimal target point for single pulse TMS. Before starting the procedure, ensure that the participant does not have any contraindications for tES24 and TMS20.
In addition, the thickness and position of the tACS electrode under the TMS coil can lead to a different RMT with respect to a standard procedure. Thus, it is important to measure the RMT when the TMS coil is already positioned over the tACS electrode.
The TMS-tACS online approach represents a technical advance for both basic research and clinical application. Since most of the tACS evidence has shown that effects are prominent during and not after the cessation of the stimulation, this approach may be useful to test online beneficial frequency-specific effects on patients with motor disease, such as essential tremor, dystonia, Parkinson's disease, and other motor diseases.
So far, this combined approach was used to investigate motor and visual cortex processes11,15. However, the tACS itself was shown to be a reliable technique to enhance cognitive functions such as memory and decision making14,16,25,26,27. In the future, the possibility of combining repetitive TMS (rTMS) together with tACS by manipulating different frequencies and targeting different cortical areas may help to investigate the mechanisms of the so-called "neuroenhancement". Likewise, it was already shown that combination of tACS with a patterned TMS protocol, such as continuous theta burst stimulation (cTBS), resulted in an enhanced plasticity effect only when cTBS was applied in-phase with the peak of the tACS-imposed activation28. Furthermore, whereas rTMS is used as a clinical tool, its combination with tACS could lead to the development of a new clinical method for neurorehabilitation.
Although this article is focused on stimulation of M1, other cortical regions may be targeted using this combined approach. However, only stimulation of the human motor system may lead to measurable motor evoked potentials (MEPs) recorded from the peripheral muscles on the contralateral side representing a compound signal from series of descending cortico-spinal volleys with different generators29. On the other hand, other electrode deployments may offer different opportunities to investigate online inter-hemispheric effects by using concomitant bilateral tACS over left and right M1 together with single-pulse TMS. In addition, the online tACS-TMS approach may be used to target different cortical regions during a behavioral task by measuring reaction times (RT) and accuracy. On the one hand, the tACS-TMS approach offers an artifact-free methodology for the investigation of human motor functions; on the other hand, a tACS-EEG approach may offer more possibilities for the study of neural correlates of different cognitive processes by targeting a variety of cortical areas, but still with a greater number of artifacts inside the signal recordings.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by Russian Science Foundation grant (contract number: 17-11-01273). Special thanks to Andrey Afanasov and colleagues from Multifunctional Innovation Centre for Television Technics (National Research University, Higher School of Economics, Moscow, Russian Federation) for video recording and video editing.
References
- Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin. Neurophysiol. 2003;114(4):589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, Struber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum. Neurosci. 2013;7:279. doi: 10.3389/fnhum.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67(1):129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen S, et al. Transcranial electric stimulation entrains cortical neuronal populations in rats. J. Neurosci. 2010;30(34):11476–11485. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, et al. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 2014;24(3):333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Bergmann TO, Karabanov A, Hartwigsen G, Thielscher A, Siebner HR. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: Current approaches and future perspectives. Neuroimage. 2016;140:4–19. doi: 10.1016/j.neuroimage.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Feher KD, Morishima Y. Concurrent Electroencephalography Recording During Transcranial Alternating Current Stimulation (tACS) J. Vis. Exp. 2016. p. e53527. [DOI] [PMC free article] [PubMed]
- Antal A, et al. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1(2):97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Struber D, Rach S, Neuling T, Herrmann CS. On the possible role of stimulation duration for after-effects of transcranial alternating current stimulation. Front Cell Neurosci. 2015;9:311. doi: 10.3389/fncel.2015.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurra M, Paulus W, Walsh V, Kanai R. Frequency specific modulation of human somatosensory cortex. Front Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurra M, et al. Frequency-dependent tuning of the human motor system induced by transcranial oscillatory potentials. J. Neurosci. 2011;31(34):12165–12170. doi: 10.1523/JNEUROSCI.0978-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurra M, Paulus W, Walsh V, Kanai R. Frequency specific modulation of human somatosensory cortex. Front Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurra M, et al. State-dependent effects of transcranial oscillatory currents on the motor system: what you think matters. J. Neurosci. 2013;33(44):17483–17489. doi: 10.1523/JNEUROSCI.1414-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurra M, Galli G, Pavone EF, Rossi A, Rossi S. Frequency-specific insight into short-term memory capacity. J. Neurophysiol. 2016;116(1):153–158. doi: 10.1152/jn.01080.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Paulus W, Walsh V. Transcranial alternating current stimulation (tACS) modulates cortical excitability as assessed by TMS-induced phosphene thresholds. Clin. Neurophysiol. 2010;121(9):1551–1554. doi: 10.1016/j.clinph.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Polania R, Moisa M, Opitz A, Grueschow M, Ruff CC. The precision of value-based choices depends causally on fronto-parietal phase coupling. Nat. Commun. 2015;6:8090. doi: 10.1038/ncomms9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, et al. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr. Biol. 2013;23(15):1449–1453. doi: 10.1016/j.cub.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, et al. Individual differences and specificity of prefrontal gamma frequency-tACS on fluid intelligence capabilities. Cortex. 2016;75:33–43. doi: 10.1016/j.cortex.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat. Neurosci. 2013;16(7):838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri P, Nitsche MA, Ekhtiari H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum. Neurosci. 2015;9:54. doi: 10.3389/fnhum.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin.Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, et al. Phase Dependency of the Human Primary Motor Cortex and Cholinergic Inhibition Cancelation During Beta tACS. Cereb. Cortex. 2016;26(10):3977–3990. doi: 10.1093/cercor/bhw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015;126(11):2181–2188. doi: 10.1016/j.clinph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Feurra M, Galli G, Rossi S. Transcranial alternating current stimulation affects decision making. Front Syst.Neurosci. 2012;6:39. doi: 10.3389/fnsys.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Sela T, Kilim A, Lavidor M. Transcranial alternating current stimulation increases risk-taking behavior in the balloon analog risk task. Front Neurosci. 2012;6 doi: 10.3389/fnins.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Vallence AM, Yang R, Pitcher JB, Ridding MC. Combined transcranial alternating current stimulation and continuous theta burst stimulation: a novel approach for neuroplasticity induction. Eur. J. Neurosci. 2016;43(4):572–579. doi: 10.1111/ejn.13142. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015;233(3):679–689. doi: 10.1007/s00221-014-4183-7. [DOI] [PubMed] [Google Scholar]


