Abstract
The protocol presents a facile, efficient, and versatile method to prepare chitosan-based hydrogels using dynamic imine chemistry. The hydrogel is prepared by mixing solutions of glycol chitosan with a synthesized benzaldehyde terminated polymer gelator, and hydrogels are efficiently obtained in several minutes at room temperature. By varying ratios between glycol chitosan, polymer gelator, and water contents, versatile hydrogels with different gelation times and stiffness are obtained. When damaged, the hydrogel can recover its appearances and modulus, due to the reversibility of the dynamic imine bonds as crosslinkages. This self-healable property enables the hydrogel to be injectable since it can be self-healed from squeezed pieces to an integral bulk hydrogel after the injection process. The hydrogel is also multi-responsive to many bio-active stimuli due to different equilibration statuses of the dynamic imine bonds. This hydrogel was confirmed as bio-compatible, and L929 mouse fibroblast cells were embedded following standard procedures and the cell proliferation was easily assessed by a 3D cell cultivation process. The hydrogel can offer an adjustable platform for different research where a physiological mimic of a 3D environment for cells is profited. Along with its multi-responsive, self-healable, and injectable properties, the hydrogels can potentially be applied as multiple carriers for drugs and cells in future bio-medical applications.
Keywords: Chemistry, Issue 127, Hydrogel, glycol chitosan, imine, self-healable hydrogel, injectable hydrogel, 3D cell culture
Introduction
Hydrogels are crosslinked polymer materials with large amounts of water and soft mechanical properties, and they have been used in many bio-medical applications1,2. Hydrogels can offer a soft and wet environment, which is very similar to the physiological surroundings for cells in vivo. Therefore, hydrogels have become one of the most popular scaffolds for 3D cell culture3,4. Compared to 2D Petri dish cell culture, 3D cell culture has advanced quickly to offer an extracellular matrix (ECM) mimicked microenvironment for cells to contact and assemble for proliferation and differentiation purposes5. Additionally, hydrogels containing natural polymers could offer bio-compatible and promoting environments for cells to proliferate and differentiate3. Hydrogels derived from synthetic polymers are preferred for their simple and clear components, which exclude complex influences like animal-origin proteins or viruses. Among all the hydrogel candidates for 3D cell culture, hydrogels that are easily prepared and have a consistent property are always preferred. The facility to adjust the hydrogel's properties to fit different research requirements is important as well6.
Here we introduce a facile preparation of a glycol chitosan-based hydrogel using dynamic imine chemistry, which becomes a versatile hydrogel platform for 3D cell culture7. In this method, well-known bio-compatible glycol chitosan are used to establish frames of the hydrogel's networks. Its amino groups are reacted with a benzaldehyde terminated polyethylene glycol as the polymer gelator to form dynamic imine bonds as crosslinkages of hydrogels8. Dynamic imine bonds can form and decompose reversibly and responsively to surroundings, endowing the hydrogels with mechanically adjustable crosslinked networks9,10,11. Due to its high water contents, bio-compatible materials, and adjustable mechanical strengths, the hydrogel is successfully applied as a scaffold for L929 cells in 3D cell culture12,13. The protocol here details the procedures, including polymer gelator synthesis, hydrogel preparation, cell embedding, and 3D cell culturing.
The hydrogel also shows several other features due to its dynamic imine crosslinkages, including its multi-responsive to various bio-stimuli (acid/pH, vitamin B6 derivative pyridoxal, protein papain, etc.), indicating that the hydrogel could be induced to decompose under physiological conditions8. The hydrogel is also self-healable and injectable, which means the hydrogel could be administrated via a minimal invasive injection method and gain an advantage in drug and cell deliveries14,15. By adding functional additives or specific predesigned polymer gelators, the hydrogel is compatible to gaining specific properties like magnetic, temperature, pH responsive, etc.16,17, which could fulfill a wide range of research requirements. Those properties reveal the hydrogel's potential capacity to be an injectable multiple carriers for drugs and cells in both in vitro and in vivo bio-medical research and applications.
Protocol
CAUTION: Please consult all relevant material safety data sheets (MSDS) before use. Please use appropriate safety practices when performing chemistry experiments, including the use of a fume hood and personal protective equipment (safety glasses, protective gloves, lab coat, etc.). The protocol requires standard cell handling techniques (sterilizing, cell recovery, cell passaging, cell freezing, cell staining, etc.).
1. Preparation of Hydrogels
- Synthesis of benzaldehyde terminated di-functionalized polyethylene glycol (DF PEG)
- Pre-desiccation of PEG polymer
- Weigh 4.00 g PEG (MW 4,000, 1.00 mmol), transfer it into a round bottom flask (250 mL), and add toluene (100 mL). Note: Other PEGs with different molecular weights can work for the hydrogel formation but will lead to different stiffness.
- Heat the solution by a heat gun (or a hot plate around 40 °C) mildly to help dissolve all the polymers. After all the polymers dissolve, use an evaporator to remove all the solvents. Repeat this dissolve and dry process two times to make the dried PEG polymers. CAUTION: This should be performed in a fume hood with extreme care. Toluene is flammable.
- Benzaldehyde termination reaction of PEG
- Add a magnetic stirrer and tetrahydrofuran (THF, 100 mL) to the flask and dissolve PEG using a stirrer. Add 4-carboxybenzaldehyde (0.90 g, 6.0 mmol) and 4-dimethylaminopyridine (0.07 g, 0.6 mmol) sequentially to the solutions to dissolve all the solids completely.
- Add N,N'-dicyclohexylcarbodiimide (DCC, 1.25 g, 6.0 mmol) to the solution, and add a drying tube that is filled with anhydrous CaCl2 to the flask. Keep the reaction under stirring at room temperature (~ 20 °C) for around 12 h.
- Post-reaction process
- Filter out the white solids generated in the solution by vacuum after the reaction is finished. Pour the solution into cold diethyl ether (500 mL) under stirring to precipitate the white solids. Gather all the white solids by filter and dry the solids in a fume hood.
- Dissolve the white solids into THF again, filter out any insoluble white solids, pour the solution into fresh cold diethyl ether to precipitate the white solids, and dry it. Repeat this process two to three times, and then place the white solids in a vacuum drying oven (20 °C, 0.1 mbar) to dry them completely. Collect the white powder as the final product: benzaldehyde terminated DF PEG.
- Preparation of hydrogels
- Weigh different amounts of DF PEG (0.11 g, 0.028 mmol; 0.22 g, 0.055 mmol; 0.44 g, 0.110 mmol) in a tube (10 mL), add deionized water (5.0 mL), and use a vortex or magnetic stirrer to help dissolve the polymer.
- Dissolve glycol chitosan (0.495 g, 6.18 x 10-3 mmol) in deionized water (15.0 mL) in a tube (50 mL), and use a vortex for a few minutes to homogenize the chitosan solution (3 wt%).
- Add the glycol chitosan solution (0.2 mL, 3 wt%) and DF PEG solutions (0.2 mL) sequentially in a tube (2.0 mL). Use a vortex to mix the solutions homogeneously to form hydrogels in several minutes. Follow the ratios in Table 1 to make hydrogels of different mechanical strengths. Note: Use the tube-inverting method to determine whether the hydrogel has already formed.
- Rheology analyses
- Carry out rheology analyses on a rotational rheometer with a parallel steel plate (diameter: 20 mm). For the gelation test, spread glycol chitosan solution (0.2 mL, 3 wt%) on a lower plate. Then, add DF PEG aqueous solutions (0.2 mL, 2 wt%) evenly dropwise onto the chitosan solution surface and mix with a pipette quickly. Lower down the upper plate and begin to test.
- For hydrogel's stiffness test, cut a hydrogel into a circle (diameter: 20 mm) and measure the storage modulus (G') values versus frequency analyses at 1% strain. Typical G' values at 6.3 rad s−1 are listed in Table 1. Note: Carry out the rheology analyses of the hydrogel 0.5 h after the hydrogel formation to ensure dynamic bond stabilization of the hydrogel.
- For the hydrogel's self-healable property test, cut a hydrogel to pieces and gather the pieces together to self-heal to an integral piece. Then cut the hydrogel piece to make a circle (diameter: 20 mm) and put it on the rheometer to carry out the rheology analysis.
2. 3D Cell Cultivation in Hydrogels
- Preparation of hydrogel gelation solutions
- Weigh DF PEG (0.44 g, 0.11 mmol) in a sterile tube (4.0 mL), add in cell culture media (RPMI-1640, 2.0 mL), and use a vortex or stirrer to help dissolve the polymer to obtain the polymer solution (20 wt%). Load the solution in a syringe (10.0 mL) and then sterilize it by passing it through a micron bacteria-retentive filter (0.22 µm).
- Weigh the glycol chitosan (0.165 g, 2.06 x 10-3 mmol) in a sterile tube (15.0 mL), add in cell culture media (RPMI-1640, 4.0 mL), and vortex to help dissolve the polymer to obtain the glycol chitosan solution (4.0 wt%). Load the solution in a syringe (10.0 mL) and filter it with a 0.22 µm bacteria-retentive filter.
- Cell cultivation in hydrogels CAUTION: Perform all cell related procedures in a tissue culture hood. Basic knowledge of sterile technique is expected.
- Preparation of cell suspension
- Culture L929 cells in RPMI-1640 medium supplemented with 10% FBS, 5% penicillin (10 mL) in a Petri dish (diameter 10 cm), and incubate at 37 °C, 5% CO2. Change the medium every day before use.
- Harvest the L929 cells with PBS containing trypsin (0.025 w/v %) and EDTA (0.01% w/v), then centrifuge (70 x g, 5 min) and re-suspend the cells in the RPMI-1640 medium (1.0 mL). Perform the cell counting using standard operation of blood-counting board. Re-suspend the cells to adjust the cell concentration to ~ 3.75 × 106 cells/mL.
- Cell encapsulation in hydrogels
- Mix the L929 cell suspensions (0.4 mL, 3.75 x 106 cells mL-1) with glycol chitosan solution (0.4 mL) in a tube (4.0 mL) by vortex. Pipette the L929/glycol chitosan solution (0.8 mL) into the center of a confocal Petri dish (diameter 2.0 cm). Pipette the DF PEG solutions (0.2 mL) into the same dish, and gently pipette to mix the solution and induce hydrogel formation. Note: Assess the hydrogel formation by tilting the petri dish.
- For direct cell culture, add additional amounts of RPMI-1640 culture media (1.0 mL) on top of the hydrogel. Put the cell embedded hydrogels (1.0 mL, 1.5 wt% glycol chitosan, 4.0 wt% DF PEG, 1.5 × 106 cells mL-1) in an incubator (37 °C, 5% CO2) and change the medium every day. Prepare for cell imaging on Day 1, 3, 5, and 7 after cell encapsulation.
- For the 3D cell post-culture in hydrogels after injection, prepare cell loaded hydrogel (1.0 mL, see step 2.2.2) in a syringe (10.0 mL, 48 G needle). After the hydrogel forms, inject the hydrogel slowly into a Petri dish for confocal imaging. Add an additional amount of culture media (1.0 mL) on top of the hydrogel and change it every day. Put the Petri dish in an incubator (37 °C, 5% CO2) and prepare for imaging afterwards. CAUTION: Please check and follow the safety operation protocol of a syringe.
- Cell viability analysis
- Confocal observation
- Rinse the hydrogels with PBS (1.0 mL) for two times. Stain the hydrogels with Fluorescein diacetate (FDA, 0.5 mL, 0.05 mg/mL) and propidium iodide (PI, 0.5 mL, 0.08 mg/mL) solutions for 15 min. After staining, remove all the solvents.
- Observe the hydrogels using a confocal microscope under excitation wavelengths of 488 nm and 543 nm to visualize live and dead cells, respectively. Take z-stacks through every 2 µm depth of the hydrogels to validate an even distribution of cells throughout. Note: FDA stains live cells while PI stains dead cells.
- Degrade the hydrogel (1.0 mL) with acetic acid (HAc, 3 v%, 1.0 mL) for 5 min and pipette into a tube (4.0 mL). Collect cells by centrifuge (70 x g, 5 min) and re-suspend the cells in RPMI-1640 cell culture medium (1.0 mL). Perform cell counting using a blood counting board.
Representative Results
A schematic presentation of this protocol on hydrogel preparation and its use as 3D cell culture is offered in Figure 1. Information of the hydrogel's contents and ratios prepared with different mechanical strengths is summarized in Table 1. The hydrogel's self-healable and rheology property presents the hydrogel's stiffness by storage modulus versus frequency test in Figure 2. The cell confocal images and cell numbers with days of culture in hydrogels are presented in Figure 3, confirming the cell viability and cell proliferation via 3D cell culture. Figure 4 presents microscopy, confocal images, and analysis of cell proliferation rates for cells embedded in hydrogels that suffered injection and post-culture. High cell viability and cell proliferation indicate that the cells embedded in the hydrogel suffered no destructive harm and could recover by post-culture, indicating the hydrogel's injectability.
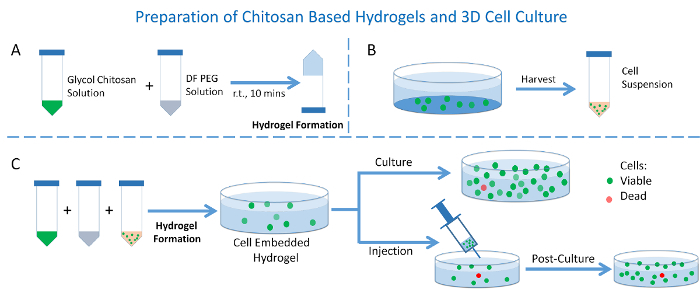
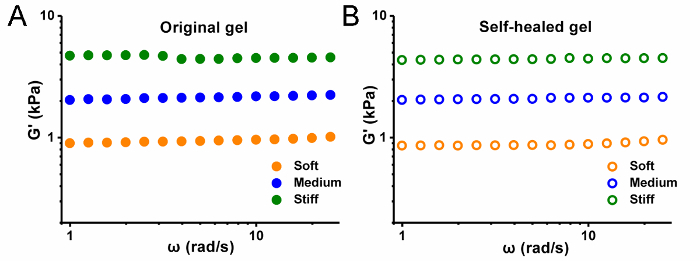
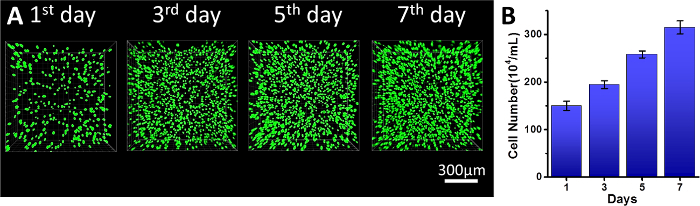
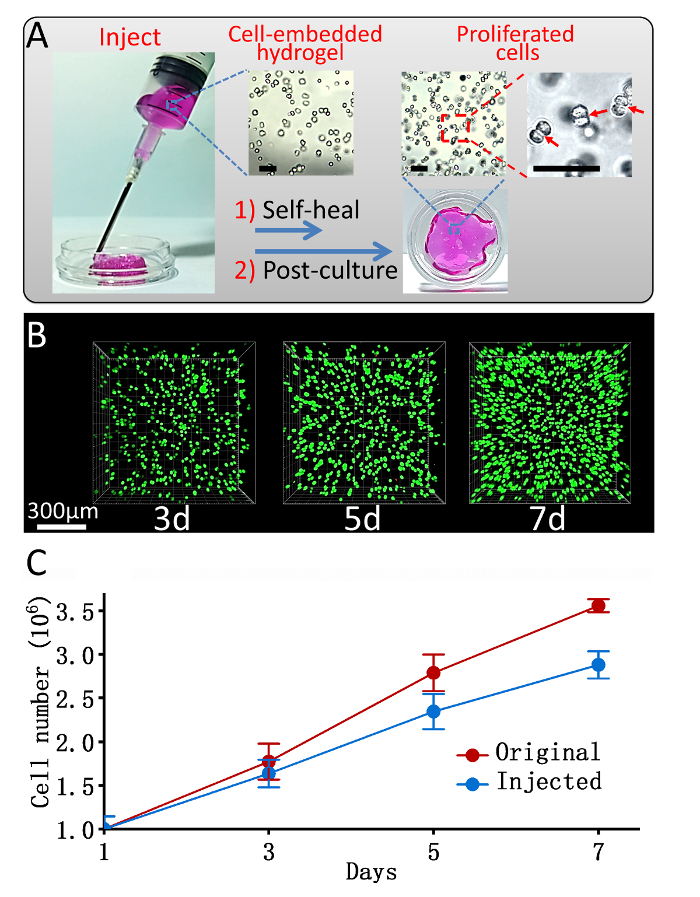
Figure 1: Preparation of the hydrogel and its use as 3D cell culture. (A) Hydrogel preparation; (B) Cell suspension preparation; (C) 3D cell culture in hydrogels with or without injection. Please click here to view a larger version of this figure.
Figure 2: Hydrogel stiffness. Storage modulus G' versus frequency of (A) original and (B) self-healed hydrogels. Please click here to view a larger version of this figure.
Figure 3: 3D cell culture in the hydrogel. (A) 3D confocal images of L929 cells embedded in stiff strength hydrogel (Green: live cells; Red: dead cells); (B) Cell counting results of L929 cells embedded in the hydrogel after cell encapsulation at denoted time. Scale bar = 300 µm. Data presented as Mean ± SD. Please click here to view a larger version of this figure.
Figure 4: 3D cell post-culture in hydrogel after injection. (A) A schematic image of injection and post-culture of a cell-loaded hydrogel. Insert: Microscopy images of cells embedded in hydrogels before injection (left) and after cell post-culture for 7 days (middle and right). Scale bar = 100 µm; (B) Confocal images of L929 cells embedded in a stiff strength hydrogel and post-cultured after injection (Green: live cells; Red: dead cells), Scale bar = 300 µm; (C) A comparison of the proliferation rate of cells cultured in hydrogels with or without injection after encapsulation. Data presented as Mean ± SD. (Adapted from reference12; Copyright © 2017 Elsevier). Please click here to view a larger version of this figure.
| Hydrogel Stiffness | Soft | Medium | Stiff |
| Glycol chitosan (mg) | 6.6 | 6.6 | 6.6 |
| DF PEG (mg) | 4.4 | 8.8 | 17.6 |
| Polymer gelator content (wt%) | 1 | 2 | 4 |
| Deionized water (mL) | 0.4 | 0.4 | 0.4 |
| Gelaion time (min) | 7.5 | 5 | 3.5 |
| G’ (Pa)* | 900 | 2100 | 4700 |
| * tested under frequency 6.3 rad s-1, strain 1% using a parallel plate (diameter 20 mm) on a rotational rheometer. |
Table 1: Information of hydrogels prepared with different mechanical strengths.
Discussion
The hydrogel presented in this protocol (Figure 1) has two main components: the natural polymer glycol chitosan and a synthetic benzaldehyde terminated polymer gelator DF PEG, which are both biocompatible materials. Synthesis of DF PEG is presented using a one-step modification reaction. PEG of molecular weight 4,000 was chosen in this protocol in concerns of solubility, modification efficiency, as well as hydrogel stiffness. A series of hydrogels with different mechanical strengths were prepared using different solid contents and ratios of glycol chitosan and DF PEG. Homogeneous hydrogels were quickly formed in minutes by mixing gelation solutions under room temperature, though the gelation speed could also slow down by dilute solutions. Rheology test was employed to evaluate the mechanical strengths of different hydrogels. The storage moduli (G') of those hydrogels were found to approximately differ from 900 Pa (soft), 2,100 Pa (medium) to 4,700 Pa (stiff) (Table 1 and Figure 2A). This is contributed to the higher crosslinking density by adding more polymer gelators in the hydrogel network, resulting in higher storage modulus (greater stiffness). The self-healable property is also confirmed by recovery of the hydrogel's modulus (Figure 2B). It is known that ECM stiffness differs for various tissues, thus the hydrogel could offer adjustable mechanical cues and meet different research requirements by varied ratios between glycol chitosan and DF PEG polymer gelators18.
L929 cells, a typical mouse fibroblast cell line with in vivo environment tissue stiffness of ~ 5,600 pa19, was used as a cell model to be embedded in the hydrogel. After the cell encapsulation in hydrogels, it can be easily observed that the cells in the hydrogels showed extremely high viability (>99%) throughout the culture process, confirming the excellent biocompatibility of the chitosan-based hydrogel. A remarkable increase of cell density in the hydrogel implied that this hydrogel could support proliferation of the L929 cells even without extra-added growth factors (Figure 3A). After 7 days in culture in the hydrogel, the cell number increased 300% (Figure 3B). It is reported that cells cultured in scaffolds could differentiate following mechanical cues20,21, which might imply further research using this 3D hydrogel culture method.
Implanting cells with injectable hydrogels has gained incomparable advantages to greatly enhance the delivery efficiency and viability of the implanted cells14. Within a dynamic imine crosslinked network, the hydrogel could self-heal after injection. The self-healable property enables the hydrogel to be injectable, which implies potential applications for the hydrogels as injectable multiple carriers. To further evaluate this hydrogel as an injectable cell carrier, an injection-mimicking and post-culturing experiment was performed. The cell embedded hydrogel was loaded in a syringe and squeezed out through a 48 G needle into a Petri dish, followed by a 7 day post-culturing process with cell viability and proliferation rate studied.
As shown in Figure 4A, the squished hydrogel pieces could self-heal to reform an integrated hydrogel about 1 h after injection, which is helpful in cell delivery. For cells in the hydrogel, when compared with the image took just after encapsulation (Figure 4A, left insert), the cell density increased dramatically after 7 days (Figure 4A, middle insert). In the enlarged vision, a more detailed cell-fissional process in which some cells are found to be divided into two (Figure 4A, right insert), directly confirmed by 3D cell proliferation in the hydrogel. Figure 4B shows the confocal images of the live-dead cell assays after injection and post-culture experiment. The density of the injected cells increased obviously with a high cell viability (>99%), demonstrating the successful cell proliferation in the 3D hydrogel after injection. To learn whether the injection affected the cell proliferation rate, a comparison of quantitative statistics analysis for the cell proliferation rates in hydrogels with or without injection was performed (Figure 4C). The cell proliferation rate after injection, although slightly decreased, remained in a high level (~75%), and the cell number increased 145% relatively after 7 days in culture in the hydrogel, indicating that the shearing force during injection has an observable limited negative influence on the cell proliferation.
We described a typical procedure of this hydrogel application but researchers can modify the protocol to fit specific research requirements. Polymer gelators have significant influences on the hydrogel and are easily modifiable. For example, the stiffness of this hydrogel could be easily manipulated by different ratios of glycol chitosan and DF PEG gelator. Meanwhile, the use DF PEG of other molecular weights could also fulfill this target. We used PEG 4K in this protocol, but PEG 2K to 10K may also work. When using other PEGs, it is important to monitor the gelation time and stiffness specifically. Other functional polymer gelators may also work if the researchers have synthesis skills to prepare specific functional benzaldehyde-terminated polymers17.
This protocol has several limitations. Firstly, due to the dynamic structure, the stiffness of this hydrogel is limited compared with a covalent bond crosslinked hydrogel. One may encounter failures to prepare hydrogels with too high of a modulus design. Secondly, this hydrogel is bio-degradable, thus limiting the long-term cell culture within the hydrogel. Thirdly, the hydrogel offers a 3D crosslinked cultivation environment that has diffusion concerns. To date, most of the bio-analyses are based on 2D culture. The 3D structure might hinder further cell related in vivo studies.
When using this protocol, there are several critical steps to follow. Firstly, during synthesis of the polymer gelator DF PEG, dehydration is very important. Secondly, when performing cell-related procedures, sterile operation is critical. Thirdly, the staining time should be well controlled in the hydrogels to get nice confocal images.
In summary, the protocol introduced a facile preparation of chitosan-based injectable hydrogels, which are applied as a platform for 3D cell culture and could offer adjustable mechanical cues for various researches. It has already been applied in areas regarding drug delivery, cell therapy, and tumor chemotherapy, which not only is a testament to its performance, but also increases its potential for biomedical applications.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This research was supported by the National Science Foundation of China (21474057 and 21604076).
References
- Hoffman AS. Hydrogels for biomedical applications. Adv. Drug. Deliver. Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki LA, Kloxin AM. Light-mediated Formation and Patterning of Hydrogels for Cell Culture Applications. J. Vis. Exp. 2016. [DOI] [PMC free article] [PubMed]
- Haycock JW. 3D cell culture: a review of current approaches and techniques. 3D Cell Culture: Methods and Protocols. 2011. pp. 1–15. [DOI] [PubMed]
- Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug. Discov. Today. 2013;18(5):240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Yang B, et al. Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier. Polym. Chem. 2012;3(12):3235–3238. [Google Scholar]
- Zhang Y, Tao L, Li S, Wei Y. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules. 2011;12(8):2894–2901. doi: 10.1021/bm200423f. [DOI] [PubMed] [Google Scholar]
- Cao L, et al. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B. 2015;3(7):1268–1280. doi: 10.1039/c4tb01705f. [DOI] [PubMed] [Google Scholar]
- Ding F, et al. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter. 2015;11(20):3971–3976. doi: 10.1039/c5sm00587f. [DOI] [PubMed] [Google Scholar]
- Wei Z, et al. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014;43(23):8114–8131. doi: 10.1039/c4cs00219a. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Modulus-regulated 3D-cell proliferation in an injectable self-healing hydrogel. Colloid. Surface. B. 2017;149:168–173. doi: 10.1016/j.colsurfb.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Tseng TC, et al. An Injectable, Self‐Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015;27(23):3518–3524. doi: 10.1002/adma.201500762. [DOI] [PubMed] [Google Scholar]
- Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008;37(8):1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. Improving Tumor Chemotherapy Effect by Using an Injectable Self-healing Hydrogel as Drug Carrier. Polym. Chem. 2017.
- Zhang Y, et al. A magnetic self-healing hydrogel. Chem. Commun. 2012;48(74):9305–9307. doi: 10.1039/c2cc34745h. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Synthesis of an injectable, self-healable and dual responsive hydrogel for drug delivery and 3D cell cultivation. Polym. Chem. 2017;8(3):537–534. [Google Scholar]
- Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13(6):645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens F. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology. 2008;45(6):677–688. [PubMed] [Google Scholar]
- Banerjee A, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008;7(10):816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]


