Abstract
The steady progess in the armamentarium of techniques available for endovascular treatment of intracranial aneurysms requires affordable and reproducable experimental animal models to test novel embolization materials such as stents and flow diverters. The aim of the present project was to design a safe, fast, and standardized surgical technique for stent assisted embolization of saccular aneurysms in a rat animal model.
Saccular aneurysms were created from an arterial graft from the descending aorta.The aneurysms were microsurgically transplanted through end-to-side anastomosis to the infrarenal abdominal aorta of a syngenic male Wistar rat weighing >500 g. Following aneurysm anastomosis, aneurysm embolization was performed using balloon expandable magnesium stents (2.5 mm x 6 mm). The stent system was retrograde introduced from the lower abdominal aorta using a modified Seldinger technique.
Following a pilot series of 6 animals, a total of 67 rats were operated according to established standard operating procedures. Mean surgery time, mean anastomosis time, and mean suturing time of the artery puncture site were 167 ± 22 min, 26 ± 6 min and 11 ± 5 min, respectively. The mortality rate was 6% (n=4). The morbidity rate was 7.5% (n=5), and in-stent thrombosis was found in 4 cases (n=2 early, n=2 late in stent thrombosis).
The results demonstrate the feasibility of standardized stent occlusion of saccular sidewall aneurysms in rats - with low rates of morbidity and mortality. This stent embolization procedure combines the opportunity to study novel concepts of stent or flow diverter based devices as well as the molecular aspects of healing.
Keywords: Neurobiology, Issue 128, Endovascular Occlusion, Intracranial Aneurysms, Biodegradable, Magnesium Stent, Animal Model, Saccular Aneurysms, End-to-Side Anastomosis, Wistar Rat
Introduction
Subarachnoid hemorrhage due to a ruptured intracranial aneurysm is associated with a high mortality rate and poor neurological outcome in many survivors. There are currently two general approaches to occlude IA: either microsurgical clipping (which requires operative exposure of the aneurysm), or endovascular occlusion. As the less invasive endovascular coil treatment of narrow-necked IA has been shown to be associated with slightly lower morbidity (especially in the posterior circulation1,2), endovascular treatment options have become the preferred modality of many neurosurgical centers. Numerous devices have been developed in order to extend the indications of endovascular treatment and overcome the main limitation of IA recurrence after coiling. Intracranial stents are especially promising to overcome these limitations, as they serve as a scaffold for neo-endothelization and coil herniation prevention, as well as protect the parent artery and improve intraluminal intraaneurysmal thrombosis caused by reduction of blood inflow. There is a need to study novel intracranial stents in a low cost animal model; at both macroscopic and molecular levels.
The aim of this study was to design a safe, fast, and standardized surgical technique for stent application in an already established saccular aneurysm model in rats3,4,5. In the present project, we evaluated the role of a biodegradable magnesium stent.
Protocol
Male Wistar rats with a mean weight of 592 g (±50 SD) and mean age of 20 weeks were housed in animal facilities at a room temperature of 22-24 °C and twelve hour light/dark cycle, with free access to tap water and a pellet diet. The animals received care from humans in accordance with institutional guidelines. The experiments were approved by the Committee for Animal Care of the Canton Bern, Switzerland (BE 102/13). We strictly followed the recommendations for Animal Research: Reporting of In Vivo Experiments (ARRIVE guidelines).
1. Laboratory equipment, consumable supplies, surgical instruments
Use a quiet, aseptic operating room and maintain the temperature at 23 ± 3 °C.
Use a surgical desk with a surface which is easy to clean and disinfect, and cover it with a surgery drape. Use a table-top surgical microscope with a camera to record the surgery and an infrared light to warm the animal before and after surgery.
- Obtain the following consumable supplies to create the sidewall aneurysm and implant the stent.
- Obtain smaller and larger sterile cellulose and gauze swabs, syringes with 18G and 26G hollow needles and a blunt tipped needle for irrigation/volume substitution.
- Obtain suture materials including non-absorbable 10-0 and 9-0 micro suture, non-absorbable 6-0 and 5-0 suture, and absorbable 3-0 suture.
- Obtain 0.9% isotonic sodium chloride solution for irrigation/volume substitution and to ensure the cleanliness of the instruments during surgery
Obtain the following instruments to implant the stent: puncture needle 19G, hydrophobic guide wire, 4 Fogaty sheath, and an inflator syringe for balloon dilatation.
- Obtain the following consumable supplies for aneurysm wall decellularization:
- Obtain microtubes for aneurysm storage at -24 °C and labels for identification, as well as a laboratory shaker to ensure full chemical treatment of the aneurysm.
- Obtain 0.1% sodium dodecyl sulfate (SDS) and phosphate buffered saline (PBS) for the proper decellularization process.
- Obtain the following standard surgery instruments:
- Obtain coarser surgical instruments: surgical scissors, soft tissue forceps, soft tissue spreader, straight and curved forceps, three Mosquito clamps, needle holder.
- Obtain surgical micro instruments: straight and curved micro-scissors, straight and curved micro-forceps, and four temporary vascular mini clips with corresponding vascular clip applicators.
2. Anesthesia
Place the rat in a gas chamber and anesthetize with a mixture of isoflurane and oxygen (4% isoflurane). Maintain inhalation until the animal loses consciousness.
Remove the anesthetized animal from the gas chamber and inject the definitive anesthesia consisting of a weight-adapted mixture of medetomidine hydrochloride (0.5 mg/kg) and ketamine hydrochloride (50 mg/kg) intraperitoneally (by injecting into the right or left lower abdomen).
Monitor the depth of anesthesia in defined intervals during surgery with a noxious toe-pinch to confirm that the rat is unresponsive and fully anesthetized. If there is a toe-pinch reflex, readminster the anesthesia with a weight-adapted intraperitoneal injection of ketamine and medetomidine. Sufficient anesthesia is required before opening the abdominal cavity, and performing the end-to-side anastomosis and stent insertion.
Place the rat under the infrared heating lamp until the intraperitoneal anesthesia takes effect, as preoperative warming effectively avoids extensive cooling during surgery.
3. Surgical preparation and positioning of the rat
Weigh the rat, extract the tongue to secure the upper airways, and apply eye ointment to avoid corneal desiccation.
Use adhesive tape to fix the rat to a small washable board and avoid any pressure or traction to the paws.
Shave the rat in the operational area with a small animal shaver and disinfect the surgical region.
Place two thick markers 2-3 cm apart under the back of the animal to cause as much lumbar and thoraco-lumbar lordosis as possible. This ensures better exposition of the retroperitoneum and easier application of the stent.
Cover the rat with a sterile aperture drape and place it under the operating microscope. Set up two supports beside the animal to facilitate a more comfortable position for the experimenter's hands.
Wash and disinfect hands and put on sterile gloves. It is useful to have an assistant present especially for the stent implantation.
Perform the surgery under aseptic conditions according to the recommendations for laboratory animals6,7.
4. Aneurysm graft harvesting
Under general anesthesia, open the abdomen below the xiphoid process. First, cut the skin in the midventral line with sharp scissors and separate the skin from the musculature. Then cut the abdominal musculature and identify the diaphragm above the liver at the insertion point of the thoracic cavity.
Cut the diaphragm and the connective tissue with sharp scissors to access the thoracic cavity and cut the ribs with large scissors one and a half centimeter to the right and left of the sternum.
When the rib cage is open, sacrifice the rat with an intracardiac overdose of ketamine hydrochloride (120 mg/kg).
Mobilize the left lung and fix it to the right side with the help of a cellulose swab.
Following appropriate exposure of the aortic arc, clamp the azygos and left cranial caval veins with two mosquito clamps above the aorta and cut in between. Leave the clamps on the cut vessel endings to avoid retrograde venous bleeding. Use the clamps to move the heart and veins to the side, allowing better access to the aortic arc and descending aorta.
Dissect the aorta using a microsurgical technique with micro-scissors and micro-forceps.
Trace the aorta upwards from the descending section to the aortic arch and identify the left subclavian artery and the first intercostal artery leaving the aorta.
Place a non-absorbable 6-0 suture just cranial of the first intercostal artery and tie it up. At the proximal end, cut the aorta right after the origin of the left subclavian artery. It is important to use a single perpendicular cut as it lowers the chance of irregularities at the cutting edge of the vascular graft. Finally, remove the newly created aneurysm with a cut just below the ligature.
After measuring the aneurysm, immediately transplant the graft into the recipient rat.
5. Aneurysm creation and stent application
- Surgical approach
- Start the skin incision in the distal third of the abdomen with sharp scissors and cut in a midventral position up to 1 cm distal of the xiphoid process. Carefully separate the skin and subcutaneous tissue from the musculature. Use the same scissors to open the abdominal muscles along the linea alba. Use forceps to raise the muscles while cutting to avoid damaging the intraabdominal organs.
- Following the laparotomy, move the small intestines, the caecum, and the colon to the right. Use a self-holding retractor to keep the abdominal cavity open and fix the organs. Place a swab beneath the liver to remove it from the surgical area and enhance retroperitoneal exposure. NOTE: If the fat mass is obstructing the view, it can be placed outside the abdominal cavity and covered with wet gauze to avoid extensive fluid depletion.
- Empty the urinary bladder to achieve maximal exposure of the infrarenal abdominal aorta. This can be accomplished either through gentle pressure to the bladder wall, or vesical discharge using a 26G hollow needle.
- Protect the bladder and testicles from dehydration and extensive manipulation during surgery by covering them with a wet sterile cellulose swab.
- Open the parietal peritoneum covering the aorta with two blunt forceps. Take care with the almost transparent ureters, testicular vessels and the superior mesenteric artery. As soon as the parietal peritoneum is open, the abdominal aorta will be located just underneath it. It is often covered by a thin layer of adipose tissue.
- Start with a blunt dissection of the abdominal aorta using two micro-forceps to release it from the surrounding retroperitoneal fat until a longer length of the vessel is visible. Continue with sharp dissection using micro-scissors and micro-forceps. Only grasp the adventitia during this procedure to avoid damage to the vessel wall.
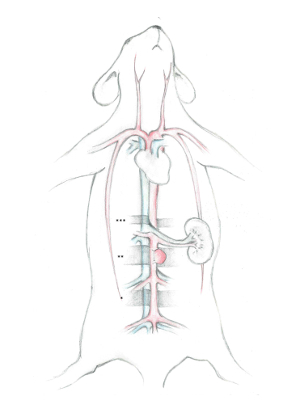
- Abdominal aorta dissection NOTE: To facilitate the description of the surgical strategy we have divided the abdominal aorta into three distinctive segments (Figure 1).
- Start the dissection in the distal segment - which is technically more challenging. NOTE: The distal segment is subdivided between the iliac bifurcation and iliolumbar vein and artery. This segment serves as the point for the distal abdominal aorta puncture and stent insertion.
- Dissect the aorta with micro-scissors and micro-forceps from the V. cava by cutting alongside the arterial adventitial layer between the two vessels. NOTE: Small lumbar arteries may arise infrequently as segmental vessels from the dorsal surface of the abdominal aorta and interfere with the procedure. Coagulation and cutting of the vessels is needed to avoid retrograde oozing during vessel suturing.
- Identify the right iliolumbar artery; its origin can vary significantly in this segment but is most frequently located more caudal than the left counterpart. NOTE: This condition rarely interferes with the planned stent insertion puncture site in the artery, but may require a puncture point farther removed or - for the most extreme anatomical variability - a vessel ligation of the right iliolumbar artery.
- After preparing the aorta in this segment, place a small colored rubber pad underneath the vessel to indicate where the arterial puncture will be performed. Avoid stretching the vessel in this segment. Tensile stretch on the vessel wall may lead to uncontrolled vessel rupture following arterial puncture, which in turn will impede the final reconstruction. It is therefore important to release the aorta from all adhesions at the artery puncture site and avoid any placement of gauze swabs beneath the aorta.
- Dissect the aorta from the V. cava in the middle segment in the same way as described before. Note: The middle segment is subdivided between the iliolumbar vein and artery and renal vein. This segment is used for the aneurysm implantation and stent positioning.
- Identify the superior mesenteric artery at its origin from the abdominal aorta and preserve it during all procedures.
- Ligate or coagulate arteries from the dorsal surface of the aorta in this section as well. To avoid damage to the lumbosacral plexus, ensure sufficient flushing and be mindful of the short coagulation time.
- Establish the location of the stent following dissection of the aorta in this segment. Anticipate possible interferences of the stent with abdominal vessels before defining the final location for the aneurysm and stent.
- After establishing the location of the aneurysm and stent, place a small piece of gauze and a colored rubber pad under the aorta for better exposure.
- Finally, dissect the proximal segment of the abdominal aorta. This is technically simpler than the same procedure in the distal and middle segments. Note: The proximal segment is located proximal to the renal vein. It is used for the application of the most proximal temporary clip during the Seldinger technique stent implantation.
- After opening the parietal peritoneum, separate the vessels with two blunt forceps.
- Following dorsal circumnavigation of the aorta with curved, blunt forceps, place a small piece of a colored rubber pad underneath it to facilitate later proximal clip application.
- Aneurysm creation
- Clamp the aorta in the middle segment by first applying the distal clamp, followed by the proximal to ensure a firm filling of the vessel and facilitates the subsequent arteriotomy.
- Remove the adventitia at the planned anastomosis site. Grasp the adventitia with micro-forceps and carefully cut with micro scissors without injuring the vessel wall.
- Lift a small piece of vessel wall with micro-forceps and perform a linear incision with micro-scissors. The arteriotomy should be the same size as the aneurysm base.
- Flush the artery in both directions with saline in a blunt tipped needle.
- Place the first two stitches of the end-to-side anastomosis, which is performed with non-absorbable 9-0 suture, at the proximal and distal end of the arteriotomy. Grasp the vessel wall as little as possible during suturing and ensure that the sutures pass through each layer of the vessel wall. They should only catch the ventral part of the aorta to avoid constriction of the artery; which could lead to vessel rupture during stent application.
- Close the incision with interrupted sutures. Start the suture on the left side and finish this before continuing onto the other side. When the back wall is finished, check the endoluminal section of the anastomosis for wrongly placed sutures. NOTE: At this point, different intra-aneurysmal therapeutics such as coil application may be introduced.
- After finishing the anastomosis, remove the distal clamp first. Place an extra suture in case of major bleeding from backflow. Manage minor oozing by gently pressing a small piece of gauze to the source of bleeding. Adipose tissue or a previously obtained adventitial layer can be useful as well. Now remove the proximal clamp.
- Rinse the anastomosis site and cut the remaining ends of ligature at the aneurysm dome.
- Observe the distal abdominal aorta patency with the direct milking test.
- Remove the small gauze swab underneath the colored rubber pad, as all segments of the aorta should be approximately at the same height before stent insertion. Avoid covering the anastomosis suture lines with adipose tissue as the stent struts must be visible while positioning the stent beneath the newly created aneurysm.
- To avoid damage from prolonged ischemia, allow for 10 minutes of reperfusion before continuing with the proximal aortic clamping and stent insertion.
- Empty the bladder before implanting the stent.
- Stent implantation NOTE: It is useful to have an assistant on hand for the following steps, especially for the final stent inflation.
- Clip the artery puncture site in the distal segment first. Begin with the distal clamp and follow with the proximal. Remove the loose connective tissue and (partially) the adventitia at the puncture site with micro-scissors.
- Place a large temporary clamp in the proximal segment to avoid major hemorrhaging during arterial puncture.
- Perform the arterial puncture in the distal segment with a 18G puncture needle. Lift a small piece of the vessel wall with micro-forceps to facilitate the puncture and reduce the risk of piercing both aortic vessel walls.
- Flush the artery thoroughly following the puncture with saline in both directions using a blunt tipped needle.
- Insert a hydrophobic guide wire into the puncture site and remove the proximal clamp in the distal segment.
- Push the guide wire forward up to the level of the clamp in the proximal segment. Insert the Teflon introducer according to the Seldinger technique using the previously inserted guide wire. NOTE: Hydrophobic guide wires are more suitable than hydrophilic ones as they have a superior grip and provide better manual control. As Teflon introducers are easier to insert than polypropylene introducers, they reduce the risk of uncontrolled rupture at the puncture site.
- Push the dilator upwards just proximal to the anastomosis site, the sheath is located about half a centimeter below.
- Remove the guide wire and dilator, and ensure the 3-way stopcock is turned off to avoid unnecessary loss of blood.
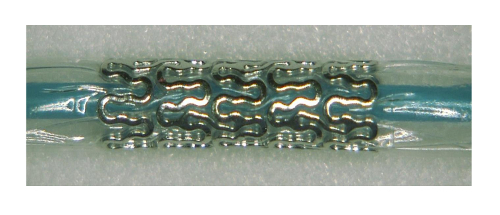
- After vacuuming the balloon system, insert the absorbable magnesium stent using the 4 Fogarty introducer. Inflate the balloon with one bar of pressure just prior to removing the introducer device to protect the endothelium of the collapsed vessel from strut-induced damage and reduce the risk of the strut becoming entangled in the previously implanted intraaneurysmal material (Figure 2).
- When the stent is in its final position, carefully inflate the balloon with 8-9 bar. Continuously check the end-to-side anastomosis sutures for possible ripping.
- After adequate stent dilation and adhesion to the aortic vessel wall, deflate and remove the balloon.
- When removing the introducer, have a temporary clip at hand for immediate application proximal to the puncture site as retrograde oozing from proximal patent arteries may be significant.
- Remove the temporary clamp in the proximal segment after placing the proximal clip in the distal segment. Observe the end-to-side anastomosis as any increase in intra-aortic blood pressure due to distal clipping may cause bleeding at the suture site.
- Flush the puncture site with saline from a blunt tipped needle in both directions. Then suture it with an interrupted technique using non-absorbable 10-0 thread. If possible, avoid grasping the vessel wall with the micro-forceps and ensure that each suture passes through all layers of the vessel wall. Suture at the outmost vessel puncture site to avoid vessel stenosis.
- Add extra sutures if major hemorrhaging occurs as a result of backflow. In case of minor oozing, secure hemostasis through gentle pressure to the bleeding site using a gauze swab or a previously preserved segment of adventitia. Small pieces of adipose tissue can be helpful as well.
- Remove the colored rubber pad underneath the puncture site. The pulsation of the aorta will be clearly visible proximal and distal to the anastomosis site. Check for distal artery patency with the direct milking test.
6. Closure
Remove all gauze swabs and gently flush the abdominal cavity with isotone saline to clear any debris and promote volume substitution.
Remove the soft-tissue spreader and return the small intestines, cecum, and fat mass back to their correct anatomical position.
Use non-absorbable 5-0 suture to join the abdominal muscles. To reduce skin traction, place some subcutaneous sutures using an absorbable 3-0 suture. Use the same 3-0 suture to close the skin.
After closure, disinfect the skin and place the animal under the infrared light to provide it with warmth.
Representative Results
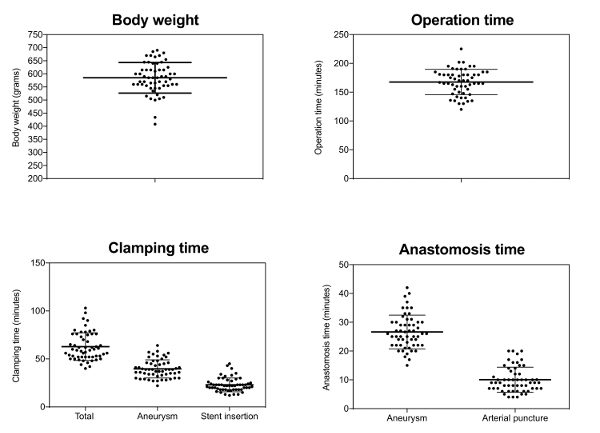
The average duration of surgery was 167 (± 22) min, 26 (± 6) min of which were needed for aneurysm creation and a further 23 (± 7) min required for stent application and reconstruction of the arteriotomy (Figure 3).
Mortality, morbidity, and macroscopic in-stent thrombosis were the primary endpoints of the study. The regular follow-up periods were 7 days (n=28), and 21 days (n=32) respectively. Mortality or eventful morbidity lead to an early termination of the study. There were no deaths during surgery. Four animals (6%) died within the first three days post-op due to early in-stent thrombosis. Five rats (7.5%) experienced postoperative complications with two caused by early in-stent thrombosis, which led to paralysis of the hind legs. These animals were euthanized due to their severe neurological deficit. In two other cases, late in-stent thrombosis was seen at day 21, after the experiment had ended. These animals had shown no neurological deficits up to that point. One rat suffered wound dehiscence of all the abdominal wall layers on the second postoperative day with consecutive loss of volume and was euthanized as a result. The combined mortality and morbidity rate was 13.4% (Figure 4 and Figure 5).
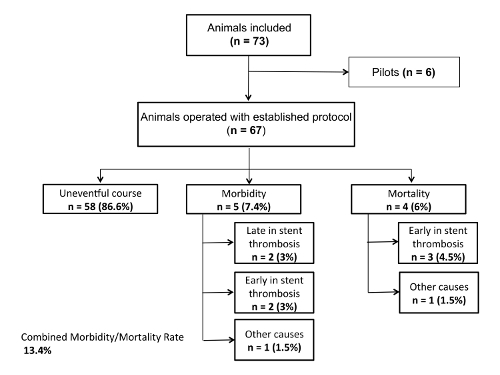
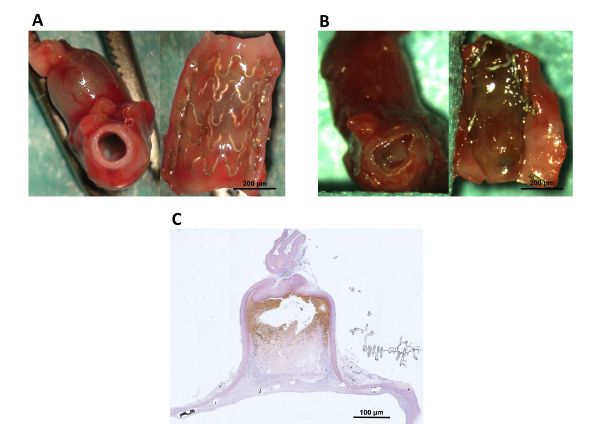
Figure 1: Illustration of the three distinctive segments of the abdominal aorta. The vena cava and their disposals are colored blue, while the abdominal aorta and their disposals are colored red. * Marks the distal segment, in which the abdominal aorta puncture and stent insertion is done. **Marks the middle segment, in which the aneurysm implantation and final stent positioning occur. ***Marks the proximal segment, which is used for the application of the most proximal temporary clip during the stent implantation. Please click here to view a larger version of this figure.
Figure 2 : Magnesium stent inflated at 1 bar Please click here to view a larger version of this figure.
Figure 3: Surgical characteristics. Each point represents one animal; mean values (long line); and standard deviation Please click here to view a larger version of this figure.
Figure 4: Flow chart. Number of animals used in the study; mortality and morbidity Please click here to view a larger version of this figure.
Figure 5: Macroscopic and microscopic (10x magnification) overview of Mg-stent treated end-to-side implanted aneurysms following tissue harvesting at 21 days. A: The photograph on the left shows a patent abdominal aorta. The photograph on the right shows the longitudinally opened aorta with the thin neointima covering the stent and the insinuated aneurysm ostium behind the stent. B: The photograph on the left shows an in-stent thrombosis of the abdominal aorta. The photograph on the right shows the longitudinally opened aorta with a thrombus covering the vessel lumen. The degenerated stent struts were caused by the thrombus. C: A microscopic overview (hematoxylin eosin stain) of the aneurysm shown in A. Please click here to view a larger version of this figure.
Discussion
Bioabsorbable stents and animal models In recent years the general trend in medicine has been away from permanent implants (which remain in the patient's body for the rest of their life) to bio-absorbable materials. Magnesium stents, in particular, are already quite established in cardiology8,9. Unfortunately these stents have not yet been tested for other applications, such as cerebrovascular diseases. For this reason we decided to study the usability of a bioabsorbable stent in saccular aneurysm treatment. The study was performed with rats as they - like other small experiment animals - are suitable due to the low associated purchase and living expenses. In addition, they can be used in different areas of research thanks to the possibility of genetic modification10. The low costs, easy handling, and relatively fast surgical procedures allow studies to be performed with a large number of animals. In order to evaluate new intravascular devices, it is crucial to use a standardized aneurysm model which allows for constant interindividual conditions; especially with regard to blood flow, wall shear stress, vessel turtuosity, aneurysm location and aneurysm size. The rat sidewall aneurysm model described provides all of these advantages. Furthermore the model allows the testing of novel endovascular approaches in different wall conditions. It presents the smallest possible animal model in which biological concepts of aneurysm healing and therapeutic applications - such as stents - can be analyzed. Thanks to its dimensions, it allows similar sized devices to be tested later for clinical use in humans. Please refer to previously published work for detailed descriptions of the advantages/disadvantages, and biological differences of preclinical extracranial aneurysm models in various species4,11.
Morbidity and mortality Previous studies have shown high rates of mortality in abdominal stent implantation. The values vary from 5.7 - 57%12,13,14,15. Most animals died of thrombosis following the intima injury during stent implantation16. Even though the mortality rate in our study was low, stent-associated thrombosis remains a significant cause of postoperative complications and fatality (7.5% early in-stent thrombosis, 3% late in-stent thrombosis). We hypothesize that the reduced contact between the stent and vessel is an important reason for the low mortality rate of 6% (5 out of 67 animals) in our study. The contact was reduced thanks to the Seldinger technique and inflation of the balloon to 1 bar prior to removing the introducer; as the balloon covers the sharp edge of the magnesium stent. Through continuous, gentle dilatation of the puncture site with a guide wire, dilatator, and sheath, the puncture site can be kept small, which in turn facilitates the reconstruction of the vessel wall and reduces the risk of iatrogenic artery constriction. The rats must weigh at least 500 g for an unproblematic Seldinger technique insertion of the 4F introducer. Antiplatelet therapy has been shown to reduce in-stent thrombosis irrespective of the mechanic intima injury caused by stent placement12,17,18,19. Furthermore, thinner-strut stent devices significantly reduce vessel stenosis following stent treatment20. Aquarius et al.12 treated sidewall aneurysms with flow diverters in rats using a similar operative technique to that described above. They reported no in-stent thrombosis in their series. The main difference to this study is that they used dual antiplatelet therapy and thinner strut stent devices compared to our magnesium stents. As our study aimed to explore the biology of aneurysm healing after stent application, we decided to conduct the experiments without drugs so as not to influence the natural intraaneurysmal coagulation and proinflammatory state. We therefore accepted the higher risk of thrombosis.
Transaortic stent placement Contrary to stent insertion through the carotid artery13,15,21,22 which sacrifices the vessel, a primary reconstruction of the puncture site (and preservation of the vessel) can be achieved by inserting the stent through a large vessel such as the abdominal aorta or the iliac artery14. Another advantage of transaortic stenting is it allows the direct visual monitoring needed for accurate stent placement and prevents damage to relevant abdominal feeders. Without visual control, false placement occurs in up to 12% of cases21. Furthermore, the size of the balloon and artery can be observed to avoid overexpansion of the vessel wall. Aneurysm creation and stent application in the abdomen allows the aneurysm to grow undisturbed, which is prevailingly seen in aneurysm with decellularized walls5. The only disadvantage of this anatomical position is the possible cellular and molecular interaction between the gastrointestinal tract and the aneurysm.
Currently there are no other translational, extracranial animal models which reflect the human intracranial aneurysm in the subarachnoid space.
Operative differences Aquarius et al.12 described a method for treating sidewall aneurysms in a rat model using flow diverters. While the time required for the stent insertion was similar to our series (24.5±6.4 vs. 26±6 minutes), they had a shorter total operating time (126±23.0 vs. 167±22 minutes) and anastomosis time (16.3±6.4 vs. 23±7 minutes). The longer surgery time in our series may be explained by the more extended abdominal aorta dissection. As we inserted the stent with the pre-extended balloon to avoid intimal injury, we were forced to place the proximal clip superior to the renal artery. A more distal application of the proximal vessel clip could lead to early impediment of the balloon, and potentially mean that the aneurysm ostium is not covered by the stent. The ischemic time was fractioned (40 versus 23 min) in the present protocol to lower the risk of hypoxic damage. The organs were reoxygenated during a reperfusion phase of at least 10 min. One single long ischemic time shortens surgery duration but may increase the risk of postoperative multi organic failure - which did not occur in any of the presented cases. After a necessary learning period, the presented procedures should be manageable even for surgeons without prior microsurgical training.
Conclusion In conclusion, this stent application technique in a rat abdominal side-wall aneurysm model proved to be expedient, with a high grade of standardization, low rate of morbidity and mortality and low costs. These characteristics make the model suitable for future study of materials intended for stent-assisted aneurysm embolization.
Disclosures
This work was supported by the research funds of the Research Council, Kantonsspital Aarau, Aarau, Switzerland. The biodegradable magnesium stents were supplied by Biotronik AG, Center for Vascular Intervention, Buelach, Switzerland. The authors are solely responsible for the design and conduct of the presented study and declare no competing interests.
Acknowledgments
We thank Eugen Hofmann and Philine Zumstein for their excellent technical assistance and for sharing their expertise in stent application procedures. We thank Majlinda Kalanderi for the anatomical drawing.
References
- Molyneux A, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- Molyneux AJ, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- Marbacher S, et al. Intraluminal cell transplantation prevents growth and rupture in a model of rupture-prone saccular aneurysms. Stroke. 2014;45(12):3684–3690. doi: 10.1161/STROKEAHA.114.006600. [DOI] [PubMed] [Google Scholar]
- Marbacher S, Marjamaa J, Abdelhameed E, Hernesniemi J, Niemela M, Frosen J. The Helsinki rat microsurgical sidewall aneurysm model. J Vis Exp. 2014. p. e51071. [DOI] [PMC free article] [PubMed]
- Marbacher S, et al. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke. 2014;45(1):248–254. doi: 10.1161/STROKEAHA.113.002745. [DOI] [PubMed] [Google Scholar]
- Pritchett-Corning KR, Luo Y, Mulder GB, White WJ. Principles of rodent surgery for the new surgeon. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Bernal J, et al. Guidelines for rodent survival surgery. J Invest Surg. 2009;22(6):445–451. doi: 10.3109/08941930903396412. [DOI] [PubMed] [Google Scholar]
- Flege C, et al. Development and characterization of a coronary polylactic acid stent prototype generated by selective laser melting. J Mater Sci Mater Med. 2013;24(1):241–255. doi: 10.1007/s10856-012-4779-z. [DOI] [PubMed] [Google Scholar]
- Zartner P, Cesnjevar R, Singer H, Weyand M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheter Cardiovasc Interv. 2005;66(4):590–594. doi: 10.1002/ccd.20520. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, et al. Green fluorescent protein-transgenic rat: a tool for organ transplantation research. Biochem Biophys Res Commun. 2001;286(4):779–785. doi: 10.1006/bbrc.2001.5452. [DOI] [PubMed] [Google Scholar]
- Bouzeghrane F, Naggara O, Kallmes DF, Berenstein A, Raymond J. International Consortium of Neuroendovascular C. In vivo experimental intracranial aneurysm models: a systematic review. AJNR Am J Neuroradiol. 2010;31(3):418–423. doi: 10.3174/ajnr.A1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquarius R, Smits D, Gounis MJ, Leenders WP, De Vries J. Flow diverter implantation in a rat model of sidewall aneurysm: a feasibility study. J Neurointerv Surg. 2017. [DOI] [PubMed]
- Indolfi C, et al. A new rat model of small vessel stenting. Basic Res Cardiol. 2000;95(3):179–185. doi: 10.1007/s003950050180. [DOI] [PubMed] [Google Scholar]
- Oyamada S, et al. Trans-iliac rat aorta stenting: a novel high throughput preclinical stent model for restenosis and thrombosis. J Surg Res. 2011;166(1):e91–e95. doi: 10.1016/j.jss.2010.11.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, et al. Comparison of bare metal stent and paclitaxel-eluting stent using a novel rat aorta stent model. J Vet Sci. 2011;12(2):143–149. doi: 10.4142/jvs.2011.12.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouer M, Meilhac O, Delbosc S, et al. A new murine model of endovascular aortic aneurysm repair. J Vis Exp. 2013. p. e50740. [DOI] [PMC free article] [PubMed]
- Hardhammar PA, et al. Reduction in thrombotic events with heparin-coated Palmaz-Schatz stents in normal porcine coronary arteries. Circulation. 1996;93(3):423–430. doi: 10.1161/01.cir.93.3.423. [DOI] [PubMed] [Google Scholar]
- Guerrero A, et al. Antioxidant effects of a single dose of acetylsalicylic acid and salicylic acid in rat brain slices subjected to oxygen-glucose deprivation in relation with its antiplatelet effect. Neurosci Lett. 2004;358(3):153–156. doi: 10.1016/j.neulet.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Niitsu Y, et al. Repeat oral dosing of prasugrel, a novel P2Y12 receptor inhibitor, results in cumulative and potent antiplatelet and antithrombotic activity in several animal species. Eur J Pharmacol. 2008;579(1-3):276–282. doi: 10.1016/j.ejphar.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Kastrati A, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103(23):2816–2821. doi: 10.1161/01.cir.103.23.2816. [DOI] [PubMed] [Google Scholar]
- Lowe HC, James B, Khachigian LM. A novel model of in-stent restenosis: rat aortic stenting. Heart. 2005;91(3):393–395. doi: 10.1136/hrt.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AV, et al. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J Vasc Res. 2002;39(5):414–425. doi: 10.1159/000064518. [DOI] [PubMed] [Google Scholar]


