Abstract
Tear film is a complex mixture of lipids, proteins and minerals which covers the external surface of the eye, thereby providing lubrication, nutrition and protection to the underlying cells. Analysis of tears is an emerging area for the identification of biomarkers for the prediction, diagnosis, and prognosis of various ocular diseases. Tears are easily accessible and their collection is non-invasive. Therefore, advancing technologies are gaining prominence for identification of multiple analytes in tears to study changes in protein or metabolite composition and its association with pathological conditions. Tear cytokines are ideal biomarkers for studying the health of the ocular surface and also help in understanding the mechanisms of different ocular surface disorders like dry eye disease and vernal conjunctivitis. Bead based multiplex assays have the capability of detecting multiple analytes in a small amount of sample with a higher sensitivity. Here we describe a standardized protocol of tear sample collection, extraction and analysis of cytokine profiling using a bead based multiplex assay.
Keywords: Immunology, Issue 128, Tears, cytokine profiling, bead based multiplex assay, cytokines, ocular surface, eye diseases
Introduction
Tears are produced by the lacrimal gland and accessory glands and coat the outer surface of the eye. Tear film consists of an outer lipid layer and an inner aqueous layer that includes soluble proteins, mucins and membrane bound mucins. Tears prevent microbial invasion, supply nutrients, and provide lubrication to the ocular surface. Tears act as an interface between the air and tissue for oxygen transport to the cornea.1 Tear film is made up of proteins, carbohydrates, lipids and electrolytes. The association between tear proteins with ocular and systemic diseases like glaucoma, dry eye disease, vernal conjunctivitis, diabetes mellitus, thyroid-associated orbitopathy and cancer has been identified in several studies.2,3,4 Tear samples can be collected by microcapillary tubes or tear flow strips (Schirmer strips). Additionally, the Schirmer's test is a standard procedure performed in the cornea and refractive surgery clinics, the results of which may be used for cytokine analysis assays. Non-invasive sample collection, accessibility of the bio-specimen, and the association of tear composition with various physiological and pathological conditions make tear film a potential source of biomarkers for several ocular and systemic diseases.4,5,6
Tear cytokines plays an important role in studying the ocular surface health and inflammatory conditions of various ocular diseases.7 Abnormal concentrations of several cytokines in tear samples were reported to be associated with dry eye disease, vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), seasonal allergic conjunctivitis, and uveitis.8,9,10,11,12,13 Tear proteins can be analyzed by traditional methods like mass spectrometry, western blotting and enzyme-linked immunosorbent assays (ELISA).14,15 However, the limitations of these methods are poor sensitivity and a larger volume of sample required for the analysis of multiple tear cytokines in each patient.16,17 Bead based multiplex assays have been developed to analyze multiple analytes in complex mixture samples and successfully applied on tear samples to analyze multiple cytokines in different diseases.6,18 A combination of sandwich ELISA and flow cytometry techniques enable these assays to become more sensitive than ELISA for the quantification of multiple analytes in a single sample.19 This method can be applied on a variety of clinical samples and cell culture supernatants and helps in the study of the immune responses in several pathophysiological conditions.20,21,22,23,24
There are several studies comparing bead based multiplex assays with ELISA and have reported a correlation between the methods. Loo et al. compared bead based multiplex assay with conventional ELISA for the detection of adiponectin, resistin, leptin and ghrelin in human serum or plasma samples and reported a strong correlation (r>0.9) between the assays.25 Dupont et al. reported a strong correlation between bead based multiplex assays and ELISA for the detection of IL-1β, IL-4, IL-5, IL-6, IL-10, IFN-ϒ, and TNF-α in phytohemagglutinin and lipopolysaccharide stimulated whole blood collected from pregnant women.26 Pickering et al. reported another strong correlation between bead based multiplex assay and ELISA for the detection of serum antibodies to Haemophilus influenza type b polysaccharide (r=0.96), toxoids of Clostridium tetani (r=0.96), and Corynebacterium diphtheriae (r=0.91).27 Biagini et al. reported a high positive correlation (r=0.852) between bead based multiplex assay and ELISA for detection of Bacillus anthracis anti-PA IgG in serum samples.28 Wang et al. reported a correlation between bead based multiplex assay and ELISA for the detection of Alzheimer's disease biomarkers amyloid-β 42 (r=0.77), total tau (r=0.94), and tau phosphorylated on amino acid 181 (r=0.82) in cerebrospinal fluid samples.29 These studies have demonstrated the applicability of bead based multiplex assays on variety of clinical samples, smaller sample volume requirements and a correlation with standard ELISA, which make bead based multiplex assays a promising alternative to traditional ELISA methods for the detection of multiple analytes in different type of samples in different disease phenotypes. Here we describe a standardized protocol for bead based multiplex assay for cytokine profiling for 41 analytes in tear samples collected from healthy subjects using Schirmer strips.
Protocol
The protocols used in this study were approved by the Institutional review board of Tan Tock Seng Hospital, Singapore.
1. Tear Cytokine Analysis
- Collection of tears:
- Ask the subject to sit comfortably on an examination chair and place his/her head against the headrest.
- Ask the subject to look up, carefully open the Schirmer strips (made of Whatman filter paper no. 41) and place the rounded end of the strip on the inferior fornix of the eye and instruct the subject to close the eyes for 5 min. While collecting the tears take great care to minimize ocular surface contact.30
- Ask the subject to open his/her eyes to remove the strip and place it in a sterile 1.5 mL microcentrifuge tube. Immediately transfer the tube to the laboratory or store at -80o C until testing for cytokine profiling.
- Elution of tear sample from tear flow strip:
- Cut the tear flow strip to a length of 0.5 cm (to standardize the quantity of tears to be tested) and place it in a sterile 1.5 mL microcentrifuge tube.
- Add 30 µL of assay buffer and incubate at room temperature for 5 min followed by centrifuging the tube at 14,000 x g for 1 min.
- Transfer the supernatant into another 1.5 mL microcentrifuge tube and discard the strip. Place the sample containing the tube on ice and use the eluted tear sample immediately for cytokine profiling.
- Preparation of reagents: NOTE: The forty one analytes to be tested in each sample by bead based multiplex assay are interleukin (IL)-1α, IL-1β, IL-Rα, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-15, IL-17A, interferon-alpha (IFN- α) 2, IFN-γ, IFN-gamma-inducible protein 10 (IP-10, CXCL10), macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP)-1α and MIP-1β, monocyte chemotactic protein (MCP)-1, MCP-3, tumor necrosis factor-alpha (TNF-α), TNF-β, growth-regulated oncogene (GRO), tumor growth factor alpha (TGF-α), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF)-2, platelet derived growth factor (PDGF)-AA, PDGF-AB/BB, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), eotaxin, fractalkine, soluble CD40 ligand (sCD40L), Fms-like tyrosine kinase 3 ligand (Flt-3L) and regulated upon activation, normal T-cell expressed and secreted protein (RANTES).
- Bring the antibody bead solution (50X) vials to room temperature (20 - 25 oC) and vortex the vials for 1 min.
- Count the number of wells required for the assay, and calculate the quantity of total antibody bead cocktail solution (25 µL per well) and each antibody bead solution (50X) required for the assay.
- Prepare the cocktail solution by adding the calculated amount of each antibody bead solution and fill to the desired final volume by adding the remaining amount of bead diluent. Ensure that the final concentration of each antibody in the cocktail is 1X. Always prepare at least 20% extra volume of cocktail solution in case of pipetting errors. NOTE: As an example, for a 96-well assay prepare 3000 µL of antibody bead cocktail solution. The required amount of each antibody bead solution = 3000/stock concentration of each antibody bead solution; 3000/50 = 60 µL of each antibody bead solution
- Add 60 µL of each of the 41 antibody bead solutions (60 X 41 = 2460 µL) into a cocktail bottle and add 540 µL of bead diluent solution to the antibody bead mixture to make 3000 µL of final working solution (1X).
- Prior to use, mix the bead cocktail solution properly. After the assay store the remaining volume at 2-8 oC for up to 30 days.
- Prepare the quality controls (QC) by adding 250 µL deionized water to QC 1 and QC 2 stocks.
- Mix properly by inverting the bottles several times and vortex for 10 s. Transfer the solution to properly labeled polypropylene microcentrifuge tubes and store the remaining solution at -20 °C which can be used for up to 30 days.
- Prepare the wash buffer by adding 270 mL deionized water to 30 mL of 10X wash buffer solution and mix well (before dilution, bring the 10X buffer to room temperature and dissolve all salt precipitates by mixing). Store the unused wash buffer (1X) at 2-8 °C which can be used for up to 30 days.
- Prepare the human cytokine standard stock (10,000 pg/mL of all analytes) by adding 250 µL deionized water to the stock vial. Mix well by inverting several times and vortex the vial for 10 s. Allow it to stand for 10 min and transfer the stock solution to properly labeled polypropylene microcentrifuge tubes. After the assay, store the remaining solution at -20 °C, which can be used for up to 30 days.
- Prepare the working human cytokine standards by 5-fold serial dilutions (50 µL -> 200 µL) with assay buffer to get 2000, 400, 80, 16, and 3.2 pg/mL.
- After standard preparation, use the working standards within 60 min and use the assay buffer as blank/background (-pg/mL).
- Cytokine profiling by bead based multiplex assay:
- Bring all reagents to room temperature and vortex for 5-10 seconds before adding them to the 96-well microtiter plate. If eluted tear samples are stored at -80oC prior to the assay, thaw the frozen tear extracts on ice and centrifuge at 1000 X g for 5 min.
- Prepare an assay work sheet in a vertical configuration for working human cytokine standards [0 (Blank), 3.2, 16, 80, 400, 2,000, and 10,000 pg/mL], QC1, QC2 and samples.
- Add 200 µL of 1X wash buffer to each well of the plate, seal it with plate sealer and keep it on a plate shaker at room temperature (20-25 oC) for 10 min.
- Decant the 1X wash buffer by inverting the plate and tap it onto absorbent towels several times to remove the any residual amount of wash buffer in the wells.
- Add 25 µL of each working human cytokine standard, QC1, QC2, blank (assay buffer) and samples into the appropriate wells.
- Add 25 µL of assay buffer into each well.
- Add 25 µL of 1X antibody bead cocktail solution into each well. As the antibody bead solution is light sensitive, seal the plate with plate sealer and cover it with aluminum foil to protect it from light during the assay.
- Incubate the plate at 4 oC overnight on a shaker.
- Place the plate on the plate rack of an automatic magnetic plate washer. Let it sit for 1 min to settle the magnetic beads at the bottom of the well and aspirate the well contents. Add 200 µL of wash buffer per well and let it sit for 1 min and then aspirate the well contents. Repeat the wash once again (for plate washing, follow the kit manufacturer's instructions).
- Add 25 µL of detection antibodies solution into each well, seal the plate, cover it with aluminum foil, and incubate at room temperature for 60 min on a shaker.
- Add 25 µL of Streptavidin-Phycoerythrin solution into each well, seal the plate, cover it with aluminum foil, and incubate at room temperature for 30 min on a shaker.
- Place the plate on a magnetic plate washer. Let it sit for 1 min and then aspirate the well contents. Add 200 µL of wash buffer per well. Let it sit for 1 min and then aspirate the well contents. Repeat the wash once again.
- Add 150 µL of sheath fluid to each well and place the plate on a shaker for 5 min at room temperature to resuspend the antibody beads.
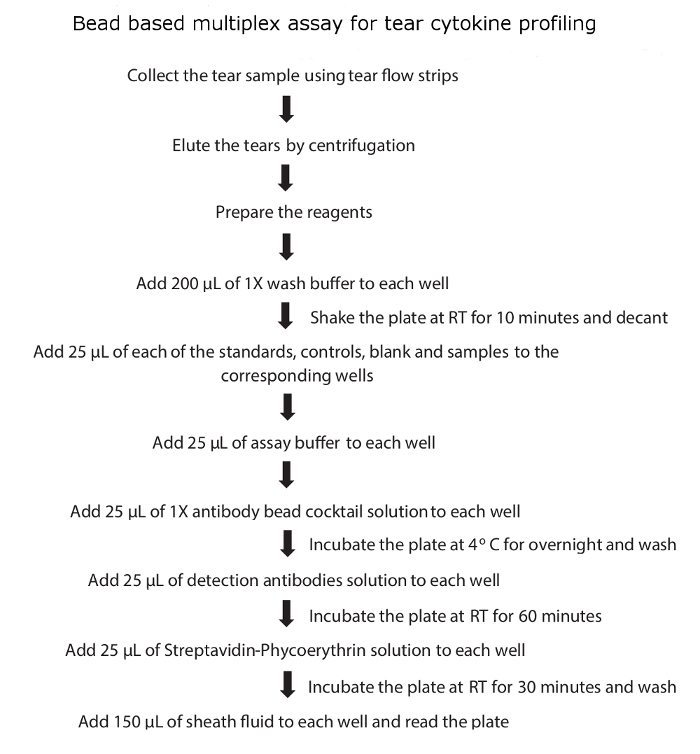
- Read the plate immediately using the bead based multiplex assay plate reader and analyze the cytokine concentrations using a 5-parameter curve-fitting algorithm. A schematic flow diagram of tear cytokine profiling is shown in Figure 1.
- Reading the assay plate (instrument set up) and data analysis:
- Switch on the bead based multiplex assay reader and pre-warm the laser for 30 min.
- Launch the bead based multiplex assay software. Under the 'Automated Maintenance' tab, select the 'Calibration-verification' option. Vortex each reagent vial of the calibration and verification beads for 30 s. Place 5 drops of each reagent into the designated wells. Fill the designated reservoirs with deionized water and 70% ethanol.
- Create a new protocol
- Open the 'Protocols' page and then the 'Protocols' tab. Click "Create New Protocol"; the 'settings' tab will open.
- In the 'Name' box, type the name of the protocol.
- Type a description in the box to the right of the 'Name' box.
- In the 'Version' box, type the version of the protocol.
- In the 'Manufacturer' box, type the manufacturer information for the protocol.
- Define settings in the 'Acquisition Settings' section as follows. Set 'Volume': 100 µL, 'Timeout': 60 s; 'DD Gating': 8,000 to 15,000; 'Reporter Gain': Standard PMT; 'Bead type': Magnetic bead.
- Define settings in the 'Analysis Settings' section, selecting 'Quantitative' as the analysis type. Set 'Number of Standards': 6; 'Number of Controls': 0; Tick the 'Mean of Replicates' box; Tick the 'Analyze results while acquiring samples' box.
- Click "Next"; the 'Analytes' tab opens, click on the desired analytes (bead ID) in the numbered analyte grid.
- Click and type the corresponding analyte name in the 'Name' column to the right of the analyte grid (analytes' names and their corresponding bead region details were mentioned in Table 1).
- Click and type the desired unit of measurement (i.e. pg/mL) in the 'Units' box to the left of the 'Apply All' button. Then click "Apply All".
- Click and type the desired bead count for each analyte (i.e. 50) in the 'Count' box. Click "Apply All".
- Click "Next"; the Plate Layout tab opens. Highlight the wells for the standards and select "2" under replicate count and click on the "S" Standard button. Repeat this step for the background "B" and samples "U" wells.
- Click "Save".
- Create New Standards/Control Lots
- Open the 'Protocols' page, and then the 'Standards & Controls' tab. Click "Create New Standard/Control Lots".
- Open the 'Select Protocol' box. Select the protocol that was created in step 1.5.3.
- Key in the information of the standards accordingly: Std/Ctrl kit number, Std/Ctrl kit name, expiration, and manufacturer.
- Key in the value of the highest standard for each analyte (i.e. 10000 pg/mL). Key in 5 under dilution and click on "Apply All" to automatically generate the expected concentrations for the rest of the standards.
- Click "Save".
- Create a New Batch from an existing protocol
- Open the 'Batches' page and click "Create a New Batch tab from an existing Protocol".
- Type the batch name in the 'Batch Name' box and type a description about the batch in the 'Enter Optional Description' box.
- Click on the protocol generated previously (in step 1.5.3).
- Click "Next"; the next tab that opens is the 'Stds & Ctrls' tab. View the details of assay standards and select "Next".
- On the 'Plate Layout' tab, assign well commands for this batch and click "Save" to save batch information to the 'Pending Batch' list.
- Load the plate into the bead based multiplex assay plate reader, shake it for 5 min and run the batch from the pending batch list. Data acquired will be saved in the .csv file format.
- Data analysis
- Convert the .csv file generated from the bead based multiplex assay software to the .rbx file (results data file) format using the rbx conversion software. Launch the conversion software, select the .csv file under select xPONENT file(s) and click on the "generate" button; the .rbx file will be saved in the selected output folder.
- Launch the analysis software and open the .rbx file.
- Optimize the standard curves using the 4PL/5PL curve fit.
- Select the following in the 'Standard Curve' tab: 'Regression Type': 'Logistic-5PL'; 'Axis Transformation': 'Log(x) - Linear(y)'; tick the 'Show Conc Range Lines' box; tick the 'Show Unknown Samples' box; Tick the 'Show Control Samples' box; Tick the 'Apply across all analytes' box; tick the 'Show report after optimization' box.
- Click the "Optimize" button for auto-optimization.
- Label the sample identities under 'Enter Sample Info' tab.
- Obtain the observed concentrations in the 'Report Table' tab and click on "export report table" to generate the data in a spreadsheet file for further analysis.
Representative Results
Tear samples were collected from 8 eyes of 4 healthy subjects using tear flow strips and cytokine levels were analyzed using the above mentioned protocol. All subjects were male and the age of the four subjects was 36, 42, 44 and 52 years respectively. Ocular surface health and dry eye disease was evaluated by clinical tests (tear film break up time, Schirmer's test, corneal staining, conjunctival staining, lid/meibomian gland examination, corneal tear signs and visual signs and symptoms) according to the International Dry Eye Workshop, 2007 guidelines and none of the subjects showed any signs and symptoms of dry eye disease.31
Out of the 41 cytokines analyzed, 31 cytokines were detected in all the tear samples, IL-17A, MIP-1β and TNF-α were detected in 7 eyes of 4 subjects, IL-4 was detected in 6 eyes of 4 subjects, IFN-γ was detected in 6 eyes of 3 subjects, IL-1A was detected in 5 eyes of 3 subjects, eotaxin and IL-9 were detected in 3 eyes of 2 subjects, IL-3 was detected in 1 eye and MIP-1α in none of the samples. The mean ± SD concentrations of 41 cytokines detected in healthy subjects using bead based multiplex assay (Figure 2) were mentioned in Table 2. Out of 41 cytokines analyzed, IL-1ra was found to be highly expressed in all the samples with the mean ± SD of 203.9 ± 52.6 ng/mL and ranged from 129.64 ng/mL to 271.7 ng/mL. Cytokines IP-10, GRO, MCP-1, PDGF-AA, Fractalkine, IL-8, EGF, PDGF-BB, VEGF and G-CSF were also highly expressed (ng/mL quantities) in the tears of healthy subjects and the remaining cytokines were expressed in pg/mL quantities (Table 2). The lowest measured concentration of tear cytokine was TNF-α with the mean ± SD of 27.25 ± 19.97 pg/mL and ranged from 10.75 pg/mL to 56.02 pg/mL. In 31 cytokines which were detected in all the samples, none showed a statistically significant difference in concentration (p>0.05) between the right and left eye by t-test (Figure 3).
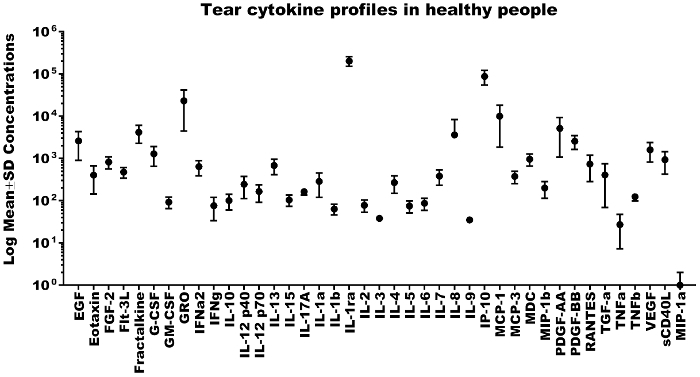
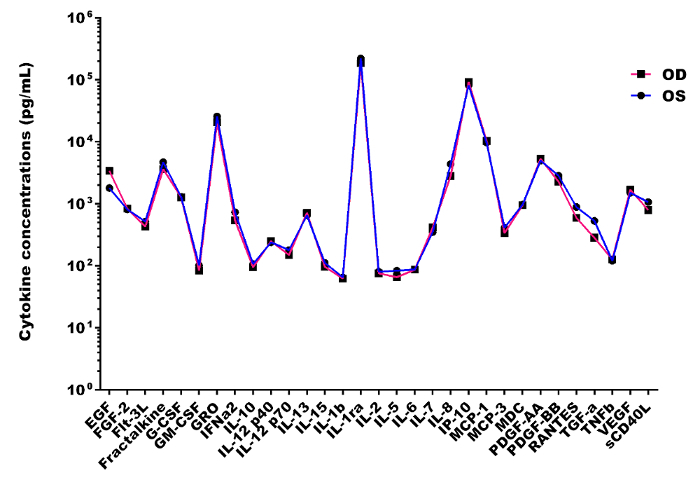
Figure 1: Schematic flow diagram of tear cytokine profiling. Please click here to view a larger version of this figure.
Figure 2: Tear cytokine profiles in healthy people. Representative scatterplot showing the log mean ± SD concentrations of 41 cytokines in tear samples collected from healthy subjects. Please click here to view a larger version of this figure.
Figure 3: Inter-eye tear cytokine profile differences in healthy subjects. Representative scatterplot showing no statistically significant difference (p>0.05) in inter-eye mean concentrations of 31 tear cytokines in healthy subjects. Please click here to view a larger version of this figure.
| S. No. | Analytes | Bead region |
| 1 | EGF | 12 |
| 2 | FGF-2 | 13 |
| 3 | Eotaxin | 14 |
| 4 | TGF-α | 15 |
| 5 | G-CSF | 18 |
| 6 | Flt-3L | 19 |
| 7 | GM-CSF | 20 |
| 8 | Fractalkine | 21 |
| 9 | IFNα2 | 22 |
| 10 | IFNγ | 25 |
| 11 | GRO | 26 |
| 12 | IL-10 | 27 |
| 13 | MCP-3 | 28 |
| 14 | IL-12P40 | 29 |
| 15 | MDC | 30 |
| 16 | IL-12P70 | 33 |
| 17 | PDGF-AA | 34 |
| 18 | IL-13 | 35 |
| 19 | PDGF-AB/BB | 36 |
| 20 | IL-15 | 37 |
| 21 | sCD40L | 38 |
| 22 | IL-17A | 39 |
| 23 | IL-1RA | 42 |
| 24 | IL-1α | 44 |
| 25 | IL-9 | 45 |
| 26 | IL-1β | 46 |
| 27 | IL-2 | 48 |
| 28 | IL-3 | 51 |
| 29 | IL-4 | 53 |
| 30 | IL-5 | 55 |
| 31 | IL-6 | 57 |
| 32 | IL-7 | 61 |
| 33 | IL-8 | 63 |
| 34 | IP-10 | 65 |
| 35 | MCP-1 | 67 |
| 36 | MIP-1α | 72 |
| 37 | MIP-1β | 73 |
| 38 | RANTES | 74 |
| 39 | TNFα | 75 |
| 40 | TNFβ | 76 |
| 41 | VEGF | 78 |
Table 1: Analytes' names and corresponding bead region details.
| S. No. | Cytokine | Number of eyes | Number of subjects | Mean concentrations | SD |
| 1 | EGF | 8 | 4 | 2610.29 | 1711.56 |
| 2 | Eotaxin | 3 | 2 | 404.73 | 260.76 |
| 3 | FGF-2 | 8 | 4 | 827.73 | 262.44 |
| 4 | Flt-3L | 8 | 4 | 475.4 | 133.6 |
| 5 | Fractalkine | 8 | 4 | 4179.08 | 1888.38 |
| 6 | G-CSF | 8 | 4 | 1287.57 | 633.16 |
| 7 | GM-CSF | 8 | 4 | 93.03 | 28.1 |
| 8 | GRO | 8 | 4 | 23176.87 | 18692.07 |
| 9 | IFNa2 | 8 | 4 | 641.4 | 253.55 |
| 10 | IFNg | 6 | 3 | 76.6 | 42.81 |
| 11 | IL-10 | 8 | 4 | 101.32 | 41.52 |
| 12 | IL-12 p40 | 8 | 4 | 245.46 | 133.01 |
| 13 | IL-12 p70 | 8 | 4 | 165.53 | 73.09 |
| 14 | IL-13 | 8 | 4 | 685.84 | 272.92 |
| 15 | IL-15 | 8 | 4 | 104.4 | 30.66 |
| 16 | IL-17A | 7 | 4 | 164.32 | 28.69 |
| 17 | IL-1a | 5 | 3 | 286.79 | 166.87 |
| 18 | IL-1b | 8 | 4 | 63.98 | 18.29 |
| 19 | IL-1ra | 8 | 4 | 203892.58 | 52593.48 |
| 20 | IL-2 | 8 | 4 | 78.35 | 25.15 |
| 21 | IL-3 | 1 | 1 | 38.2 | 0 |
| 22 | IL-4 | 6 | 4 | 268.94 | 120.84 |
| 23 | IL-5 | 8 | 4 | 74.96 | 23.8 |
| 24 | IL-6 | 8 | 4 | 86.97 | 27.78 |
| 25 | IL-7 | 8 | 4 | 384.08 | 153.57 |
| 26 | IL-8 | 8 | 4 | 3615.21 | 4677.5 |
| 27 | IL-9 | 3 | 2 | 35.22 | 5.11 |
| 28 | IP-10 | 8 | 4 | 87546.26 | 33256.53 |
| 29 | MCP-1 | 8 | 4 | 10074.73 | 8205.5 |
| 30 | MCP-3 | 8 | 4 | 376.13 | 120.68 |
| 31 | MDC | 8 | 4 | 968.08 | 309.39 |
| 32 | MIP-1b | 7 | 4 | 199.72 | 84.79 |
| 33 | PDGF-AA | 8 | 4 | 5174.06 | 4103.97 |
| 34 | PDGF-BB | 8 | 4 | 2567.61 | 919.01 |
| 35 | RANTES | 8 | 4 | 742.41 | 457.57 |
| 36 | TGF-a | 8 | 4 | 407.82 | 339.12 |
| 37 | TNFa | 7 | 4 | 27.25 | 19.97 |
| 38 | TNFb | 8 | 4 | 124 | 24.16 |
| 39 | VEGF | 8 | 4 | 1601.2 | 776.85 |
| 40 | sCD40L | 8 | 4 | 932.1 | 509.27 |
| 41 | MIP-1a | 0 | 0 | 0 | 0 |
Table 2: Tear cytokine profiles in healthy subjects. Mean ± SD concentrations of 41 cytokines detected in healthy subjects using bead based multiplex assay.
Discussion
Cytokines are small cellular secreted proteins and potent immune modulators regulating immune responses.32 The expression profiles of various cytokines in tear samples are related to various pathological conditions of the eye and studies on cytokine profiles help to understand the mechanisms of disease pathogenesis, determine the state of ocular health, disease severity, diagnosis and progression.2,5 Lower protein concentrations and small sample volumes are the main challenges of traditional methods, limiting the use of assays like ELISA for the analysis of tear cytokine profiles.33 Bead based multiplex assays are immunoassays, which have distinct advantages over ELISA. Bead based multiplex assays require smaller sample volumes for analysis of multiple analytes and are highly sensitive, capable of detecting picogram concentrations of proteins in biological fluids.34
Here we report a standardized protocol for the quantitative analysis of 41 tear cytokines in healthy people using bead based multiplex assay. Out of the 41 cytokines in the panel, bead based multiplex assay detected 40 cytokines in tear samples and could not detect MIP-1α. This negative result might be because there were undetectable levels of MIP-1α in healthy tear samples or due to a smaller volume of tears. Tear samples collected using Schirmer strips have an advantage over other collection methods like microcapillary tube and sponges. For instance, in several clinical conditions, tear fluid volume can be measured by Schirmer strips and the tear fluid can be eluted from the same strip after measurement and used for cytokine analysis.35 Though microcapillary tubes are routinely used to collect tears and produce more consistent tear profiles, the procedure takes more time than the strip method, is more tedious, and may be uncomfortable for children and other patients. Moreover, this method may also induce reflex tear production by touching the conjunctiva and produce variable results of cytokine profiles compared to basal tears, thereby affecting the reproducibility of the assay.36 In some clinical conditions like dry eye disease, tear sample collection by Schirmer strips is reliable for protein analysis and helps in the identification of biomarkers.37,38
Bead based multiplex assays are a combination of flow cytometry and multiplex ELISA in which analytes in the sample are sandwiched between the specific antibody coated and color coded magnetic beads or microspheres and biotinylated detection antibody. These complexes can be detected by adding the reporter molecule Streptavidin-Phycoerythrin (PE) conjugate and subsequent exposure to a dual laser system in a modified flow cytometry-based instrument.39 In a dual laser system, one laser excites the internal dyes and its signal represents the coated specific antibody. The second laser excites the reporting molecule PE conjugate bound to the detection antibody complex and the intensity of the signals represent the concentration of analyte.
The capability of the microspheres to be coded with dyes of varying intensities enable the assay to detect up to 100 different analytes in a small volume of sample in a single well.39 Though bead based multiplex assays are rapid, reproducible, and comparable to standard ELISA assays,34,40 this method requires dedicated instruments, evaluation of kits and standardization of protocols for various biological specimens.39 The presence of auto-antibodies in the clinical samples may affect the bead based multiplex results.34,39 For routine use of these assays in clinical settings, it is recommended to use the assay kits from one specific manufacturer. The sensitivity, detection of absolute concentrations of cytokines, and reproducibility were reported to vary with the different kits.41 Antibody microarrays or membrane microarrays are highly sensitive, inexpensive alternative high throughput techniques which detect multiple analytes in a small volume of samples and can be applied successfully on tear samples for the analysis of cytokines and other proteins.35,42 However, numerous limitations exist, as these assays are time consuming, semi quantitative, exhibit a high signal-to-noise ratio, and lack standardized protocols.19,43
Disclosures
The authors have nothing to disclose. No financial interest or conflict of interest.
Acknowledgments
The research work was supported by the Centre Grant from Tan Tock Seng Hospital Personalised Seed Funding Program 2015; Singapore Eye Research Institute Pilot Grant and Tan Tock Seng Hospital Pitch for Fund grant.
References
- Conrady CD, Joos ZP, Patel BC. Review: The Lacrimal Gland and Its Role in Dry Eye. J Ophthalmol. 2016;7542929 doi: 10.1155/2016/7542929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Thun Und Hohenstein-Blaul N, Funke S, Grus FH. Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res. 2013. pp. 126–137. [DOI] [PubMed]
- Pieragostino D, D'Alessandro M, di Ioia M, Di Ilio C, Sacchetta P, Del Boccio P. Unraveling the molecular repertoire of tears as a source of biomarkers: beyond ocular diseases. Proteomics Clin Appl. 2015;9(1-2):169–186. doi: 10.1002/prca.201400084. [DOI] [PubMed] [Google Scholar]
- Azkargorta M, Soria J, Acera A, Iloro I, Elortza F. Human tear proteomics and peptidomics in ophthalmology: Toward the translation of proteomic biomarkers into clinical practice. J Proteomics. 2017;150:359–367. doi: 10.1016/j.jprot.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7:15. doi: 10.1186/s13167-016-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentka A, et al. Membrane array and multiplex bead analysis of tear cytokines in systemic sclerosis. Immunol Res. 2016;64(2):619–626. doi: 10.1007/s12026-015-8763-9. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31(6):527–550. doi: 10.1016/j.preteyeres.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Jackson DC, et al. Tear Interferon-Gamma as a Biomarker for Evaporative Dry Eye Disease. Invest Ophthalmol Vis Sci. 2016;57(11):4824–4830. doi: 10.1167/iovs.16-19757. [DOI] [PubMed] [Google Scholar]
- Agrawal R, et al. A distinct cytokines profile in tear film of dry eye disease (DED) patients with HIV infection. Cytokine. 2016;88:77–84. doi: 10.1016/j.cyto.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Uchio E, Ono SY, Ikezawa Z, Ohno S. Tear levels of interferon-gamma, interleukin (IL) -2, IL-4 and IL-5 in patients with vernal keratoconjunctivitis, atopic keratoconjunctivitis and allergic conjunctivitis. Clin Exp Allergy. 2000;30(1):103–109. doi: 10.1046/j.1365-2222.2000.00699.x. [DOI] [PubMed] [Google Scholar]
- Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36(6):777–784. doi: 10.1111/j.1365-2222.2006.02499.x. [DOI] [PubMed] [Google Scholar]
- Enríquez-de-Salamanca A, Calonge M. Cytokines and chemokines in immune-based ocular surface inflammation. Expert Rev Clin Immunol. 2008;4(4):457–467. doi: 10.1586/1744666X.4.4.457. [DOI] [PubMed] [Google Scholar]
- Carreño E, et al. Cytokine and chemokine tear levels in patients with uveitis. Acta Ophthalmol. 2016. [DOI] [PubMed]
- Bjerrum KB, Prause JU. Collection and concentration of tear proteins studied by SDS gel electrophoresis. Presentation of a new method with special reference to dry eye patients. Graefes Arch Clin Exp Ophthalmol. 1994;232(7):402–405. doi: 10.1007/BF00186580. [DOI] [PubMed] [Google Scholar]
- Saijyothi AV, et al. Two dimensional electrophoretic analysis of human tears: collection method in dry eye syndrome. Electrophoresis. 2010;31(20):3420–3427. doi: 10.1002/elps.201000271. [DOI] [PubMed] [Google Scholar]
- Thakur A, Willcox MD. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998;67(1):9–19. doi: 10.1006/exer.1998.0480. [DOI] [PubMed] [Google Scholar]
- Wei Y, Gadaria-Rathod N, Epstein S, Asbell P. Tear cytokine profile as a noninvasive biomarker of inflammation for ocular surface diseases: standard operating procedures. Invest Ophthalmol Vis Sci. 2013;54(13):8327–8336. doi: 10.1167/iovs.13-12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu-Yilmaz P, et al. Determination of tear and serum inflammatory cytokines in patients with rosacea using multiplex bead technology. Ocul Immunol Inflamm. 2013;21(5):351–359. doi: 10.3109/09273948.2013.795229. [DOI] [PubMed] [Google Scholar]
- Hagan S, Tomlinson A. Tear fluid biomarker profiling: a review of multiplex bead analysis. Ocul Surf. 2013;11(4):219–235. doi: 10.1016/j.jtos.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Choi R, et al. Serum inflammatory profiles in pulmonary tuberculosis and their association with treatment response. J Proteomics. 2016;149:23–30. doi: 10.1016/j.jprot.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Kang JH, Vanderstichele H, Trojanowski JQ, Shaw LM. Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease. Methods. 2012;56(4):484–493. doi: 10.1016/j.ymeth.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Staples E, Ingram RJ, Atherton JC, Robinson K. Optimising the quantification of cytokines present at low concentrations in small human mucosal tissue samples using Luminex assays. J Immunol Methods. 2013;394(1-2):1–9. doi: 10.1016/j.jim.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inic-Kanada A, et al. Comparison of ophthalmic sponges and extraction buffers for quantifying cytokine profiles in tears using Luminex technology. Mol Vis. 2012;18:2717–2725. [PMC free article] [PubMed] [Google Scholar]
- Moncunill G, Aponte JJ, Nhabomba AJ, Dobaño C. Performance of multiplex commercial kits to quantify cytokine and chemokine responses in culture supernatants from Plasmodium falciparum stimulations. PLoS One. 2013;8(1):e52587. doi: 10.1371/journal.pone.0052587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo BM, Marniemi J, Jula A. Evaluation of multiplex immunoassays, used for determination of adiponectin, resistin, leptin, and ghrelin from human blood samples, in comparison to ELISA assays. Scand J Clin Lab Invest. 2011;71(3):221–226. doi: 10.3109/00365513.2011.554996. [DOI] [PubMed] [Google Scholar]
- Dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66(2):175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering JW, Martins TB, Schroder MC, Hill HR. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for auantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin Diagn Lab Immunol. 2002;9(4):872–876. doi: 10.1128/CDLI.9.4.872-876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini RE, Sammons DL, Smith JP, MacKenzie BA, Striley CA, et al. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin Diagn Lab Immunol. 2004;11(1):50–55. doi: 10.1128/CDLI.11.1.50-55.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Leung YY, Chang SK, Leight S, Knapik-Czajka M, et al. Comparison of xMAP and ELISA assays for detecting cerebrospinal fluid biomarkers of Alzheimer's disease. J Alzheimers Dis. 2012;31(2):439–445. doi: 10.3233/JAD-2012-120082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsow CM, Reindel WT, Merchea MM, Bateman KM, Barr JT. Tear cytokine response to multipurpose solutions for contact lenses. Clin Ophthalmol. 2013;7:1291–1302. doi: 10.2147/OPTH.S44642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) The ocular surface. 2007;5(2)(2007):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Stenger S, Röllinghoff M. Role of cytokines in the innate immune response to intracellular pathogens. Ann Rheum Dis. 2001;60 doi: 10.1136/ard.60.90003.iii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. Antibody protein array analysis of the tear film cytokines. Optom Vis Sci. 2008;85(8):653–660. doi: 10.1097/OPX.0b013e3181824e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe , Negm O, Todd I, Fairclough L. Utility, reliability and reproducibility of immunoassay multiplex kits. Methods. 2013;61(1):23–29. doi: 10.1016/j.ymeth.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Dionne K, Redfern RL, Nichols JJ, Nichols KK. Analysis of tear inflammatory mediators: A comparison between the microarray and Luminex methods. Mol Vis. 2016;22:177–188. [PMC free article] [PubMed] [Google Scholar]
- Sitaramamma T, Shivaji S, Rao GN. HPLC analysis of closed, open, and reflex eye tear proteins. Indian J Ophthalmol. 1998;46(4):239–245. [PubMed] [Google Scholar]
- Saijyothi AV, et al. Two dimensional electrophoretic analysis of human tears: collection method in dry eye syndrome. Electrophoresis. 2010;31(20):3420–3427. doi: 10.1002/elps.201000271. [DOI] [PubMed] [Google Scholar]
- VanDerMeid KR, Su SP, Krenzer KL, Ward KW, Zhang JZ. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011;17:1056–1063. [PMC free article] [PubMed] [Google Scholar]
- Khalifian S, Raimondi G, Brandacher G. The use of luminex assays to measure cytokines. J Invest Dermatol. 2015;135(4):e31. doi: 10.1038/jid.2015.36. [DOI] [PubMed] [Google Scholar]
- Richens JL, Urbanowicz RA, Metcalf R, Corne J, O'Shea P, Fairclough L. Quantitative validation and comparison of multiplex cytokine kits. J Biomol Screen. 2010;15(5):562–568. doi: 10.1177/1087057110362099. [DOI] [PubMed] [Google Scholar]
- Berthoud TK, et al. Comparison of commercial kits to measure cytokine responses to Plasmodium falciparum by multiplex microsphere suspension array technology. Malar J. 2011;10:115. doi: 10.1186/1475-2875-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52(10):7725–7730. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- Sack R, Conradi L, Beaton A, Sathe S, McNamara N, Leonardi A. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res. 2007;85(4):528–538. doi: 10.1016/j.exer.2007.07.004. [DOI] [PubMed] [Google Scholar]


