Abstract
Coronary artery disease (CAD) is a leading cause of morbidity and mortality worldwide. Atherosclerosis, a leading cause of CAD, is initiated by the transmigration of innate immune monocytes to inflammatory sites of deposited lipid called fatty streaks, which are present in arterial walls of medium to large arteries. The key pathogenic feature of lesions at this early stage of atherosclerosis is the maturation of monocytes which migrate into arteries to form foam cells or lipid-laden macrophages. Considerable evidence supports the hypothesis that risk of atherosclerosis is increased by chronic inflammatory conditions accompanying diseases such as rheumatoid arthritis and HIV, as well as general ageing, and that this risk is predicted by monocyte activation. While mouse models provide a good platform to investigate the role of monocytes in atherogenesis in vivo, they require genetic alteration of natural cholesterol metabolism and drastic alteration of normal mouse diets, and have limited suitability for the study of atherogenic influences of human comorbid diseases. This motivated us to develop a human in vitro model to measure the atherogenic potential of monocytes isolated from individuals with defined disease states. Currently, human in vitro models are limiting in that they evaluate monocyte transmigration and foam cell formation in isolation. Here we describe a protocol in which monocytes isolated from patient blood transmigrate across human endothelial cells into a type 1 collagen matrix, and their propensity to mature into foam cells in the presence or absence of exogenous lipid is measured. The protocol has been validated for the use of human monocytes purified from individuals with HIV infection and elderly HIV uninfected individuals. This model is versatile and allows monocyte transmigration and foam cell formation to be evaluated using either microscopy or flow cytometry as well as allowing the assessment of atherogenic factors present in serum or plasma.
Keywords: Immunology, Issue 128, Atherosclerosis, inflammation, monocytes, endothelium, transmigration, foam cells
Introduction
Monocyte transmigration is a crucial step in the development of atherosclerotic plaque that may lead to thrombosis, stroke and myocardial infarction. Atherosclerotic plaques develop from fatty streaks, generally present at sites of low oscillatory blood flow in medium to large arteries, where deposited lipid contributes to endothelial activation and localized inflammation1. Monocytes are recruited to endothelial cells in fatty streaks via monocyte chemotactic proteins (such as CCL2) and transmigrate into the intima2. Following transmigration, monocytes may form atherogenic, lipid-laden macrophages called foam cells as a consequence of lipid uptake, lipid synthesis, down-regulation of cholesterol efflux or a combination of the above factors. Monocytes may also accumulate lipids in the circulation and have a 'foamy' phenotype, possibly predisposing cells for foam cell formation3,4. Foam cells are the defining feature of fatty streaks and early-stage atherosclerotic plaques and their formation is influenced by both lipid and inflammatory mediators5. Alternatively, monocytes have the ability to reverse transmigrate from the artery into the bloodstream6, thereby removing lipid from the intima and acting to maintain the health of the artery.
Determining the propensity of monocytes to transmigrate across arterial endothelium and form foam cells in the intima, or to reverse transmigrate and carry lipid out of the plaque, is a key requirement for understanding the role of monocyte activation in increasing atherosclerotic risk. Mouse models of CADs such as atherosclerosis are important in elucidating real-time in vivo information on fatty streak/atherosclerotic plaque development. However, these models require a genetic alteration of the natural cholesterol processing abilities of these animals usually coupled with drastic alterations in diet (such as the ApoE-/- Western-type diet model)7,8, thereby, inducing non-physiological accumulation of circulating lipid levels which drive plaque development. These models may have limited relevance to chronic inflammatory human conditions such as HIV infection which are not associated with increased circulating cholesterol or low-density lipoprotein (LDL) levels. Furthermore, differences in monocyte biology between humans and mice make the testing of immunological questions regarding the relevance of subpopulations of monocytes (such as intermediate monocytes (CD14++CD16+))9 difficult. This is important when studying the mechanisms driving cardiovascular disease as intermediate monocyte counts independently predict cardiovascular events10,11. While assays exist to sequentially measure either monocyte transmigration or foam cell formation in isolation, no in vitro assay has been validated for quantifying both aspects of early atherogenesis using the same cells from clinical cohorts. Transwell models utilize a modified Boyden two-chamber system whereby cells are loaded into the top chamber and transmigrate across a porous plastic barrier or cell monolayer into a lower chamber that typically contains media with chemoattractant12,13. Whilst widely used for analyzing leukocyte transmigration, these models do not generally incorporate a layer representing the intima, resulting in transmigrated cells migrating into solution, and do not allow for the measurement of foam cell formation or reverse transmigration of the same cells. Conversely, models of foam cell formation do not account for any transmigratory-induced changes to monocytes or effects of endothelial activation which is known to contribute to foam cell formation14. Furthermore, these systems induce foam cell formation from macrophages adhered to cell culture plates by the addition of saturating concentrations of exogenous oxidized low-density lipoprotein (oxLDL)15,16, a key inducer of foam cell formation. LDL used in these models is often oxidized by non-physiologically-relevant processes such as CuSO4 treatment17, therefore, questioning the physiological importance of studies using these models.
Here we describe an assay that quantifies monocyte transmigration and foam cell formation of the same cells which does not require the addition of exogenous oxLDL, thus better modelling the role of monocytes in foam cell formation. This model was originally developed by Professor William Muller (Northwestern University, Chicago)18, and has been further refined in our laboratory to assess ex vivo the atherogenicity of monocytes isolated under non-activating conditions from individuals with underlying inflammatory conditions accompanying diseases such as HIV infection19 as well as ageing20, that are associated with an increased risk of atherosclerosis. This model also provides a platform for answering basic biological questions regarding the propensity of different monocyte subsets to form foam cells20, the influence of endothelial activation by cytokines such as TNF on foam cell formation14, and the migratory properties of monocytes such as the depth and speed of transmigration in gels19. Furthermore, monocyte transmigration and foam cell formation can be quantified using standard microscopy, live cell imaging, flow cytometry and imaging flow cytometry, therefore, providing a versatile method to evaluate the role of monocytes in atherogenesis.
Protocol
NOTE: All experiments using human biological samples were performed with ethics approval from the Alfred Hospital Human Ethics Committee, Melbourne. All experiments were performed in Class II Biosafety cabinets unless specified. "Prewarmed" refers to reagents warmed to 37 °C in a waterbath.
1. Preparation of Type I Fibrous Collagen Gels: Day 1
Prepare polymerized collagen gels by sequentially adding and mixing 35.7 mM NaOH, 0.71 x M199, 4.58 mM acetic acid and 1.71 mg/mL type I fibrous collagen into a 5 mL polystyrene tube as per Table 1. NOTE: Ensure that the collagen is well-mixed by gently pipetting up and down 5 times in order to stop the formation of collagen 'pockets' in the gel mixture. Prepare 4-6 gels per test condition for microscopy or 15 gels per test condition for flow cytometry.
Once well mixed, aliquot 50 µL of collagen gel mixture into each well of a sterile flat-bottomed 96-well tissue culture plate. Do not use the outside rows and columns, but fill these with 200 µL of 1x Dubecco's phosphate buffered saline (PBS) to protect the gels from desiccation. Incubate plates at 37 °C/5% CO2 for 2 h to allow the collagen to polymerize. Note: Place the plates directly on clean metal racks in the incubator to ensure even heat distribution.
Following incubation, overlay the gels with 150 µL of 1x supplemented M199 (see Table of Materials) and incubate for 5 days until use.
2. Expansion of Stored HUVEC: Day 1
Label and coat a 10 cm diameter Petri dish with 1 mL of 50 µg/mL fibronectin diluted in 1x PBS and incubate at room temperature for 10 min.
Thaw aliquots of cryopreserved primary human umbilical vein endothelial cells (HUVEC, 1.0 x 106 cells) in 10 mL M20. NOTE: HUVEC should be prepared as previously described21 and used at a low passage number (< passage 4). HUVEC may be replaced with human coronary artery endothelial cells here.
Resuspend HUVEC in 10 mL M20 and add to fibronectin coated dish, aspirating excess fibronectin prior to addition of cells, and culture to confluence (approximately 5 days), replacing the media on Day 3.
3. Culturing HUVEC Monolayer on Collagen Gels: Day 5
On Day 5, detach HUVEC by aspirating culture supernatant from the Petri dish and washing away serum-containing media with 10 mL of serum-free M199.
Add 5 mL 0.05% trypsin/0.53 EDTA in M199 to HUVEC and incubate for 1-2 min at room temperature, shaking gently until the cells detach.
Once detached, quickly rinse Petri dish with 5 mL M20 and transfer the media containing cells to a 10 mL tube.
Centrifuge samples at 300 x g for 5 min at room temperature, aspirate supernatant, resuspend cells in 200 µL of M20, and count cells using a hemocytometer.
Resuspend the cells to 2.0 x 105 cells/mL in M20, aspirate the M199 on the gels from the 96-well plate (step 1.3) and add 100 µL of resuspended HUVEC (2.0 x 104 cells) to each collagen gel prepared above (step 1.2). Culture plates for a further 3 days at 37 °C/5% CO2. NOTE: The integrity of the HUVEC monolayer may be confirmed after 3 days by silver nitrate staining22 or immunohistochemistry for tight junction proteins23 at this stage. Additional gels must be prepared for this purpose.
4. Activation of HUVEC Monolayer and Isolation/Activation of Monocytes for Transmigration: Day 8
On Day 8, activate each HUVEC monolayer prior to the addition of monocytes by aspirating the M20 media overlaying the gels and adding 100 µL of 10 ng/mL human TNFα in M20 per gel. Incubate at 37 °C/5% CO2 for 4 h. NOTE: Non-activated HUVEC conditions may also be included if required as controls.
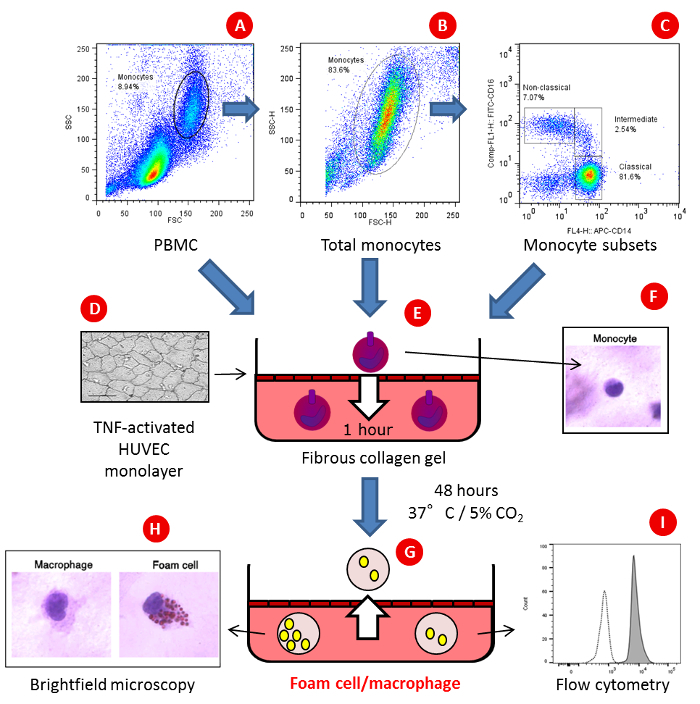
During the 4 h HUVEC activation step, isolate monocytes from PBMCs using magnetic bead techniques to negatively select for cells as per the manufacturer's instructions. A minimum of 6.5 x 106 PBMCs should be used at this step in order to reliably recover 3.0 x 105 monocytes required for one condition, as monocytes typically account for approximately 10-15% of PBMCs. NOTE: To evaluate the effect of monocyte activation prior to monocyte transmigration and foam cell formation, monocytes can be activated at this stage. Monocytes can be isolated from thawed PBMCs if required (i.e., stored patient samples). Monocyte subsets isolated by FACS sorting can be prepared for addition to gels (Figure 1).
5. Transmigration of Primary Human Monocytes: Day 8
To measure monocyte transmigration, remove TNFα-containing media and wash gels twice with 100 µL M199 by adding and removing media. Following washing, add 2.0 x 105 freshly isolated or thawed and washed cryopreserved PBMCs or 5.0 x 104 freshly isolated monocytes or purified monocyte subsets to HUVEC monolayers in 100 µL M20. Incubate for 1 h at 37 °C and 5% CO2 to allow the forward transmigration of monocytes into the gel. It is best to allow 6 wells for every experimental condition examined. NOTE: To evaluate the effect of autologous serum on transmigration and foam cell formation, incubate cells with M20 containing the desired concentration of heat-inactivated donor serum and compare to conditions with a pooled human serum control. Leukocytes other than monocytes may be added at this stage. If investigating the impact of specific lipids/lipid species (e.g., HDL, oxHDL), perform all following steps with serum-free media containing 20-50 µg/mL lipids.
After 1 h of forward transmigration, collect the non-transmigrated cells by washing gels twice with 100 µL of prewarmed 1 mM EGTA in 1x PBS, and once with 100 µL M199 (same centrifuge conditions), pooling the supernatants from each well of the same experimental condition (usually 6 wells) into a 1.5 mL microcentrifuge tube on ice. Centrifuge the non-transmigrated cells at 4 °C, 300 x g for 5 min and resuspend in 30 µL 1x PBS.
Count the cells collected in the supernatant to determine the number of forward transmigrated cells.
The percentage of forward transmigrated cells is determined as follows:
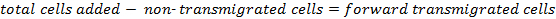
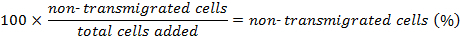
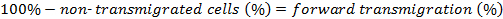
Cover the washed gels containing transmigrated cells with 100 µL M20 and incubate for a further 48 h.
After 48 h, collect the supernatant and wash non-transmigrated cells twice with 100 µL prewarmed 1 mM EGTA/PBS, collecting and pooling the supernatant from each condition as in step 5.2. NOTE: The phenotype of reverse transmigrated cells may be tested on these cells following standard flow cytometry staining protocols.
Centrifuge cells at 4 °C, 300 x g for 5 min and resuspend cells in 30 µL 1x PBS. Count cells and determine the viability via trypan blue staining.
Determine the percentage of reverse transmigrated cells by using the following equation:
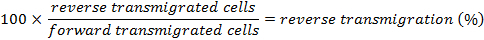 NOTE: Accounting for the total number of forward transmigrated cells gives a more accurate indication of reverse transmigration as it is unlikely forward transmigration will be 100%.
NOTE: Accounting for the total number of forward transmigrated cells gives a more accurate indication of reverse transmigration as it is unlikely forward transmigration will be 100%.For analysis by microscopy, fix the gels by adding 100 µL 2% formaldehyde (final concentration) to each well, cover the plates in aluminum foil and store at 4 °C until analysis. For flow cytometry, do not fix the cells at this stage. See the protocols below for microscopy (section 6) or flow cytometry (section 7) analysis. CAUTION: Formaldehyde is toxic and corrosive; use personal protective equipment (PPE). Care should be taken when adding formaldehyde, however, this step does not need to be performed in a fume hood due to the low concentration used.
6. Quantitation of Foam Cells and Macrophages by Microscopy (Oil-Red O Stain)
Remove the formaldehyde and wash the gels with 100 µL of 50% (v/v) methanol for 5 min and then 100 µL 78% (v/v) methanol for 15 min. NOTE: The staining steps may be performed on the laboratory bench and not in a Class II Biohazard cabinet.
Stain for lipid droplets by adding 100 µL of freshly prepared 0.2% (w/v) Oil-Red O (see Table of Materials for details) for 1 h at room temperature.
Remove excess stain by washing the gels 4 times with 100 µL of 78% (v/v) methanol, aspirating and discarding the supernatant after each wash.
Counterstain for 15 min at room temperature with 100 µL of Giemsa, diluted 1:10 in distilled water.
Aspirate the Giemsa stain and wash gels once with water.
To detach the gels from the plate, rim the wells using a 21 G needle, with the bevel facing outwards, around the edge of the gel.
- To mount the gels on a microscope slide, punch two holes (6.35 mm diameter) in a 2.54 cm x 1.5 cm strip of double-sided tape. Fix the tape to a standard microscope side and remove the protective coating from the top.
- Add a drop of water to the holes in the tape using a transfer pipette. Using tweezers, gently transfer gels to the holes in the tape and cover with a size 1.5 glass coverslip. Press gently on the coverslip to adhere it to the tape.
- Examine with differential interference contrast bright-field microscopy using 40X objective on an inverted microscope.
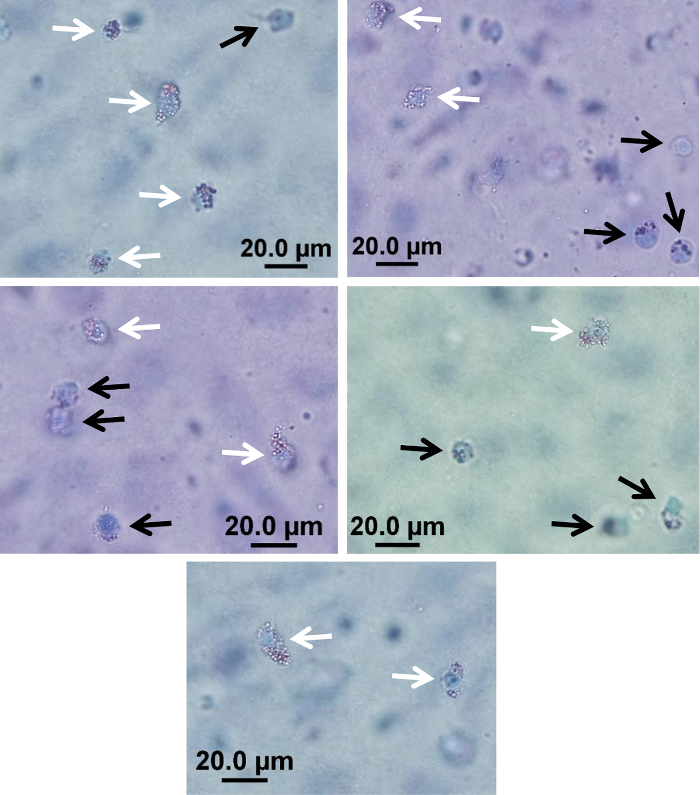
- Bring the HUVEC monolayer into focus at 40X magnification and scroll 'into' the gel, counting macrophages/foam cells throughout the entire depth of the gel. Repeat for three distinct fields of view located at similar distances from the edge of the gel in order to minimize possible edge effects. This will ensure gel depth is consistent between counting regions. NOTE: Score cells in the gel as either foam cells (defined as cells containing >1/3 of their cytoplasm as Oil-Red O stained lipid droplets) or macrophages within 2 h of mounting on slides (Figure 2). Foam cell formation is expressed as the percentage of foam cells relative to the total number of migrated cells and is expressed as the median counts in 3 fields of view examined for 6 gels per condition, therefore a median of 18 measurements per condition. An example of raw cell counts from a typical experiment is shown in Table 2. NOTE: Classifying a cell as a foam cell vs macrophage by microscopy is subjective and consequently can be investigator-dependent. In clinical studies, where experiments are performed over an extended period of time, it is important for all counting to be performed blinded by a single investigator. Examples of foam cells and macrophages in gels are provided in Figure 2.
7. Analysis of Transmigrated Cells by Flow Cytometry
To phenotypically characterize foam cells and macrophages following transendothelial migration, digest the gels by adding 100 µL of 37 °C prewarmed 1 mg/mL collagenase D diluted in M199 to each well and incubate for 20 min at 37 °C/5% CO2. NOTE: Place the 96-well plates directly on clean metal racks in the incubator to ensure even heat distribution.
Following incubation, macerate the gels using a 200 µL pipette tip and incubate for a further 20 min at 37 °C/5% CO2.
Once fully digested, filter and pool the digested replicate gels through 35 µm nylon mesh capped FACS tubes placed on ice, and wash once with FACS wash (see Table of Materials) at 4 °C, 300 x g. Resuspend the cells in 100 µL of FACS wash.
Incubate the resulting cells with fluorophore-conjugated antibodies specific for CD45, a live/dead cell marker and surface/intracellular phenotypic or functional markers of interest using standard flow cytometry protocols. NOTE: Prepare control tubes for compensation using the appropriate fluorophores and Ig compensation beads. Extracted cells may also be collected onto glass slides for immunofluorescence microscopy by cytospinning. Alternatively, we have successfully collected cells using this protocol for assessment via imaging flow cytometry.
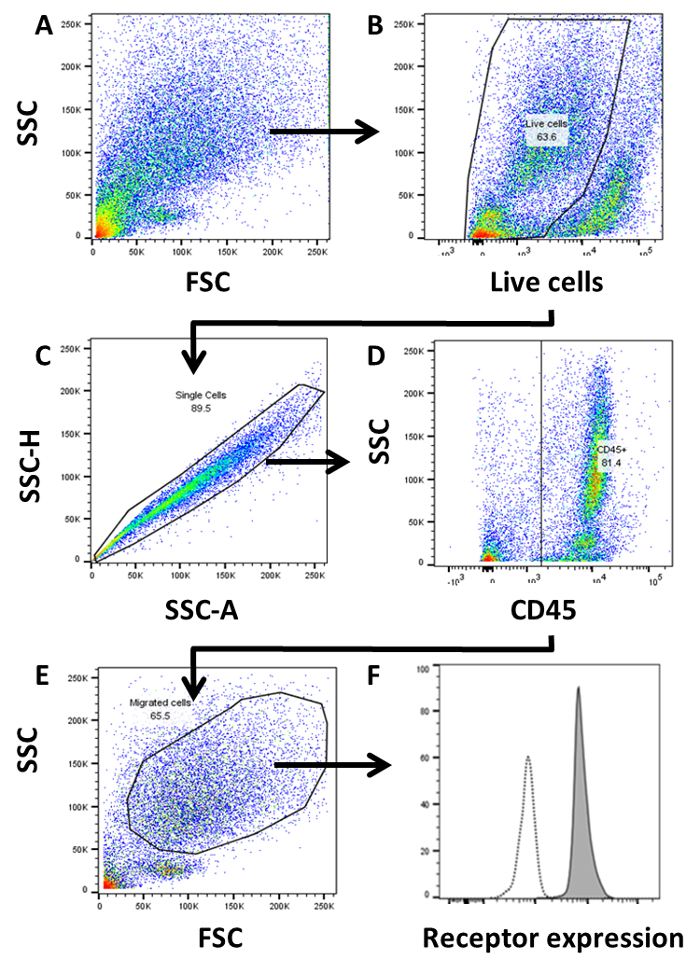
Acquire cells using a flow cytometer, and perform compensation as required. NOTE: As foam cells/macrophages may not form individual populations using light scatter properties and may differ considerably in size and shape, set liberal forward scatter (FSC) and side scatter (SSC) gates in order to capture all migrated cells as shown in Figure 3A.
Following acquisition, perform the data analysis using flow cytometry software. To identify migrated cells, first select cells as negative for the live/dead marker as shown in Figure 3B. Next, perform a single-cell discrimination by creating a tight gate around the cells shown on a SSC-area vs SSC-height plot.
Select CD45+ cells and gate the major population of migrated cells present in a FSC/SSC plot as these are the macrophages/foam cells.
Representative Results
Quantifying monocyte transmigration
Monocytes are added to the model as described in Figure 1, and six gels are prepared for each condition. Monocytes for 6 gels per donor (i.e., 5.0 x 104 monocytes per gel 6 gels = 3.0 x 105 monocytes per donor) are resuspended to a final volume of 600 µL of M199 media containing the required serum/isolated lipid. Cells (i.e, 100 µL per gel = 5.0 x 104 monocytes) are then aliquoted onto gels and incubated as above for 1 h to facilitate monocyte transmigration. Following transmigration, non-transmigrated cells are counted as above. In this example, 1.5 x 104 non-transmigrated cells were recovered. The percentage of forward transmigration was determined using the formulas described in step 5.4:
![]()
![]()
Following 48 h of incubation, 3.6 x 104 reverse transmigrated cells were recovered as above. The percentage of reverse transmigration was determined using the formula in step 5.8:
![]()
Forward and reverse transmigration may be compared between conditions using the appropriate statistical tests and monocytes prepared from multiple independent donors to determine whether the monocyte transmigration ability is altered between experimental conditions.
Evaluating foam cell formation
Brightfield microscopy
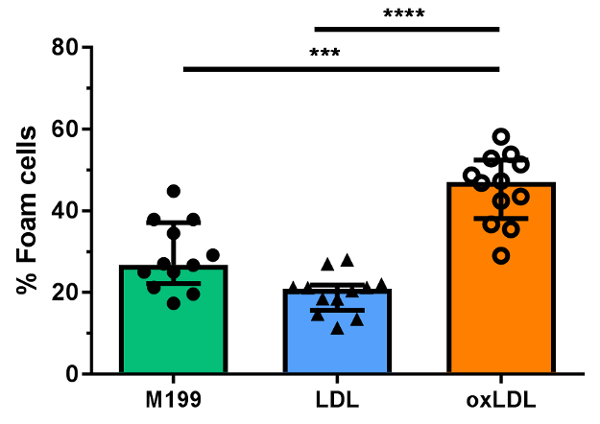
Oxidized lipoproteins are known to promote monocyte-derived foam cell formation in vitro in comparison to unoxidized lipids15. Here we confirm that the treatment of monocytes with oxLDL, commonly used to induce foam cell formation in conventional foam cell induction models, enhances monocyte-derived foam cell formation in this model of transendothelial migration and foam cell formation in comparison to native LDL. Following monocyte transmigration and foam cell formation in gels incubated with M199 containing 50 µg/mL native or CuSO4-oxidized LDL (see step 5.1, Figure 1), cells were analyzed by microscopy. Examples of foam cells and macrophages observed in representative experiments are shown in Figure 2. Cells were counted in three different fields per gel, by focusing at the HUVEC monolayer and progressively focusing deeper into the gel. The cell counts are recorded for each donor as shown in Table 2 for a single donor, to compare the percentage of foam cells formed in response to oxidized and native LDL. Aggregate data from n = 12 independent counts are shown in Figure 4 to confirm that incubation of monocytes with oxLDL in this model induces more foam cell formation than conditions where monocytes are incubated with native LDL or M199 media alone.
Flow cytometry
To determine the phenotype of migrated cells, the cells are extracted from digested gels and labeled using a standard flow cytometry panel. Cells were labeled with a live/dead cell marker and antibodies specific for CD45 and other surface phenotype markers using standard flow cytometry protocols. Compensation controls must be also prepared for full compensation as per standard flow cytometry techniques. Migrated cells are gated as shown in Figure 3 in plots representative of 10 independent experiments. Live cells are gated using live/dead cell viability assays (Figure 3B) and doublets are excluded by single cell analysis (Figure 3C). Cells are then gated as CD45+ in order to exclude HUVEC (as these cells are CD45-, Figure 3D). The major population is then gated by forward scatter/side scatter discrimination (Figure 3E) and the mean fluorescence intensity (MFI) of receptors of interest are determined in comparison to either fluorescence minus one (FMO) or isotype control (Figure 3F) to determine expression (ΔMFI) or percentage of positive cells.
Figure 1: An in vitro model of monocyte transmigration and foam cell formation. (A) PBMC,(B) total monocytes or(C) FACS-sorted monocyte subsets are added to type I fibrous collagen gels formed in 96-well plates and overlaid with a monolayer of activated primary human umbilical vein endothelial cells (HUVEC), whose integrity may be assessed by silver staining (D) and allowed to (E)transmigrate for 1 h in the presence of serum or lipid containing media. Non-transmigrated cells are counted(F) and gels are incubated for a further 48 h with the same serum/lipid containing media. Following incubation, (G)reverse migrated cells are counted and the percentage of foam cells vs. macrophages in the gel is determined by(H) phase contrast microscopy following staining with Oil-Red O or the phenotype of migrated cells is determined by(I) flow cytometry. Please click here to view a larger version of this figure.
Figure 2: Examples of foam cells and macrophages in an in vitro model of monocyte transmigration and foam cell formation. Representative examples of cells scored as foam cells (white arrows) and macrophages (black arrows) after Giemsa staining to visualize cells and Oil-Red O staining to visualize lipid droplets as determined by phase contrast microscopy of extracted gels using an inverted microscope at 40X magnification. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Figure 3: Gating strategy of transmigrated cells by flow cytometry. Transmigrated cells are phenotypically characterized by flow cytometry following extraction of cells from gels. Cells are gated by (A) FSC/SSC, (B) live cells, (C) single cells, (D) CD45+, (E) FSC/SSC and (F) expression of receptor compared to isotype or fluorescence minus one (FMO) control. Expression of receptors of interest are defined as mean fluorescence intensity (MFI) and expressed with respect to levels of isotype. ΔMFI = Stain (MFI) minus isotype/FMO control (MFI). Please click here to view a larger version of this figure.
Figure 4: Effect of exogenously added oxLDL and LDL on monocyte-derived foam cell formation. Foam cell formation is determined under conditions where monocytes from a single donor are incubated with media (M199) or 50 µg/mL unoxidized low-density lipoprotein (LDL) or copper (II) sulphate oxidized LDL (oxLDL) added to gels post-transmigration. Counts for 12 distinct sites are shown. Foam cells are expressed as the percentage of total migrated cells. Median and interquartile ranges are shown in bar graphs and comparisons were made using non-parametric Mann-Whitney U tests. ***p <0.001, ****p <0.0001. Please click here to view a larger version of this figure.
| Reagent | [Stock] | [Final] | Volume for 60 gels (µL) |
| NaOH | 100 mM | 35.7 mM | 1071 |
| M199 | 10x | 0.71x | 213 |
| AcCOOH | 20 mM | 4.58 mM | 687 |
| Collagen | 5 mg/mL | 1.71 mg/mL | 1029 |
| Total volume | - | - | 3000 |
Table 1: Type I fibrous collagen gel preparation
| LDL | oxLDL | |||||||
| Gel | Count | Foam cells1 | Migrated cells1 | Foam cells (%)2 | Foam cells1 | Migrated cells1 | Foam cells (%)2 | |
| 1 | 1 | 5 | 44 | 11.4 | 26 | 55 | 47.3 | |
| 2 | 5 | 27 | 18.5 | 10 | 23 | 43.5 | ||
| 3 | 11 | 52 | 21.2 | 19 | 39 | 48.7 | ||
| 2 | 1 | 5 | 34 | 14.7 | 9 | 31 | 29 | |
| 2 | 14 | 50 | 28 | 32 | 55 | 58.2 | ||
| 3 | 5 | 37 | 13.5 | 19 | 37 | 51.4 | ||
| 3 | 1 | 10 | 37 | 27 | 28 | 52 | 53.8 | |
| 2 | 7 | 33 | 21.2 | 11 | 31 | 35.5 | ||
| 3 | 7 | 38 | 18.4 | 28 | 53 | 52.8 | ||
| 4 | 1 | 9 | 44 | 20.5 | 14 | 33 | 42.4 | |
| 2 | 7 | 33 | 21.2 | 22 | 47 | 46.8 | ||
| 3 | 11 | 50 | 22 | 11 | 30 | 36.7 | ||
| Median foam cells (%) | 20.8 | 47 | ||||||
| Average foam cells (%) | 19.8 | 45.5 |
Table 2: Raw cell counts from comparison of monocyte-derived foam cells following LDL vs oxLDL treatments. 1Cell counts per field of view in gel. 2Percentage of foam cells = foam cell counts/total migrated cell counts x 100. *Data are representative of one experiment using monocytes from a single donor
Discussion
The protocol described here offers a versatile and physiologically relevant method for assessing the atherogenicity of monocytes from human clinical cohorts, by combining both monocyte transmigration and foam cell formation. This model offers advantages over alternative methods of foam cell formation as it takes into account the effect of monocyte transmigration on foam cell formation and allows the measurement of reverse transmigration6 in addition to the inherent propensity of monocytes to mature into foam cells in the presence or absence of exogenous factors. Furthermore, the role of endothelial activation is also taken into account as we have shown that endothelial activation upregulates oxidation of lipid species that is associated with foam cell formation14. Finally, the phenotype of monocytes that undergo egress (a property associated with plaque regression) can be quantified and characterized by microscopy and flow cytometry.
Due to the three-dimensional nature of transmigration into gels, identifying and scoring the transmigrated cells as foam cells or macrophages can be difficult as cells transmigrate to different levels in the gel19. To aid the identification of foam cells, fresh Oil-Red O stain must always be used. It is also of note that different ex vivo disease states or in vitro conditions may alter monocyte transmigration in the gel. We have used live cell imaging to identify that monocytes from HIV-infected individuals tend to transmigrate to shallower points in gels and at different speeds than those from uninfected individuals19. Furthermore, foam cell formation is associated with less monocyte motility in the gel so cells tend to move in circles at a particular z-section in comparison to cells from HIV-uninfected individuals which tend to migrate in a relatively straight line towards the bottom of the gel. These findings raise the possibility of a similar phenomenon occurring in experiments using human patient samples from other disease states associated with increased monocyte atherogenicity.
When performing flow cytometry-based analyses it is common to observe a significant proportion of dead cells in the recovered cell population when assessing viability using live/dead cell markers (Figure 4B). These cells are predominantly CD45- HUVEC that are damaged during the collagen digestion/maceration step required to extract monocyte-derived cells from the collagen matrix. This process is particularly harsh on the HUVEC monolayer, but is not detrimental to phenotyping the transmigrated cells.
This model differs from others in that no artificially oxidized exogenous lipid is required in the culture media to drive monocyte-derived foam cell formation. Instead, foam cell formation in this assay is influenced by the serum present in the culture media, allowing for the discrete effects of soluble factors from clinical samples to be evaluated. As such, we have shown that incubation of control monocytes with serum from either young HIV-infected19 or elderly HIV-uninfected20 individuals promotes foam cell formation, indicating that soluble factors can independently promote foam cell formation. As the assay is influenced by soluble components, a single batch of pooled human serum must be used for experiments aimed at determining the inherent difference of atherogenic properties of monocytes derived from different individuals in order to standardize control conditions. This assay may also be used to evaluate the role of specific lipid species from clinical samples (Figure 3). For example, we have found that incubation of monocytes with media containing isolated lipid species such as high-density lipoproteins from individuals with known lipid dysfunction24 also promotes foam cell formation. In this case, monocytes from a single healthy individual or group of individuals can be used in the presence of serum or serum factors derived from test subjects. Therefore, this model allows for specific questions to be asked regarding the discrete effects of both soluble and cellular components on foam cell formation.
This assay utilizes HUVEC as a model of coronary artery endothelium due to the practical difficulty in obtaining large numbers of low passage number primary coronary artery cells. However, we have compared outcomes from assays using both human coronary artery endothelial cells or HUVEC and observed little difference in the monocyte transmigration or foam cell formation (data not shown), indicating that the use of HUVECs in this system is an acceptable substitute. Manual counting of foam cells is a limiting factor in this model as it may introduce operator bias. Therefore, all samples mounted on slides must be blinded to the operator in order to remove the potential of bias. We have, however, compared foam cell formation as measured by manual counting and by imaging flow cytometry and found that these methods give similar results19. A key limitation of this model is that monocyte adhesion and transmigration occur in the absence of shear forces associated with physiological blood flow in vivo. We hypothesize that shear flow will predominantly affect monocyte transmigration and not subsequent foam cell formation within the matrix; however, this must be considered when interpreting results from this assay. This assay also does not model the influence of smooth muscle cells in monocyte-derived foam cell formation which is a physiological limitation of the system; however, this is consistent in all conditions allowing the discrete atherogenic potential of monocytes to be compared between different disease states.
In summary, this model provides a versatile and physiologically relevant method for quantifying monocyte transmigration and foam cell formation from human samples in different disease states ex vivo. This model has further applications for evaluating the propensity of monocytes to form foam cells in conditions where monocyte atherogenicity is associated with increased risk of CAD such as obesity25, diabetes26 and chronic kidney disease27. Therefore, this model may also be optimized for use in disease states other than atherosclerosis where cellular transmigration may influence disease pathogenesis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors gratefully acknowledge the work of Prof. William Muller and Dr. Clare Westhorpe for their key role in development of earlier iterations of this model. The authors would also like to thank the AMREP Flow Cytometry core for the sorting of monocyte subsets and the Alfred Hospital Infectious Disease Unit clinical research nurses for the recruitment of HIV+ individuals for some studies. The authors gratefully acknowledge the contribution to this work of the Victoria Operational Infrastructure Support Program received by the Burnet Institute. TAA is supported by an RMIT University Vice-Chancellor's Postdoctoral Fellowship. This work was supported by NHMRC project grant 1108792 awarded to AJ and AH. TK is supported by NIH grants NIH K08AI08272, NIH/NCATS Grant # UL1TR000124.
References
- Napoli C, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100(11):2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, et al. Foamy Monocytes Form Early and Contribute to Nascent Atherosclerosis in Mice With Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2015;35(8):1787–1797. doi: 10.1161/ATVBAHA.115.305609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WD, Weinrich TW, Woollard KJ. Very-low and low-density lipoproteins induce neutral lipid accumulation and impair migration in monocyte subsets. Scientific Reports. 2016;6:20038. doi: 10.1038/srep20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelovich TA, Hearps AC, Jaworowski A. Inflammation-induced foam cell formation in chronic inflammatory disease. Immunol. Cell. Biol. 2015;93(8):683–693. doi: 10.1038/icb.2015.26. [DOI] [PubMed] [Google Scholar]
- Llodrá J, et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl. Acad. Sci. 2004;101(32):11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal Models of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32(5):1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir KS, Leitersdorf E. Atherosclerosis in the Apolipoprotein E-Deficient Mouse. A Decade of Progress. Arterioscler. Thromb. Vasc. Biol. 2004;24(6):1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- Hilgendorf I, Swirski FK. Making a difference: Monocyte Heterogeneity in Cardiovascular Disease. Curr. Atheroscler. Rep. 2012;14(5):450–459. doi: 10.1007/s11883-012-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogacev KS, et al. Lower Apo A-I and Lower HDL-C Levels Are Associated With Higher Intermediate CD14++CD16+ Monocyte Counts That Predict Cardiovascular Events in Chronic Kidney Disease. Arterioscler. Thromb. Vasc. Biol. 2014;34(9):2120–2127. doi: 10.1161/ATVBAHA.114.304172. [DOI] [PubMed] [Google Scholar]
- Rogacev KS, et al. CD14++CD16+ Monocytes Independently Predict Cardiovascular Events: A Cohort Study of 951 Patients Referred for Elective Coronary Angiography. J. Am. Coll. Cardiol. 2012;60(16):1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014. p. e51046. [DOI] [PMC free article] [PubMed]
- Boyden S. The Chemotactic Effect Of Mixtures Of Antibody And Antigen On Polymorphonuclear Leucocytes. J. Exp. Med. 1962;115(3):453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhorpe CLV, et al. Endothelial cell activation promotes foam cell formation by monocytes following transendothelial migration in an in vitro model. Exp. Mol. Pathol. 2012;93:220–226. doi: 10.1016/j.yexmp.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. 1987;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 1983;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized Low-Density Lipoprotein. Methods Mol. Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J. Exp. Med. 1992;176(3):819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisa A, et al. Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. AIDS. 2015;29(12):1445–1457. doi: 10.1097/QAD.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelovich TA, et al. Ex vivo foam cell formation is enhanced in monocytes from older individuals by both extrinsic and intrinsic mechanisms. Exp. Gerontol. 2016;80:17–26. doi: 10.1016/j.exger.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Westhorpe CLV, et al. Effects of HIV-1 infection in vitro on transendothelial migration by monocytes and monocyte-derived macrophages. J. Leukoc. Biol. 2009;85(6):1027–1035. doi: 10.1189/jlb.0808501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie MB, Cramer EB, Naprstek BL, Silverstein SC. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J. Cell Biol. 1984;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller AM, et al. Expression of the Endothelial Markers PECAM-1, vWf, and CD34 in Vivo and in Vitro. Exp. Mol. Pathol. 2002;72(3):221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- Kelesidis T, et al. Predictors of impaired HDL function in HIV-1 infected compared to uninfected individuals. J. Acquir. Immune Defic. Syndr. 2017. [DOI] [PMC free article] [PubMed]
- Rogacev KS, et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. European Heart Journal. 2010;31(3):369–376. doi: 10.1093/eurheartj/ehp308. [DOI] [PubMed] [Google Scholar]
- Gacka M, et al. Proinflammatory and atherogenic activity of monocytes in Type 2 diabetes. J. Diabetes Complications. 2010;24(1):1–8. doi: 10.1016/j.jdiacomp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Rogacev KS, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 2011;32(1):84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]


