Abstract
Deficiency of hepatic Nogo-B receptor (NgBR) expression activates liver X receptor α (LXRα) in an adenosine monophosphate-activated protein kinase α (AMPKα)-dependent manner, thereby inducing severe hepatic lipid accumulation and hypertriglyceridemia. Statins have been demonstrated non-cholesterol lowering effects including anti-nonalcoholic fatty liver disease (NAFLD). Herein, we investigated if the anti-NAFLD function of statins depends on activation of NgBR expression. In vivo, atorvastatin protected apoE deficient or NgBR floxed, but not hepatic NgBR deficient mice, against Western diet (WD)-increased triglyceride levels in liver and serum. In vitro, statins reduced lipid accumulation in nonsilencing small hairpin RNA-transfected (shNSi), but not in NgBR small hairpin RNA-transfected (shNgBRi) HepG2 cells. Inhibition of cellular lipid accumulation by atorvastatin is related to activation of AMPKα, and inactivation of LXRα and lipogenic genes. Statin also inhibited expression of oxysterol producing enzymes. Associated with changes of hepatic lipid levels by WD or atorvastatin, NgBR expression was inversely regulated. At cellular levels, statins increased NgBR mRNA and protein expression, and NgBR protein stability. In contrast to reduced cellular cholesterol levels by statin or β-cyclodextrin, increased cellular cholesterol levels decreased NgBR expression suggesting cholesterol or its synthesis intermediates inhibit NgBR expression. Indeed, mevalonate, geranylgeraniol or geranylgeranyl pyrophosphate, but not farnesyl pyrophosphate or farnesol, blocked atorvastatin-induced NgBR expression. Furthermore, we determined that induction of hepatic NgBR expression by atorvastatin mainly depended on inactivation of extracellular signal-regulated kinases 1/2 (ERK1/2) and protein kinase B (Akt). Taken together, our study demonstrates that statins inhibit NAFLD mainly through activation of NgBR expression.
Keywords: Akt, AMPKα, ERK1/2, Liver steatosis, NgBR, Statin
1. Introduction
In general, the nonalcoholic fatty liver disease (NAFLD) is characterized by accumulation of excessive triglycerides (TG) in hepatocytes of the patients without alcohol intake, chronic viral hepatitis or other liver diseases. NAFLD is one of the most common chronic liver diseases worldwide. The spectrum of NAFLD covers from the simple steatosis, a benign and non-progressive condition, to nonalcoholic steatohepatitis (NASH) which is characterized by hepatocellular necroinflammation and ballooning, and may further progress to liver fibrosis, cirrhosis and in some cases hepatocellular carcinoma [1]. Although about 20–30% of the general population may have NAFLD, a much higher prevalence of NAFLD (> 75%) can be found in patients with insulin resistance [2]. NAFLD is also considered as an independent risk factor for cardiovascular diseases [3]. The excessive lipid accumulation in the liver of NAFLD patients can be attributed to multiple factors, such as the increased uptake of plasma non-esterified fatty acids and de novo lipogenesis [1]. Statins, a class of medicines used for treatment of patients with hypercholesterolemia, can reduce cardiovascular diseases by 30–40%. Statins reduce serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels by, 1) inhibiting activity of 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR), the rate-limiting enzyme which catalyzes the conversion of HMG—CoA into mevalonate, thereby reducing de novo cholesterol synthesis; 2) activating hepatic LDL receptor (LDLR) expression which enhances cholesterol catabolism and excretion [4]. In plasma, TG is mainly presented in different lipoproteins, particularly in very low-density lipoprotein (VLDL) and LDL. In addition to cholesterol-lowering effects, statins can decrease TG levels in hypertriglyceridemic patients, even with a greater effect than that on LDL-C levels [5]. For instance, atorvastatin does not affect TG distribution but consistently reduces it in all lipoprotein fractions in hypertriglyceridemic patients. The epidemiological studies have demonstrated that statins can reduce NAFLD in overweight individuals. The clinical observational studies have shown positive outcomes of statin treatment on NAFLD patients [6–10]. However, the mechanisms by which statins protect patients against NAFLD have not been fully elucidated.
Nogo-B, also known as Reticulon 4B, is a member of the family of reticulon proteins. High expression of Nogo-B can be determined in caveolin-1 enriched microdomains or/and lipid rafts of endothelial cells (ECs) and vascular smooth muscle cells [11]. NgBR is a receptor specific for Nogo-B [12]. So far, several biological functions of NgBR have been identified. NgBR expression is necessary for Nogo-B stimulated chemotaxis and morphogenesis of ECs [12]. In zebrafish, NgBR expression is essential for angiogenesis [13]. Niemann-Pick type C2 (NPC2) is a protein for cholesterol traficking between cellular membranes. The interaction between NgBR and NPC2 increases stability of NPC2 protein, thereby enhancing NPC2-mediated intracellular cholesterol trafficking. Thus, NgBR can play an important role in lipid metabolism [14]. We recently reported that deficiency of hepatic NgBR expression activates liver X receptor α (LXRα) through an adenosine monophosphate-activated protein kinase α (AMPKα)-dependent pathway, thereby resulting in liver steatosis which is due to TG accumulation [15]. Because of the anti-liver steatosis properties of statins and the importance of NgBR in hepatic lipogenesis, we hypothesized that inhibition of NAFLD by statins is completed, at least in part, by activating hepatic NgBR expression.
2. Materials and methods
2.1. Materials
Rabbit anti-NgBR antibody was generated as described [13]. Rabbit anti-extracellular signal-regulated kinases 1/2 (ERK1/2), phosphorylated ERK1/2 (pi-ERK1/2), protein kinase B (Akt) and phosphorylated Akt (pi-Akt) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Rabbit anti-GAPDH, fatty acid synthase (FASN), AMPKα, phosphorylated AMPKα (pi-AMPKα), sterol-regulatory element binding protein 2 (SREBP2) and HMGCR antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-LXRα and Lamin A polyclonal antibodies were purchased from Proteintech Group, Inc. (Rosemont, IL). LDL was purchased from Athens Research & Technology, Inc. (Athens, Georgia). Manumycin A and GGTI-286 were purchased from Merck Millipore (Nottingham, UK). PD98059, U0126, LY294002, SP600125 and SB203580 were purchased from Selleck Chemicals (Houston, TX). Atorvastatin was purchased from AK Scientific Inc. (Palo Alto, CA). Farnesyl pyrophosphate (FPP), geranylgeranylpyrophosphate (GGPP), geranylgeraniol (GGOH), farnesol (FOH) and water soluble-cholesterol (Chol/CD) were purchased from Sigma-Aldrich (St Louis, MO).
PTEN expression vector (C2-PTEN) was prepared as follows: the cDNA encoding mouse PTEN was generated by reverse transcription with total cellular RNA isolated from differentiated 3 T3-L1 adipocytes and an oligo(dT)18 primer, followed by PCR with the forward primer (5′-GGGAATTCATGACAGCCATCATCAAAGAGATCG-3′) and the backward primer (5′-CGGGATCCTCAGACTTTTGTAATTTGTGAATGC-3′). After the sequence was confirmed, the PCR product was digested with EcoRI and BamHI followed by ligation into the pEGFP-C2 expression vector. Mature SREBP2, MKK1* and Akt1* expression vectors (They are also ligated into the pEGFP-C2 vector) were purchased from Addgene (Cambridge, MA).
2.2. In vivo studies
The protocols for animal study were approved by the Ethics Committees from Nankai University and Medical College of Wisconsin, respectively, and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (NIH), USA.
Liver specific NgBR deficient (NgBRLKO) and the littermate control (NgBR floxed, NgBRfl/fl) mice were generated as described [15]. ApoE deficient (apoE−/−) mice were purchased from the Animal Center of Nanjing University (Nanjing, China). ApoE−/−, NgBRLKO and NgBRfl/fl mice (males, ~8-week old) were randomly divided into 3 groups (6 mice/group), and fed normal chow, Western diet (WD, 21% fat and 0.5% cholesterol) or WD containing atorvastatin [15 mg/day/kg bodyweight (mpk)] for 2 weeks, respectively. During the treatment, we routinely checked the food intake, body weight gain and water drinking, and did not observe difference between control mice and mice receiving atorvastatin treatment, indicating the high safety with atorvastatin at this dose. At the end of experiment, all the mice were anesthetized and euthanized by i.p injection of 2,2,2-tribromoethanol (640 mg/kg bodyweight) followed by collection of liver and serum samples. Serum TG and TC levels were analyzed by enzymatic methods with an automatic biochemical analyzer (Model 7020; Hitachi, Tokyo, Japan).
2.3. Cell culture
HepG2 cells, a human hepatic cell line (ATCC, Manassas, VA), were cultured in MEM medium containing 10% fetal calf serum, 50 μg/ml penicillin/streptomycin and 2 mM glutamine. Both nonsilencing small hairpin RNA-transfected (shNSi) cells and NgBR small hairpin RNA-transfected (shNgBRi) cells were established as described [15]. Cells received treatment at ~85% confluence. HepG2 cells lacking AMPKα or LXRα expression were established using the CRISPR-Cas9 technology, respectively. The selection of mutated clonal cell lines was completed using the standard protocol, and the knockout of target gene expression was confirmed by Western blot. The cells lacking AMPKα or LXRα expression were named as Cas9-AMPKα or Cas9-LXRα cells, and the corresponding control cells as Cas9-NS cells.
Mouse primary hepatocytes were isolated from C57BL/6 mice (males, ~8-week old, also purchased from the Animal Center of Nanjing University) by a collagenase perfusion method. Briefly, after anesthetized the midline laparotomy was performed, and the inferior vena cava was cannulated with an angiocatheter. The liver was then perfused with 1 ml heparin (320 U/ml), 40 ml solution I (Kreb’s solution containing 0.1 mM EGTA) and 30 ml solution II (Kreb’s solution containing 2.74 mM CaCl2 and 0.05% collagenase I) at 37 °C, sequentially. The perfused liver was then passed through a 400 μm screening size filter by flushing with the cold DMEM medium. The isolated he-patocytes were collected after centrifuge for 5 min at 50 g, re-suspended with DMEM medium and plated in 6-well plates (the cell density is ~1 × 106 cells/well). The viability of the isolated hepatocytes was ~90% which was determined by the method of trypan blue exclusion.
2.4. Determination of NgBR promoter activity
A human NgBR promoter (from −1918 to +220) was constructed using PCR with genomic DNA extracted from HepG2 cells and the following primers: forward, 5′-TGTACTCGAGAAACCCCGCCTCTACTAAA AAC-3′; and backward, 5′-CGCGAAGCTTCATACTCTTGTGGCCCT CAG-3′. After the sequence was confirmed, the PCR product was digested with XhoI and HindIII followed by ligation with the pGL4 luciferase reporter vector, and the promoter was named as pNgBR. To analyze the pNgBR activity, human embryonic kidney (HEK)-293T cells (ATCC) in 24-well plates (~3 × 105 cells/well) were transfected with DNA for pNgBR, Renilla luciferase (for internal normalization), pSREBP2-C2 or the empty C2 vector using lipofectamine 2000. After 24 h of transfection plus treatment, cells were lysed with the lysate buffer provided in the dual-luciferase reporter assay kit purchased from Promega (Madison, WI) according to the manufacturer’s instruction. The cellular lysate was then used to determine activities of firefly and Renilla luciferases.
2.5. Transfection of HepG2 cells with PTEN, mature SREBP2, MKK1* or Akt1* expression vector
HepG2 cells in 12-well plates (~6 × 105 cells/well) were switched into serum- and antibiotic-free medium for 2 h. Cells were then transfected with C2-PTEN, pSREBP2-C2, MKK1* or Akt1* expression vector at 1 μg/well or indicated concentrations, or the corresponding empty vector for 16 h, respectively. The DNA was pre-mixed well with 3 μl/well lipofectamine 2000 in 100 μl/well OPTI-MEM medium and incubated for 25 min at room temperature (RT) before transfection. The cells were then cultured for 24 h after C2-PTEN transfection or for 16 h after pSREBP2-C2, MKK1* or Akt1* transfection, respectively.
2.6. Determination of lipid content
The lipid content in the liver or HepG2 cells was determined by both Oil Red O staining and TG/TC quantitative assay. After treatment, a piece of liver was removed followed by preparation of cryosections with a standard protocol. HepG2 cells were washed twice with PBS and then fixed in 4% paraformaldehyde (PFA)/PBS for 5 min. Both fixed HepG2 cells on slides and liver cryosections were initially stained with Oil Red O solution (0.3% Oil Red O in 60% isopropanol) for 45 min at RT, and then washed twice with distilled water. The samples were then counterstained with haematoxylin solution for 30 s, kept in water for 5 min. All the slides were observed under a Leica DM5000B microscope (Wetzlar, Germany), and then the images were photographed. Meanwhile, total lipid was extracted from HepG2 cells or a piece of liver followed by quantitative analysis of TG or TC levels using commercially available enzymatic kits (BioSino, Beijing, China).
2.7. Determination of protein and mRNA expression by western blot and by real time RT-PCR, respectively
After treatment, total cellular proteins were extracted from cells or a piece of liver followed by determination of NgBR, FASN, AMPKα, pi-AMPKα, LXRα, HMGCR, ERK1/2, pi-ERK1/2, SREBP2, Akt and pi-Akt protein expression by Western blot with quantitative assay of band density based on three repeated experiments as described [16].
To determine mRNA expression, total RNA was extracted as described [16]. The cDNA was synthesized with 1 μg total RNA using the reverse transcription kit (New England Biolabs, Ipswich, MA). The real time PCR was then performed using a SYBR green PCR master mix (Bio—Rad, Los Angeles, CA) with the primers listed in Table 1. Expression of NgBR, FASN, stearoyl-CoA desaturase 1 (SCD1), SREBP1c, acetyl-CoA carboxylase 1 (ACC1), cytochrome P450 family 46 subfamily A member 1 (cholesterol 24-hydroxylase, CYP46A1), cholesterol 25-hydroxylase (CH25H), cytochrome P450 family 27 subfamily A member 1 (cholesterol 27-hydroxylase, CYP27A1), geranylgeranyl di-phosphate synthase 1 (GGPS1) and NPC2 mRNA was normalized by GAPDH mRNA in the corresponding samples.
Table 1.
Sequences of primers for real time RT-PCR assay.
| Gene | Species | Sense | Anti-sense |
|---|---|---|---|
| ACC1 | Human | 5′-GCCATTGGGATTGGGGCTTAC-3′ | 5′-CCCGCCCGAGGACTTTGTTG-3′ |
| Mouse | 5′-GAAGTCAGAGCCACGGCACA-3′ | 5′-GGCAATCTCAGTTCAAGCCAG-3′ | |
| ATGL | Human | 5′-AGCATCTGCCAGTACCTGGTGAT-3′ | 5′-ACCTGCTCCGGCAGCCTGG-3′ |
| Mouse | 5′-GAGCCCCGGGGTGGAACAAGAT-3′ | 5′-AAAAGGTGGTGGGCAGGAGTA-3′ | |
| CGI-58 | Human | 5′-GATCAGCAAGGTCTGGTCGT-3′ | 5′-TAGGTGGATTCTTGGCTGCT-3′ |
| Mouse | 5′-TGTGCAGGACTCTTACTTGGCAGT-3′ | 5′-GTTTCTTTGGGCAGACCGGTTT-3′ | |
| CH25H | Human | 5′-ATGTTGACCACGTGGAAGGT-3′ | 5′-TGGGAACTGTTTTCTTTGGG-3′ |
| Mouse | 5′-TGGAGTCAATGACACTGGGA-3′ | 5′-TGTTGTTTTTGCAGCCTGAC-3′ | |
| CYP27A1 | Human | 5′-AGCTGCGCTTCTTCTTTCAG-3′ | 5′-GGCCCTAAGTAGGACATCCA-3′ |
| Mouse | 5′-GGGCACTAGCCAGATTCACA-3′ | 5′-CTATGTGCTGCACTTGCCC-3′ | |
| CYP46A1 | Human | 5′-CTGTCCCAGGCAGTGAAACT-3′ | 5′-AATGCTCTCCCGGACCTC-3′ |
| Mouse | 5′-AACACATCTTGGAGCACACG -3′ | 5′-GCTATGAGCACATCCCCG-3′ | |
| FASN | Human | 5′-AACTCCAAGGACACAGTCACCAT-3′ | 5′-CAGCTGCTCCACGAACTCAAA-3′ |
| Mouse | 5′-CTGCGATGAAGAGCATGGTTT-3′ | 5′-CCATAGGCGATTTCTGGGAC-3′ | |
| GGPS1 | Human | 5′-GCCCACAGCATCTATGGAAT-3′ | 5′-GCGGGTAAAAAGCTTCACTG-3′ |
| GAPDH | Human | 5′-GGTGGTCTCCTCTGACTTCAACA-3′ | 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ |
| Mouse | 5′-ACCCAGAAGACTGTGGATGG-3′ | 5′-ACACATTGGGGGTAGGAACA-3′ | |
| HSL | Human | 5′-GAAGCCTTTGAGATGCCACT-3′ | 5′-AGATGAGCCTGACGAGGAC-3′ |
| Mouse | 5′-GCCGGTGACGCTGAAAGTGGT-3′ | 5′-CGCGCAGATGGGAGCAAGAG-3′ | |
| NgBR | Human | 5′-AGCCTCGTGGTGTGGTGTA-3′ | 5′-GCCCAGAAGTTCTTGCTGTT-3′ |
| NPC2 | Human | 5′-TGGGCTCACATTCACTTCCT-3′ | 5′-AGCTACATTCCTGCTCCTGG-3′ |
| SCD1 | Human | 5′-TGGGTTGGCTGCTTGTG-3′ | 5′-GCGTGGGCAGGATGAAG-3′ |
| Mouse | 5′-TGGGTTGGCTGCTTGTG-3′ | 5′-GCGTGGGCAGGATGAAG-3′ | |
| SREBP1c | Human | 5′-GGATTGCACTTTCGAAGACATG-3′ | 5′-AGCATAGGGTGGGTCAAATAGG-3′ |
| Mouse | 5′-GCGCCATGGACGAGCTG-3′ | 5′-TTGGCACCTGGGCTGCT-3′ | |
| TGH | Human | 5′-GCTCCAGCATCTCTGTGGTT-3′ | 5′-GGAACAGACGACACTGTCAAA-3′ |
| Mouse | 5′-TCCTGGGGTCTATCGTCTGA-3′ | 5′-CCCATCTGTAATCGTGTCCC-3′ | |
| LDLR | Mouse | 5′-GAGGAACTGGCGGCTGAA-3′ | 5′-GTGCTGGATGGGGAGGTCT-3′ |
| MTTP | Mouse | 5′-CTGGATCTCCATATTGGCCT-3′ | 5′-TGATGTCCAAAATGCTGTCG-3′ |
| VLDLR | Mouse | 5′-TGCACACGAAGTCAGACTCA-3′ | 5′-CCTGCTGTGGAAATGTGATG-3′ |
ACC1: acetyl-CoA carboxylase 1; ATGL: adipose triglyceride lipase; CGI-58: comparative gene identification-58; CH25H: cholesterol 25-hydroxylase; CYP27A1: cytochrome P450 family 27 subfamily A member 1 (cholesterol 27-hydroxylase); CYP46A1: cytochrome P450 family 46 subfamily A member 1 (cholesterol 24-hydroxylase); FASN: fatty acid synthase; GGPS1: geranylgeranyl diphosphate synthase 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; HSL: hormone-sensitive lipase; LDLR: low density lipoprotein receptor; MTTP: microsomal triglyceride transfer protein; NgBR: Nogo-B receptor; NPC2: Niemann-Pick type C2; SCD-1: stearoyl-CoA desaturase 1; SREBP1c: sterol-responsive element binding protein 1c; TGH: triacylglycerol hydrolase; VLDLR: very low density lipoprotein receptor.
2.8. Determination of NgBR protein by immunofluorescent staining
Cells cultured on cover slips in 24-well plates (~2.5 × 105 cells/well) were washed twice with PBS and then fixed in 4% PFA/PBS for 30 min at RT. After removal of PFA by aspiration and washing with PBS, cells were blocked with 2% BSA for 2 h, and then incubated with rabbit anti-NgBR antibody (1:200 dilution) for 16 h at 4 °C. Cells were re-incubated with FITC-conjugated goat anti-rabbit IgG (1:1000 dilution) for 2 h at RT. After washing with PBS again, the slips were stained with DAPI solution (1:1000 dilution) for nuclei. The slips were observed under a fluorescence microscope (Leica), and the images of cells were photographed.
2.9. Inhibition of HMGCR, NPC2 and MEK1/2 expression by siRNA and determination of cellular free cholesterol by filipin staining
All the siRNAs were purchased from Santa Cruz Biotechnology (Dallas, TX). HepG2 cells plated on cover slips in 12-well plates (for filipin staining, ~6 × 105 cells/well) or 6-well plates (for Western blot or real time RT-PCR assays, ~1.5 × 106 cells/well) were transfected with siRNA using Lipofectamine® RNAiMAX Transfection Reagent (Invitrogen). After 24 h transfection, cells were switched to complete medium and cultured for another 24 h followed by atorvastatin treatment for 5 h. After treatment, cells except those transfected with siNPC2 were lysed, and cellular proteins were used to determine HMGCR, ERK1/2, pi-ERK1/2 or NgBR protein expression by Western blot. The NPC2 siRNA-transfected cells were used to collect total RNA followed by determination of NPC2 or NgBR mRNA expression by real time RT-PCR. The cellular free cholesterol in the cells on cover slips was determined by filipin staining as follows: after washing 3 times with PBS, cells were fixed in 4% PFA/PBS for 1 h followed by washing with PBS containing 1.5 mg/ml glycine for 10 min. Cells were then incubated with a filipin working solution (50 μg/ml: a stock solution at 25 mg/ml in DMSO was diluted with PBS just prior to use) for 2 h. The stained cells were observed and photographed with a microscope.
2.10. Data analysis
All experiments were repeated at least three times, and the representative results were presented. Data were presented as mean ± standard errors. All the data were initially subject to a normal distribution analysis with SPSS software (1-sample K-S of non-parametric test). The data in normal distribution was then analyzed by a parametric statistic, post-hoc test of one-way ANOVA. The significant difference was considered if P < 0.05 (n ≥ 3).
3. Results
3.1. Atorvastatin inhibits hepatic TG accumulation in an NgBR-dependent manner
Reduction of NgBR expression in shNgBRi HepG2 cells results in severe cellular TG accumulation while NgBRLKO mice have liver steatosis [15]. Clinical observational studies also indicate that statins can reduce TG levels in both serum and liver suggesting the anti-NAFLD function of statins [17–19]. However, the underlying mechanisms need more investigations. We hypothesized that statin-reduced hepatic TG accumulation is contributed, at least in part, by activation of hepatic NgBR expression. To determine it, we initially fed apoE−/− mice normal chow, WD or WD containing atorvastatin (15 mpk), respectively, for 2 weeks. After treatment, we found WD substantially increased lipid accumulation and TG levels in apoE−/− mouse liver, compared with the animals fed normal chow. However, the increases were totally blocked by atorvastatin (Fig. 1A; B, left panel). Similarly, liver TC levels were increased by WD which were also substantially reduced by atorvastatin (Fig. 1B, right panel). Meanwhile, we determined that serum TG and TC levels (Fig. 1C) were changed by WD or atorvastatin at the same trends to that in the liver. Taken together, the results above demonstrate that atorvastatin not only reduces hypercholesterolemia, but also inhibits liver steatosis.
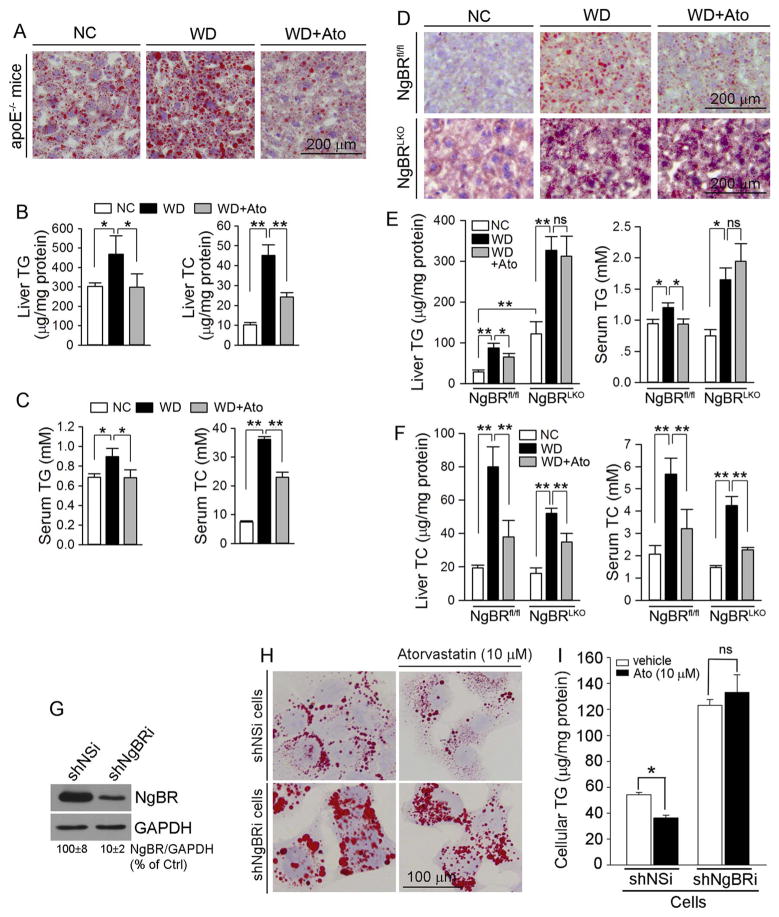
Fig. 1.
Atorvastatin inhibits hepatic and serum TG accumulation in an NgBR-dependent manner
ApoE−/− (A–C), NgBRfl/fl or NgBRLKO mice (D–F) in 3 groups were fed normal chow (NC), Western diet (WD) or WD containing atorvastatin (WD + Ato, 15 mpk), respectively, for 2 weeks. After treatment, liver and serum samples were collected for lipid content assay by Oil Red O staining with liver frozen sections (A, D), and TG or TC quantitative assays (B, C, E, F), respectively; G–I: after the reduction of NgBR expression in shNgBRi cells was confirmed by Western blot (G), both shNSi and shNgBRi cells were treated with atorvastatin (10 μM) for 12 h followed by determination of lipid content by Oil Red O staining (H) and TG quantitative assay (I). *: P < 0.05; **: P < 0.01; ns: not significantly different. The in vivo studies (A–F) were completed with 6 mice in each group. The studies with cell culture (G–I) were repeated 3 times.
To define the role of NgBR in statin-inhibited liver steatosis, NgBRLKO and NgBRfl/fl mice received the same treatment as that to apoE−/− mice. Similar to our previous report [15], compared with NgBRfl/fl mice, a severe lipid accumulation was determined in NgBRLKO mouse liver at the basal levels (normal chow feeding: Fig. 1D, left lower panel; > 4-fold in TG levels, Fig. 1E, left panel). WD increased hepatic lipid levels in both NgBRfl/fl and NgBRLKO mice (Fig. 1D, middle panel; Fig. 1E, left panel). However, it is similar to apoE−/− mice (Fig. 1A, B) that atorvastatin reduced WD-induced hepatic and serum TG accumulation in NgBRfl/fl mice (Fig. 1D, right upper panel; Fig. 1E). In contrast, atorvastatin had little effect on WD-induced hepatic and serum TG accumulation in NgBRLKO mice (Fig. 1D, right lower panel; Fig. 1E). Therefore, NgBR expression is critical for statin-inhibited liver steatosis. Interestingly, WD-increased TC levels in both liver and serum were reduced by atorvastatin in both NgBRfl/fl and NgBRLKO mice (Fig. 1F), indicating that the cholesterol-lowering effect of statins is independent of NgBR expression.
In vitro, we established shNSi and shNgBRi HepG2 cell lines (Fig. 1G) and treated them with atorvastatin, respectively. Compared with shNSi cells, reduced NgBR expression increased cellular lipid content at the basal levels (Fig. 1H, left panel; 1I). Similar to in vivo study, atorvastatin decreased cellular lipid content in shNSi cells (Fig. 1H, upper panel; Fig. 1I, left panel), but not in shNgBRi cells (Fig. 1H, lower panel; Fig. 1I, right panel), which further confirms the importance of NgBR expression in statin-inhibited hepatic lipid accumulation.
3.2. Atorvastatin-inhibited hepatic lipogenesis is related to activation of AMPKα, inactivation of LXRα, and inhibition of oxysterol producing enzyme expression
Our previous report demonstrates that activation of hepatic lipo-genesis by NgBR deficiency depends on activation of LXRα in an AMPKα-dependent manner [15]. To determine if AMPKα or/and LXRα is also involved in atorvastatin-inhibited lipogenesis, we treated HepG2 cells with atorvastatin, and determined that cellular NgBR expression was increased, which was associated with increased AMPKα phosphorylation (pi-AMPKα) (Fig. 2A, upper panel), indicating AMPKα activation. Meanwhile, we observed that LXRα in total cellular protein extract was not changed (Fig. 2A, upper panel), while LXRα in nuclear protein extract was reduced (Fig. 2A, lower panel) by atorvastatin treatment. These results suggest that LXRα overall expression is not affected, but LXRα nuclear translocation is decreased indicating inactivation of LXRα by atorvastatin. Consequently, expression of FASN, the LXRα target gene for lipogenesis, was inhibited by atorvastatin (Fig. 2A, upper panel).
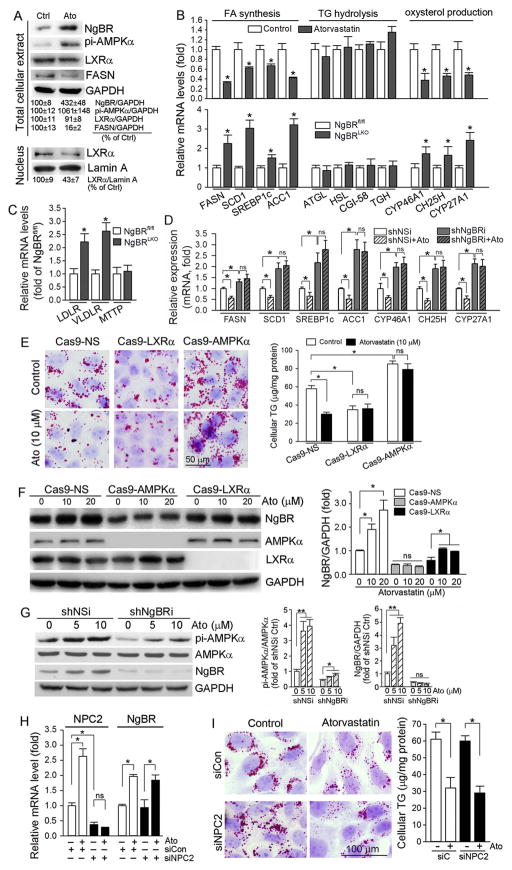
Fig. 2.
Activation of AMPKα, inactivation of LXRα, and inhibition of oxysetrol producing enzymes are involved in atorvastatin-inhibited hepatic lipogenesis
A: HepG2 cells were treated with atorvastatin (10 μM) for 8 h followed by extraction of total cellular proteins and nuclear proteins, separately. Expression of NgBR, pi-AMPKα, LXRα and FASN in total cellular protein extract, and LXRα in nuclear protein extract was determined by Western blot, respectively; B–D: HepG2 cells (B), shNSi or shNgBRi cells (D) were treated with atorvastatin for 8 h. After treatment, total RNA was extracted from cells. Meanwhile, a piece of liver from NgBRfl/fl or NgBRLKO mice was used to extract total RNA. Expression of mRNA of the genes for lipogenesis (B, D: FASN, SCD1, SREBP1c and ACC1), the genes for TG hydrolysis (B: ATGL, HSL, CGI-58 and TGH), the genes for oxysterol production (B, D: CYP46A1, CH25H and CYP27A1), and the genes for TG-rich lipoprotein metabolism (C: LDLR, VLDLR and MTTP) was determined by real time RT-PCR, respectively; E, F: Cas9-NS, Cas9-AMPKα and Cas9-LXRα cells were treated with atorvastatin at the indicated concentrations for 16. Cellular lipid content was determined by Oil Red O staining and TG quantitative analysis (E). Expression of NgBR, AMPKα and LXRα was determined by Western blot (F); G: shNSi and shNgBRi cells were treated with atorvastatin for 8 h. Expression of AMPKα, pi-AMPKα and NgBR was determined by Western blot; H, I: HepG2 cells in serum-free medium were transfected with scrambled siRNA (siCon) or NPC2 siRNA (siNPC2) for 24 h. Cells were then switched to complete medium and cultured for another 24 h followed by treatment with atorvastatin (10 μM) for 8 h. Expression of NPC2 and NgBR mRNA was determined by real time RT-PCR (H). Cellular lipid content was determined by Oil Red O staining and TG quantitative analysis (I). *: P < 0.05 vs. control or as indicated in the corresponding group; ns: not significantly different. All the experiments were repeated 3 times.
Furthermore, we determined mRNA expression of the genes for fatty acid synthesis in HepG2 cells in response to atorvastatin treatment, and found that besides FASN, expression of other genes involved in fatty acid synthesis, such as SCD1, SREBP1c and ACC1, was also reduced (Fig. 2B, left upper panel). In contrast to atorvastatin treatment, we determined that NgBR deficiency increased expression of the genes for lipogenesis (FASN, SCD1, SREBP1c and ACC1) in NgBRLKO mouse liver (Fig. 2B, left lower panel). Compared with the effects on expression of the genes for fatty acid synthesis, neither atorvastatin treatment nor NgBR deficiency had effect on expression of the genes responsible for TG hydrolysis, such as adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), comparative gene identification-58 (CGI-58) and triacylglycerol hydrolase (TGH) (Fig. 2B, middle panel).
LXRα is a ligand-activated transcription factor. Several oxysterols, such 24-, 25- and 27-hydroxycholesterol, can serve as endogenous LXRα agonists [20]. We determined that expression of CYP46A1, CH25H and CYP27A1, the enzymes are responsive for production of 24-, 25- and 27-hydrocholesterol, respectively, in HepG2 cells was reduced by atorvastatin (Fig. 2B, right upper panel). In contrast, deficiency of NgBR expression activated expression of these genes in mouse liver (Fig. 2B, right lower panel).
In this study, we observed that deficiency of NgBR expression increased TG levels in the liver at a much higher degree than that in the serum when the animals were fed either normal chow or WD (Fig. 1E). The non-proportional changes between hepatic and plasma TG levels might be due to changes of TG-rich lipoprotein metabolism, therefore, we determined expression of LDLR, VLDL receptor (VLDLR) and microsomal triglyceride transfer protein (MTTP, a molecule enhancing synthesis of chylomicrons and VLDL) in mouse liver. We found that NgBR deficiency increased expression of LDLR and VLDLR while had no effect on MTTP expression (Fig. 2C), suggesting enhanced TG-rich lipoprotein metabolism, not TG-rich lipoprotein synthesis, by NgBR deficiency.
Our above results demonstrate that NgBR deficiency enhances, while atorvastatin treatment reduces hepatic lipogenesis (Fig. 2B) which is associated with activation of NgBR expression. Therefore, it is possible that reduced NgBR expression may impair the effect of atorvastatin on lipogenesis. In fact, in addition to that the inhibitory effect of atorvastatin on lipid content in NgBRLKO mouse liver or shNgBR HepG2 cells was blunted (Fig. 1D, E, H and I), we determined that atorvastatin had little effect on expression of the genes for lipogenesis (FASN, SCD1, SREBP1c and ACC1) and the oxysterol producing enzymes (CYP46A1, CH25H and CYP27A1) in shNgBRi cells (Fig. 2D). Taken together, the results in Fig. 2B–D suggest that regulation of hepatic lipid levels by either atorvastatin treatment or NgBR deficiency is mainly contributed by changing lipogenesis which is linked to regulation of oxysterol producing enzymes expression.
To further confirm the involvement of AMPKα and LXRα in statin-inhibited lipogenesis, we established AMPKα and LXRα knockout HepG2 cell lines (Cas9-AMPKα and Cas9-LXRα), respectively. As shown in Fig. 2E, cellular lipid content was reduced by deficiency of LXRα expression, but increased by deficiency of AMPKα expression, indicating the pro- and anti-lipogenic properties of LXRα and AMPKα, respectively. Similar to normal HepG2 cells, atorvastatin inhibited lipid accumulation in Cas9-NS cells, but had little effect on either Cas9-LXRα or Cas9-AMPKα cells (Fig. 2E). The results of Western blot also demonstrate that atorvastatin induced NgBR expression in Cas9-NS cells, and the induction was attenuated in Cas9-LXRα cells (Fig. 2F). In addition, we determined that lack of AMPKα expression substantially reduced NgBR expression at basal levels, and blocked atorvastatin-induced NgBR expression (Fig. 2F), suggesting the interaction between NgBR and AMPKα since our previous report demonstrates that lack of NgBR expression inactivates AMPKα [15]. Although reduction of NgBR expression can decrease AMPKα activity at the basal levels, atorvastatin is still able to activate AMPKα moderately (Fig. 2G), suggesting NgBR is important, but not essential for activation of AMPKα. Taken together, the results above suggest that atorvastatin-inhibited lipid accumulation is involved by activation of AMPKα and inactivation of LXRα.
To elucidate if NPC2 expression is also involved in statin-inhibited cellular lipid accumulation, we transfected HepG2 cells with NPC2 siRNA (Fig. 2H, left panel), and treated the transfected cells with atorvastatin. Fig. 2H shows that atorvastatin increased NPC2 expression in control siRNA-transfected (siCon) cells. However, reduced NPC2 expression had no effect on atorvastatin-induced NgBR expression (Fig. 2H, right panel) indicating that the effect of atorvastatin on NgBR expression is independent of NPC2 expression. The results of cellular lipid content assay also confirm that atorvastatin reduced cellular lipid content in an NPC2-independent manner (Fig. 2I).
3.3. Statin induces NgBR expression both in vivo and in vitro
Atorvastatin inhibited WD-induced lipid accumulation in NgBRfl/fl, but not in NgBRLKO mouse liver (Fig. 1D, E), which implies that the effect of WD or atorvastatin on hepatic lipid content might be attributed to regulation of NgBR expression. In fact, compared with the animals fed normal chow, WD inhibited NgBR expression in apoE−/− mouse liver, whereas administration of atorvastatin totally restored WD-inhibited NgBR expression (Fig. 3A). Similarly, in NgBRfl/fl mice, the WD-inhibited hepatic NgBR expression was totally blocked by atorvastatin (Fig. 3B, left panel). The simultaneous induction of hepatic NgBR expression and inhibition of lipid accumulation by atorvastatin suggest that induction of NgBR expression may play an important role in anti-liver steatosis by statins. Indeed, atorvastatin had no effect on WD-induced TG accumulation in NgBRLKO mouse liver and serum (Fig. 1D, E).
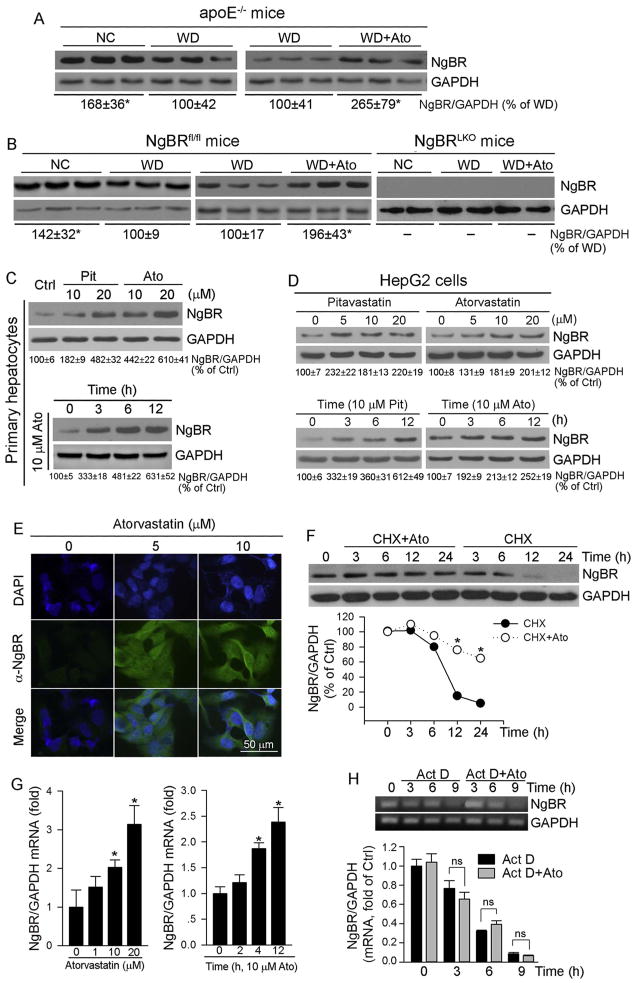
Fig. 3.
Statins induce NgBR expression both in vivo and in vitro
Total proteins were extracted from a piece of apoE−/− (A), NgBRfl/fl or NgBRLKO mouse (B) liver used in Fig. 1. Primary hepatocytes isolated from C57BL/6 mice (C) and HepG2 cells (D) were treated with pitavastatin (Pit) and atorvastatin (Ato) at the indicated concentrations for 5 h or with 10 μM atorvastatin or pitavastatin for the indicated times, respectively; E: HepG2 cells were treated with atorvastatin (10 μM) for 5 h; F: HepG2 cells were treated with cycloheximide (CHX, 2 μg/ml) or CHX plus atorvastatin (10 μM) for the indicated times; G: HepG2 cells were treated with atorvastatin at the indicated concentrations for 5 h or 10 μM atorvastatin for the indicated times; H: HepG2 cells were treated with actinomycin D (Act D, 2 μM) or Act D plus atorvastatin (10 μM) for the indicated times. Expression of NgBR protein was determined by Western blot (A–D, F) or immunofluorescent staining (E). Expression of NgBR mRNA was determined by real time RT-PCR (G, lower panel of H) and RT-PCR followed by gel electrophoresis (H, upper panel). *: P < 0.05 vs. control in the corresponding group; ns: not significantly different. All the experiments were repeated 3 times.
To determine the regulation of NgBR expression by statins in detail, we initially treated primary hepatocytes isolated from C57BL/6 mice with pitavastatin and atorvastatin. Both of them induced NgBR expression in a concentration-dependent manner with a greater effect by atorvastatin (Fig. 3C, upper panel). In addition, atorvastatin induced NgBR expression in primary hepatocytes quickly and the induction lasted for 12 h after treatment (Fig. 3C, lower panel). In HepG2 cells, pitavastatin and atorvastatin also induced NgBR protein expression at a broad concentration range and quickly (Fig. 3D) indicating that the induction of hepatic NgBR expression by statins is in a species-independent manner. The results of immunofluorescent staining further confirm induction of NgBR protein expression by atorvastatin in cytosol and plasma membrane of HepG2 cells (Fig. 3E).
To determine if atorvastatin can affect NgBR protein stability, we treated HepG2 cells with cycloheximide to arrest cellular protein synthesis, in the absence or presence of atorvastatin. Fig. 3F shows that NgBR protein degraded at a rate of t1/2–10 h. However, atorvastatin substantially reduced NgBR protein degradation suggesting it increases NgBR protein stability.
Associated with increased NgBR protein expression, NgBR mRNA expression was increased by atorvastatin in both concentration- and time-dependent manners (Fig. 3G). However, atorvastatin had little effect on NgBR mRNA degradation/stability (Fig. 3H), suggesting that statins may activate NgBR transcription.
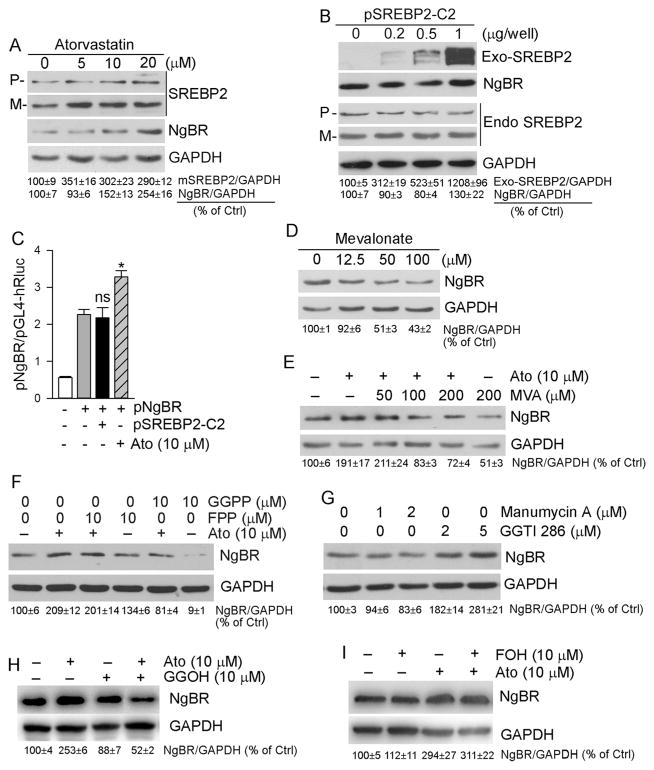
3.4. Cellular cholesterol negatively regulates NgBR expression
The effect of WD or atorvastatin on liver or serum TC levels (Fig. 1B, C, F) and hepatic NgBR expression (Fig. 3A, B) in apoE−/− or NgBRfl/fl mice implies that cellular cholesterol can negatively regulate NgBR expression. To determine it, we treated HepG2 cells cultured in either complete (10% serum) or serum-free medium with atorvastatin, and determined that atorvastatin decreased cellular TC levels under different culture conditions, in a dose-dependent manner (Fig. 4A). Reciprocally, atorvastatin increased NgBR expression (Fig. 4B). Next, we treated HepG2 cells with β-cyclodextrin (β-CD), a vehicle for solubilization of cholesterol in aqueous solution reducing cellular TC levels. Associated with decreased cellular TC levels (Fig. 4C), NgBR expression was increased by β-CD (Fig. 4D). In contrast, loading cholesterol to HepG2 cells with LDL or water soluble cholesterol (Chol/CD) reduced NgBR expression (Fig. 4E). In addition, the β-CD-induced NgBR expression was blocked by Chol/CD (Fig. 4F). Furthermore, we determined that increased cellular TC levels by Chol/CD activated expression of GGPS1, the enzyme responsible for GGPP synthesis (Fig. 4G, left panel). In contrast, reduced cellular TC levels by β-CD inhibited GGPS1 expression (Fig. 4G, right panel). The effects of cellular cholesterol levels on GGPS1 expression may also influence NgBR expression.
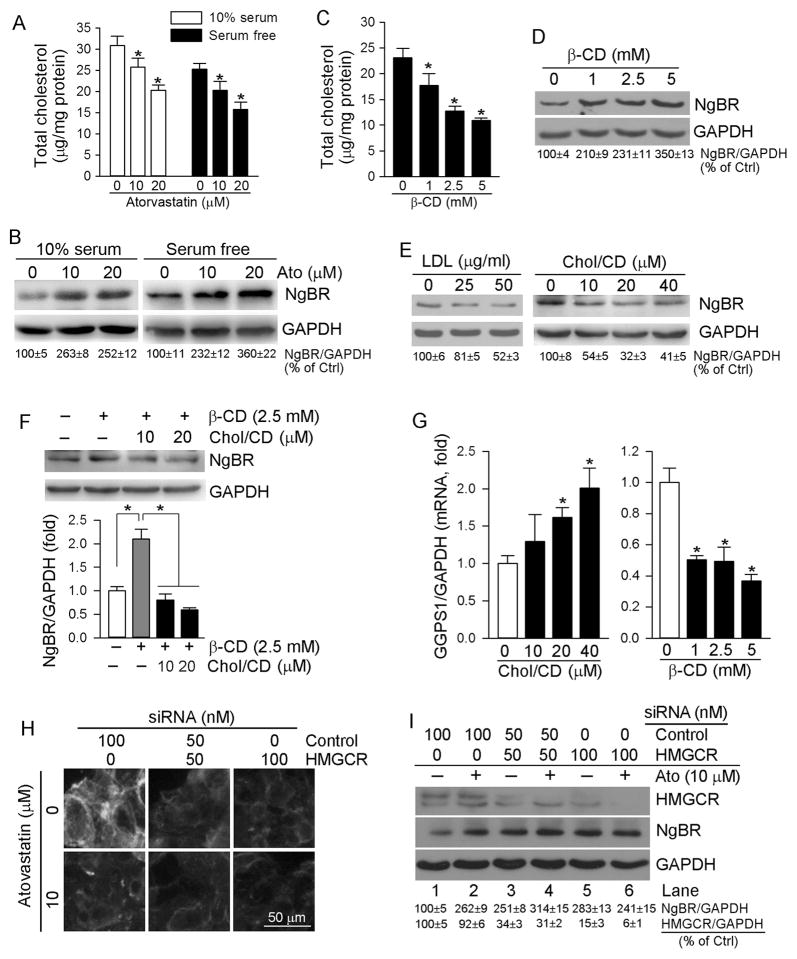
Fig. 4.
Cellular cholesterol negatively regulates NgBR expression
A, B: HepG2 cells in serum-free and complete (10% serum) medium received atorvastatin treatment at the indicated concentrations for 16 h, respectively; C–G: HepG2 cells in serum-free medium received the following treatment for 16 h: β-cyclodextrin (β-CD) (C, D), LDL or water soluble cholesterol (Chol/CD) (E), β-CD (2.5 mM) or β-CD plus Chol/CD (F), and β-CD or Chol/CD (G), at the indicated concentrations. Cellular TC levels were determined by TC assay kit (A, C). Expression of NgBR protein was determined by Western blot (B, D–F). Expression of GGPS1 mRNA was determined by real time RT-PCR (G). *: P < 0.05 vs. control or as indicated in the corresponding group; H, I: HepG2 cells were transfected with scrambled siRNA (Control) or HMGCR siRNA (HMGCR) followed by treatment with atorvastatin (10 μM) for 5 h. Cellular free cholesterol and expression of HMGCR and NgBR protein were determined by filipin staining and Western blot, respectively. All the experiments were repeated 3 times.
To directly link hepatic NgBR expression to cholesterol synthesis, we transfected HepG2 cells with HMGCR siRNA followed by atorvastatin treatment. The results of filipin staining demonstrate that inhibition of HMGCR expression by siRNA decreased cellular free cholesterol levels (Fig. 4H, upper panel). Atorvastatin also reduced cellular free cholesterol levels in control cells (Fig. 4H, left lower panel). Correspondingly, HMGCR siRNA increased NgBR expression at the basal levels (Fig. 4I, lane 3 or 5 vs. lane 1). Atorvastatin induced NgBR expression in scrambled siRNA-transfected cells (Fig. 4I, lane 2 vs. lane 1). However, atorvastatin had little effect on NgBR expression in HMGCR siRNA-transfected cells (Fig. 4I, lane 4 vs. lane 3, and lane 6 vs. lane 5) that might be due to no further reduction of cellular cholesterol or cholesterol synthesis intermediates by atorvastatin in HMGCR siRNA-transfected cells (Fig. 4H, lower panel). Taken together, Fig. 4 suggests that NgBR expression is negatively regulated by cellular cholesterol levels.
3.5. Atorvastatin induces NgBR expression in a geranylgeranylation-dependent manner
To determine if statin-induced NgBR expression is related to activation of SREBP2, HepG2 cells were treated with atorvastatin followed by determination of SREBP2 expression and maturation. Fig. 5A shows that atorvastatin increased both SREBP2 precursor (P) and mature SREBP2 (M) which was associated with a dose dependent increased NgBR expression (Fig. 5A).
Fig. 5.
Cholesterol synthesis intermediates regulate NgBR expression
HepG2 cells in serum-free medium received the following treatment. A: atorvastatin at the indicated concentrations for 8 h; B: transfection with mature SREBP2 expression vector (pSREBP2-C2) for 16 h; C: HEK-293 T cells in 24-well plates were transfected with DNA for pNgBR promoter and pSREBP2 expression vector or empty vector, plus the DNA for Renilla luciferase (for internal control). After 4 h of transfection, cells were treated with 10 μM atorvastatin for 16 h followed by determination of activities of firefly and Renilla luciferases. *: P < 0.05; ns: not significantly different vs. pNgBR alone; D, E: mevalonate alone for 16 h (D) or mevalonate for 16 h and then plus atorvastatin for 5 h (E); F: FPP or GGPP at the indicated concentrations for 16 h and then FPP or GGPP plus atorvastatin for 5 h; G: manumycin A or GGTI-286 at the indicated concentrations for 5 h; H, I: GGOH or FOH (10 μM) for 16 h and then GGOH or FOH plus atorvastatin (10 μM) for 5 h. Expression of NgBR protein (A, B, D–I), endogenous SREBP2 precursor (P), mature SREBP2 (M), exogenous SREPB2 protein was determined by Western blot. All the experiments were repeated 3 times.
To obtain the direct evidence that SREBP2 is involved in statin-induced NgBR expression or not, we initially transfected HepG2 cells with mature SREBP2 expression vector. We determined that high expressing mature SREBP2 did not affect endogenous SREBP2 expression or maturation, and it had no effect on NgBR expression either (Fig. 5B). We then constructed an NgBR promoter (pNgBR), and found that atorvastatin induced pNgBR activity. However, high expressing mature SREBP2 had no effect on it (Fig. 5C). Therefore, the results in Fig. 5B and C indicate that statins induce NgBR expression at a transcriptional level but the induction is unrelated to activation of SREBP2.
We next dissected the effect of cholesterol synthesis intermediates on NgBR expression. As shown in Fig. 5D, mevalonate, the immediate product of HMGCR, reduced NgBR expression in a concentration-dependent manner, and blocked atorvastatin-induced NgBR expression (Fig. 5E).
Isoprenoids, FPP and GGPP, are also cholesterol synthesis intermediates and involved in the posttranslational prenylation of several proteins (e.g., Ras, Rho and Rac). Therefore, both FPP and GGPP play important roles in a variety of cellular processes [21,22]. To determine the effect of these intermediates on NgBR expression, we treated HepG2 cells with FPP, GGPP or plus atorvastatin. GGPP, but not FPP, not only inhibited NgBR basal levels, but also attenuated atorvastatin-induced NgBR expression (Fig. 5F). GGTI-286, a CAAX-mimetic peptide which specifically inhibits geranylgeranylation, induced NgBR expression, while manumycin A, an antibiotic that acts as a selective and vigorous inhibitor of Ras farnesyltransferase, had no inductive effect (Fig. 5G). Meanwhile, we found that GGOH, which can be converted into GGPP within cells, blocked atorvastatin-induced NgBR expression (Fig. 5H). In contrast, treatment of HepG2 cells with FOH, the precursor of FPP, had little effect on NgBR expression (Fig. 5I). Therefore, Fig. 5 suggests that induction of NgBR expression by statins is related to reduction of cholesterol synthesis intermediates, particularly of GGPP.
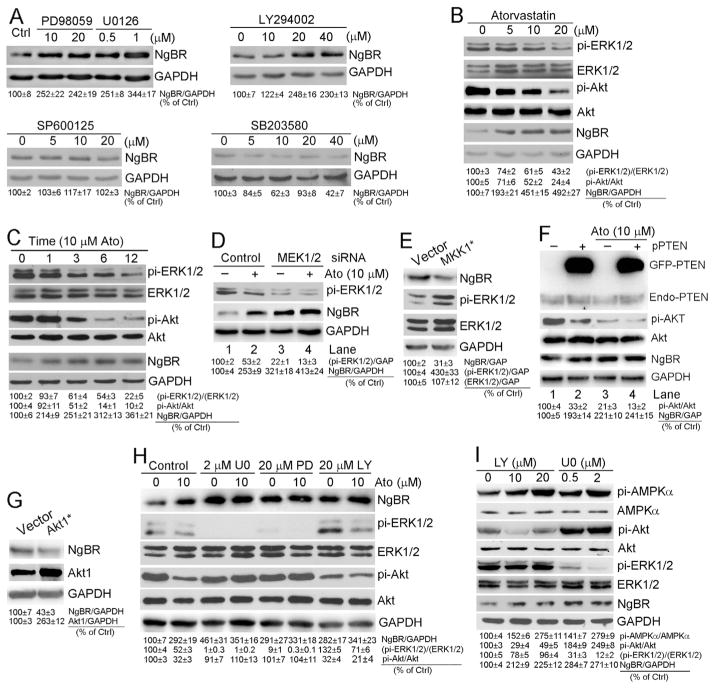
3.6. Induction of hepatic NgBR expression by atorvastatin depends on inactivation of ERK1/2 and Akt
Geranylgeranylation of Rho GTPase can activate MAP kinase and PI3K pathways through different strategies [23]. To further identify the downstream protein of geranylgeranylation which is involved in atorvastatin-induced NgBR expression, we initially treated HepG2 cells with inhibitors of different kinases. Fig. 6A shows that both ERK1/2 inhibitor (PD98059 or U0126) and PI3K inhibitor (LY294002) induced NgBR expression in a dose-dependent manner (upper panel), while neither p38 MAP kinase inhibitor (SP600125) nor JNK inhibitor (SB203580) had effects on NgBR expression (lower panel). Therefore, the induction of NgBR expression by statins might be due to statin-inhibited ERK1/2 or/and Akt.
Fig. 6.
Induction of NgBR expression by atorvastatin depends on inactivation of ERK1/2 and Akt
HepG2 cells received the following treatment. A: PD98059 or U0126, LY294002, SP600125 or SB203580 at the indicated concentrations for 4 h; B, C: atorvastatin at the indicated concentrations for 5 h (B) or 10 μM atorvastatin for the indicated times (C); D, F: transfection with scrambled siRNA (control) and MEK1/2 siRNA (MEK1/2) (D), or empty vector and PTEN expression vector (pPTEN) (F), respectively, in serum-free medium for 24 h. Cells were then switched to complete medium and cultured for another 24 h followed by treatment with atorvastatin (10 μM) for 5 h; E, G: transfection with empty vector (vector) or constitutively active MKK1 expression vector (MKK1*) (E) or Akt1 expression vector (Akt1*) (G) for 16 h; H: U0126 (U0, 2 μM), PD98059 (PD, 20 μM), LY294002 (LY, 20 μM) in the absence or presence of atorvastatin (10 μM) for 8 h; I: LY294002 or U0126 at the indicated concentrations for 4 h. Expression of NgBR (A–I), ERK1/2 (B, C, E, H, I), pi-ERK1/2 (B–E, H, I), Akt (B, C, F–I) and pi-Akt (B, C, F, H, I) was determined by Western blot, respectively. All the experiments were repeated 3 times.
Indeed, associated with induction of NgBR expression, atorvastatin substantially reduced phosphorylation of ERK1/2 (pi-ERK1/2) and Akt (pi-Akt) in a dose-dependent manner while slightly affecting total ERK1/2 and Akt levels (Fig. 6B). Similarly, atorvastatin inhibited pi-ERK1/2 or pi-Akt while activating NgBR expression in a time-dependent manner (Fig. 6C).
To further determine the effect of ERK1/2 activity on NgBR expression, we transfected HepG2 cells with MEK1/2 siRNA and then treated transfected cells with atorvastatin. Similar to inhibitors, MEK1/2 siRNA also substantially inhibited pi-ERK1/2 while activating NgBR expression (Fig. 6D, lane 3 vs. lane 1). Although atorvastatin potently inhibited pi-ERK1/2 and activated NgBR expression in control siRNA-transfected cells (Fig. 6D, lane 2 vs. lane 1), it slightly influenced either pi-ERK1/2 or NgBR expression in si-MEK1/2-transfected cells (Fig. 6D, lane 4 vs. lane 3). In contrast, transfection of HepG2 cells with constitutively active mutant MKK1 [MKK1*, DN3 (deleted residues 32–51), S218D, S222D] increased pi-ERK1/2 while inhibiting NgBR expression (Fig. 6E).
Besides chemical inhibitors, pi-Akt can be inhibited by PTEN. We determined that high expressing PTEN reduced pi-Akt while activating NgBR expression (Fig. 6F, lane 2 vs. lane 1). However, in the presence of atorvastatin, the effects of PTEN on pi-Akt and NgBR expression was substantially attenuated (Fig. 6F, lane 4 vs. lane 3). Reciprocally, transfection of HepG2 cells with constitutively active mutant Akt1 (Akt1*) inhibited NgBR expression (Fig. 6G). In addition, induction of NgBR expression by ERK1/2 inhibitor (U0126 or PD98059) or Akt inhibitor (LY294002) was slightly affected by atorvastatin either (Fig. 6H) suggesting that activation of NgBR expression by atorvastatin is completed by inactivating ERK1/2 or Akt, and no further inhibition of ERK1/2 or Akt can be achieved by co-treatment of atorvastatin with U0126 (PD98059) or LY294002 than U0126 (PD98059) or LY294002 alone. Finally, we determined if the induction of NgBR expression by inhibition of ERK1/2 or Akt is related to activation of AMPKα. We treated HepG2 cells with LY294002 or U0126 at different concentrations, and determined that associated with induction of NgBR expression or inhibition of either Akt or ERK1/2, AMPKα was activated by LY294002 or U0126 in a concentration dependent manner (Fig. 6I).
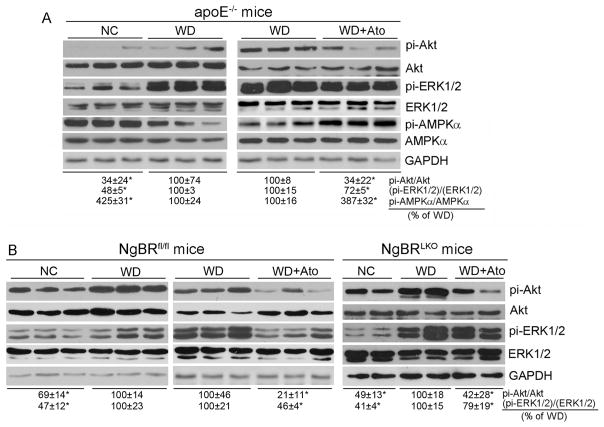
We also confirmed the inverse correlation between NgBR expression and activity of ERK1/2 and Akt in vivo. In apoE−/− or NgBRfl/fl mouse liver, WD feeding increased pi-ERK1/2 and pi-Akt while administration of atorvastatin substantially attenuated the activations (Fig. 7A; Fig. 7B, left panel). In contrast to ERK1/2 or Akt, WD feeding reduced AMPKα activity which was restored to normal by atorvastatin treatment (Fig. 7A). In addition, we observed that the WD-activated ERK1/2 or Akt was still reduced by atorvastatin treatment in NgBRLKO mouse liver (Fig. 7B, right panel), which suggests that regulation of ERK1/2 or Akt activity is not impaired by deficiency of NgBR expression, and NgBR should be a downstream molecule in ERK1/2 and Akt pathways. Taken together, the results in Figs. 6 and 7 suggest that induction of NgBR expression by statins depends on inactivation of ERK1/2 and Akt.
Fig. 7.
Regulation of NgBR expression by atorvastatin and WD in vivo is related to ERK1/2 and Akt activity
Total proteins were extracted from a piece of liver of apoE−/− (A), NgBRfl/fl or NgBRLKO mouse (B) used in Fig. 1. Expression of ERK1/2, pi-ERK1/2, pi-AMPKα, AMPKα, Akt and pi-Akt was determined by Western blot, respectively. *: P < 0.05 vs. WD in the corresponding group. All the experiments were completed with 3 samples randomly selected from the 6 samples used in Fig. 1.
4. Discussion
Several biological functions of NgBR have been reported [13–15,24]. In current study, we demonstrate that the WD-induced fatty liver can be blocked by statins in an NgBR-dependent manner. Our study not only confirms the physiological role of NgBR, but also unveils that NgBR is involved in anti-NAFLD function of statins.
The signaling pathways for regulation of NgBR expression have not been fully investigated. In our study, we initially demonstrated that hepatic NgBR expression was inversely regulated by cholesterol levels with the following evidence: 1) in vivo, NgBR expression in the liver was decreased by WD feeding which was associated with increased cholesterol levels in liver and serum (Figs. 1F, 3A, B). However, WD-inhibited NgBR expression was restored by atorvastatin treatment (Fig. 3A, B); 2) in vitro, besides by statin treatment (Figs. 3C–E, 4A, B), NgBR expression was activated by decreased cellular cholesterol levels with β-CD treatment (Fig. 4C, D). In contrast, loading cells with cholesterol inhibited NgBR expression (Fig. 4E, F); 3) direct inhibition of HMGCR expression and cholesterol synthesis by siRNA also increased NgBR expression (Fig. 4H, I).
We next disclosed that the following signaling pathways can play an important role in statin-induced NgBR expression. Statin treatment can reduce cholesterol and cholesterol synthesis intermediates. However, we observed that not all the cholesterol synthesis intermediates can influence NgBR expression. In addition to mevalonate, GGPP but not FPP, reduced NgBR expression (Fig. 5D–I). Because the geranylger-anylation of Rho GTPase can activate MAP kinase and PI3K pathways, so we dissected and identified that inhibition of PI3K and ERK1/2 activated NgBR expression (Fig. 6A). We then used different approaches demonstrating that induction of NgBR expression by statins depends on inactivation of ERK1/2 and Akt. Direct inhibition of ERK1/2 by siRNA or inhibition of Akt by high expressing PTEN induced NgBR expression, and attenuated the inductive effect of atorvastatin on NgBR expression (Fig. 6D, F) indicating the overlap between the regulatory pathways on NgBR expression by statins and by ERK1/2 or Akt. Correspondingly, the constitutively activated MKK1 and Akt mutants inhibited NgBR expression (Fig. 6E, G). In addition, atorvastatin did not further enhance ERK1/2 inhibitor- or Akt inhibitor-induced NgBR expression (Fig. 6H).
In our previous report, we demonstrated that lack of NgBR expression inactivates AMPKα which activates LXRα by inducing its nuclear translocation. Thus, LXRα plays a central role in NgBR-inhibited NAFLD. LXRα activity is determined by its nuclear levels and the levels of LXR agonists. In this study, we determined that statin treatment activated AMPKα while inactivating LXRα. Several oxysterols can function as LXRα endogenous agonists. It has been reported that oxy-sterol levels in patients with NAFLD are higher than healthy controls. The clinical studies indicate that statin treatment can reduce plasma levels of oxysterols, such as 24-, 25- and 27-hydroxycholesterol, in patients with NAFLD, gallstone, diabetes and Alzheimer disease [25–28]. In vitro, statins reduced oxysterol ligand for LXR in macrophages, thereby reducing expression of LXR target genes and cholesterol eflux [29]. In this study, we determined that lack of NgBR expression activated expression of oxysterol producing enzymes, such as CYP46A1, CH25H and CYP27A1, the enzymes responsible for production of 24-, 25- and 27-hydroxycholesterol, respectively (Fig. 2B, right lower panel). In contrast, atorvastatin reduced expression of these genes but the reduction was attenuated by reduced NgBR expression (Fig. 2D). These results not only suggest another mechanism by which statins inactivate LXRα, but also implies that reduced oxysterol production and activated NgBR expression by statins may work cooperatively to inactivate LXRα and inhibit NAFLD.
In addition, we speculate that inhibition of enzyme expression for oxysterol production, such as 25-hydroxycholesterol, might be a mechanism protecting cellular cholesterol homeostasis in the context of statin treatment. Statins inhibit HMGCR activity thereby reducing de novo cholesterol synthesis. HMGCR expression can be activated by SREBP2, the transcription factor which must be pre-activated through maturation in the Golgi apparatus. In the ER, SREBP2 precursor forms a complex with SREBP cleavage-activating protein (SCAP), and then the complex of SCAP/SREBP2 moves to the Golgi apparatus, where the SREBP2 precursor is cleaved into mature SREBP2. 25-Hydroxycholesterol enhances the binding of SCAP with Insigs, thereby blocking formation and movement of SCAP/SREBP2 [30]. Therefore, the reduction of 25-hydroxycholesterol production by statins can enhance SREBP2 maturation and consequently activate HMGCR expression [21,31]. In addition, oxysterols function as LXR agonists to enhance cellular cholesterol eflux. The reduced oxysterol levels by statin treatment may reduce cholesterol eflux also. Working together, activation of HMGCR expression and inhibition of cholesterol eflux by statin treatment may keep the reduction of cholesterol by statins under control.
Synthetic LXR ligand, such as T0901317, can activate macrophage ATP-binding cassette transporter A1 (ABCA1) expression to enhance reverse cholesterol transport (RCT), thereby inhibiting the development of atherosclerosis. However, the induction of hepatic lipogenesis by synthetic LXR ligand limits the application of LXR ligand for treatment of atherosclerosis. We previously determined that co-administration of U0126 and T0901317 can eficaciously block T0901317-induced fatty liver in apoE−/− mice [32]. The inhibition of LXR-induced lipogenesis by U0126 is completed by inhibiting TG synthesis while activating TG hydrolysis and fatty acid oxidation [32]. Therefore, inhibition of ERK1/2 synergizes LXR-reduced atherosclerosis while eliminating LXR-induced adverse effects of LXR ligand [32]. In this study, we observed that statin inhibits lipogenesis by activating NgBR expression through inhibition of ERK1/2, therefore, unveiled another novel mechanism for anti-lipogenic action of ERK1/2 inhibition. In addition, we did not observe changes in cell proliferation by U0126 or PD98059 indicating a high safety of U0126 used at low concentrations. Hepassocin (HPS), a hepatokine, is elevated in serum of NAFLD patients. High expressing HPS induces NAFLD in transgenic mice, and facilitates lipid accumulation in HepG2 cells, in an ERK1/2-dependent manner [33]. It has been reported that liver specific deletion of PTEN expression activates Akt and enhances the development of fatty liver [34]. Similarly, we demonstrate in this study that inhibition of Akt also plays an important role in atorvastatin-inhibited lipogenesis.
Several clinical observational studies have shown the anti-NAFLD properties of statin treatment [35–39]. Similarly, in animal studies it has been reported that rosuvastatin ameliorates WD-induced liver steatosis in rats by decreasing TNF-α, IL-6, TGF-β and type-1 pro-collagen expression [40]. The reverse of WD-induced fibrotic NASH in obese, diabetic mice by atorvastatin is believed to be related to inactivation of JNK and reduction of hepatocyte apoptosis and inflammatory recruitment [41]. Compared with the studies above, we demonstrate that statins inhibit NAFLD in an NgBR-dependent manner.
We previously reported that deficiency of NgBR expression inactivates AMPKα [15]. Reciprocally, in this study, we observed that deficiency of AMPKα resulted in reduced NgBR expression and impaired atorvastatin-activated NgBR expression (Fig. 2F), which suggests the interaction between NgBR and AMPKα. However, similar to the effect on Akt or ERK1/2 activity, we observed that reduced NgBR expression did not impair activation of AMPKα by atorvastatin (data not shown).
In summary, in this study, we found that inhibition of hepatic steatosis by statin treatment is completed, at least in part, by activating hepatic NgBR expression, which further demonstrates the physiological role of NgBR expression in regulation of lipogenesis. Mechanically, we determined that activation of NgBR expression by statins is mediated by inactivating ERK1/2 and Akt which is related to the reduced generation of cholesterol synthesis intermediates, in particular of GGPP. The induced NgBR expression activates AMPKα or enhances interaction between NgBR and AMPKα. Meanwhile, statins reduce production of LXR agonists, working together with the activated NgBR/AMPKα to inactivate LXRα and consequently reduce NAFLD substantially (Graphical abstract).
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grants 81473204 and 81773727 to J Han, 31770863 to Y Chen, 81573427 and 81722046 to Y Duan, the Program for Changjiang Scholars and Innovative Research Team in University (IRT13023) and 111 Project (B08011) to J Han; the International Science & Technology Cooperation Program of China 2015DFA30430 to JH, YD and YC; China Postdoctoral Science Foundation Grant 2015M570226 to YC; NIH 1R01DK112971-01 to QRM.
Footnotes
Conflict of interest
None declared.
Transparency document
The http://dx.doi.org/10.1016/j.bbalip.2017.12.002 associated with this article can be found, in online version.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Alberts AW. Discovery, biochemistry and biology of lovastatin. Am J Cardiol. 1988;62:10J–15J. doi: 10.1016/0002-9149(88)90002-1. [DOI] [PubMed] [Google Scholar]
- 5.Stein EA, Lane M, Laskarzewski P. Comparison of statins in hypertriglyceridemia. Am J Cardiol. 1998;81:66B–69B. doi: 10.1016/s0002-9149(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 6.Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, Keilson LM, Brown WV, Miller VT, Shurzinske LJ, Black DM. Eficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA. 1996;275:128–133. [PubMed] [Google Scholar]
- 7.Ekstedt M, Franzen LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135–141. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, Kakela P, Wiklund O, Mozzi E, Grimaudo S, Kaminska D, Rametta R, Craxi A, Fargion S, Nobili V, Romeo S, Pihlajamaki J, Valenti L. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis. 2004;174:193–196. doi: 10.1016/j.atherosclerosis.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.de Keyser CE, Koehler EM, Schouten JN, Visser LE, Hofman A, Janssen HL, Stricker BH. Statin therapy is associated with a reduced risk of non-alcoholic fatty liver in overweight individuals. Dig Liver Dis. 2014;46:720–725. doi: 10.1016/j.dld.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, Strittmatter SM, Sessa WC. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 12.Miao RQ, Gao Y, Harrison KD, Prendergast J, Acevedo LM, Yu J, Hu F, Strittmatter SM, Sessa WC. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad Sci U S A. 2006;103:10997–11002. doi: 10.1073/pnas.0602427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao B, Chu C, Liu Z, Horswill MA, Pramanik K, Wilkinson GA, Ramchandran R, Miao RQ. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116:5423–5433. doi: 10.1182/blood-2010-02-271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison KD, Miao RQ, Fernandez-Hernando C, Suarez Y, Davalos A, Sessa WC. Nogo-B receptor stabilizes Niemann-Pick type C2 protein and regulates intracellular cholesterol traficking. Cell Metab. 2009;10:208–218. doi: 10.1016/j.cmet.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu W, Zhang W, Chen Y, Rana U, Teng RJ, Duan Y, Liu Z, Zhao B, Foeckler J, Weiler H, Kallinger RE, Thomas MJ, Zhang K, Han J, Miao RQ. Nogo-B receptor deficiency increases LXRα nuclear translocation and hepatic lipogenesis via an AMPKa-dependent pathway. Hepatology. 2016;64:1559–1576. doi: 10.1002/hep.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan Y, Chen Y, Hu W, Li X, Yang X, Zhou X, Yin Z, Kong D, Yao Z, Hajjar DP, Liu L, Liu Q, Han J. Peroxisome proliferator-activated receptor gamma activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J Biol Chem. 2012;287:23667–23677. doi: 10.1074/jbc.M112.350181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talavera JO, Martinez G, Cervantes JL, Marin JA, Rodriguez-Briones I, Gonzalez JG, Ocampo R, Sanchez-Mijangos H, Bernal-Rosales LP, Polanco A. A double-blind, double-dummy, randomized, placebo-controlled trial to evaluate the effect of statin therapy on triglyceride levels in Mexican hypertriglyceridemic patients. Curr Med Res Opin. 2013;29:379–386. doi: 10.1185/03007995.2013.766590. [DOI] [PubMed] [Google Scholar]
- 18.Adams SP, Sekhon SS, Wright JM. Lipid-lowering eficacy of rosuvastatin. Cochrane Database Syst Rev. 2014;11:CD010254. doi: 10.1002/14651858.CD010254.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams SP, Tsang M, Wright JM. Lipid-lowering eficacy of atorvastatin. Cochrane Database Syst Rev. 2015;3:CD008226. doi: 10.1002/14651858.CD008226.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutemberezi V, Guillemot-Legris O, Muccioli GG. Oxysterols: from cholesterol metabolites to key mediators. Prog Lipid Res. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 22.Maltese WA. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990;4:3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- 23.Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773:1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Harrison KD, Park EJ, Gao N, Kuo A, Rush JS, Waechter CJ, Lehrman MA, Sessa WC. Nogo-B receptor is necessary for cellular dolichol biosynthesis and protein N-glycosylation. EMBO J. 2011;30:2490–2500. doi: 10.1038/emboj.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikegami T, Hyogo H, Honda A, Miyazaki T, Tokushige K, Hashimoto E, Inui K, Matsuzaki Y, Tazuma S. Increased serum liver X receptor ligand oxysterols in patients with non-alcoholic fatty liver disease. J Gastroenterol. 2012;47:1257–1266. doi: 10.1007/s00535-012-0585-0. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkhem-Bergman L, Nylen H, Eriksson M, Parini P, Diczfalusy U. Effect of statin treatment on plasma 4β-hydroxycholesterol concentrations. Basic Clin Pharmacol Toxicol. 2016;118:499–502. doi: 10.1111/bcpt.12537. [DOI] [PubMed] [Google Scholar]
- 27.Ferderbar S, Pereira EC, Apolinario E, Bertolami MC, Faludi A, Monte O, Calliari LE, Sales JE, Gagliardi AR, Xavier HT, Abdalla DS. Cholesterol oxides as biomarkers of oxidative stress in type 1 and type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:35–42. doi: 10.1002/dmrr.645. [DOI] [PubMed] [Google Scholar]
- 28.Vega GL, Weiner MF, Lipton AM, Von Bergmann K, Lutjohann D, Moore C, Svetlik D. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch Neurol. 2003;60:510–515. doi: 10.1001/archneur.60.4.510. [DOI] [PubMed] [Google Scholar]
- 29.Wong J, Quinn CM, Brown AJ. Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. Arterioscler Thromb Vasc Biol. 2004;24:2365–2371. doi: 10.1161/01.ATV.0000148707.93054.7d. [DOI] [PubMed] [Google Scholar]
- 30.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279:52772–52780. doi: 10.1074/jbc.M410302200. [DOI] [PubMed] [Google Scholar]
- 31.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, Casciola-Rosen LA. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Duan Y, Yang X, Sun L, Liu M, Wang Q, Ma X, Zhang W, Li X, Hu W, Miao RQ, Xiang, Hajjar DP, Han J. Inhibition of ERK1/2 and activation of LXR synergistically reduce atherosclerotic lesions in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2015;35:948–959. doi: 10.1161/ATVBAHA.114.305116. [DOI] [PubMed] [Google Scholar]
- 33.Wu HT, Lu FH, Ou HY, Su YC, Hung HC, Wu JS, Yang YC, Wu CL, Chang CJ. The role of hepassocin in the development of non-alcoholic fatty liver disease. J Hepatol. 2013;59:1065–1072. doi: 10.1016/j.jhep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, Wu H. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci U S A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Dominguez E, Gisbert JP, Moreno-Monteagudo JA, Garcia-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643–1647. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 36.Nseir W, Mograbi J, Ghali M. Lipid-lowering agents in nonalcoholic fatty liver disease and steatohepatitis: human studies. Dig Dis Sci. 2012;57:1773–1781. doi: 10.1007/s10620-012-2118-3. [DOI] [PubMed] [Google Scholar]
- 37.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP, Group GSC. Safety and eficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 38.Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, Gwon HC, Bae JH, Hong BK, Choi DH, Han KR. Evaluation of short-term safety and eficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage) J Clin Lipidol. 2012;6:340–351. doi: 10.1016/j.jacl.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Onofrei MD, Butler KL, Fuke DC, Miller HB. Safety of statin therapy in patients with preexisting liver disease. Pharmacotherapy. 2008;28:522–529. doi: 10.1592/phco.28.4.522. [DOI] [PubMed] [Google Scholar]
- 40.Okada Y, Yamaguchi K, Nakajima T, Jo T, Nishikawa M, Mitsumoto Y, Kimura H, Nishimura T, Tochiki N, Yasui K, Mitsuyoshi H, Minami M, Kagawa K, Okanoue T, Itoh Y. Rosuvastatin ameliorates high-fat and high-cholesterol diet-induced nonalcoholic steatohepatitis in rats. Liver Int. 2013;33:301–311. doi: 10.1111/liv.12033. [DOI] [PubMed] [Google Scholar]
- 41.Van Rooyen DM, Gan LT, Yeh MM, Haigh WG, Larter CZ, Ioannou G, Teoh NC, Farrell GC. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol. 2013;59:144–152. doi: 10.1016/j.jhep.2013.02.024. [DOI] [PubMed] [Google Scholar]